Abstract
Background:
Normothermic ex vivo liver perfusion (NEVLP) is a novel system for organ preservation which may improve over static cold storage (CS) clinically and offers the chance for graft modification prior to transplantation. Although recent studies have shown the presence of inflammatory molecules during perfusion, none have yet shown the effects of NEVLP on liver-resident immune cell activation. We investigated the effects of NEVLP on liver-resident immune cell activation and assessed the ability of anti-inflammatory cytokines IL-10 and TGF-β to improve organ function and reduce immune activation during perfusion.
Methods:
Rat livers were perfused for 4 hours at 37°C with or without the addition of 20ng/mL each IL-10 and TGF-β (n=7). Naïve and cold storage (4 hours at 4°C) livers served as controls (n=4). Following preservation, gene expression profiles were assessed through single cell RNA sequencing, dendritic cell and macrophage activation was measured by flow cytometry, and cytokine production was assessed by ELISA.
Results:
NEVLP induced a global inflammatory gene expression signature, most notably in liver-resident macrophages and dendritic cells, which was accompanied by an increase in cell-surface levels of MHC II, CD40, and CD86. Immune activation was partially ameliorated by IL-10 and TGF-β treatment, but no changes were observed in inflammatory cytokine production. Overall levels of liver damage and cellular apoptosis from perfusion were low, and liver function was improved with IL-10 and TGF-β treatment.
Conclusions:
This is the first study to demonstrate that liver-resident immune cells gain an activated phenotype during NEVLP on both the gene and protein level and that this activation can be reduced through therapeutic intervention with IL-10 and TGF-β.
Keywords: Dendritic cell, Macrophage, Organ preservation, Allograft tolerance, Transplantation
Introduction
Normothermic ex vivo liver perfusion (NEVLP) provides an exciting system for improved organ preservation. An improved organ preservation method has been necessitated by the increasing use of marginal organs which poorly tolerate static cold storage (CS) and are associated with inferior clinical outcomes.(1, 2) Unlike CS, preservation at physiological temperature maintains metabolic processes which can prevent the accumulation of toxic metabolites(3), allows assessment of graft condition prior to transplantation, and makes possible the delivery of therapeutic medications to the donor liver prior to transplantation. Previous investigations have shown that machine perfusion improves clinical outcomes for marginal organs,(4-6) and a large prospective trial has already demonstrated the efficacy of NEVLP clinically.(7)
Despite the preliminary clinical successes of NEVLP, many recent investigations have demonstrated the inherent inflammatory nature of machine perfusion. During perfusion, inflammatory mediators accumulate, including damage associated molecular patterns(8, 9) and inflammatory cytokines.(8, 10) Several groups have tested novel therapeutic interventions to counter-act inflammatory processes during perfusion, including treatment with stem cells(11) or stem cell-derived extracellular vesicles,(12) active gaseous components,(10) and inhibitory RNA sequences.(13, 14) However, these prior studies have not investigated the effects of NEVLP on liver-resident immune cells or tested anti-inflammatory cytokines as a novel therapeutic intervention during NEVLP.
Anti-inflammatory cytokines interleukin (IL)-10 and transforming growth factor (TGF)-β are known to generate a tolerogenic immune environment.(15, 16) IL-10 has been previously demonstrated to generate immature, regulatory phenotype dendritic cells (DCs) with a reduced capacity to activate allogeneic T-cells, and TGF-β is known to inhibit DC maturation.(17, 18) Tolerogenic DCs(19) and TGF-β(20, 21) play a role in the induction of regulatory T-cells which are known to inhibit effector T-cell activity. The generation of tolerogenic immune cells within the organ itself may serve as a novel therapeutic option to dampen the inflammatory environment, decreasing organ damage and potentially allograft dysfunction following transplantation.
In this study, we performed NEVLP of the Lewis rat liver and observed activation of liver-resident immune cells. We then hypothesized that treatment with anti-inflammatory cytokines IL-10 and TGF-β would polarize liver-resident immune cells to a tolerogenic phenotype and reduce inflammatory signaling. This approach provides a novel system to test immunomodulatory agents which could generate a tolerogenic environment in the donor liver prior to transplantation.
Materials and Methods
Animals
Male Lewis rats (Charles River Laboratories, Wilmington, MA) aged 8-12 weeks weighing 331g ± 16 (mean ± standard error of the mean [SEM]) were used in all experiments. Animals were housed in specific pathogen-free conditions in animal care facilities at the University of Wisconsin (UW)-Madison in accordance with institutional guidelines. The study protocol was approved by the Institutional Animal Care and Use Committee at the UW-Madison, and all animals were treated ethically.
Experiment Design
Rats were randomly assigned to one of four treatment groups: naïve (n=4), CS (n=4), NEVLP (n=7), and NEVLP with anti-inflammatory cytokines (NEVLP-Cyt, n=7) (Fig. 1). Naïve livers were collected without any storage period or intervention. CS livers were flushed with NaCl saline solution (Baxter, Deerfield, IL) then stored in phosphate buffered saline (PBS, Mediatech, Inc., Manassas, VA) on ice for 4 hours. NEVLP livers were flushed with NaCl saline solution and machine perfused at 37°C for 4 hours. NEVLP-Cyt livers were prepared in a similar fashion to the NEVLP livers, except with the addition of 20ng/mL each rat IL-10 and TGF-β (Sino Biological, Beijing, China) during perfusion. The concentrations of IL-10 and TGF-β were selected based on their in vitro effects (Fig. S1).
Figure 1. Experiment design.
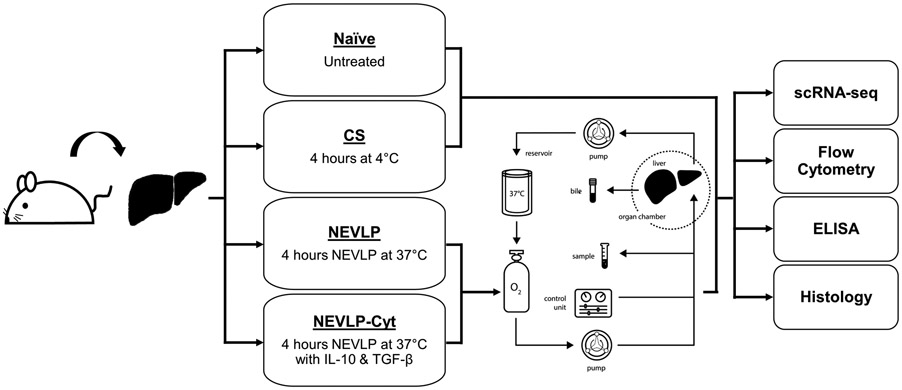
Rat livers were randomly divided into four groups: naïve, CS, NEVLP, or NEVLP-Cyt. NEVLP and NEVLP-Cyt livers were perfused on an oxygenated circuit heated to 37°C with flow rate regulation based on liver mass and pressure monitored throughout. Perfusate samples were collected once hourly for assessment of lactate, liver damage molecule, and cytokine concentrations. Following completion of perfusion, livers were partitioned for histological analysis and collagenase digestion. NPCs were isolated from digested livers for analysis by scRNA-seq and flow cytometry.
Animal Surgery and Liver Procurement
All surgeries were performed under cone mask anesthesia with inhaled 5% isoflurane (Phoenix, St. Joseph, MO) for induction and 2-3% isoflurane for maintenance with 2L/minute oxygen flow. After disinfection with betadine (Purdue Products L.P., Stamford, CT), the abdominal cavity was opened by a midline and transverse incision and the portal vein was exposed by moving the intestine laterally to the left of the abdomen. A 24-gauge angiocatheter (BD, Sandy, Utah) was inserted into the common bile duct and secured with ties. The gastrosplenic and duodenopancreatic branches of the portal vein were isolated and tied off, and the hepatic artery was tied off. Heparin (400U, Fresenius Kabi, Lake Zurich, IL) was injected through the inferior vena cava with a 27-gauge needle and allowed to circulate for 5 minutes. The portal vein was cannulated by insertion of a 1.3mm miniball cannula with basket tip (Harvard Aparatus, Holliston, MA) and immediately flushed with 20mL of NaCl saline. The liver was then explanted, weighed, and transferred to a low-rimmed petri dish on ice for either 4 hours of CS or for the short duration between liver procurement and initiation of perfusion. Livers in the NEVLP and NEVLP-Cyt treatment groups were stored on ice for no longer than 5 minutes prior to connection to the machine perfusion circuit.
Machine Perfusion
Ex vivo machine perfusion of the liver was performed in a recirculating system (Fig. 1) made up of a perfusate reservoir, leukocyte filter, peristaltic pump, oxygenator, heater, bubble trap, and moist chamber (Harvard Apparatus). The perfusate was stored in a jacketed glass reservoir and maintained at a continuous flow rate of 1.8mL/min/g liver by a peristaltic pump. Flow rate and pressure were monitored throughout perfusion (Hugo Sachs Elektronic, Harvard Apparatus). A 45μm polypropylene particle filter (Merck Millipore Ltd., Tullagreen, Carrigtwohill, Ireland) fitted within a leukocyte filter captured circulating inflammatory cells. A tubing oxygenator supplied by 95% O2 and 5% CO2 gas maintained oxygen saturation of the perfusate at >95%, and a tubing heat exchanger supplied by a thermocirculator heating bath (Harvard Apparatus) maintained the perfusate temperature at 37°C. The petri dish containing the cannulated liver was placed in the center of an autoclavable moist chamber, preceded in the circuit by a bubble trap to prevent air embolism. The perfusate entered the portal vein and exited the vena cava. Perfusate outflow from the vena cava was recirculated to the perfusate reservoir, completing the circuit.
The perfusate was made up of 65mL William’s E Media (Quality Biological, Gaithersburg, MD), 3,250U each Penicillin/Streptomycin (Life Technologies Corporation, Grand Island, NY), 0.65mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO), 1.30mM L-glutamine (Sigma-Aldrich), 1% human albumin (Baxter), 500U heparin, 15mg papaverine (American Regent, Inc., Shirley, NY), 1mg insulin (Sigma-Aldrich), 1.25mg hydrocortisone (Pfizer, New York, NY), and 30mL of leukoreduced, packed red blood cells. All perfusion experiments used human red blood cells (American Red Cross, Madison, WI) with the exception of perfusion experiments performed for single cell RNA sequencing (scRNA-seq) which used packed Lewis rat red blood cells.
Liver Digestion
Following organ preservation (or immediately for naïve livers), 100mL of digestion solution I (containing 1X Hank’s balanced salt solution [HBSS, Worthington, Lakewood, NJ], 25mM HEPES [Sigma-Aldrich], 4.2mM NaHCO3 [Sigma-Aldrich], and 83μM EDTA [Sigma-Aldrich]) was pre-warmed to 37°C and perfused through the portal vein of the liver into a waste container to chelate free Ca2+ ions for disruption of cell-cell tight junctions. Subsequently, 100mL of digestion solution II (containing 1X HBSS, 25mM HEPES, 4.2mM NaHCO3, 1.26mM CaCl2 [Sigma-Aldrich], 490μM MgCl2 [Sigma-Aldrich], 406μM MgSO4 [Sigma-Aldrich], and 50mg of Collagenase Type IV [Worthington]) was pre-warmed to 37°C and perfused through the liver for 15 minutes to disrupt the extracellular matrix.
Perfusate Analysis
Blood gas analysis was performed hourly on inflow perfusate samples using an i-STAT point-of-care analyzer (CG4+ cartridges, Abbott Point of Care Inc., Abbott Park, IL), and measurements included lactate, pH, and pO2. Samples were collected hourly from the inflow and outflow ports, spun at >20,000g for 15 minutes, and the cell-free supernatants were stored at −80°C for subsequent analysis.
Transaminase Release
Aspartate transaminase (AST) and alanine transaminase (ALT) levels in the perfusate were measured on an Architect i2000 (Abbott Point of Care Inc.) according to manufacturer protocols.
Histopathology and Immunohistochemistry
Left median lobe liver sections were fixed in 4% paraformaldehyde after storage but prior to collagenase digestion. Fixed tissue was embedded in paraffin. Slides were cut at 4μm thickness and stained with hematoxylin and eosin (H&E) or an anti-activated caspase 3 antibody (Asp175, Cell Signaling Technology, Inc., Danvers, MA). Severity of histologic damage was blindly scored by an experienced liver pathologist (Y.L.) according to the Suzuki criteria.(22) Images for quantification were acquired from 15-20 random fields within each slide at appropriate magnification using an Olympus DP73 equipped microscope (Olympus, Tokyo, Japan), and staining was quantified using ImageJ software (NIH, Bethesda, MD) with color-separation, automatic-threshold, and particle-analysis algorithms.
Immune Cell Isolation
Collagenase-digested livers were crushed and passed through a 100μM cell strainer into 50mL William’s E media containing 2mM L-glutamine, 1mM sodium pyruvate, and 50U/mL each Penicillin/Streptomycin. Hepatocytes were pelleted using low-density centrifugation at 70g for 3 minutes, and non-parenchymal cells (NPCs) in the supernatant were transferred to a fresh tube. Hepatocyte removal was repeated once and the resulting NPC fraction was pelleted at 300g for 5 minutes. Red blood cells were lysed by incubating the NPC fraction with 2mL of ACK buffer (Sigma-Aldrich) for 2 minutes at room temperature then washed out with 1X PBS and centrifugation at 300g for 5 minutes.
A Percoll gradient was prepared following a modified version of the protocol published by Bourgognon et al. for density-based purification of liver-resident immune cells.(23) Briefly, Stock Isotonic Percoll was generated from Percoll (Sigma-Aldrich) and Ca++ Mg++ free 10X PBS (Mediatech, Inc.), diluted to 50% and 25% with 1X PBS, and gently overlayed. Isolated NPCs were resuspended in 10mL RPMI (Life Technologies, Bleiswijk, The Netherlands, containing 10% heat-inactivated fetal bovine serum [Life Technologies, Grand Island, NY], 1mM sodium pyruvate, 50U/mL each Penicillin/Streptomycin, 10mM HEPES) and overlayed onto the prepared Percoll gradient. The gradient was centrifuged at 1100g for 20 minutes at 4°C with slow acceleration and no brake. Purified immune cells were collected at the 25%/50% Percoll interface using a transfer pipet, washed once with 1X PBS to remove remaining Percoll, counted with Trypan Blue, and stained for flow cytometry.
Single cell RNA sequencing
scRNA-seq was performed by the UW-Madison Biotechnology Center Gene Expression Center. The 10X Genomics Chromium Next GEM Single Cell 3’ Library (v3.1, PN-1000121) Single Cell Gene Expression Assay was used to target 6,000 or 10,000 cells per sample with a depth of 70,000 reads per cell according to the manufacturer’s recommendations (10x Genomics, Pleasanton, CA). Cell Ranger (v5.0, 10x Genomics) pipelines were used to align reads and perform clustering which were then imported to Loupe Browser (v5.0.0, 10x Genomics) for analysis of gene expression. Cell cluster identification was performed by filtering on expected gene expression patterns and cross-referencing the identified clusters using the PanglaoDB and CellMarker databases.(24, 25)
Flow Cytometry
Percoll-purified NPCs were incubated for 5 minutes at room temperature with 2μL mouse anti-rat α-CD32 Fc block (BD, San Jose, CA). After Fc block, cells were stained with fluorochrome-labeled monoclonal antibodies (Table S1) for 30 minutes at 4°C. Unbound antibodies were washed away and cells were fixed in Cytofix (BD) then stored for up to 24 hours. Data was acquired with an Attune Nxt instrument (BD) using single color controls generated with UltraComp eBeads (eBioscience, San Diego, CA) for compensation and rainbow calibration particles (Spherotech, Lake Forest, IL) to standardize instrument settings between experiments. Data analysis was performed in FlowJo v10.7.1 using fluorescence minus one (FMO) controls to set gates.
ELISA
The frozen cell-free perfusate supernatants were thawed and briefly centrifuged to remove aggregates. For cytokine analysis by enzyme-linked immunosorbent assay (ELISA), perfusate samples were plated in the Milliplex MAP Rat Cytokine/Chemokine Magnetic Bead Panel (Millipore Sigma, Burlington, MA) containing 7 analytes: Interferon (IFN)-γ, IL-1β, IL-2, IL-4, IL-6, IL-12p70, and Tumor necrosis factor (TNF)-α. For liver damage marker analysis, perfusate samples were plated in the Milliplex MAP Rat Liver Injury Panel – Toxicity Multiplex Assay (Millipore Sigma) containing 5 analytes: liver-type arginase 1 (ARG1), α-glutathione S-transferase (GSTα), sorbitol dehydrogenase (SDH), 5’-nucleotidase (5’-NT), and glutamic-oxaloacetic transaminase 1 (GOT1). GOT1 was omitted from analysis due to standard curve errors. Data was acquired on the Luminex MAGPIX (Austin, TX) and analyzed in the Belysa software package v1.0.19.
Statistical Analysis
All statistical analyses were performed in GraphPad Prism v.8.3.1. One-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test or two-tailed t-tests were used as appropriate unless otherwise indicated. A P value of <0.05 was considered significant.
Results
Surgical procedures showed low variability between groups with an average surgical time of 32 ± 3 minutes from anesthesia induction to liver explant. The perfusion apparatus and a representative image of a perfused rat liver are shown (Fig. 2A-B).
Figure 2. IL-10 and TGF-β treatment improves lactate clearance during machine perfusion.
A: The small animal perfusion apparatus used for all NEVLP experiments. B: A representative image of a rat liver during NEVLP. C: Pressure was monitored throughout perfusion. No significant differences in pressure were found between treatment groups (NEVLP & NEVLP-Cyt n=7). D: Bile was collected throughout perfusion and total volume was assessed at the conclusion of each experiment. Total bile volume was divided by hours of perfusion to determine bile production per hour. No differences were found in bile production between livers (NEVLP & NEVLP-Cyt n=5). E: Perfusate lactate concentration. F: Perfusate lactate normalized to the baseline within each experiment. There was a statistically significant difference in lactate fold change over baseline at 180 and 240 minutes of perfusion between NEVLP and NEVLP-Cyt livers. *P<0.05 by a two-tailed student’s t-test (NEVLP & NEVLP-Cyt n=7). Data are shown as mean ± SEM.
IL-10 and TGF-β improved lactate clearance during perfusion.
Liver perfusion quality and liver function were assessed throughout each experiment (Fig. 2). Oxygen saturation of the perfusate was maintained at >95% throughout the duration of each perfusion (data not shown), and no significant difference was found in pressure between NEVLP and NEVLP-Cyt livers (Fig. 2C).
Bile production and clearance of lactate from the perfusate indicate liver metabolic activity and were measured to assess graft performance.(26) Total bile production was measured at the conclusion of most experiments (Fig. 2D) and only excluded from analysis if the bile duct was not cannulated due to technical error. The majority of livers produced bile during perfusion, and no significant differences were found in bile production per hour between treatment groups. We assessed lactate concentrations in the perfusate hourly throughout perfusion. Due to the differences in baseline lactate concentrations between experiments (Fig. 2E), we normalized each hourly concentration to the baseline within each experiment and grouped these fold changes over baseline for analysis (Fig. 2F). There was a statistically significant difference between the perfusate lactate in the NEVLP and NEVLP-Cyt treatment groups at 180 minutes of perfusion (2.3 ± 0.6 vs. 1.1 ± 0.1) and 240 minutes of perfusion (2.3 ± 0.4 vs. 1.0 ± 0.1), P<0.05. Bile production and lactate clearance demonstrated liver function during perfusion, with IL-10 and TGF-β treatment improving lactate clearance.
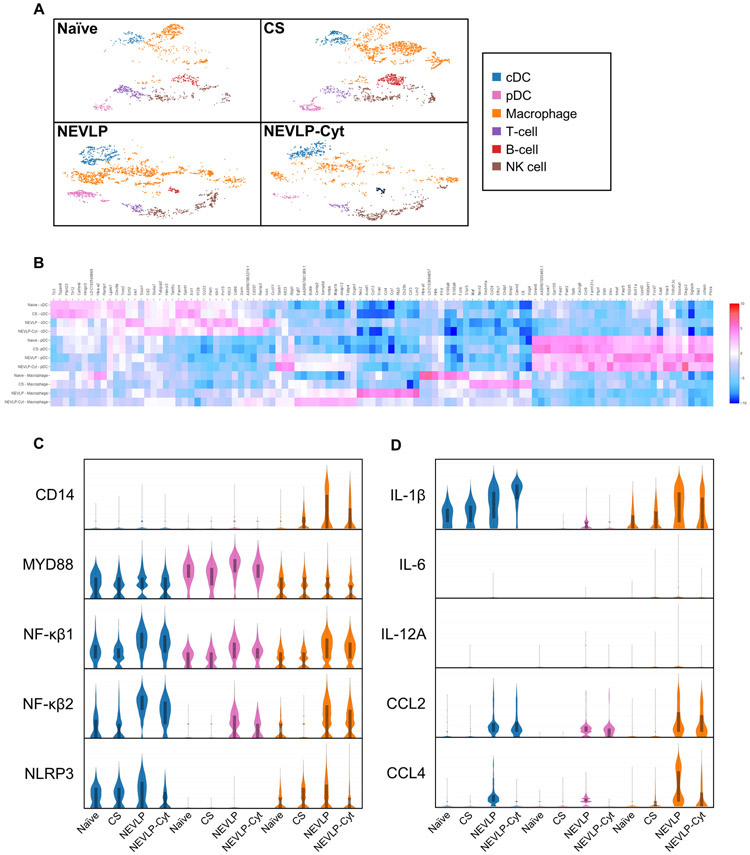
scRNA-seq revealed changes in the inflammatory gene signature following NEVLP
To further investigate the potential mechanisms underlying the observed functional differences in livers between treatment groups (Fig. 2F), we performed a comprehensive analysis of gene expression signatures using scRNA-seq (Fig. 3). Liver NPCs were isolated fresh for the naïve liver or after 4 hours of storage for the CS, NEVLP, and NEVLP-Cyt livers, and gene expression analyses were performed as described. Immune cell clustering was similar between NEVLP and NEVLP-Cyt livers but different from CS and freshly isolated naïve controls (Fig. 3A). To explore the gene expression signatures underlying these differences, we grouped several immune cell subsets for comparison of differences between cell subsets and treatment groups. We focused our gene expression analyses on macrophages, classical DCs (cDC), and plasmacytoid DCs (pDC) due to the relatively high abundance of macrophages among liver-resident immune cells which play a key role in maintaining liver tolerogenicity (27) and the well-described role for DCs in the control of allograft tolerance vs. rejection in the transplantation arena.(28) In this study, macrophages encompassed Kupffer cells and migratory macrophages.
Figure 3. scRNA-seq reveals an inflammatory gene expression signature during NEVLP which is partially reduced through IL-10 and TGF-β treatment.
A: tSNE plots revealed differing clustering patterns for immune cells isolated from naïve and CS vs. NEVLP and NEVLP-Cyt livers. B: A heat map of the top 50 differentially expressed genes for macrophages, cDC, and pDCs between treatment groups. C: Inflammatory genes involved in toll-like receptor signaling and inflammasome formation. D: Cytokines and chemokines upregulated by machine perfusion (naïve, CS, NEVLP, & NEVLP-Cyt n=1).
An inflammatory gene expression signature was observed in macrophages, cDCs, and pDCs isolated from perfused livers (Fig. 3B). Violin plots are shown for select inflammatory mediators of interest (Fig. 3C-D). Genes upregulated by NEVLP included the toll-like receptor-associated proteins CD14, myeloid differentiation primary response 88 (MYD88), nuclear factor kappa B (NF-κβ) 1, and NF-κβ2 as well as the inflammasome protein NLR family pyrin domain containing 3 (NLRP3) (Fig. 3C). Other upregulated inflammatory mediators included cytokines and chemokines such as IL-1β, C-C motif chemokine ligand (CCL) 2, and CCL4 (Fig. 3D). Some inflammatory cytokines, including IL-6 and IL-12A (Fig. 3D) experienced low levels of upregulation from perfusion. The differences in the gene expression signatures between treatment groups indicated that NEVLP induced transcription of pro-inflammatory mediators which was in part ameliorated by treatment with IL-10 and TGF-β.
IL-10 and TGF-β treatment reduced cell-surface macrophage and DC activation marker levels
The inflammatory gene expression signature findings were followed by a multi-color flow cytometric analysis of liver-resident macrophages and DCs to determine cell-surface expression levels of immune activation markers. Liver NPCs were isolated and stained as described freshly for naïve livers and after 4 hours of CS or perfusion. Following debris, doublet, dead cell, and lymphocyte exclusion, macrophages and DCs were gated on by selecting the CD11bc+/CD103− and CD11bc+/CD103+ populations, respectively (Fig. S2). DCs encompassed both cDC and pDC subsets in this analysis.
The median fluorescence intensity (MFI) of MHC II, CD40, CD86, and PD-L1 was determined for liver-resident macrophages (Fig. 4A) and DCs (Fig. 4B). There was a significant increase in liver-resident macrophage expression of MHC II in NEVLP livers (25,841 ± 7,525) in comparison to naïve (3942 ± 1660), CS (3831 ± 2104), and NEVLP-Cyt livers (7903 ± 2182), P<0.05. In DCs, there was a significant difference in MHC II expression between the naïve (36351 ± 2439) and CS (42728 ± 4723) livers in comparison to NEVLP (164,616 ± 10,229) and NEVLP-Cyt (133,587 ± 2,540) livers, P<0.0001 as well as a difference between NEVLP and NEVLP-Cyt livers, P<0.05. There was also a significant difference in CD40 expression between the CS (319 ± 145) and NEVLP (2163 ± 390) livers as well as a difference in CD86 expression between the CS (1274 ± 273) and perfused livers, NEVLP (2496 ± 413) and NEVLP-Cyt (3808 ± 398), P<0.001.
Figure 4. NEVLP upregulates expression of macrophage and DC activation markers which is partially ameliorated through IL-10 and TGF-β treatment.
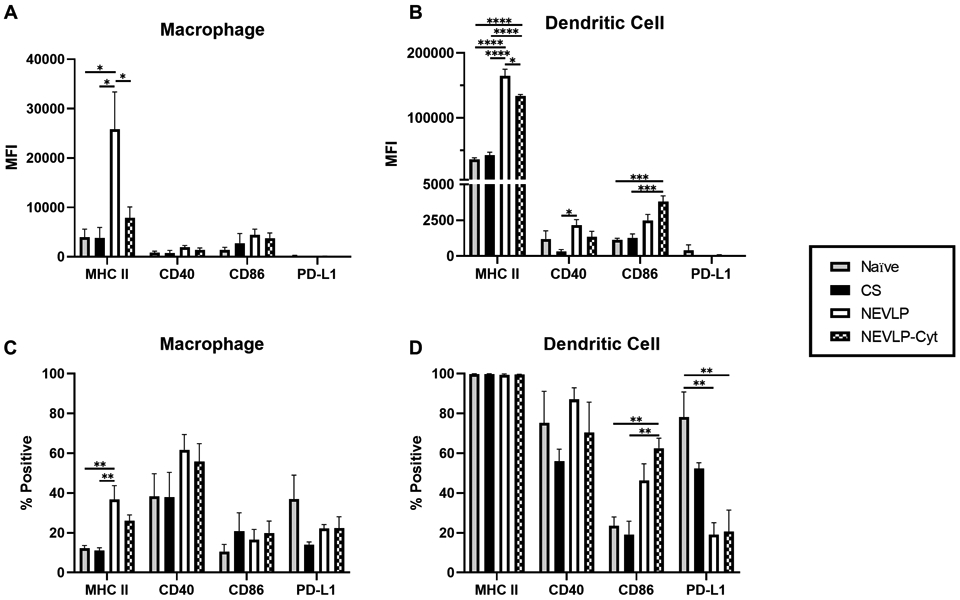
A-B: Liver NPCs were isolated and stained for multi-color flow cytometry to assess immune activation marker expression levels. Median fluorescence intensity (MFI) of MHC II, CD40, CD86, and PD-L1 on liver-resident macrophages (A) and DCs (B) was assessed. C-D: The percentage of macrophages and DCs positive for each marker evaluated using FMO controls to set positive gates (naïve n=4, CS n=4, NEVLP & NEVLP-Cyt n=5). *P<0.05, **P<0.01, ***P<0.001, & ****P<0.0001 by one-way ANOVA followed by Tukey’s multiple comparisons test. Data are shown as mean ± SEM.
Positive gates were set using FMO controls to determine the percentage of macrophages and DCs expressing each marker analyzed (Fig. 4C-D). There was a significant difference in the percentage of macrophages expressing MHC II between naïve (12 ± 1) and CS (11 ± 1) in comparison to NEVLP (37 ± 7) livers, P<0.01 (Fig. 4C). There were also significant differences in the percentage of DCs expressing CD86 and PD-L1 between treatment groups (Fig. 4D). There was a significant increase in the percentage of DCs expressing CD86 between naïve (24 ± 4) and CS (19 ± 7) in comparison to NEVLP-Cyt (63 ± 5) livers, P<0.01, and a significant decrease in the percentage of DCs expressing PD-L1 in comparison of naïve (78 ± 13) to NEVLP (19 ± 6) and NEVLP-Cyt (21 ± 11) livers, P<0.01. These differences indicated that NEVLP induced higher levels of cell-surface macrophage and DC activation markers which were reduced by treatment with IL-10 and TGF-β.
Cytokine production during perfusion was not altered by IL-10 and TGF-β treatment
Based on our findings that IL-10 and TGF-β treatment during perfusion reduced expression of inflammatory genes and proteins, we postulated that anti-inflammatory cytokine treatment may decrease inflammatory cytokine secretion during perfusion. We assessed cytokine secretion with multiplex ELISA for NEVLP and NEVLP-Cyt livers using cell-free outflow perfusate supernatants from 0, 60, and 240 minutes of perfusion (Fig 5). Although inflammatory cytokine concentration in the perfusate trended higher for NEVLP than NEVLP-Cyt livers by the end of perfusion for some cytokines, most notably for IL-1β (5.9 ± 2.8 vs. 0.9 ± 0.4 pg/mL/g liver), P=0.12, and IL-6 (1,199.0 ± 417.9 vs. 774.1 ± 134.3 pg/mL/g liver), P=0.14, these differences were small and did not reach significance. Thus, IL-10 and TGF-β treatment during perfusion did not influence inflammatory cytokine concentrations in the perfusate.
Figure 5. No differences were found in cytokine production between treatment groups.

Cell-free perfusate supernatants were thawed, and cytokine concentrations were assessed by multiplex ELISA. Data was normalized to liver mass within each experiment (NEVLP & NEVLP-Cyt n=4). Data are shown as mean ± SEM.
IL-10 and TGF-β treatment does not significantly reduce liver damage molecule production during perfusion
To evaluate liver damage caused by perfusion, we analyzed perfusate samples to determine levels of liver damage molecules. Although the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) trended higher in NEVLP compared to NEVLP-Cyt livers by 240 minutes of perfusion (AST: 26.5 ± 6.2 vs. 16.9 ± 3.6 U/L/g liver, P=0.22; ALT: 17.2 ± 5.1 vs. 12.6 ± 3.1 U/L/g liver, P=0.46), these trends were not significant (Fig. 6A-B). A multiplex analysis of ARG1, GSTα, SDH, and 5’-NT concentrations in the perfusate also did not reveal any significant differences between groups (Fig. 6C-F) although there was a trend toward higher 5’-NT production at 60 and 240 minutes in NEVLP compared to NEVLP-Cyt livers which did not reach significance. Overall, the production of liver damage molecules increased throughout perfusion but was low in both treatment groups.
Figure 6. Low levels of liver damage molecule production were observed throughout perfusion.

A-B: Liver perfusate samples were assessed hourly for AST and ALT concentrations which were normalized to liver mass within each experiment. C-F: Liver perfusate samples were assessed at the baseline, 60 minutes of perfusion, and 240 minutes of perfusion for ARG1, GSTα, 5’-NT, and SDH liver damage molecule concentrations. (NEVLP & NEVLP-Cyt n=4). Data are shown as mean ± SEM.
Liver architecture damage and cellular apoptosis following perfusion
To determine if a reduction in immune activation during machine perfusion could confer hepatoprotective effects on the liver graft, we performed histopathological analysis of liver architecture damage following the Suzuki criteria(22) and immunohistochemistry of activated caspase 3 for analysis of cellular apoptosis. Representative images of H&E and activated caspase 3 stained livers are shown (Fig. 7A). A significant difference was found in the Suzuki score between naïve and NEVLP-Cyt livers (1.4 ± 0.7 vs. 6.6 ± 0.8), P<0.01, along with a trend toward a difference between naïve and NEVLP (5.0 ± 0.8) livers, P=0.12 (Fig. 7B). Significant differences were also found between naïve (0.03 ± 0.02) and CS (0.07 ± 0.04) groups in comparison to NEVLP (0.61 ± 0.10) and NEVLP-Cyt (0.75 ± 0.16) groups in the percentage of cells positive for activated caspase 3 (Fig. 7C). These data suggest that machine perfusion induced liver architecture damage and low levels of cellular apoptosis which were not reduced by treatment with IL-10 and TGF-β.
Figure 7. Liver architecture damage and cellular apoptosis were not reduced by IL-10 and TGF-β treatment.
A: Representative micrographs of H&E and activated caspase 3 staining showing the grade of tissue injury. B: Quantitative analysis of liver damage using the Suzuki criteria. A significant difference was found between naïve and NEVLP-Cyt livers. C: Quantitative analysis of cellular apoptosis by the percentage of cells positive for activated (cleaved) caspase 3. Significant differences were found between control and perfused livers. *P<0.05, **P<0.01. Data are shown as mean ± SEM.
Discussion
NEVLP has received revitalized interest in recent years,(7, 29, 30) especially due to the opportunity for graft conditioning prior to transplantation.(31) There are many therapeutic opportunities which include organ defatting,(32) treatment of infectious diseases like Hepatitis C Virus,(33) improvement of mitochondrial function,(34) and global anti-inflammatory therapies.(10-12) Recent studies have begun to investigate the biological effects of perfusion on graft condition and secretion of inflammatory factors,(8, 35) but none prior to this study had examined the effects of perfusion on the phenotype of liver-resident immune cells. Our team characterized the graft-resident immune activation induced by NEVLP and harnessed the anti-inflammatory properties of cytokines IL-10 and TGF-β to counteract the inflammatory nature of machine perfusion.
We selected IL-10 and TGF-β for anti-inflammatory cytokine therapy due to their previously demonstrated ability to generate tolerogenic phenotype DCs(17) and use in other pre-clinical models.(36) Tolerogenic DCs, by definition, have low expression levels of MHC II, CD80, CD86, and CD40 with a reduced antigen presentation capacity.(37) They can secrete anti-inflammatory mediators which, in concert with TGF-β, polarize CD4+ T-helper cells to a FoxP3+ regulatory T-cell subset.(38) Together, regulatory DCs and T-cells can play a key role in dampening the inflammatory response and potentially decreasing donor-specific sensitization to prevent allograft rejection.(39) Prior to our perfusion experiments, we tested IL-10 and TGF-β in vitro to optimize dosing and confirm that the cytokines could polarize immune cells to a regulatory phenotype prior to perfusion experiments (Fig. S1).
In this study, we observed the inflammatory nature of machine perfusion and utilized anti-inflammatory cytokines IL-10 and TGF-β to improve liver function and reduce immune activation during perfusion. We performed scRNA-seq analysis of naïve, CS, NEVLP, and NEVLP-Cyt livers which, to our knowledge, is the first report of scRNA-seq on perfused liver-resident immune cells. We showed that machine perfusion induces an inflammatory gene expression signature which can be partially ameliorated through treatment with IL-10 and TGF-β (Fig. 3B-D). We focused our analyses of specific genes involved in inflammatory processes to macrophages and DCs due to the high abundance of liver-resident macrophages(27) and the postulated role of DCs in allograft tolerance through T-cell mediated mechanisms.(17, 19, 28) We found an upregulation of inflammatory mediators in NEVLP livers, including CD14, NF-κβ1, NF-κβ2, MYD88, NLRP3, and IL-1β which indicates toll-like receptor activity (40) as well as chemoattractants CCL2 and CCL4. The upregulation of these genes was partially, but not fully, ameliorated by treatment with IL-10 and TGF-β during perfusion. These results implied immune activation mediated by toll-like receptor activity, consistent with previous reports of damage-associated molecular pattern release during machine perfusion,(8) and provide novel targets for future therapeutic attempts.
These inflammatory changes in gene expression patterns were also observed on the protein level. Flow cytometry was used to identify macrophage and DC expression of antigen presentation machinery MHC II, CD40, CD86, and PD-L1 which are upregulated following activation. We found that NEVLP led to a significant increase in MHC II and CD40 expression levels which was partially or fully ameliorated by treatment with IL-10 and TGF-β. Unexpectedly, we found an increase in CD86 expression on cytokine treated livers which opposes previous reports that IL-10 and TGF-β inhibit DC maturation(16, 17). Further investigations are required to determine the molecular mechanisms underpinning this observed phenomenon.
Despite the reduction observed in the expression of inflammatory genes and protein levels of molecules involved in antigen presentation, no differences were observed in secretion of inflammatory cytokines between treatment groups (Fig. 5). Although we observed a trend toward higher levels of IL-1β and IL-6 secreted by NEVLP than NEVLP-Cyt livers, consistent with the observation that NEVLP-Cyt livers had lower gene expression levels of these inflammatory cytokines (Fig. 3D), these values did not reach significance. Pre-formed intracellular inflammatory cytokines can be released during inflammatory events,(41) and it is possible that treatment with IL-10 and TGF-β is not sufficient to prevent these processes. Future studies would be required to elucidate the mechanisms underlying early inflammatory cytokine release in the machine perfusion setting.
The observed decrease in immune activation was associated with improved liver function and overall low levels of liver damage. Lactate clearance was improved in NEVLP-Cyt livers (Fig. 2C) which indicates improved liver function, and the level of liver damage marker release was low in all treatment groups (Fig. 6). The low levels of damage marker release may be related to the short duration of perfusion, and future investigations with longer duration of organ storage may reveal differences in liver damage between treatment groups. We also observed that IL-10 and TGF-β treated livers experienced similar levels of liver architecture damage in comparison to NEVLP livers, demonstrating that while anti-inflammatory cytokines may decrease immune activation, they are not sufficient to prevent tissue damage. Combinatory treatments in future studies may demonstrate the ability to prevent both immune activation and liver damage during perfusion. However, the low levels of damage marker production observed during perfusion itself are encouraging and further support the use of NEVLP as a novel organ preservation method.
This study has some inherent limitations. The short perfusion period of 240 minutes was sufficient to observe differences in gene expression signatures (Fig. 3) and immune cell activation (Fig. 4); however, this is much shorter than the average clinical machine perfusion time of 548 minutes reported by Nasralla et al.(7) A longer preservation period and the utilization of larger animal and human models would be required to generate clinically translational results. We also did not assess the functionality of isolated macrophages or DCs following NEVLP. Future studies in allogeneic models are required to investigate the functional immunogenicity of liver-resident immune cells following machine perfusion.
In conclusion, we have provided the first report of scRNA-seq analysis of perfused liver-resident immune cells and demonstrated for the first time the phenotypic changes induced by NEVLP onto liver-resident immune cells. Treatment with the anti-inflammatory cytokines IL-10 and TGF-β improved liver function and partially ameliorated immune activation during NEVLP, providing a proof-of-concept for the performance of immunological organ modification prior to transplantation. These results are critical to enhance the understanding of the inflammatory processes induced by NEVLP as clinical application increases, and further investigations are required to comprehensively demonstrate these immune profiles and functionally assess organ immunogenicity following therapeutic intervention during NEVLP.
Supplementary Material
Acknowledgements
We would like to thank the UW Carbone Cancer Center Flow Cytometry Laboratory and Experimental Animal Pathology Laboratory, who are supported by NIH/NCI P30 CA014520, and the Wisconsin Institutes for Medical Research small animal vivarium for their support. The authors utilized the UW-Madison Biotechnology Center’s Gene Expression Center Core Facility (Research Resource Identifier – RRID:SCR_017757) for the single cell RNA library preparation, the DNA sequencing facility (RRID:SCR_017759) for sequencing, and the UW-Madison Biotechnology Center Bioinformatics Resource Center Core Facility (RRID:SCR_017799) for analysis services. The author(s) also thank the UW-Madison, Department of Surgery, Histology Core Lab, Dr. Susan Thibeault PhD, CCC-SLP, PI of the DOS Histology Core, along with certified Histotechnician, Sierra Raglin HTL (ASCP), and Lab Supervisor, Sara Dutton Sackett, PhD for their assistance in histopathology and immunohistochemistry.
Funding:
This work was supported in part by the St. Baldrick’s-Stand Up to Cancer Pediatric Dream Team Translational Research Grant SU2C-AACR-DT-27-17 and NIH/NCI R01 CA215461 (C.M.C), and a Society for Surgery of the Alimentary Tract Career Development Award (D.P.A). Stand Up to Cancer is a division of the Entertainment Industry Foundation. Research grants are administered by the American Association for Cancer Research, the scientific partner of SU2C. The contents of this article do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. None of these funding sources had any input in the study design, analysis, manuscript preparation or decision to submit for publication.
Abbreviations
- 5’-NT
5’-nucleotidase
- ARG1
Liver-type arginase 1
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- CCL
C-C motif chemokine ligand
- cDC
Classical dendritic cell
- CS
Cold storage
- CXCL
Chemokine (C-X-C motif) ligand
- DC
Dendritic cell
- ELISA
Enzyme-linked immunosorbent assay
- FMO
Fluorescence minus one
- GOT1
Glutamic-oxaloacetic transaminase 1
- GSTα
α-glutathione S-transferase
- H&E
Hematoxylin and eosin
- HBSS
Hank’s balanced salt solution
- IFN
Interferon
- IL
Interleukin
- MFI
Median fluorescence intensity
- MHC
Major histocompatibility complex
- MYD88
Myeloid differentiation protein 88
- NEVLP
Normothermic ex vivo liver perfusion
- NEVLP-Cyt
NEVLP with cytokines
- NF-κβ
Nuclear factor kappa B
- NLRP3
NLR family pyrin domain containing 3
- NPCs
Non-parenchymal cells
- PBS
Phosphate buffered saline
- pDC
Plasmacytoid dendritic cell
- scRNA-seq
Single cell RNA sequencing
- SDH
Sorbitol dehydrogenase
- SEM
Standard error of the mean
- TGF-β
Transforming growth factor-β
- TNF
Tumor necrosis factor
- UW
University of Wisconsin
Footnotes
Disclosure: C.M.C. reports honorarium from Nektar Therapeutics, who had no input in the study design, analysis, manuscript preparation, or decision to submit for publication. No other relevant conflicts of interest are reported.
References
- 1.Busquets J, Xiol X, Figueras J, Jaurrieta E, Torras J, Ramos E et al. The impact of donor age on liver transplantation: influence of donor age on early liver function and on subsequent patient and graft survival. Transplantation 2001;71(12):1765–1771. [DOI] [PubMed] [Google Scholar]
- 2.Foley DP, Fernandez LA, Leverson G, Anderson M, Mezrich J, Sollinger HW et al. Biliary complications after liver transplantation from donation after cardiac death donors: an analysis of risk factors and long-term outcomes from a single center. Ann Surg 2011;253(4):817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014;515(7527):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boehnert MU, Yeung JC, Knaak JM, Selzner N, Selzner M. Normothermic acellular ex vivo liver perfusion (NEVLP) reduces liver and bile duct in DCD liver grafts. Am J Transplant 2013;13(12):3290. [DOI] [PubMed] [Google Scholar]
- 5.Tolboom H, Pouw RE, Izamis ML, Milwid JM, Sharma N, Soto-Gutierrez A et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation 2009;87(2):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasteiger S, Berchtold V, Bösmüller C, Dostal L, Ulmer H, Bogensperger C et al. A Retrospective Propensity Score Matched Analysis Reveals Superiority of Hypothermic Machine Perfusion over Static Cold Storage in Deceased Donor Kidney Transplantation. J Clin Med 2020;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasralla D, Coussios CC, Mergental H, Akhtar MZ, Butler AJ, Ceresa CDL et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018;557(7703):50–56. [DOI] [PubMed] [Google Scholar]
- 8.Scheuermann U, Zhu M, Song M, Yerxa J, Gao Q, Davis RP et al. Damage-Associated Molecular Patterns Induce Inflammatory Injury During Machine Preservation of the Liver: Potential Targets to Enhance a Promising Technology. Liver Transpl 2019;25(4):610–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashimoto K, Cypel M, Juvet S, Saito T, Zamel R, Machuca TN et al. Higher M30 and high mobility group box 1 protein levels in ex vivo lung perfusate are associated with primary graft dysfunction after human lung transplantation. J Heart Lung Transplant 2017. [DOI] [PubMed] [Google Scholar]
- 10.Goldaracena N, Echeverri J, Spetzler VN, Kaths JM, Barbas AS, Louis KS et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transpl 2016;22(11):1573–1583. [DOI] [PubMed] [Google Scholar]
- 11.Laing RW, Stubblefield S, Wallace L, Roobrouck VD, Bhogal RH, Schlegel A et al. The Delivery of Multipotent Adult Progenitor Cells to Extended Criteria Human Donor Livers Using Normothermic Machine Perfusion. Front Immunol 2020;11:1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigo F, De Stefano N, Navarro-Tableros V, David E, Rizza G, Catalano G et al. Extracellular Vesicles from Human Liver Stem Cells Reduce Injury in an Ex Vivo Normothermic Hypoxic Rat Liver Perfusion Model. Transplantation 2018;102(5):e205–e210. [DOI] [PubMed] [Google Scholar]
- 13.Thijssen MF, Brüggenwirth IMA, Gillooly A, Khvorova A, Kowalik TF, Martins PN. Gene Silencing With siRNA (RNA Interference): A New Therapeutic Option During Ex Vivo Machine Liver Perfusion Preservation. Liver Transpl 2019;25(1):140–151. [DOI] [PubMed] [Google Scholar]
- 14.Yuzefovych Y, Valdivia E, Rong S, Hack F, Rother T, Schmitz J et al. Genetic Engineering of the Kidney to Permanently Silence MHC Transcripts During ex vivo Organ Perfusion. Front Immunol 2020;11:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 16.Esebanmen GE, Langridge WHR. The role of TGF-beta signaling in dendritic cell tolerance. Immunol Res 2017;65(5):987–994. [DOI] [PubMed] [Google Scholar]
- 17.Torres-Aguilar H, Aguilar-Ruiz SR, González-Pérez G, Munguía R, Bajaña S, Meraz-Ríos MA et al. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol 2010;184(4):1765–1775. [DOI] [PubMed] [Google Scholar]
- 18.Fogel-Petrovic M, Long JA, Misso NL, Foster PS, Bhoola KD, Thompson PJ. Physiological concentrations of transforming growth factor beta1 selectively inhibit human dendritic cell function. Int Immunopharmacol 2007;7(14):1924–1933. [DOI] [PubMed] [Google Scholar]
- 19.Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clin Immunol 2012;142(3):332–342. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt A, Éliás S, Joshi RN, Tegnér J. In Vitro Differentiation of Human CD4+FOXP3+ Induced Regulatory T Cells (iTregs) from Naïve CD4+ T Cells Using a TGF-β-containing Protocol. J Vis Exp 2016(118). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Espinosa JR, Samy KP, Kirk AD. Memory T cells in organ transplantation: progress and challenges. Nat Rev Nephrol 2016;12(6):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993;55(6):1265–1272. [DOI] [PubMed] [Google Scholar]
- 23.Bourgognon M, Klippstein R, Al-Jamal KT. Kupffer Cell Isolation for Nanoparticle Toxicity Testing. J Vis Exp 2015(102):e52989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzén O, Gan LM, Björkegren JLM. PanglaoDB: a web server for exploration of mouse and human single-cell RNA sequencing data. Database (Oxford) 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X, Lan Y, Xu J, Quan F, Zhao E, Deng C et al. CellMarker: a manually curated resource of cell markers in human and mouse. Nucleic Acids Res 2019;47(D1):D721–d728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhoeven CJ, Farid WR, de Jonge J, Metselaar HJ, Kazemier G, van der Laan LJ. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J Hepatol 2014;61(3):672–684. [DOI] [PubMed] [Google Scholar]
- 27.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009;27:147–163. [DOI] [PubMed] [Google Scholar]
- 28.Rosen SJ, Harris PE, Hardy MA. State of the Art: Role of the Dendritic Cell in Induction of Allograft Tolerance. Transplantation 2018;102(10):1603–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beal EW, Dumond C, Kim JL, Akateh C, Eren E, Maynard K et al. A Small Animal Model of Ex Vivo Normothermic Liver Perfusion. J Vis Exp 2018(136). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nösser M, Gassner J, Moosburner S, Wyrwal D, Claussen F, Hillebrandt KH et al. Development of a Rat Liver Machine Perfusion System for Normothermic and Subnormothermic Conditions. Tissue Eng Part A 2020;26(1-2):57–65. [DOI] [PubMed] [Google Scholar]
- 31.Carlson K, Kink J, Hematti P, Al-Adra DP. Extracellular Vesicles as a Novel Therapeutic Option in Liver Transplantation. Liver Transpl 2020;26(11):1522–1531. [DOI] [PubMed] [Google Scholar]
- 32.Boteon YL, Attard J, Boteon A, Wallace L, Reynolds G, Hubscher S et al. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transpl 2019;25(7):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldaracena N, Spetzler VN, Echeverri J, Kaths JM, Cherepanov V, Persson R et al. Inducing Hepatitis C Virus Resistance After Pig Liver Transplantation-A Proof of Concept of Liver Graft Modification Using Warm Ex Vivo Perfusion. Am J Transplant 2017;17(4):970–978. [DOI] [PubMed] [Google Scholar]
- 34.Beal EW, Kim JL, Reader BF, Akateh C, Maynard K, Washburn WK et al. [D-Ala(2), D-Leu(5)] Enkephalin Improves Liver Preservation During Normothermic Ex Vivo Perfusion. J Surg Res 2019;241:323–335. [DOI] [PubMed] [Google Scholar]
- 35.de Vries RJ, Pendexter CA, Cronin SEJ, Marques B, Hafiz EOA, Muzikansky A et al. Cell release during perfusion reflects cold ischemic injury in rat livers. Sci Rep 2020;10(1):1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machuca TN, Cypel M, Bonato R, Yeung JC, Chun YM, Juvet S et al. Safety and Efficacy of Ex Vivo Donor Lung Adenoviral IL-10 Gene Therapy in a Large Animal Lung Transplant Survival Model. Hum Gene Ther 2017;28(9):757–765. [DOI] [PubMed] [Google Scholar]
- 37.Raïch-Regué D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett 2014;161(2):216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity 2003;18(5):605–617. [DOI] [PubMed] [Google Scholar]
- 39.Nouri-Shirazi M, Guinet E. Direct and indirect cross-tolerance of alloreactive T cells by dendritic cells retained in the immature stage. Transplantation 2002;74(7):1035–1044. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Zhou H, Ni M, Wang X, Busuttil R, Kupiec-Weglinski J et al. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation 2016;100(12):2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shurety W, Merino-Trigo A, Brown D, Hume DA, Stow JL. Localization and post-Golgi trafficking of tumor necrosis factor-alpha in macrophages. J Interferon Cytokine Res 2000;20(4):427–438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.