Abstract
Background
To prevent the invasion and transmission of SARS-CoV-2, mRNA-based vaccines, non-replicating viral vector vaccines, and inactivated vaccines have been developed. The European Medicines Agency (EMA) authorized the use of the anti-SARS-CoV-2 vaccine in January 2021, the date on which the vaccination program began in Spain and across Europe. The aim of this study is to monitor the safety of anti-SARS-CoV-2 vaccines and report any cases of undesirable effects that have occurred, that are not included in the health profile of mRNA-based vaccines for commercialisation in humans. Furthermore, a brief review is given of the mechanism of action of the anti-SARS-CoV-2 vaccine on the host's immune system in triggering the reactivation of the herpes varicella-zoster infection.
Methods
Follow-up of patients under the care of the southern health district of Seville of the SAS (Andalusian Health Service) during the Spanish state of alarm over the COVID-19 pandemic.
Results
Two patients, a 79-year-old man and a 56-year-old woman, are reported who, after 4 and 16 days respectively of receiving the Pfizer-BNT162b2 vaccine against SARS-CoV-2, presented a state of reactivation of herpes varicella-zoster virus (VZV).
Discussion
The immunosenescence of the reported patients, together with the immunomodulation generated by administering the anti-SARS-CoV-2 vaccines, that depress certain cell subpopulations, could explain the awakening of VZV latency.
Keywords: The anti-SARS-CoV-2 vaccines, mRNA-based vaccines, Herpes zoster infection, Immune system
Abbreviations: APC, Professional antigen presenting cells. COVID-19; Coronavirus Disease 2019. HLA, Human leukocyte antigens. IACE; Angiotensin-Converting Enzyme Inhibitor. RT-PCR, Reverse transcriptase polymerase chain reaction. S protein: Transmembrane spike glycoprotein. SARS-CoV-2; Severe Acute Respiratory Syndrome Coronavirus. VZV, Herpes virus varicella zoster
Graphical abstract
Highlights
-
•
Two cases of possible reactivation of latent viral infections of the Herpesviridae family after Pfizer vaccination against SARS-CoV-2.
-
•
It is important to be alert to the undesirable effects of anti-SARS-CoV-2 vaccines since they are vaccines for which there are no references.
-
•
Inactive vaccines against SARS-CoV-2 immunomodulate the host's immune system by activating some compartments and inhibiting others.
-
•
The immunosenescence of the host's immune system would explain the different immune response to the inactive vaccine against SARS-CoV-2.
1. Introduction
Since the declaration of a pandemic of the SARS-CoV2 virus, by the World Health Organization on March 11, 2020, the international scientific community has dedicated a vast amount of human and financial resources to the achievement of a vaccine as, an instrument capable of preventing, both the incidence of COVID-19 cases and the number of deaths. The results have led to 4 vaccines approved by the European Medicines Agency EMA for use in Europe, these are the vaccines of: Moderna (mRNA-1273 vaccine), Pfizer-BioNTech (mRNA vaccine BNT162b2), AstraZeneca (vector vaccine) and Johnson & Johnson (vector vaccine). The Johnson & Johnson vaccine requires only one dose, while all the others require two doses. Never in the history of humankind, have such rapid achievements been made, this is a true example of scientific innovation and collaboration between the public and private sectors around the world. Developing a vaccine takes between 10 and 15 years on average, but the anti-SARS-CoV-2 vaccines have been developed in a mere a year and a half. All vaccines approved by the EMA have passed the clinical trials and quality controls established for their use in humans. However, like any other vaccine or drug, they are not exempt or free from presenting undesirable or adverse effects (Walter et al., 1999; Salmon et al., 2015). It is therefore, necessary to closely monitor these vaccines. Hitherto, the most common undesirable effects described after the administration of these vaccines have been: wheals and soreness at the vaccine injection site; fever and chills; general malady with muscle and joint fatigue; headache; digestive symptoms such as nausea, vomiting and/or diarrhoea (WHO 2019). However, clinical states of thrombosis, serious anaphylaxis-type allergic reactions, other severe adverse events, and unknown long-term effects of the vaccinations have also been reported, which have caused a number of the population to be reluctant to be vaccinated (Rzymski et al., 2021; Kerr et al., 2021).
Herpes viruses (Herpesviridae family) are among the microorganisms that cause the most infections in the world population, ranking third behind the influenza virus and rhinovirus (‘flu and common cold viruses) (Cantan et al., 2019). Since the COVID-19 pandemic began, many articles have pointed to an increase in the incidence of these herpes virus infections compared to previous years without a pandemic (Verdoni et al., 2020; Dursun and Temiz, 2020). These cases are probably due to a reactivation of these viruses triggered by the invasion of SARS-CoV-2 itself and its role in depressing some branches of the host's immune system and/or due to the psychological stress generated by the pandemic period. The bibliography of this pandemic includes cases of herpes virus infections before (Elsaie et al., 2020; Maldonado et al., 2021), during (Tartari et al., 2020; Ferreira et al., 2020), and after (Brambilla et al., 2020) suffering from the SARS-CoV-2 infection.
Chickenpox is an infectious disease that usually occurs, for the first time, in childhood due to the herpes varicella-zoster virus (VZV). After recovery from the infection, the virus does not disappear, but remains quartered in the neuronal ganglia of different nerve roots as a silent resident for life, hiding inside human neurons and other cells. From this hiding place, the VZV can be reactivated at any time, and can affect different dermatomes, thereby giving a state of infection with the formation of very itchy and sometimes very painful erythematous vesicles (Madavaraju et al., 2020). The causes for the reactivation of this virus remain unknown, but there are certain risk factors that can favour them, such as: advanced age, insomnia, heat stroke, stress, taking immunosuppressive drugs, and debilitating therapies (chemotherapy and radiotherapy). Neutralising antibodies, effector CD4 and CD8 T lymphocytes and memory T cells are responsible for humoral and cellular protection against VZV, and all the aforementioned factors, contribute towards a decrease in the host's defences that affects both the innate, and the adaptive immune systems (Weinberg and Levin, 2010).
In this article, two patients are reported who developed a clinical situation consistent with the diagnosis of herpes zoster virus infection after having received the Pfizer anti-SARS-CoV-2 vaccine. We have reviewed the literature, on other reported cases, of herpes virus reactivation after the administration of vaccines against SARS-CoV-2, which speculate on the mechanism of action of the vaccine on the host's immune system, in that it alters the system and favour the reactivation of VZV. It is hoped that this article of reported cases with the literature review will trigger studies that improve the health profile of mRNA-based vaccines.
2. Material and methods
These involve the follow-up of patients under the care of the southern health district of Seville of the SAS (Andalusian Health Service) during the Spanish state of alarm over the COVID-19 pandemic.
2.1. Presentation of case 1: herpes zoster infection after having received the anti-SARS-CoV-2 vaccine
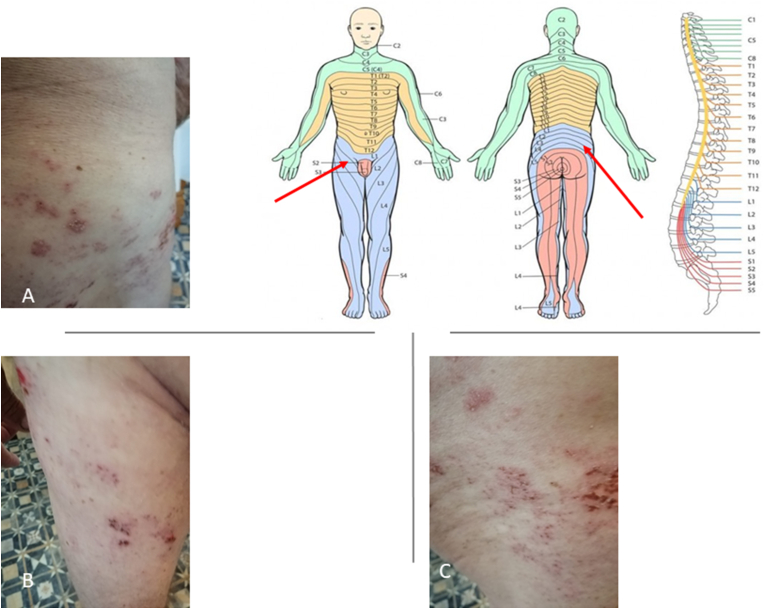
A 79-year-old man was notified by the Health Services of his Autonomous Community (Andalusia-Spain) to receive his first dose of the Pfizer-BNT162b2 anti-SARS-CoV-2 vaccine on April 1, 2021. Until then, the patient had been in good health with an active life that included gardening as a hobby. Regarding his personal medical history, he reported having hypercholesterolemia, which was well controlled with 20 mg/day of sinvastatin; hyperuricemia, treated with 100 mg/day of allopurinol; hypertension, treated with 50/12,5 mg day of losartan/hydrochlorothiazide. The patient also specified that a pacemaker had been implanted two years previously to which he was very well adapted. According to the agreed date, the patient received his first dose of the anti-SARS-CoV-2 vaccine, and four days later, he went to his Health Centre for presenting elevated erythematous lesions with vesicles on his right-hand-side lumbar area that quickly spread to his lower back, hip, groin, and right-hand-side front and inner thigh (Fig. 1) corresponding to L1, L2 and L3 dermatomes. The area around these blisters was warm and tender to the touch with slight pain. The dermatological lesions were not accompanied by any other noteworthy symptoms. The diagnosis was herpes zoster infection and treatment was initiated with: 800 mg/day of acliclovir taken orally for one week; 50 mg of acyclovir applied topically on the vesicles; and non-steroidal anti-inflammatory drugs to reduce pain and inflammation. On questioning, he emphasised that he had not had a fever before, that he was not a drug addict, that he drank no alcoholic beverages, that to the best of his knowledge, he had not been exposed to hepatotoxins, or immunosuppressive drugs, or chemotherapy, and that he had not travelled. The evolution of the patient, with the administered treatment, was good with improvement in the dermatological lesions and general clinical state. On April 22, 2021, the patient received his second dose of the Pfizer-BNT162b2 anti-SARS-CoV-2 vaccine without symptoms.
Fig. 1.
Tthe macroscopic examination showed some scabby and erythematous lesions with vesicles on the right-hand-side lumbar area and gluteus. Images B and C) show lesions on the anterior part of the right thing corresponding to the L1-L3 dermatomes.
2.2. Presentation of case 2: herpes zoster virus after having received the anti-SARS-CoV-2 vaccine
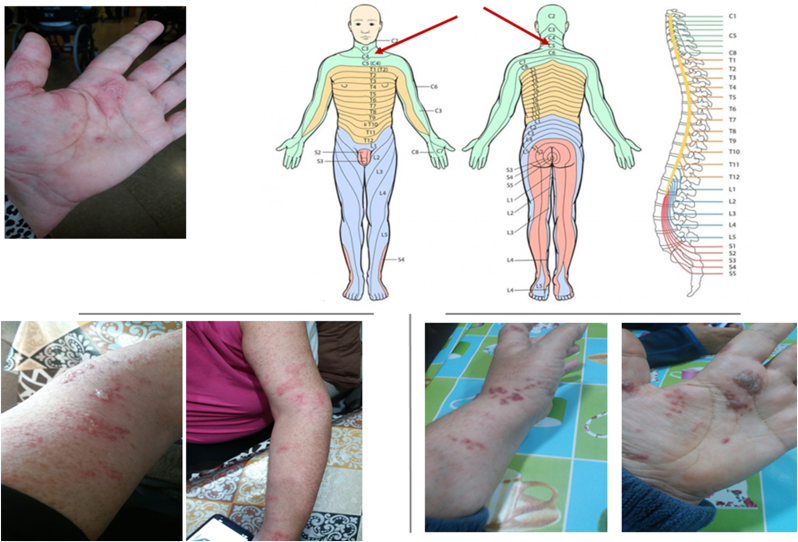
Our second case is of a 56-year-old Spanish woman diagnosed with COVID-19 by RT-PCR on October 29, 2020 with a symptomatic state of general malady, fever, and generalised muscle and joint pain. She was treated with symptomatic treatment at home and in isolation for the whole month of November. The patient was discharged and went back to work. Her personal medical history reports that she is hypertensive, a condition well controlled with enalapril [(IACE) Angiotensin-Converting Enzyme Inhibitor]. She received her first dose of the Pfizer-BNT162b2 vaccine on January 28, 2021, with no mention of reactions. On February 18, 2021, she received her second dose of the Pfizer vaccine, and on March 6, 2021, a state of fever began, with haemorrhagic vesicles upon an erythematous base spreading on her arm, hand, and left side of her chest, with chest pain, and pain in her arm on the same side, that forced her to be taken in the hospital emergency room. The patient took sick leave from work and was diagnosed with herpes zoster infection. After recovering from herpetic dermatological lesions, she suffered, during the following 5 months, postherpetic neuralgia (Fig. 2) treated with 400 mg/8 h of gabapentin and 25 mg/12 h of a vitamin B complex administered orally.
Fig. 2.
Dermatological photographs showed some crusted and aggregated vesicles upon an erythematous base spreading over an area corresponding to the C3–C5 dermatomes on the arm, hand and left chest.
3. Discussion
In recent months, due to the massive administration of anti-SARS-CoV-2 vaccines to the population, a series of adverse effects have been observed that were not initially described in the safety data sheet of these vaccines or had not been detected in previous clinical trials for its commercialisation in humans. For example, the AstraZeneca vaccine can cause thrombosis in certain people. Fortunately, it is a highly unusual reaction and the benefits of the vaccine far outweigh the risks involved (Ledford, 2021). In relation to the Pfizer-BioNTech vaccine, cases have been described of myocarditis in vaccinated patients especially with the second dose (Ammariti et al., 2021). It is difficult to establish a link between the vaccine and these conditions of cardiac inflammation, which in turn can be caused by different viruses and subsequently may disappear without complications.
One of the most common causes of reluctance, of the general population, and even of healthcare personnel, to having the anti-SARS-CoV-2 vaccine was the adverse effects that these could generate. Anti-SARS-CoV-2 vaccine refusal has been triggered by outbreaks of reactivation of the herpes virus family (Beekmann et al., 2021). As with other undesirable effects of vaccines, it is difficult to establish the link between the administration of the vaccine and the appearance of these infectious outbreaks due to herpes viruses (Walter et al., 1999). Hence, authors such as Bostan and Yalici-Armagan, 2021 from Turkey, question whether the appearance of the herpes zoster virus following inactivated anti-SARS-CoV-2 vaccine is a coexistence or whether it is simply a coincidence. These authors reported a clinical case of a 78-year-old man, hypertensive and with vascular problems, who five days after receiving the anti-SARS-CoV-2 vaccine, presented haemorrhagic vesicles occupying an area corresponding to T3-T4 dermatomes, diagnosed as herpes zoster. Furer et al. (2021) from Israel reported 6 cases of female patients with stable rheumatological autoimmune diseases, who showed clinical states of VZV reactivation after vaccination against COVID-19 (BNT162b2 mRNA vaccine). Five of these women, presented symptoms after the first vaccine dose and one woman after the second dose. All cases evolved favourably and received their full vaccination. Psichogiou et al. (2021) from Greece, describe 7 cases of immunocompetent patients over 50 years of age who presented a state of VZV infection in an average of 9 days (range 7–20 days) after having received the Pfizer vaccine against SARS-CoV-2. Tessas and Kluger, 2021 from Finland, provide a new case of ipsilateral herpes zoster virus after the first dose of BNT162b2 mRNA anti-SARS-CoV-2 vaccine in a 44-year-old male patient that affected C5–C6 dermatomes. An increasing number of clinicians, from different parts of the world, are reporting cases of herpes virus infections after the administration of the anti-SARS-CoV-2 vaccine. For this reason, the possibility should be considered that this may constitute, one more undesirable effect of the vaccine, and anti-herpes treatment should be made available (acyclovir and anti-inflammatories) as soon as possible, within 24–48 h, for its greater effectiveness.
The mechanism of action by which mRNA-based vaccines act consists of the administration of a transcript mRNA, wrapped in a lipid capsule, that encodes one or more immunogens [spike glycoprotein (S- protein)] in the cytoplasm of the host cell. This is where translation generates immunogenic proteins of SARS-CoV-2 itself, which are subsequently phagocytosed and processed by professional antigen presenting cells (APC) and are presented, together with HLA-II or HLA-I molecules to Th or Tc lymphocytes respectively, to activate and produce the corresponding cellular and humoral defence (Kowalzik et al., 2021).
We provide two clinical cases that presented dermatological lesions, days after receiving the vaccines. In the first of our cases (Fig. 1), it seems that the determinant of VZV reactivation was the administration of the first dose of the Pfizer mRNA-based vaccine, which stimulated the host's immune system, thereby awakening cellular and humoral immunity and leaving protection memory. Although we cannot fully confirm the association between the vaccine and the reactivation of VZV in our patient in case 1, it is known that, until the administration of the vaccine, the patient had been in good health, which suggests a causal link.
In the second of our clinical cases (Fig. 2), there are other factors, in addition to the administration of the 2nd dose of the Pfizer mRNA-based vaccine that could explain the reactivation of VZV. For example, the patient suffered from a SARS-CoV-2 infection in October 2020 with two positive RT-PCR at different times and symptoms that, without being serious, kept the patient confined to her home for the entire month of November under symptomatic treatment. Therefore, although the herpes zoster virus appeared after the second dose of the vaccine, the initial infection by SARS-CoV-2 could have caused an altered immune system in the patient. Indeed, cases of herpes virus reactivation in the post-COVID-19 period have already been described in the literature (Brambilla et al., 2020).
The exact mechanism by which our patients' immune systems are depressed, after vaccination, remains difficult to understand. The mRNA vaccines against the SARS-CoV-2 virus activate the host's immune system, by mimicking the action of the virus but without generating infection. This supposes an immunomodulation of the host's immune system in which some branches or compartments of immunity are activated, while others are inhibited. Thus, for example, for the humoral immune response, Th2 lymphocytes, B lymphocytes and antibody-releasing plasma cells are activated, but other subpopulations, such as Th1 and Th17 lymphocytes, are inhibited. Furthermore, the reactivation of the herpes virus latency is more frequent in the elderly, although not exclusively. People with an ageing immune system have different characteristics to those of middle adulthood. The elderly therefore tends to present a decrease in naïve lymphocyte production, lymphocyte repertoire diversity, effector cell functionality, lymphocyte proliferation, and post-vaccination antibody titers; concomitantly, there is an increase in populations of differentiated memory cells, lymph node fibrosis, and altered cytokine production. This immunosenescence has been associated with a decreased response to vaccines and an increased susceptibility to viral infections (Crooke et al., 2019). Hence, debates and discrepancies are taking place in relation to the administration of the various vaccines according to the age of the population.
Our work has certain limitations. This potentially causal link between vaccines and the reactivation of the varicella-zoster virus should be tested in larger cohorts of patients who have received the SARS-CoV-2 vaccination. Indeed, it is very difficult to confirm the link of a particular vaccine to an observed undesirable effect. Nevertheless, these types of clinical states should be monitored closely, and vigilance must be maintained since these new vaccines have yet to be tested in long-term that have and therefore no references are available for comparison purposes.
4. Ethics statement
Informed consent was obtained from all subjects involved in the study, according to the specifications established by the Ethics Committee of the University of Seville for the publication of clinical cases.
5. Data availability statements
Data supporting the findings of this study are available at SAS (Andalusian Health Service Spain). Restrictions apply to the availability of these data, which were used under license for this study. The data have been made available, to the authors, with the permission of affected patients.
Authors’ contributions
Maldonado MD conducted the literature review, interpreted the immunological and clinical data of the patients and was the major contributor to the manuscript. Romero-Aibar J participated in the care and analytical follow-up of the patients as a senior laboratory technician and helped in revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by Seville University (Immunology area), Department of Medical Biochemistry, Molecular Biology, and Immunology; Also, by the Ministry of Economy, Industry and Competitiveness (MINECO 2017), Reference: BFU2017-85832-R.
References
- Ammariti E., Cavalotti C., Milazzo A., Pedrotti P., Soriano F. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. IJC Heart & Vasculature. 2021;34:100774. doi: 10.1016/j.ijcha.2021.100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekmann S.E., Babcock H.M., Rasnake M.S., Talbot T.R., Polgreen P.M. COVID-19 vaccination preparedness policies in U.S. Hospitals. Infect. Control Hosp. Epidemiol. 2021:1–13. doi: 10.1017/ice.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan E., Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J. Cosmet. Dermatol. 2021:1–2. doi: 10.1111/jocd.14035. 00. [DOI] [PubMed] [Google Scholar]
- Brambilla L., Maronese A.C., Tourlaki A., Veraldi S. Herpes zoster following COVID-19: a report of three cases. EJD. 2020;6:754–756. doi: 10.1684/ejd.2020.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantan B., Luyt ChE., Martin-Loeches I. Influenza infections and emergent viral infections in intensive care unit. Semin. Respir. Crit. Care Med. 2019;40:488–497. doi: 10.1055/s-0039-1693497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S.N., Ovsyannikova I.G., Poland G.A., Kennedy R.B. Immunosenescence and human vaccine immune responses. Immun. Ageing. 2019;16:25. doi: 10.1186/s12979-019-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursun R., Temiz S.A. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaie M., Youssef E.A., Nada H.A. Herpes zoster might be an indicator for latent COVID 19 infections. Dermatol. Ther. 2020;33 doi: 10.1111/dth.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F., Romao T.T., Macedo Y.S., Pupe C., Nascimento O.J. COVID-19 and herpes zoster co-infection presenting with trigeminal neuropathy. Eur. J. Neurol. 2020;27:1748–1750. doi: 10.1111/ene.14361. 24:10.1111/ene.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furer V., Zisman D., Kibari A., Rimar D., Paran Y., Elkayam O. Herpes zoster following BNT162b2 mRNA vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021 doi: 10.1093/rheumatology/keab345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J.R., Freeman A.L., Marteau T.M., Linden S. Effect of information about COVID-19 vaccine effectiveness and side effects on behavioural intentions: two online experiments. 2021. Vaccines. 9:379. [DOI] [PMC free article] [PubMed]
- Kowalzik F., Schreiner D., Jensen Ch, Teschner D., Gehring S., Zepp F. mRNA-Based Vaccines. Vaccines. 2021;390(9) doi: 10.3390/vaccines9040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford H. Researchers are studying possible links between rare clots and the Oxford-AstraZeneca COVID-19 vaccine. Nature. 2021;592:334–335. [Google Scholar]
- Madavaraju K., Koganti R., Volety I., Yadavalli T., Shukla D. Herpes simplex virus cell entry mechanisms: an update. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2020.617578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado M.D., Romero-Aibar J., Pérez-San-Gregorio M. COVID-19 pandemic as a risk factor for the reactivation of herpesviruses. Epidemiol. Infect. 2021;149(e145):1–5. doi: 10.1017/S0950268821001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psichogiou M., Samarkos M., Mikos N., Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS-CoV-2. Vaccines. 2021;9:572. doi: 10.3390/vaccines9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski P., Zeyland J., Poniedziałek B., Małecka I., Wysocki J. The perception and attitudes toward COVID-19 vaccines: a cross-sectional study in Poland. Vaccines. 2021;9:382. doi: 10.3390/vaccines9040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D.A., Dudley M.Z., Glanz J.M., Omer S.B. Vaccine hesitancy. Causes, consequences, and a call to action. Am. J. Prev. Med. 2015;49:S391–S398. doi: 10.1016/j.amepre.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Tartari F., Spadotto A., Zengarini C., Zanoni R., Guglielmo A., Adorno A., Valzania C., Pileri A. Herpes zoster in COVID-19-positive patients. Int. J. Dermatol. 2020;59:1028–1029. doi: 10.1111/ijd.15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessas I., Kluger N. Ipsilateral Herpes Zoster after the first dose of BNT162b2 mRNA COVID-19 vaccine. JEADV. 2021 doi: 10.1111/jdv.17422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R., Hartmann K., Fleisch F., Reinhart W.H., Kuhn M. Reactivation of herpesvirus infections after vaccinations. Lancet. 1999;353:810. doi: 10.1016/S0140-6736(99)00623-6. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Levin M.J. VZV T cell-mediated immunity. Curr. Top. Microbiol. Immunol. 2010;342:341–357. doi: 10.1007/82_2010_31. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Coronavirus disease (COVID-19) pandemic. Vaccines. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings of this study are available at SAS (Andalusian Health Service Spain). Restrictions apply to the availability of these data, which were used under license for this study. The data have been made available, to the authors, with the permission of affected patients.