Abstract
Introduction:
A critical appraisal of the literature regarding female urethral function and dysfunction is needed in light of recent evidence showing the urethra’s role in causing stress and urge urinary incontinence.
Methods:
An evidence assessment was conducted using selected articles from the literature that contained mechanistic data on factors affecting urethral function and failure.
Results:
Maximal urethral closure pressure (MUCP) is 40% lower in stress urinary incontinence (SUI) than normal controls. Evidence from 5 women shows relatively equal contributions to MUCP from striated/smooth muscle, vascular-plexus, connective tissue. MUCP varies 2-fold in individuals of similar age and declines 15% per decade even in nulliparous women. Age explains 57% of variance in MUCP. This parallels with striated/smooth muscle loss and reduced nerve density. Factors influencing pressure variation minute-to-minute and decade-to-decade are poorly understood. Connective tissue changes have not been investigated. MUCP in de novo SUI persisting 9-months postpartum is 25% less than in age and parity matched controls. Longitudinal studies do not show significant changes in urethral function after vaginal birth suggesting that changes in urethral support from birth may unmask pre-existing sphincter weakness and precipitate SUI. Mechanisms of interaction between support injury, pre-existing urethral weakness and neuropathy are unclear.
Conclusion:
Urethral failure is the predominant cause of SUI and a contributing factor for UUI; potentially explaining why mixed symptoms predominate in epidemiological studies. Age-related striated muscle loss and differences between women of similar age are prominent features of poor urethral closure. Yet, connective tissue changes, vasculature function and complex interactions among factors are poorly understood.
Keywords: urinary incontinence, urge incontinence, stress incontinence, urethral function, urethra anatomy
1. Introduction
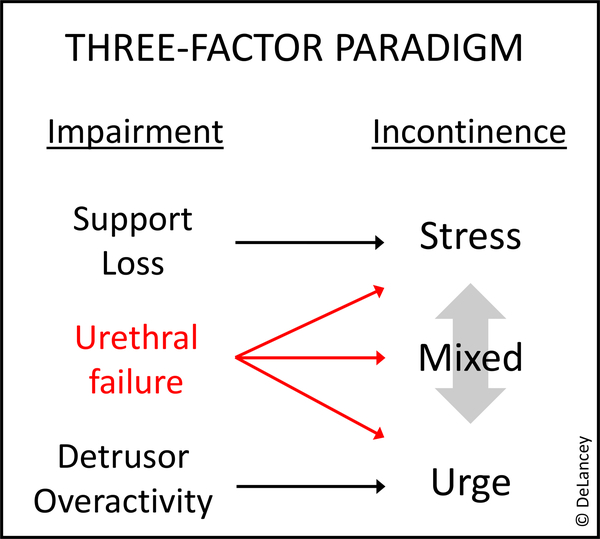
For many years, urethral failure - the reduced urethral closure pressure - was not thought to be a primary factor causing urinary incontinence. Broadly speaking, a two-factor paradigm has been widely used for understanding incontinence: stress urinary incontinence (SUI) as a result of defective urethral support – provided by the suburethral connective tissue and their muscular connections that are regulated by pelvic floor reflexes and voluntary control1 - and urge urinary incontinence (UUI) as caused by detrusor overactivity. However, more recently, large case-control studies that evaluate both urethral function and support have shown that urethral failure is the factor most responsible for SUI,2 and epidemiological studies show it is also related to UUI.3 This suggests revising the two-factor paradigm to a three-factor paradigm illustrated in Figure 1 which fits better with the observation that the majority of women experience mixed symptoms.4 This proposed shift in paradigm highlights the need to understand urethral function and failure, a topic that has lagged behind evaluation of support and detrusor function.5
Figure 1.
Three-factor Paradigm: Urethral function as an important component of stress and urge urinary incontinence.
Success for current treatment strategies targeting the detrusor dysfunction and urethral mobility has plateaued at levels that leave many treated women with continued incontinence. Careful studies of mid-urethral slings, for example, show a 20% objective and 40% subjective failure rate.6 Also, the impact of urethral support surgery and bulking injections on the urethral function has been reported. No statistically significant change was found in rest MUCP after retropubic and transobturator mid-urethral slings and bulking agents.7–9 If, as we show in this review, urethral failure is the dominant causal factor for SUI and operations do not change this, some incontinence would be expected postoperatively. This suggests that, in the absence of interventions targeting low urethral closure pressure, surgical results from support operations are bound to remain imperfect. Similarly, although there are many pharmacologic treatments for UUI that target the bladder, they reduce incontinent episodes from, for example, 2.5 episodes a day to 1.5 episodes a day.10 While this is a welcome improvement for women, it is curious that the women that meet success endpoints in these well-conducted clinical trials, still have enough incontinence to meet the inclusion criteria of the study.10
Since urethral function is important to continence, and our treatments do not address this factor, gaining a scientific understanding of the mechanisms determining urethral function in a way that can suggest novel treatment and prevention strategies is justified. To begin this process, a broad review of the factors known about urethral structure and function would be helpful in forming a scientific basis for studying this important, though long-neglected organ.
Consistent with the Neurourology and Urodynamics’ goal for review articles to provide an “editorial point of view rather than a litany of dogma or an exhaustive compilation of all prior work in that field”, we consider here the scientific foundation examining urethral function and failure.
2. Anatomy
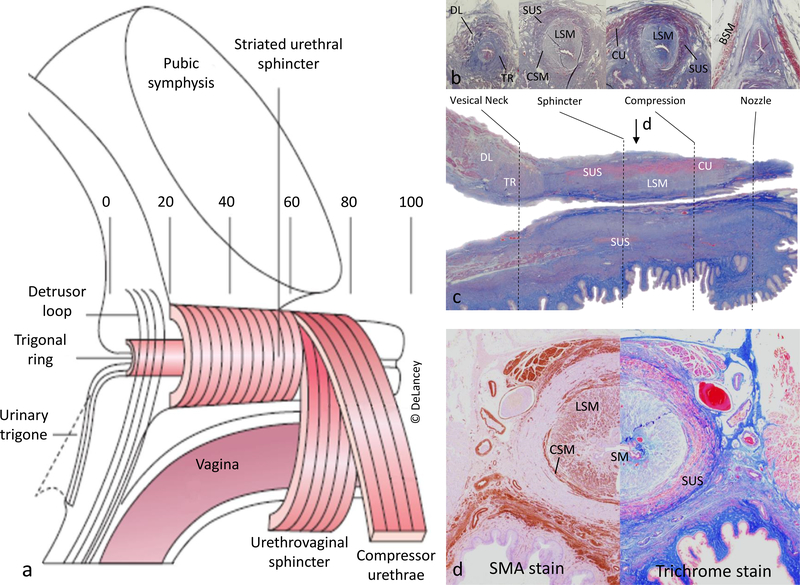
Knowing the anatomy of the structural components that generate urethral closure pressure (Figure 2) is necessary to understand urethral function. The urethra can be divided into 5 segments each of approximately one fifth of luminal length.11 This first fifth – where the urethra traverses the bladder base - is surrounded by a trigonal ring of smooth muscle that is contiguous with the trigonal muscle and distinctly different than the detrusor muscle, and a detrusor loop that passes lateral to and in front of (ventral to) the lumen 12 (commonly referred to as internal urethral sphincter). The next two-fifths are a multilayered tube with an outer circular striated muscle surrounding a thin circular, thicker longitudinal muscle, and vascular plexus. In the fourth segment, an arch of striated muscle passes over the urethra (compressor urethra and urethrovaginal sphincter). This third and fourth segments that contain striated muscle are often referred to as the “external urethral sphincter”. The distal portion is a fibrous “nozzle” devoid of muscle. The urethral lumen is filled along its length with a prominent and highly specialized vascular/mucosal core with arteriovenous communications with a sealing function.12
Figure 2.
Anatomy of the female urethra. (a) Illustration of the urethra in mid-sagittal view and divided in fifths: from 0 (internal urethral meatus) to 100 (external urethral meatus). (b) Examples of representative regions in axial trichrome histological section. (c) Mid-sagittal trichrome histologic section; arrow indicates cross-section level for panel d. (d) Mid-urethra axial section with SMA stain (left) and trichrome stain (right). Abrevv.: DL, Detrusor loop; TR, Trigonal ring; SUS, Striated urethral sphincter; LSM, Longitudinal smooth muscle; CSM, Circular smooth muscle; CU Compressor urethrae; BSM, Bulbospongiosus muscle; SM, Submucosa; V, Vagina.
3. Neurophysiology
A detailed review of urethral neurophysiology is beyond the scope of this review and has been summarized elsewhere.13,14 However, a brief outline of contributing factors is necessary for context. The lower urinary tract is innervated by afferent, and efferent axons of 3 sets of peripheral nerves: 1) Sacral parasympathetic nerves (S2-S4), which excite the bladder and relax the urethra - in humans, parasympathetic postganglionic neurons are in the detrusor wall, as well as in the pelvic plexus; 2) Lumbar sympathetic nerves (T10-L2), which inhibit the bladder body and excite the bladder neck and urethral smooth muscle; and 3) Pudendal somatic nerves from the sacral ventral horn (S2-S4), which excite the external urethral sphincter. The central governance of these systems comes from the pontine micturition and storage centers, and evaluation of their electrophysiological coordination is complex.
As described above, the active structures in the urethra are controlled by autonomic and somatic nerves. For the study of urinary incontinence, the pudendal nerve has to be assessed in its full length – as to say, from the Onuf’s nucleus in the spinal cord to its most distal perineal branches.15 Currently, available electrophysiology data have limited applicability to clinical practice, since it is difficult to determine “normalcy” and studies usually assess only part of the problem.16
4. Urethral function: Current state of science
It is a hydraulic fact that the closure pressure in the urethra must exceed the pressure in the bladder for continence to be maintained. Therefore, this “continence margin” depends on the actions of the muscles, nerves, blood vessels and connective tissue that surround the urethral lumen. Understanding how the structures work together to achieve this continence margin is needed. Although pressure transmission – that is, the rise in urethral pressure that is simultaneous with and of a similar magnitude to rises in intravesical pressure17 - plays a role in continence, this review focuses on the function of the urethra itself.
4.1. The contribution of urethral function to SUI and UUI
In a case-control study comparing SUI women to continent controls with groups matched for age, parity, race, and hysterectomy status maximum urethral closure pressure (MUCP) is, by far, the most important factor in maintaining SUI.2 It was 42% lower in incontinent women and with an effect size of 1.47. None of the support factors had an effect size of greater than 0.6 (Table 1).
Table 1.
Comparison of Clinical Pelvic Floor Measures Between Stress Urinary Incontinent Women and Continent Volunteers. (Adapted from DeLancey, 2008)
| Stress Incontinent n=103 | Continent n=108 | Effect Size (d) | P | |
|---|---|---|---|---|
| URETHRAL FUNCTION | ||||
| Maximum Urethral Closure Pressure (cmH2O) | 40.8 ± 17.1 | 70.2 ± 22.4 | 1.47 | <.0001 |
| URETHRAL SUPPORT | ||||
| Cotton-tipped swab-rest (degrees) | −0.8 ± 11.8 | −6.3 ± 15.1 | 0.41 | 0.004 |
| Cotton-tipped swab axis-Muscle Contraction (degrees) | −11.6 ± 14.9 | −21.0 ± 17.4 | 0.58 | <.0001 |
| POP-Q point Aa (cm) | −0.6 ± 0.8 | −1.0 ± 0.8 | 0.5 | <.0001 |
| Genital hiatus (cm) | 4.0 ± 1.0 | 3.4 ± 1.0 | 0.6 | <.0001 |
Data are reported as mean ± standard deviation
In regression models, MUCP explained 50% of SUI cases. To put into perspective, the most predictive support parameter, point Aa in the Pelvic Organ Prolapse Quantification assessment, only added 16%.2 To assure that it was not inadequate measurement strategies that resulted in an underestimate of urethral support’s role, the ultrasound recordings that showed urethrovesical movement dynamics during a cough were reviewed by 5 experts who were blinded to continence status; they were successful in identifying the women with SUI in 57% of subjects, just 7% better than random chance.18
A population-based study on continence mechanism differences between black and white women analyzed three different groups: SUI, UUI, and continent women.3 In white women, both SUI and UUI subjects had average MUCP that were significantly lower than continent controls, and white women with UUI had only half the ability to increase urethral closure during pelvic muscle contraction. This finding supports what many women with UUI say, “I just can’t hold it in when I have the urge to go”. The authors hypothesized that SUI and UUI both have weak urethras but UUI still has good support and SUI has not. They also found that this similarity between stress and urge incontinent women’s urethral pressure did not occur in black women. In addition, black women were half as likely to be incontinent and had overall 22% higher urethral pressure than white women.
Although many other factors have been discussed in the pathophysiology of overactive bladder and UUI,19 urethral dysfunction is certainly one that has not been satisfactorily addressed in the literature.
4.2. Contributing components to urethral closure pressure
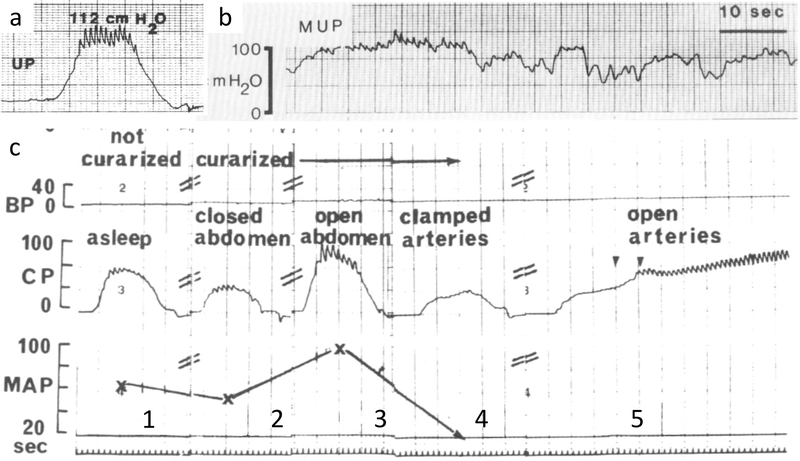
Surprisingly, few studies have sought to determine the relative contribution of the different components to urethral function. Only a single study of five women undergoing pelvic surgery measuring urethral pressure at different time points (before anesthesia induction, after neuromuscular blockade, and after temporary vascular clamping) provides any data.20 The striated muscle and the vascular plexus were responsible for 41% and 31% of the urethra closure pressure, respectively. The remaining 28% were attributed to the smooth muscle and surrounding connective tissue (Figure 3c). Although limited by its very small sample size, it provides an important conceptual insight on how the continence mechanism may be composed by multiple and symbiotic components rather than it mainly relying on urethral support, as believed for many years.
Figure 3.
Urethral pressure variations.
(a) Normal urethral pressure profile showing arterial pulsations in high pressure zone synchronous with EKG (not shown). (b) Normal variations in urethral pressure. (c) Urethral closure pressure profiles during different steps of the Rud experiment: 1) before curarization, 2) after curarization, 3) after abdomen was open, 4) after arterial clamping, 5) and reopened arteries (continuous tracing). UP, urethral pressure; BP, bladder pressure; CP, closure pressure; MAP, mean arterial pressure.
(a) From Kulseng-Hanssen, 1983. (b&c) From Rud, 1980.
The inner diameter of the muscular tube that forms the urethra is approximately 5mm (15 Fr.) in width.21 Therefore, in the absence of some sealing mechanism inside the muscular tube, urine would pour out of the bladder. There is a remarkable submucosal vascular plexus filling the center of the muscular tube with specialized arteriovenous anastomoses that can control the amount of venous filling,12 and loss of vascular filling in the experiment noted above20 greatly reduced closure pressure. Recent ultrasound studies have addressed vascular components of the continence system, although knowledge on this topic is still in its early stages. For instance, multiparous women show significant reduction in vascular parameters on doppler ultrasound parameters in mid-urethra when compared to nulliparous controls.22 Similar results were found when incontinent and postmenopausal were compared to continent and premenopausal women, respectively.23
4.3. Intra- and inter-subject urethral pressure variation
It has been traditional to report urethral function as a single MUCP obtained in a static situation (Figure 3a), which is a very limited assessment of a potentially complex physiology. Urethral closure pressure is dynamic and is constantly being modulated to adjust to the needs being placed on the urethra (Figure 3b), which translates into within individual variability of urethral pressure. The simple action of willful relaxation of the pelvic floor muscle, for instance, yields a urethral pressure 15% lower than rest measurements, and voluntary pelvic floor contraction elevates it by 30%.24 Minute-to-minute measurement of urethral pressure during ambulatory urodynamics (Figure 3) shows that over 50% of normal healthy women were found to have pressures that vary by more than 20 cm H2O at rest.25 Both findings illustrate the dynamic nature of urethral pressure and strongly suggest that urethral pressure variation is a physiologic rather than pathologic phenomenon. This dynamic nature of urethral pressure might be partly explained by the pre-activation of pelvic floor muscles before intra-abdominal pressure increases.26 As yet, the exact regulatory mechanism for these variations remains poorly understood.
Urethral pressure also varies greatly from one woman to another (Figure 4) at any given age even in nulliparas. For example, one woman in her early 20’s has a urethral closure pressure of 75 cm H2O while another has a closure pressure of 120 cm H2O, a 60% difference, and at 50, there are individuals with 40 cm H2O and 100 cm H2O, a 250% difference. 27
Figure 4.
Age effect on the urethra. Panel a shows the decrease in urethral closure pressure per decade, which is associated to decrease in striated muscle fiber density (panel b) and nerve density (panel c). Panels d and e show the decrease in striated muscle cells from young (d) to old (e).
(a) From Trowbridge 2008. (b) From Perucchini 2002. (c) From Pandit Obstet Gynecol 2000. (d&e) From samples collected for Perucchini 2002.
5. Factors affecting urethral function
5.1. Age
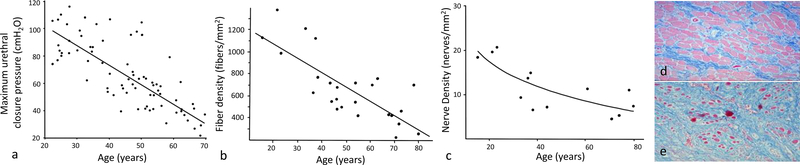
Urethral closure pressure declines substantially with advancing age (Figure 4) as documented in a study evaluating pressures from childhood to old age in 169 healthy women.28 When compared to 21–25-year-old women, the 36–40 and the 61–65-year-old groups had 25% and 50% lower urethral closure pressure, respectively. After the age of 45 years, subjects also showed progressive decrease in urethral functional length.
A study of nulliparous women allowed age to be analyzed without the effect of parity and found that with increasing age MUCP declines 15 cmH2O per decade.27 Age alone explained 57.4% of variance in MUCP in linear regression modeling.
Some changes that could contribute to this decline in closure pressure have been studied. Striated muscle cell counts were found to decrease by 50% from age of 20 to 80 years.29 When smooth muscle is analyzed in another study, muscle fiber density was 25–50% lower in women aged 70–89 years compared with those aged 20–39 years.30 Striated muscle loss with advancing age was found to have a characteristic pattern: muscle cells disappeared first in the upper urethra and was preserved distally as seen in sagittal section, and on the vaginal side of the urethra being preserved on the pubic aspect as seen in cross-section (Figure 5).21,31
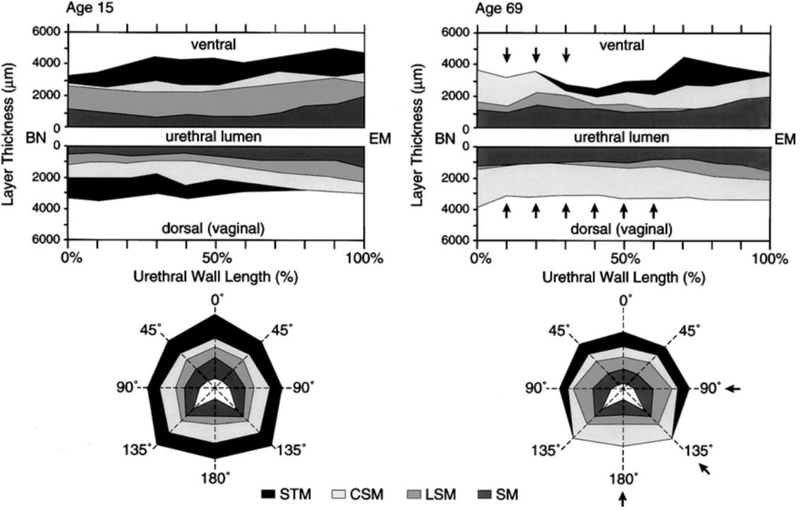
Figure 5.
Graphic illustration of the muscle loss pattern of urethral wall with aging based on histologic findings. Upper plots show the layer thickness in mid-sagittal section. Lower plots are the corresponding mid-urethral cross-sections. Note the proximal loss (arrows) in the thickness of the striated muscle in both the dorsal wall and the mid-urethral cross-section with age. BN, Bladder neck. EM, external meatus. STM, Striated muscle. CSM, Circular smooth muscle. LSM, Longitudinal smooth muscle. SM, Submucosa (from Perucchini, 2002).
The reason for muscle loss is also not entirely known but it might be partially related to nerve damage, as the quantity and distribution of intramuscular nerves within the striated urethral sphincter declines with age (Figure 4) and women with sparse intramuscular nerves show fewer striated muscle cells.32 Also, the pattern of nerve loss mirrors the pattern of muscle loss with the nerves in the more peripheral areas being lost in the regions where striated muscle loss was seen. Somatic innervation to the urethra comes from the pudendal nerve that enters at the perineal membrane where the compressor urethra and urethrovaginal sphincter are found. This is consistent with the nerves in this area having the highest numbers of axons.32 The areas of striated muscle loss are farthest from this point suggesting that the smaller terminal nerve fibers are most vulnerable.
There is also well-established electrophysiology data showing neuropathy in women with incontinence. An electrophysiology study compared SUI patients to normal controls and found pudendal nerve terminal motor latency (PNTML) to be significantly greater in the disease group (3.9 vs 2.0ms, p<0.001).33 In addition, evidence of partial pelvic floor denervation and subsequent re-innervation as assessed by a 20% increase in unit fiber density (ratio of muscle fibers to nerve fibers) in SUI women has been reported.34
Connective tissue changes with advancing age, similar to changes in the rest of the body, could also play a role in the incontinence symptoms. However, to our knowledge, the correlation between extracellular matrix components in women – in particular collagen and elastin – and urethral function has not been determined and should be topic of future research.
5.2. Childbirth
Childbirth is established as a major risk factor for future urinary incontinence. In a large Norwegian epidemiological study, women who delivered vaginally had 2.3 higher odds for reporting urinary incontinence, followed by cesarean deliveries (OR 1.5) when compared to nulliparous women.35
A case-control study compared primiparous women with de novo SUI to continent primiparas (positive controls) and continent nulliparas (negative controls) with groups matched for age and race.36 MUCP was 25% lower in incontinent women (effect size .91) while the strongest effect size for any of several support parameters was 0.76 (Table 2). These findings show that urethral failure is the most important causal factor for SUI. However, they do not address the important question of whether differences occur because of birth-induced changes or pre-existing urethral weakness.
Table 2.
Urethral function and support comparison between changes associated with birth and those associated with stress urinary incontinence (Adapted from DeLancey, 2007)
| Nulliparous Continent (NC) n=80 |
Primiparous Continent (PC) n=80 |
Primiparous Incontinent (PI) n=80 |
“SUI” PC vs. PI |
“Birth” NC vs. PC |
|
|---|---|---|---|---|---|
|
| |||||
| Effect size* |
|||||
| URETHRAL FUNCTION | |||||
| Maximal urethral closure pressure (cmH2O) | 90.3 ± 25.0 | 83.9 ± 21.0 | 62.9 ± 25.2 | 0.91 | - |
| Length of striated urethral sphincter (mm) on MRI | 9.8 ± 3.8 | 10.2 ± 3.9 | 8.4 ± 4.6 | 0.42 | - |
| Length of urethra (mm) on MRI | 29.4 ± 3.3 | 30.2 ± 3.7 | 28.7 ± 5.7 | 0.31 | - |
| URETHRAL SUPPORT | |||||
| Urethral Mobility: Supine Posture (Q-tip) (degrees) | |||||
| Rest | −3.0 ± 8.9 | −0.2 ± 9.5 | 5.4 ± 13.3 | 0.48 | - |
| Valsalva | 27.2 ± 17.8 | 39.1 ± 17.5 | 51.0 ± 17.3 | 0.68 | 0.67 |
| Kegel | −16.1 ± 12.1 | −14.6 ± 13.0 | −8.2 ± 17.2 | 0.42 | - |
SUI, stress urinary incontinence; MRI, magnetic resonance imaging.
Data presented as mean and standard deviation.
Effect size (Cohen’s d) listed when P<.05.
When investigating urethral pressure profile of primiparous women 8 weeks postpartum, a study found that vaginal delivery subjects had a drop of approximately 6% when compared to the same women at 36 weeks of gestation and of approximately 17% when compared to a pre-pregnancy cohort values (p 0.01).37 This decrease was not observed in the cesarean section group, although caution is required when analyzing those findings as sample size was small. It is not clear whether the drop in the mean occurred in all women or whether the mean difference occurred because of a small number of women who sustain significant sphincter damage. Care has to be taken in interpreting such findings as another longitudinal study did not find a significant drop in urethral closure pressure in a vaginal delivery cohort five days postpartum.38
One potential reason for poor urethral function is pelvic floor denervation seen after childbirth. Pudendal nerve studies39 showed prolonged PNTML in 42% of women 48–72h after vaginal delivery, but in none of the ones who had delivered by cesarean. Of the women with increased PNTML, 60% returned to normal values 2 months after delivery. In another study of nulliparous and parous asymptomatic women, no difference was found in terms of conduction time to the striated urethral sphincter.40 In a longitudinal study 80% of women who had delivered vaginally were found to have evidence of reinnervation of the pelvic floor muscles on EMG.41 Such evidence was more pronounced in subjects who had longer second stages of labor (>83.2 min, p 0.0008) or heavier babies (>3410g, p 0.02). However, although evidence of prolonged PNTML as an etiology has been evaluated in the literature, a longitudinal study failed to correlate such changes to urinary incontinence symptoms later in life,42 which leaves their role in the childbirth-related stress incontinence unclear.
The studies mentioned above are aligned with the current evidence that maternal, fetal and obstetric factors determine the likelihood of pelvic floor trauma during childbirth and that different types of injury can occur (muscle, connective tissue, nerves). However, it is not known whether those women already were more susceptible to urinary incontinence – determined by genetics and behavior – and such weakness was only unmasked by damage to the pelvic floor support, or if delivery damages the urethra itself, or both. Also, comparing means in groups does not distinguish between small changes that happen to most women from large changes (injuries) that happen to a few, and selection bias for those having cesarean section may play a role as well.43
5.3. Pregnancy
According to observational studies, more than half of women experience urinary incontinence during pregnancy.44 When they do, they are more likely to be incontinent at 6 months postpartum (relative risk, 2.3).45 Whether this happens because women with weak urethras before pregnancy are more prone to leakage caused by the mechanical burden of a growing baby and uterus or because pregnancy somehow affects urethral function is an important issue.
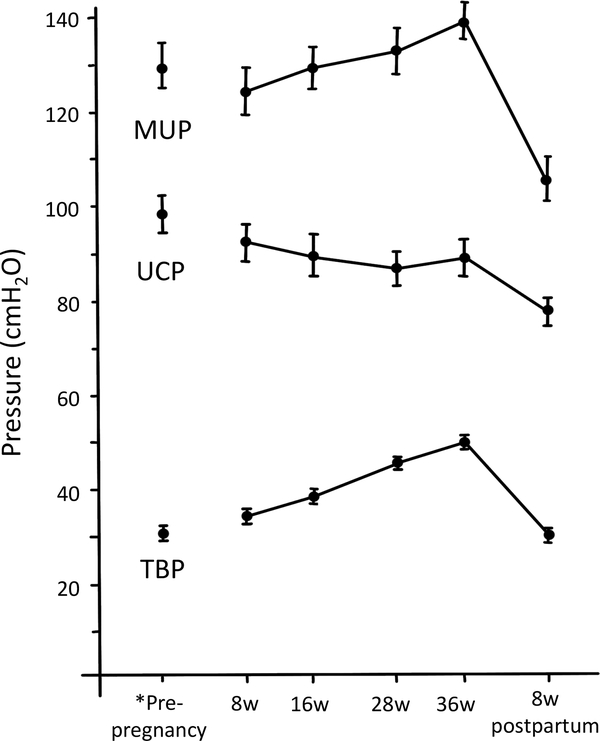
When studying urethral pressure profile during pregnancy and the puerperium, total bladder pressure and maximum urethral pressure, from 8 to 36 weeks, increased in average by 12±0.7 and 10±2.2 cmH2O, respectively. As values rose throughout pregnancy almost in a parallel fashion, no significant difference was seen in urethral closure pressure at any point (Figure 4).37
Changes in urethral sphincter neuromuscular function during pregnancy might also help elucidate how pregnancy itself becomes a contributing factor for urinary incontinence. Weidner et al.46 compared electromyographic patterns between nulliparous women and third-trimester primigravidas. In this study, the pregnant women group had decreased turns/second and amplitude values by around 50% and 15%, respectively.
Considering the available literature, however, current evidence is consistent with the hypothesis that women who inherently have poor urethral function leak during pregnancy as the sphincter weakness is revealed by the increased mechanical loads on the bladder by the pregnant uterus. They are also more likely to have incontinence afterward as the possible toll on the support system may also unmask this preexisting sphincter weakness. To date, there is no indication that pregnancy itself in the absence of vaginal birth (or second stage events) damages the continence mechanism.
5.4. Obesity and SUI
Epidemiological studies show that SUI is more common in women with obesity,47,48 raising the question of whether it is associated with urethral failure. Structural equation modeling of the relative contribution of BMI to SUI showed that the relationship was primarily caused by maximal cough pressure (indirect effect, p = 0.04), but not through MUCP (indirect effect, p = 0.2) or urethral support (POP-Q point Aa) (indirect effect, p = 0.4). Obese women (BMI ≥30 kg/m2) had higher Valsalva leak point pressures, as well as higher intravesical and intra-abdominal pressures at rest49 suggesting that they actually have stronger urethra but not strong enough to handle the increased pressures generated during cough in the obese.
6. Clinical relevance / Conclusion
Urethral failure - the inability to provide adequate closure pressure within the urethra - is the predominant cause of SUI and a contributing factor for UUI; potentially explaining why mixed symptoms predominate in epidemiological studies. As we look for novel therapies to improve treatment success rates that have been stagnant for decades, advancing our knowledge of urethral function and failure has the potential to uncover novel therapeutic targets. Important knowledge gaps exist in understanding the relative contributions of striated and smooth muscle, the rich vascular plexus and the largely unstudied connective tissue. Our understanding of the afferent aspects of neural control mechanisms that modulate urethral closure on a minute by minute and hour by hour basis remains incomplete. Given the easy accessibility of this small organ that has a relatively simple structure, it provides an ideal target for important mechanistic studies that can fill existing knowledge gaps.
Figure 6.
Changes in urethral pressure during pregnancy and postpartum; sitting measures. *Pre-pregnancy values are from nulliparous women provided for comparison. MUP, Maximal urethral pressure. UCP, urethral closure pressure. TBP, total bladder pressure. Data presented as mean and standard error. (Graph redrawn from van Geelen, 1982)
Acknowledgments
Disclosure/Funding: University of Michigan receives partial salary support for Dr. DeLancey for research. This work was partially supported by NIH grant RC2 DK122379
REFERENCES
- 1.DeLancey JOL. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet Gynecol 1994;170(6):1713–23. [DOI] [PubMed] [Google Scholar]
- 2.DeLancey JOL, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress Urinary Incontinence: Relative Importance of Urethral Support and Urethral Closure Pressure. J Urol 2008;179:2286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLancey JOL, Fenner DE, Guire K, Divya;, Patel A, Howard D, et al. Differences in continence system between community-dwelling black and white women with and without urinary incontinence in the EPI study. YMOB. 2010;202:584.e1–584.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: A population-based study. Vol. 165, Archives of Internal Medicine. Arch Intern Med; 2005. p. 537–42. [DOI] [PubMed] [Google Scholar]
- 5.Falah-Hassani K, Reeves J, Shiri R, Hickling D, Mclean L. The pathophysiology of stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J 2021;32:501–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richter HE, Albo ME, Zyczynski HM, Kenton K, Norton PA, Sirls LT, et al. Retropubic versus Transobturator Midurethral Slings for Stress Incontinence. N Engl J Med 2010;362(22):2066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasenda EJ, Kirby AC, Lukacz ES, Charles |, Nager W, Nager CW. The female continence mechanism measured by high resolution manometry: Urethral bulking versus midurethral sling. 2018; [DOI] [PMC free article] [PubMed]
- 8.Kirby AC, Tan-Kim J, Nager CW. Introduction to a new technology for measuring urethral pressures: 3D high-resolution manometry. Int Urogynecol J 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saaby M-L, Klarskov N, Lose G. The impact of tension-free vaginal tape on the urethral closure function: Mechanism of action. Neurourol Urodyn 2015. January 1;34(1):50–4. [DOI] [PubMed] [Google Scholar]
- 10.Herschorn S, Barkin J, Castro-Diaz D, Frankel JM, Espuna-Pons M, Gousse AE, et al. A phase iii, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the β3 adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82(2):313–20. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JOL. Correlative Study of Paraurethral Anatomy. ACOG; 1986. [PubMed] [Google Scholar]
- 12.Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contr Gynec Obs 1983;10:1–31. [PubMed] [Google Scholar]
- 13.De Groat WC, Fraser MO, Yoshiyama M, Smerin S, Tai C, Chancellor MB, et al. Neural Control of the Urethra. Scand J Urol Nephrol 2001;35:35–43. [DOI] [PubMed] [Google Scholar]
- 14.Birder L, De Groat W, Mills I, Morrison J, Thor K, Drake M. Neural Control of the Lower Urinary Tract: Peripheral and Spinal Mechanisms. Neurourol Urodyn 2010;29:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Brien C, O’Herlihy C, O’Connell PR, Olsen AL, Ross MA. Pudendal neuropathy is best determined by full neurophysiologic assessment. Am J Obstet Gynecol. 2004;191(5):1836–7. [DOI] [PubMed] [Google Scholar]
- 16.Olsen AL, Ross M, Stansfield RB, Kreiter C. Pelvic floor nerve conduction studies: Establishing clinically relevant normative data. Am J Obs Gynecol 2003;189(4):1114–9. [DOI] [PubMed] [Google Scholar]
- 17.Enhorning G. Simultaneous recording of intravesical and intra-urethral pressure. A study on urethral closure in normal and stress incontinent women. Acta Chir Scand Suppl 1961;Suppl 276:1–68. [PubMed] [Google Scholar]
- 18.Lewicky-Gaupp C, Blaivas J, Clark A, McGuire EJ, Schaer G, Tumbarello J, et al. “The cough game”: Are there characteristic urethrovesical movement patterns associated with stress incontinence? Int Urogynecol J 2009. October 11;20(2):171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross J, Vetter JM, Lai HH. Clustering of patients with overactive bladder syndrome. BMC Urol 2021. December 1;21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rud T, Andersson K-E, Asmussen M, Hunting A, Ulmsten U. Factors maintaining the intraurethral pressure in women. Invest Urol 1980;17(4):343–7. [PubMed] [Google Scholar]
- 21.Perucchini D, DeLancey JOL, Ashton-Miller JA, Galecki A, Schaer GN. Age effects on urethral striated muscle: II. Anatomic location of muscle loss. Am J Obstet Gynecol 2002;186(3):356–60. [DOI] [PubMed] [Google Scholar]
- 22.Lone F, Sultan AH, Stankiewicz A, Thakar R, Wieczorek AP. Vascularity of the urethra in continent women using colour doppler high-frequency endovaginal ultrasonography. Springerplus. 2014. October 20;3(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang CC, Chang SD, Chang YL, Wei TY, Wu HM, Chao AS. Three-dimensional power Doppler measurement of perfusion of the periurethral tissue in incontinent women - A preliminary report. Acta Obstet Gynecol Scand 2006;85(5):608–13. [DOI] [PubMed] [Google Scholar]
- 24.Baessler K, Miska K, Draths R, Schuessler B. Effects of voluntary pelvic floor contraction and relaxation on the urethral closure pressure. Int Urogynecol J 2005;16(3):187–91. [DOI] [PubMed] [Google Scholar]
- 25.Kulseng-Hanssen S, Klevmark B. Ambulatory urethro-cysto-rectometry: A new technique. Neurourol Urodyn 1988;7(2):119–30. [Google Scholar]
- 26.Deffieux X, Raibaut P, Rene-Corail P, Katz R, Perrigot M, Sheikh Ismael S, et al. External Anal Sphincter Contraction During Cough: Not a Simple Spinal Re£ex. Neurourol Urodyn 2006;25:782–7. [DOI] [PubMed] [Google Scholar]
- 27.Trowbridge ER, Wei JT, Fenner DE, Ashton-Miller JA, DeLancey JOL. Effects of aging on lower urinary tract and pelvic floor function in nulliparous women. Obstet Gynecol 2007;109(3):715–20. [DOI] [PubMed] [Google Scholar]
- 28.Rud T. Urethral pressure profile in continent women from childhood to old age. Acta Obstet Gynecol Scand 1980;59(4):331–5. [DOI] [PubMed] [Google Scholar]
- 29.Carlile A, Davies I, Rigby A, Brocklehurst JC. Age changes in the human female urethra: A morphometric study. J Urol 1988;139(3):532–5. [DOI] [PubMed] [Google Scholar]
- 30.Clobes A, DeLancey JOL, Morgan DM. Urethral circular smooth muscle in young and old women. Am J Obstet Gynecol 2008;587:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perucchini D, DeLancey JOL, Ashton-Miller JA, Galecki A, Schaer GN. Age effects on urethral striated muscle I. Change in number and diameter of striated muscle fibers in ventral urethra. Am J Obs Gynecol 2002;186(3):356–60. [DOI] [PubMed] [Google Scholar]
- 32.Pandit M, DeLancey JOL, Ashton-Miller JA, Iyengar J, Blaivas MILA, Perucchini D. Quantification of intramuscular nerves within the female striated urogenital sphincter muscle. Obstet Gynecol 2000;95(6):797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snooks SJ, Badenoch DF, Tiptaft RC, Swash M. Perineal Nerve Damage in Genuine Stress Urinary Incontinence; An Electrophysiological Study. Br J Urol 1985;57(4):422–6. [DOI] [PubMed] [Google Scholar]
- 34.Smith ARB, Hosker GL, Warrell DW. The role of partial denervation of the pelvic floor in the aetiology of genitourinary prolapse and stress incontinence of urine. A neurophysiological study. BJOG An Int J Obstet Gynaecol 1989;96(1):24–8. [DOI] [PubMed] [Google Scholar]
- 35.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Urinary Incontinence after Vaginal Delivery or Cesarean Section. Vol. 10, N Engl J Med 2003. [DOI] [PubMed] [Google Scholar]
- 36.DeLancey JOL, Miller JM, Kearney R, Howard D, Reddy P, Umek W, et al. Vaginal birth and de novo stress incontinence: Relative contributions of urethral dysfunction and mobility. Obstet Gynecol 2007;110(2 I):354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Geelen JM, Lemmens W, Eskes T, Martin CB. The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obs Gynecol 1982;144:636–49. [DOI] [PubMed] [Google Scholar]
- 38.Le Coutour X, Jouffroy C, Beuscart R, Renaud R. Influence de la grossesse et de l’accouchement sur la fonction de clôture cervico-urétrale. I. Etude prospective chez 27 femmes [Effect of pregnancy and delivery on the function of the cervico-urethral closure. I. Prospective study of 27 women]. J Gynecol Obs Biol Reprod 1984;13(7):771–4. [PubMed] [Google Scholar]
- 39.Snooks SJ, Swash M, Setchell M, Henry MM. Injury To Innervation of Pelvic Floor Sphincter Musculature in Childbirth. Lancet. 1984;324(8402):546–50. [DOI] [PubMed] [Google Scholar]
- 40.Smith ARB, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. BJOG An Int J Obstet Gynaecol 1989;96(1):29–32. [DOI] [PubMed] [Google Scholar]
- 41.Allen RE, Hosker GL, Smith ARB, Warrell DW. Pelvic floor damage and childbirth: A neurophysiological study. Obstet Gynecol Surv 1991;46(4):209–10. [DOI] [PubMed] [Google Scholar]
- 42.Dolan LM, Hosker GL, Mallett VT, Allen RE, Smith ARB. Stress incontinence and pelvic floor neurophysiology 15 years after the first delivery. BJOG An Int J Obstet Gynaecol 2003. December 1;110(12):1107–14. [PubMed] [Google Scholar]
- 43.Gachon B, De Tayrac R, Schmitz T, Mahmood T, Nizard J, Fritel X. Should we advise women that pre-labor caesarean section prevents pelvic floor dysfunction? Eur J Obstet Gynecol Reprod Biol 2020;244:31–4. [DOI] [PubMed] [Google Scholar]
- 44.Wesnes SL, Rortveit G, Bo K, Hunskaar S. Urinary incontinence during pregnancy. Am J Obs Gynecol 2007; [DOI] [PubMed] [Google Scholar]
- 45.Wesnes SL, Hunskaar S, Bo K, Rortveit G. The effect of urinary incontinence status during pregnancy and delivery mode on incontinence postpartum. A cohort study*. BJOG. 2009;116:700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weidner AC, South MMT, Sanders DB, Stinnett SS. Change in urethral sphincter neuromuscular function during pregnancy persists after delivery. 2009; [DOI] [PMC free article] [PubMed]
- 47.Subak LL, Richter HE, Hunskaar S. Obesity and Urinary Incontinence: Epidemiology and Clinical Research Update. J Urol 2009;182(6 SUPPL.):S2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vaughan CP, Auvinen A, Cartwright R, Johnson TM, Tähtinen RM, Ala-Lipasti MA, et al. Impact of obesity on urinary storage symptoms: Results from the FINNO study. J Urol 2013;189(4):1377–82. [DOI] [PubMed] [Google Scholar]
- 49.Swenson CW, Kolenic GE, Trowbridge ER, Berger MB, Lewicky-Gaupp C, Margulies RU, et al. Obesity and stress urinary incontinence in women: compromised continence mechanism or excess bladder pressure during cough? Int Urogynecol J 2017;28(9):1377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]