Abstract
Introduction
Over 100 million adults in the United States have hypertension. The DASH (Dietary Approaches to Stop Hypertension) eating pattern is an evidence-based first-line treatment option for hypertension; however, adherence to the DASH eating pattern at a population level remains low. To address this gap, we will implement Nourish, a randomized controlled efficacy trial that will leverage a commercially-available smartphone application and evidence-based behavior change principles to improve adherence to the DASH eating pattern among adults with hypertension.
Methods
The Nourish trial is a two-arm, 12-month randomized control trial that will enroll adults (N=300) with hypertension, defined as a systolic blood pressure of 120-159 mmHg; a diastolic blood pressure of 80-99 mmHg; and/or adults on blood pressure-lowering medication. Nourish will test the efficacy of a digital health intervention, as compared to the attention control arm, on DASH eating pattern adherence and blood pressure. Intervention components will include skills training, self-monitoring, personalized feedback, and responsive coaching. The primary outcome of the trial is 6-month changes in adherence to the DASH eating pattern, as measured by 24-hour dietary recalls.
Discussion
Millions of Americans remain in need of effective behavioral interventions to manage and improve their hypertension and its adverse consequences. The ubiquity of smartphones offers a promising approach to disseminate the DASH eating pattern. By leveraging these widely used smartphone applications, combined with evidence-based behavior change principles and the DASH eating plan, Nourish will demonstrate the effectiveness of a digital health intervention to improve DASH adherence, and ultimately, to reduce blood pressure.
Keywords: hypertension, dietary quality, mHealth, randomized controlled trial, nutrition, high blood pressure
Introduction
Nearly one in two adults has hypertension in the United States (U.S.).1 Hypertension is a leading risk factor for stroke, kidney disease, heart disease, and premature mortality.2-4 Lifestyle treatment approaches, including dietary interventions, are recommended for the 108 million adults with hypertension, regardless of pharmacological therapy recommendations.1,5 The American College of Cardiology and the American Heart Association recommends the DASH (Dietary Approaches to Stop Hypertension) eating pattern as a nonpharmacological intervention for hypertension that can be implemented on its own or in combination with hypertensive medications.5
DASH has been shown to significantly lower blood pressure in various populations and is particularly effective for individuals with hypertension.6 In fact, among those with hypertension in a seminal DASH trial, the DASH eating pattern lowered systolic and diastolic blood pressure by 10.7 mmHg and 4.7 mmHg, respectively, compared to a typical U.S. diet.7 Importantly, the positive effects of DASH have been observed among adults from diverse backgrounds, including Black Americans and persons who are socioeconomically disadvantaged.6,7 Despite the efficacy of DASH, adherence to the eating pattern in behavioral trials is lower than in feeding trials8 and national data indicate that adoption of DASH at a population level is suboptimal.9 Innovative efforts are needed to disseminate DASH and increase adherence rates across the U.S. to ultimately lower blood pressure.
The proliferation of smartphone ownership offers a promising dissemination channel to encourage DASH eating pattern engagement. Around 81% of U.S. adults own a smartphone, with widespread use across different sociodemographic groups, including Black (80%) and Hispanic (79%) individuals; individuals earning less than $30,000 annually (71%); and individuals living in rural areas (71%).10 Smartphone owners have access to mobile applications (apps), including the top diet tracking apps, which are downloaded and used by millions of users each year.11 These publicly-available apps offer sophisticated tracking interfaces that are linked to robust nutritional databases, enabling users to quickly track their dietary intake and obtain detailed nutrition information. Despite the popularity and advanced user interface of these apps, they are often not supported by evidence-based behavior change principles, limiting the potential of their impact.12-14 Moreover, there is limited research regarding the effectiveness of using these apps, in combination with evidence-based behavior change principles, to achieve dietary behavior change for the self-management of hypertension.12
Combining the large-scale reach of smartphone apps with evidence-based behavior change techniques to improve adoption of the evidence-based DASH eating pattern increases the potential to reduce blood pressure on a population level. As such, we describe the Nourish trial, a 12-month randomized controlled efficacy trial that combines a dietary smartphone app and evidence-based behavior change techniques,15 including DASH education and skills training,16,17 nutrient goal setting,18,19 daily dietary tracking/self-monitoring,18,20,21 text message feedback,19,22,23 and responsive health coaching,24,25 to improve adherence to the DASH eating pattern among U.S. adults with hypertension.
Methods
The Nourish trial is a two-arm, 12-month randomized control trial (N=300) that tests the efficacy of a digital health intervention, as compared to an attention control arm, on DASH eating pattern adherence, blood pressure, and other clinical, behavioral, and psychosocial outcomes. The primary outcome of the trial is 6-month change in adherence to the DASH eating pattern as measured by 24-hour dietary recalls. The secondary outcome is 6-month change in blood pressure as measured during online study visits. Initial approvals from the Duke Health Institutional Review Board were obtained in 2019.
Population
The Nourish trial will enroll adults (≥18 years of age) with hypertension, defined as a systolic blood pressure of 120-159 mmHg; a diastolic blood pressure of 80-99 mmHg;5 and/or adults on blood pressure-lowering medications. Individuals with a systolic or diastolic blood pressure above these ranges will not be enrolled out of concern for participant safety. Because this is a digital health trial, eligible participants must have a smartphone with a data plan and an active email address; be willing to receive daily text messages; and be able to participate in online video conferencing visits. Eligible participants must also have a BMI ≥18.5 kg/m2 and reside in the U.S. Lastly, due to budgetary and time constraints to adapt intervention materials, participants must be fluent in English to enroll in Nourish.
Individuals will be excluded from Nourish if they have: a cardiovascular disease event in the previous 6 months; active cancer; bariatric surgery in the previous 12 months or planned bariatric surgery within the next 12 months; recent hospitalization due to a psychiatric condition or event; documented dementia; or are pregnant or planning to be pregnant during the study period. Individuals who are currently participating in another related research study will also be excluded from Nourish.
Intervention Delivery
The intervention will be delivered remotely using data collected through a smartphone app, which contains dietary tracking capability and educational content. The app is hosted by Pattern Health, a software company, and will provide text message feedback about adherence to the DASH eating pattern; motivational messages to support behavior change; and skills training. There will be no in-person research visits required during the intervention period.
Participant recruitment
Our target enrollment is 300 randomized participants. To ensure a diverse sample with broad generalizability, we aim to recruit 120 participants (40% of our total sample) who identify as a member of a racial and/or ethnic group that is underrepresented in research.26,27 We also aim to recruit at least 90 participants (30% of our total sample) who identify as male. In order to do so, we will use a multi-pronged approach. First, we will list Nourish on clinical trial websites hosted by Duke University and the National Institutes of Health. Second, we will leverage social media platforms, such as Facebook, Twitter, and Reddit, to disseminate trial information. Third, we will use ResearchMatch, a national health volunteer registry, to identify and contact adults residing in the U.S. with hypertension. ResearchMatch has a large population of volunteers who have consented to be contacted by researchers about health studies for which they may be eligible. Fourth, we will identify patients at Duke Health with hypertension using a computable phenotype in the electronic health record. Individuals who have active patient portals (logged into within the last 12 months) will be contacted through patient portal messaging and individuals who lack an active patient portal will be contacted via email. The third and fourth recruitment methods are especially critical to enroll a wide array of participants with varying sociodemographic characteristics (e.g., race, ethnicity, geographic location, gender, etc.). Fifth, we will leverage networks within community organizations, such as local churches, health departments, and other community-based organizations with a health focus. Lastly, we will recruit Black and African American individuals participating in DIP (Digital Intervention Participation) in DASH (NCT04515303), a supplemental formative research grant that aims to elicit the barriers and facilitators to hypertension management for Black and African American adults.
All recruitment sources will direct individuals to visit the Nourish trial website which will include details regarding the trial and trial participation. The website also includes contact information for study staff and a link to complete a pre-screening online eligibility survey.
Participant screening and pre-randomization assessments
The pre-screening eligibility survey will be administered via REDCap (Research Electronic Data Capture), a secure, web-based software platform designed to support data capture and management for research studies.28,29 The survey will include questions regarding major eligibility criteria, as described above. If eligible, the potential participant will be directed to an electronic consent form and an additional online evaluation survey. Following completion of these steps, the participant will be asked to complete two 24-hour dietary recalls using the Automated Self-Administered 24-hour (ASA24®) recall tool developed by the National Cancer Institute. The ASA24 provides comprehensive nutrient data on all foods and beverages consumed during the previous 24-hour period. If both ASA24s are completed, the participant is invited to participate in a secure video conference visit (visit 1), conducted via Zoom, a cloud-based videoconferencing service.30 During visit 1, study staff will orient the participant to the study, the Nourish dietary tracking app, and use of the at-home blood pressure monitor to be used during future data collection visits. Following completion of the first video conference visit, participants will be asked to track their daily intake for the next 7 days using the smartphone app. Daily dietary tracking is considered complete if the participant tracks ≥600 calories in one day, as this is typically the minimum level of calories needed to determine if the data represent a complete day of tracking.31
If all of the above tasks are completed, visit 2 is confirmed and participants are shipped study materials, which includes a measuring tape and an at-home digital blood pressure monitor (Omron® Series 3, Model BP7100). Final eligibility is determined during visit 2 based on the average of three blood pressure readings. Additional detail on the measurement of blood pressure is described below (see Data collection and outcome assessments). Participants with an average systolic blood pressure ≥160 mm Hg or a diastolic blood pressure ≥100 mm Hg are excluded at this point. See Figure 1 for visit flow.
Figure 1. Nourish study diagram and participant workflow.
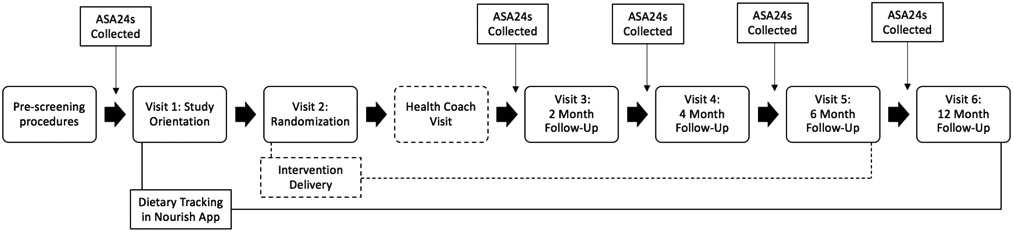
Note: Dashed line indicates intervention only event
Randomization
We will randomize eligible participants at visit 2 to one of two arms using a stratified block randomization method. Participants will be stratified by their baseline blood pressure risk category and blood pressure medication status to minimize the imbalance of these important prognostic factors between study arms. Blood pressure risk categories, including elevated blood pressure, hypertension stage 1 and hypertension stage 2, will be defined using the current American Heart Association guidelines. Allocation tables with random block sizes of 2, 4, 6 or 8 will be created by the study statistician using an online randomization service (www.sealedenvelope.com)32 and stored securely within REDCap. The random assignment will be revealed to the study staff and participant during visit 2. Trial investigators will be blind to participant allocation status.
Treatment arms
Participants will be assigned to one of two treatment arms: the intervention arm or the attention control arm. Both treatment arms are asked to track what they eat and drink every day using the Nourish smartphone application (Nourish app). Both treatment arms also receive educational materials and skills training on the DASH eating pattern within the Nourish app. The intervention arm receives daily nutrients goals, daily text messages and personalized feedback, a health coach visit, and may also receive responsive coaching, an algorithmic coaching approach that varies based on progress toward DASH adherence. The intervention components are described in greater detail below.
Health coach visit
All intervention arm participants will be onboarded at an initial videoconferencing visit with a Nourish registered dietitian shortly after randomization. This visit is expected to last 20 minutes. During this visit, the registered dietitian will discuss the DASH eating plan, answer any nutrition-related questions, and use motivational interviewing principles to elicit intrinsic motivation, engage in problem-solving, and facilitate increased engagement with using the Nourish smartphone app and adopting the DASH eating pattern.
Follow-up visits
There will be a total of 4 follow-up visits after randomization, as depicted in Figure 1. All follow-up visits will be held in video conference format via Zoom. These will be held at 2-months, 4-months, 6-months and 12-months post-randomization.
Intervention components
Skills training
Both treatment arms will have access to several skills training videos and informational documents within the Nourish app. These videos and documents will cover a variety of topics, including an overview of the DASH nutrients and daily targets; adapting the DASH eating pattern, including menu-planning while following DASH; dining out on the DASH eating pattern; and adapting the DASH eating pattern on a budget. Evidence-based behavioral principles will be used to develop additional skills training on dietary behavior change including intuitive eating, managing stress, self-monitoring, and improving social support. Electronic versions of government-created materials, such as the NHLBI booklet describing the DASH eating pattern and recipes for the DASH eating plan, will also be electronically available within the Nourish app. Intervention curriculum is outlined in Supplementary Table 1.
Daily dietary tracking
As described above, both treatment arms will be asked to track their daily dietary intake using the Nourish app, which is powered by a nutritional database created and maintained by Nutritionix (www.nutritionix.com). Nutritionix has a comprehensive database that includes all data available from the USDA Food Composition Database, data from restaurant chains, and foods added by registered dietitians at Nutritionix. The Nourish app will store foods and drinks that a participant regularly logs for convenience and quick tracking. It will also contain a “recipe” feature in which a participant can add favorite recipes; these recipes will be locally stored within the participant’s app and will be available for the participant to track. For foods in the Nutritionix database with incomplete nutrient data, particularly nutrients recommended in the DASH eating pattern (e.g., magnesium, potassium) registered dietitians on the Nourish staff will manually populate these data for the intervention.
Goals
The intervention arm will receive daily goals to encourage dietary tracking and adherence to the DASH eating pattern (see Table 1) during the active 6-month intervention phase. These goals will occur over four modules, detailed in Table 1. Module one will focus on dietary tracking; modules two and three will focus on achieving target ranges for micro and macro nutrients important for DASH adherence as noted by Mellen et al.9 These include potassium, fiber, calcium, magnesium, sodium, saturated fat and protein. Each week will focus on an individual nutrient and in module four, a DASH adherence summary score of these seven nutrients will be generated; the participant’s goal is to achieve a score based on target nutrient ranges. We chose not to include intervention goals for total fat or cholesterol. The 2015-2020 Dietary Guidelines for Americans, which were current at the time of study development,33 do not include explicit guidance for dietary cholesterol, a notion supported by the American Heart Association and the American College of Cardiology, or restrictions on total fat intake.34 Evidence supporting the need to reduce dietary cholesterol for blood pressure reduction is limited and cholesterol is not considered a nutrient of concern for overconsumption.35 Additionally, the DASH eating pattern naturally supports limited consumption of dietary cholesterol, which coexists with food sources of saturated fat, and increased consumption of unsaturated fatty acids, found in nuts, seafood and plant foods.
Table 1.
Nourish Intervention Goals and Feedback Schedule
| Topic | Module 1 | Module 2 | Module 3 | Module 4 |
|---|---|---|---|---|
| Dietary tracking | Weeks 1 and 2 | --- | --- | |
| Potassium | --- | Week 3 | Week 10 | --- |
| Fiber | --- | Week 4 | Week 11 | --- |
| Calcium | --- | Week 5 | Week 12 | --- |
| Sodium | --- | Week 6 | Week 13 | --- |
| Magnesium | --- | Week 7 | Week 14 | --- |
| Saturated Fat | --- | Week 8 | Week 15 | --- |
| Protein | --- | Week 9 | Week 16 | --- |
| All nutrients | --- | --- | --- | Weeks 17 to 24 |
Feedback
Intervention arm participants will receive daily responsive feedback, based on their dietary tracking data during the active 6-month intervention phase, and tailored to the goal for the corresponding week of the intervention. Specifically, our system will calculate participant adherence to the DASH eating pattern using target ranges for the micro and macro nutrients. Participants will receive tailored feedback messages that will begin with a personalized greeting (e.g., Hello, [name]) and include the participant’s DASH adherence from the previous day (e.g., Nice job working on potassium this week. Your average potassium intake was X.).
Self-identified participant characteristics (e.g., having young children in the home; working full-time; eating out often; dietary restrictions, etc.) will also be incorporated into the daily automated text message feedback. Figure 2 illustrates how personal characteristics will be used to determine automated feedback for two different participants in the study who have the same nutrient intake. In addition to scores for DASH adherence, our system will calculate changes in scores over time, such as change from the previous day, week or month, to inform feedback.
Figure 2.
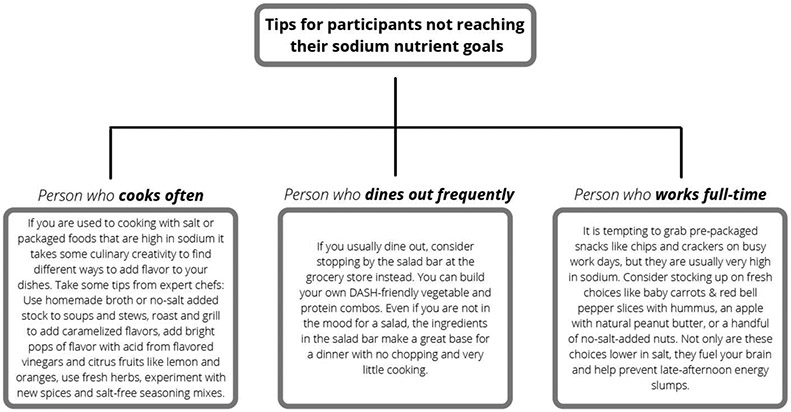
Personalized feedback for Nourish intervention-arm participants
Participants allocated to the intervention arm will also receive motivational messages based on core principles of Social Cognitive Theory and the Social Ecological Model.36 Examples of these motivational messages can be found in Figure 3.
Figure 3.
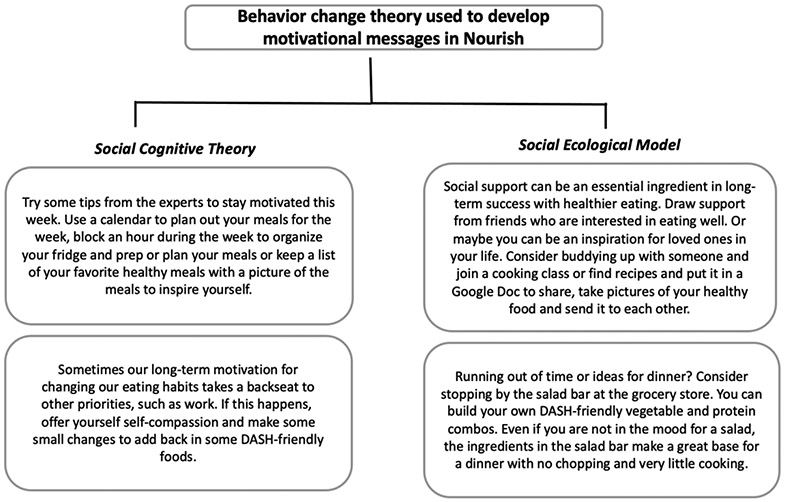
Behavior change theory used to develop motivational messages for Nourish intervention-arm participants
Responsive coaching
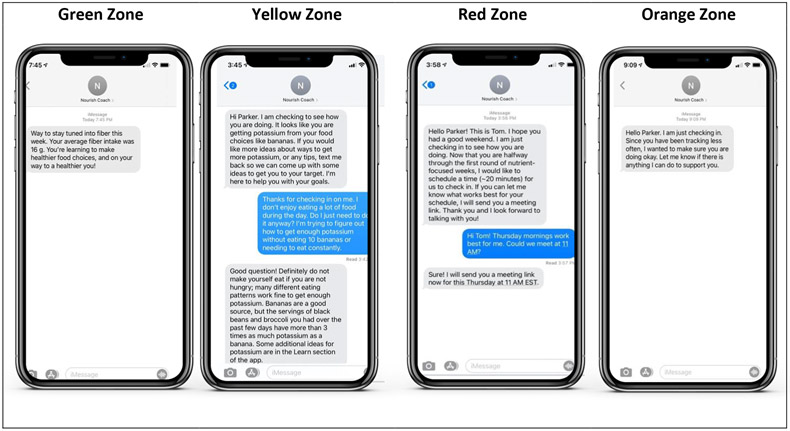
During the active 6-month intervention phase, participants allocated to the intervention arm may also receive responsive coaching from a Nourish registered dietitian depending on their level of engagement with the Nourish app and how well they are meeting DASH eating pattern targets. The Nourish registered dietitian is trained in nutritional counseling, motivational interviewing, and intuitive eating principles. Responsive coaching will be delivered via text message, video and/or phone-based modalities and will be informed by an algorithm that utilizes dietary tracking data and participant characteristics, as described above. Our system will use the dietary tracking data to automatically categorize participants into one of four progress zones: orange, green, yellow, or red. Zones will be determined based upon adherence to DASH nutrient goals and engagement in dietary tracking. The zones inform the frequency, intensity, and mode of the responsive coaching. All coaching interactions are captured and deployed by computer algorithms in a custom server behind the Nourish app. See Figure 4 for zone description and sample messages.
Figure 4.
Responsive coaching for Nourish intervention-arm participants
Orange zone
Participants will be automatically categorized into the orange zone if they do not engage in dietary tracking in the Nourish app by tracking ≥600 calories for at least one day per week. Orange zone participants will be sent automated texts encouraging tracking. If participants do not engage for more than seven days consecutively, a custom text will be sent by the registered dietitian. After over 14 days of consecutively not tracking, the registered dietitian will make an additional attempt with the participant to employ motivational interviewing to identify barriers and facilitators to re-engagement.
Green zone
Participants will be categorized into the green zone if they track ≥600 calories per day for three or more days of the week and meet weekly targets for specific DASH nutrients (Table 1) on at least half of those days. Green zone participants will receive no responsive coaching; they will receive automated text messages congratulating them on their adherence.
Yellow zone
Participants will be categorized into the yellow zone if they track ≥600 calories per day for three or more days of the week and they are not meeting their weekly nutrient target on more than half of those days. Yellow zone participants will receive brief coaching through text messages from the registered dietitian. The purpose of the texting is to provide psychoeducation about changes in dietary intake, to enhance motivation and efficacy for behavior change, and/or to engage in problem-solving.
Red zone
Participants will be automatically categorized into the red zone if they do not meet their nutrient target ranges (are in the yellow zone) for three consecutive weeks. When a participant enters the red zone, the registered dietitian will contact the participant to schedule a coaching call. These calls will be approximately 15-20 minutes and will be performed using the participant’s preferred modality of choice, either video or phone. These sessions are designed to assess and enhance motivation and self-efficacy for behavior change, deliver in-depth behavioral skills training, and provide social support. On each call, the registered dietitian will: 1) review changes in DASH nutrient adherence and self-monitoring data; 2) discuss problem-solving strategies; 3) deliver skills training content; and 4) discuss community resources, if applicable. Participants who do not meet nutrient goals in subsequent weeks after being categorized in the red zone will remain in the red zone until they complete a nutrient-focused week where they meet a nutrient goal on at least half of the days they track ≥600 calories.
Data collection and outcome assessments
Self-reported sociodemographic characteristics, including age, race, ethnicity, gender identity, health insurance status, income, employment status, education, household size, eating habits, and technology usage, will be solicited prior to randomization through REDCap online surveys. Outcome assessments, detailed below, will occur throughout the course of the trial period. Additional details regarding outcome assessments can be found in Table 2.
Table 2.
Nourish Schedule of Outcome Assessments
| Measure | Screening | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 |
|---|---|---|---|---|---|---|---|
| Primary Outcome: | |||||||
| ASA 24 Dietary Tracking | ASA-24 sent after baseline survey | - | - | ASA-24 sent two weeks prior | ASA-24 sent two weeks prior | ASA-24 sent two weeks prior | ASA-24 sent two weeks prior |
| Secondary Outcome: | |||||||
| Blood Pressure | - | - | ✓ | ✓ | ✓ | ✓ | ✓ |
| Clinical, Behavioral, & Psychosocial Outcome | |||||||
| Self-Reported Weight | ✓ | - | - | - | - | - | - |
| Blood Pressure Medication | ✓ | - | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blood Pressure Adherence | ✓ | - | - | - | - | ✓ | ✓ |
| Physical Activity | ✓ | - | - | - | - | ✓ | ✓ |
| Self-Efficacy | ✓ | - | - | - | - | ✓ | ✓ |
| Intuitive Eating | ✓ | - | - | - | - | ✓ | ✓ |
| Depressive Symptoms | ✓ | - | - | - | - | ✓ | ✓ |
| Everyday Discrimination | ✓ | - | - | - | - | - | - |
| Experiences of Discrimination | ✓ | - | - | - | - | - | - |
DASH adherence
The primary outcome of the trial is 6-month change in DASH adherence. To assess change in adherence to the DASH eating pattern, dietary intake will be measured using the ASA24® recall tool. The ASA24 is an automated tool that uses USDA’s validated multiple pass method to obtain total intake of all food and beverages throughout a given day.37 Participants will be asked via email to complete 2 separate dietary recalls (1 weekday and 1 weekend day) within a 14-day period prior to randomization and at 2-months, 4-months, 6-months and 12-months post-randomization. These data will be used to calculate a nutrient-based DASH score, using a validated scoring index developed by Mellen and colleagues.9 The Mellen et al. index uses a 9-point scale based on the previous day’s intake for potassium, sodium, magnesium, calcium, saturated fat, total fat, total protein, cholesterol, and fiber. Nutrient targets are standardized to total calorie intake. A score of 0, 0.5, or 1 will be applied to each nutrient based on distance from the recommended target. Individual nutrient scores are then summed to calculate a total DASH adherence score. The range is from 0 to 9; higher scores indicate greater adherence to the DASH eating pattern with 9 representing full adherence.
Blood pressure
At-home blood pressure measurements will occur during select video conference visits (Table 2). A standardized protocol will be followed by trained research staff to guide participants in taking their blood pressure during video conference visits. Participants will be instructed to be on-camera and observed by the research staff for the duration of the visit, including during blood pressure measurements. Prior to randomization, an Omron® Series 3 blood pressure monitor and a measuring tape will be shipped to the participant’s home. Participants will also be shown a video made by the American Heart Association to orient them to taking an accurate at-home blood pressure measurement.38 Participants will be asked to measure their upper arm circumference at the randomization visit. This measurement will inform placement of blood pressure cuff (upper arm vs. forearm); a forearm measurement will be taken if the upper arm circumference is greater than 17.5 inches. After guiding the participant through the arm measurement and cuff placement, trained research staff will ask the participant to sit with their back supported and feet flat on the floor. The first blood pressure reading will occur after an initial five-minute quiet sitting period and the following two readings will take place after one-minute quiet sitting intervals. After each reading, the participant will be asked to hold up the blood pressure device to the camera, showing the research staff each blood pressure reading. An average of the three readings is used to establish eligibility for participant randomization. However, only the second and third readings will be averaged for analysis of a secondary outcome: blood pressure change between Visit 2 (randomization), Visit 5 (6-months post-randomization) and Visit 6 (12-months post-randomization).
Clinical, behavioral and psychosocial outcomes
Physical activity, self-efficacy for healthy eating, intuitive eating behaviors, and depressive symptoms will be collected via online surveys administered using REDCap at baseline screening and at visits 5 and 6, i.e., 6 and 12 months. Perceived discrimination will be assessed at baseline screening. All prescription medication (including names, dosage etc.) will be self-reported in the online survey at baseline screening and at 6 and 12 months; blood pressure medication adherence will also be assessed at these timepoints via survey. Prescriptions will also be confirmed and recorded by the study staff at each videoconference visit. Additional details on all study measures are displayed in Table 2.
Engagement
Intervention engagement will be calculated by dividing the number of days with valid dietary tracking data (numerator) over the total number of possible tracking days (denominator). We will calculate and compare engagement rates by week and intervention period (i.e., months 0 to 6 verse months 6 to 12). Using typical standards for dietary assessment, if the daily calorie intake is <600 calories, that tracking day will be considered incomplete and will be marked as invalid.37,39
Analytic Approach
Power and Sample Size
We hypothesize that the intervention will lead to an increase in average DASH score of 2 units at 6 months and the control arm will achieve a 1-unit increase in DASH score. These estimates are based on the pilot study we conducted where both intervention and control arms achieved a 1-unit increase in DASH adherence from baseline to 3 months.40 Given the Nourish intervention is more intensive than what was delivered in the pilot, we anticipate a greater increase in DASH adherence. We estimate a standard deviation of 2 units for both intervention and control arms. This results in an effect size of 0.5, which is considered a medium effect size by Cohen’s d.41 With 90% power and a type I error rate (alpha) of .01, a sample size of 242 will allow us to detect a difference of 1 unit in DASH adherence mean change score between study arms. We conservatively assume 20% attrition for this length of follow-up, resulting in the final analytic sample size of 300 total participants. With this sample size, we will also have power to examine intervention effects in systolic and diastolic blood pressure. Using data from the pilot study we anticipate systolic blood pressure changes in intervention vs control as follows: −3.4 (SD=8.6) mmHg and −2 (SD=9.3) mmHg respectively, which results in an effect size of 0.6. The effect size for diastolic blood pressure is 0.75 based on changes in intervention vs control of −3.8 (SD=7.9) mmHg vs. −1.6 (SD=6.4) mmHg, respectively. With 90% power and a type I error rate (alpha) of .01, we can detect the expected effect size with the proposed sample size of 300.
Statistical analysis plan
Demographic and baseline data will be examined for integrity of randomization scheme and the homogeneity of study arms. If any demographic and baseline data are not balanced between the two study arms and are believed to affect the outcome, we will consider adjusting these variables in the modeling process. All analyses will be based on intention-to-treat (ITT) principles.
We will assess the overall intervention effect on change of DASH score from baseline to 6 months by a two-sample t-test. To understand how DASH score improves over time from baseline to 6 months, and sustains through 12-month follow-up between study arms, we will plot observed DASH score vs. time (baseline, 2-month, 4- month, 6-month, 12-month) by two study arms. Linear mixed models will be built to test the primary hypotheses. If we observe a linear trend in the previous plot, our model will contain fixed effects including a group variable (intervention vs. control), time, and intervention by time interaction. To account for the correlations within the same participant over time, we will include a random intercept and random slope for time in the model. If there is a quadratic trend in time, we will also include a quadratic term for time in the model. To account for the stratified block randomization design, our models will also include our randomization stratification variables, blood pressure risk category and blood pressure medication status.42 If any other demographic or baseline variables are statistically different between the two study arms, then these variables will be included in a stepwise model selection process for the linear mixed model. We will evaluate the primary hypotheses by testing the null hypothesis that the time by intervention parameter is 0. Similar analysis will be conducted for changes in blood pressure and relevant clinical, behavioral and psychosocial variables. If the distribution of any of the outcomes is heavily skewed, then we will either appropriately transform the data so it’s normally distributed or use a generalized linear mixed model with an appropriate link function.
Our linear mixed models will be fitted with a full maximum likelihood estimation using all available data to address missing data. These models allow for responses to be missing at random (MAR), where the missing mechanism may be related to either observed covariates or response variables, but not related to the unobserved data. Linear mixed models provide valid estimates of the intervention effect under the MAR mechanism and allow for a broad array of possible reasons for missingness.
Discussion
The burden of hypertension cannot be overstated. As an efficacious first-line treatment option, increased adherence to the DASH eating pattern could have a substantial impact on the prevention and management of hypertension. However, innovative and accessible strategies to disseminate DASH are critically needed for the majority of Americans who do not follow the DASH eating pattern. The current trial addresses this need by testing the efficacy of a 6-month digital-health intervention on improving DASH adherence and blood pressure in U.S. adults with hypertension.
Like many other chronic diseases and conditions, stark disparities exist in rates of hypertension across racial, ethnic, and socioeconomic groups.43,44 Black Americans experience higher rates of hypertension compared to their White counterparts.44 They also possess lower rates of hypertension control. Similarly, individuals with lower income and those residing in rural areas experience higher rates of hypertension compared to their higher income and urban-residing counterparts.43,45 Unfortunately, these groups often face myriad barriers to obtaining healthcare and hypertension treatments,46 such as distance from and/or transportation challenges to access healthcare providers and facilities.47 The ubiquity of smartphone apps offers a unique opportunity to reach the populations that are disproportionately affected by hypertension and overcome frequently faced barriers. In fact, smartphone ownership continues to grow in the U.S., with ownership rates exceeding 70% across most age groups, racial and ethnic groups, socioeconomic levels, and among individuals residing in urban, suburban and rural areas.10 Likewise, the use of health and fitness apps continues to climb annually, reaching 87.4 million users in 2020.11 Nourish will leverage these widely used digital technologies to test a remotely delivered intervention to adults from all sectors of the U.S. looking to manage their hypertension. If efficacious, the intervention can be scaled to positively impact population health.
In effort to further increase the inclusivity of Nourish, we will adopt several principles from the Health at Every Size (HAES),48 which disentangles health from weight status and allows for a more holistic and multifaceted definition of health at any size, to offer a weight-inclusive approach to hypertension management.49 In doing so, Nourish will de-emphasize messaging about weight loss or caloric monitoring in recruitment materials and intervention content. For instance, the Nourish app will be designed to omit caloric monitoring completely, making it more applicable to HAES principles. Further, the DASH scoring approach and personalized feedback will be nutrient-focused, focusing on the addition of health-promoting DASH-congruent foods, rather than dietary restriction, and will encourage intuitive eating principles50 (i.e., honoring internal hunger and fullness cues). Study staff, including registered dietitians, will be trained to deliver content in a weight-inclusive manner and every effort will be made to depict a wide range of body sizes in recruitment and intervention materials (e.g., website, instructional videos). Structural barriers will be modified to allow Nourish to recruit individuals at all body sizes, such as implementing a robust protocol for blood pressure measurement using the forearm if blood pressure cuffs are too small to accommodate a participant’s upper arm. We believe this approach allows for increased acceptability of Nourish across populations that are often overlooked in traditional weight-normative approaches to hypertension management.
Although we chose to use a nutrient-based scoring approach for this intervention,9 we recognize that there are alternative ways to measure DASH adherence, such as the food-group-based approach,51 that would be valuable to the participant and to the interpret the results. Due to the limitations of the automated feedback algorithms, we are unable to create a summary score based on food-level data or provide automated feedback on specific food intake. We will, however, incorporate suggestions within the personalized text feedback and health coaching sessions regarding which foods participants can consume/limit to increase/decrease certain nutrients, and thus, improve their DASH scores.
The COVID-19 pandemic necessitated innovative modifications to maintain the health and safety of trial participants and research personnel alike. The original Nourish protocol included in-person recruitment, research visits and data collection, however, as a response to the pandemic, we adapted our protocol to be fully remote. There are limitations to these modifications. First, it necessitated using predominantly online recruitment approach, which may have implications on the generalizability of our trial sample. Online recruitment methods, such as patient portal messaging, have been shown to reach predominately White and non-Hispanic populations.52,53 Second, it limited our ability to collect urine and blood specimens, in addition to in-person blood pressure measurements. Third, due to the pandemic, we will not be able to perform a verification of our at-home blood pressure protocol or the Omron® Series 3 devices against a clinic-based gold standard measurement for each participant.
There were also strengths to adopting a fully remote protocol. Instead of limiting participation to individuals residing within a commutable distance of our study center, it allowed us to open enrollment to adults across the U.S., thus, increasing the representativeness of our sample population and generalizability of our results. Second, if successful, these remote protocols can be adapted in future trials to increase the accessibility of our interventions and findings to adults living across the U.S. from diverse groups.
As the absolute burden of hypertension continues to increase in the U.S., sustainable and scalable efforts aimed at prevention and control are critically needed.54 The use of publicly available smartphone apps, in conjunction with evidence-based behavior change principles, offers a scalable approach to treat and prevent chronic diseases, like hypertension, and its related co-morbidities. This trial offers a widely available treatment option for the millions of Americans who remain in need of effective behavioral interventions to manage and improve their hypertension and its adverse consequences.
Supplementary Material
Acknowledgements
This trial is funded by the National Heart, Lung and Blood Institute at the National Institutes of Health (R01HL146768). The funder had no role in study design, data collection, data analysis and interpretation of data, in the writing of the report, and in the decision to submit this article for publication.
We wish to express our gratitude to the following collaborators: Jeff Cohen at Software for Research, Pattern Health, all community-based organizations, and the Duke Clinical and Translational Science Institute Community Engaged Research Initiative (CERI).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ostchega Y, Fryar CD, Nwankwo T, Nguyen DT. Hypertension Prevalence Among Adults Aged 18 and Over: United States, 2017-2018. NCHS Data Brief. 2020;(364):1–8. [PubMed] [Google Scholar]
- 2.Health Threats From High Blood Pressure. The American Heart Association. https://www.heart.org/en/health-topics/high-blood-pressure/health-threats-from-high-blood-pressure. Published 2016. Accessed January 11, 2021. [Google Scholar]
- 3.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. 2018;3(7):572–581. doi: 10.1001/jamacardio.2018.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray CJL, Atkinson C, Bhalla K, et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task. Hypertens (Dallas, Tex 1979). 2018;71(6):1269–1324. doi: 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 7.Svetkey LP, Simons-Morton D, Vollmer WM, et al. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;159(3):285–293. doi: 10.1001/archinte.159.3.285 [DOI] [PubMed] [Google Scholar]
- 8.Kwan MW-M, Wong MC-S, Wang HH-X, et al. Compliance with the Dietary Approaches to Stop Hypertension (DASH) diet: a systematic review. PLoS One. 2013;8(10):e78412. doi: 10.1371/journal.pone.0078412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellen PB, Gao SK, Vitolins MZ, Goff DCJ. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168(3):308–314. doi: 10.1001/archinternmed.2007.119 [DOI] [PubMed] [Google Scholar]
- 10.Pew Research Center. Mobile Fact Sheet. https://www.pewresearch.org/internet/fact-sheet/mobile/. Published 2021. Accessed June 21, 2021. [Google Scholar]
- 11.Statista Survey. Number of health and fitness app users in the United States from 2018 to 2022. https://www.statista.com/statistics/1154994/number-us-fitness-health-app-users/. Published 2020. Accessed March 23, 2021. [Google Scholar]
- 12.Alessa T, Hawley MS, Hock ES, de Witte L. Smartphone Apps to Support Self-Management of Hypertension: Review and Content Analysis. JMIR mHealth uHealth. 2019;7(5):e13645. doi: 10.2196/13645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breland JY, Yeh VM, Yu J. Adherence to evidence-based guidelines among diabetes self-management apps. Transl Behav Med. 2013;3(3):277–286. doi: 10.1007/s13142-013-0205-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagoto S, Schneider K, Jojic M, DeBiasse M, Mann D. Evidence-based strategies in weight-loss mobile apps. Am J Prev Med. 2013;45(5):576–582. doi: 10.1016/j.amepre.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 15.Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour change techniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess. 2015;19(99):1–188. doi: 10.3310/hta19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svetkey LP, Harsha DW, Vollmer WM, et al. Premier: a clinical trial of comprehensive lifestyle modification for blood pressure control: rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–471. http://www.ncbi.nlm.nih.gov/pubmed/12875806. [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083 [DOI] [PubMed] [Google Scholar]
- 18.Samdal GB, Eide GE, Barth T, Williams G, Meland E. Effective behaviour change techniques for physical activity and healthy eating in overweight and obese adults; systematic review and meta-regression analyses. Int J Behav Nutr Phys Act. 2017;14(1):42. doi: 10.1186/s12966-017-0494-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lara J, Evans EH, O’Brien N, et al. Association of behaviour change techniques with effectiveness of dietary interventions among adults of retirement age: a systematic review and meta-analysis of randomised controlled trials. BMC Med. 2014;12:177. doi: 10.1186/s12916-014-0177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakih El Khoury C, Karavetian M, Halfens RJG, Crutzen R, Khoja L, Schols JMGA. The Effects of Dietary Mobile Apps on Nutritional Outcomes in Adults with Chronic Diseases: A Systematic Review and Meta-Analysis. J Acad Nutr Diet. 2019;119(4):626–651. doi: 10.1016/j.jand.2018.11.010 [DOI] [PubMed] [Google Scholar]
- 21.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Heal Psychol Off J Div Heal Psychol Am Psychol Assoc. 2009;28(6):690–701. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 22.de Vries H, Kremers SPJ, Smeets T, Brug J, Eijmael K. The effectiveness of tailored feedback and action plans in an intervention addressing multiple health behaviors. Am J Health Promot. 2008;22(6):417–425. doi: 10.4278/ajhp.22.6.417 [DOI] [PubMed] [Google Scholar]
- 23.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Heal Psychol Off J Div Heal Psychol Am Psychol Assoc. 2008;27(3):379–387. doi: 10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- 24.Olsen JM, Nesbitt BJ. Health coaching to improve healthy lifestyle behaviors: an integrative review. Am J Health Promot. 2010;25(1):e1–e12. doi: 10.4278/ajhp.090313-LIT-101 [DOI] [PubMed] [Google Scholar]
- 25.Kivelä K, Elo S, Kyngäs H, Kääriäinen M. The effects of health coaching on adult patients with chronic diseases: a systematic review. Patient Educ Couns. 2014;97(2):147–157. doi: 10.1016/j.pec.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 26.U.S Census Bureau; American Community Survey; Table PST045219. generated using census.gov/quickfacts. https://www.census.gov/quickfacts/fact/table/US/PST045219. Published 2019. Accessed December 4, 2020. [Google Scholar]
- 27.Khan MS, Shahid I, Siddiqi TJ, et al. Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent US Food and Drug Administration Approval of Novel Cardiometabolic Drugs. J Am Heart Assoc. 2020;9(11):e015594. doi: 10.1161/JAHA.119.015594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zoom Video Communications Inc. Security Guide.; 2016. [Google Scholar]
- 31.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089 [DOI] [PubMed] [Google Scholar]
- 32.Sealed Envelope Ltd. Create a blocked randomisation list. https://www.sealedenvelope.com/simple-randomiser/v1/lists. Published 2020. Accessed August 16, 2020. [Google Scholar]
- 33.USDA. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Part B. Chapter 1: Introduction. Dep Heal Hum Serv. 2015:1–571. https://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf. [Google Scholar]
- 34.Carson JAS, Lichtenstein AH, Anderson CAM, et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circulation. 2020;141(3):e39–e53. doi: 10.1161/CIR.0000000000000743 [DOI] [PubMed] [Google Scholar]
- 35.Allison MA, Aragaki AK, Ray RM, et al. A Randomized Trial of a Low-Fat Diet Intervention on Blood Pressure and Hypertension: Tertiary Analysis of the WHI Dietary Modification Trial. Am J Hypertens. 2016;29(8):959–968. doi: 10.1093/ajh/hpv196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glanz K, Rimer BK, Viswanath K. Health Behavior: Theory, Research, and Pracice (5th Ed.). 5th ed. Jossey-Bass/Wiley.; 2015. [Google Scholar]
- 37.Subar AF, Kirkpatrick SI, Mittl B, et al. The Automated Self-Administered 24-hour dietary recall (ASA24): a resource for researchers, clinicians, and educators from the National Cancer Institute. J Acad Nutr Diet. 2012;112(8):1134–1137. doi: 10.1016/j.jand.2012.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Heart Association News. How to accurately measure blood pressure at home. [Google Scholar]
- 39.Rhee JJ, Sampson L, Cho E, Hughes MD, Hu FB, Willett WC. Comparison of methods to account for implausible reporting of energy intake in epidemiologic studies. Am J Epidemiol. 2015;181(4):225–233. doi: 10.1093/aje/kwu308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinberg DM, Kay MC, Svetkey LP, et al. Feasibility of a Digital Health Intervention to Improve Diet Quality Among Women With High Blood Pressure: Randomized Controlled Feasibility Trial. JMIR mHealth uHealth. 2020;8(12):e17536. doi: 10.2196/17536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen J Statistical Power Analysis. Curr Dir Psychol Sci. 1992;1(3):98–101. doi: 10.1111/1467-8721.ep10768783 [DOI] [Google Scholar]
- 42.Kahan BC, Morris TP. Improper analysis of trials randomised using stratified blocks or minimisation. Stat Med. 2012;31(4):328–340. doi: 10.1002/sim.4431 [DOI] [PubMed] [Google Scholar]
- 43.Samanic CM, Barbour KE, Liu Y, et al. Prevalence of Self-Reported Hypertension and Antihypertensive Medication Use by County and Rural-Urban Classification — United States, 2017. MMWR Morb Mortal Wkly Rep. 2020;69(18):533–539. doi: 10.15585/mmwr.mm6918a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Facts About Hypertension. [Google Scholar]
- 45.Anstey DE, Christian J, Shimbo D. Income Inequality and Hypertension Control. J Am Heart Assoc. 2019;8(15):1–3. doi: 10.1161/JAHA.119.013636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazar M, Davenport L. Barriers to Health Care Access for Low Income Families: A Review of Literature. J Community Health Nurs. 2018;35(1):28–37. doi: 10.1080/07370016.2018.1404832 [DOI] [PubMed] [Google Scholar]
- 47.Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. J Rural Heal Off J Am Rural Heal Assoc Natl Rural Heal Care Assoc. 2006;22(2):140–146. doi: 10.1111/j.1748-0361.2006.00022.x [DOI] [PubMed] [Google Scholar]
- 48.Bacon L, Aphramor L. Weight science: Evaluating the evidence for a paradigm shift. Clin Nutr Interface Between Metab Diet, Dis. 2013:335–366. doi: 10.1201/b16308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tylka TL, Annunziato RA, Burgard D, et al. The weight-inclusive versus weight-normative approach to health: evaluating the evidence for prioritizing well-being over weight loss. J Obes. 2014;2014:983495. doi: 10.1155/2014/983495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tribole E, Resch E. The Intuitive Eating Workbook: Ten Principles for Nourishing a Healthy Relationship with Food. Media Alternatives, Incorporated; 2017. https://books.google.com/books?id=TrdLxQEACAAJ. [Google Scholar]
- 51.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-Style Diet and Risk of Coronary Heart Disease and Stroke in Women. Arch Intern Med. 2008;168(7):713–720. doi: 10.1001/archinte.168.7.713 [DOI] [PubMed] [Google Scholar]
- 52.Miller HN, Charleston J, Wu B, et al. Use of electronic recruitment methods in a clinical trial of adults with gout. Clin Trials. September 2020:1740774520956969. doi: 10.1177/1740774520956969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller HN, Gleason KT, Juraschek SP, et al. Electronic medical record-based cohort selection and direct-to-patient, targeted recruitment: Early efficacy and lessons learned. J Am Med Informatics Assoc. 2019;26(11):1209–1217. doi: 10.1093/jamia/ocz168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dorans KS, Mills KT, Liu Y, He J. Trends in prevalence and control of hypertension according to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guideline. J Am Heart Assoc. 2018;7(11):1–11. doi: 10.1161/JAHA.118.008888 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.