Abstract
Background
Subsequent cancers (SC) are a significant cause of morbidity and mortality in long-term survivors after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Chronic graft-versus-host disease (cGVHD) and treatment-related immunosuppression have been recognized as risk factors for SC.
Objective
This study sought to investigate the incidence and risk factors for SC in patients with established cGVHD, assessed separately for onset of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC)—categorized into non-melanoma skin cancers (NMSC)—and all cancers other than NMSC.
Study Design
Two-hundred-and-four patients were enrolled in the prospective cross-sectional cGVHD Natural History Study and underwent comprehensive clinical evaluation. Patients were followed up with annual survey. Cumulative incidence of NMSC and cancers other than NMSC with competing risks was estimated separately, and transplant- and cGVHD-related factors were assessed for association with outcomes using Grey’s test and multivariable Cox models. Time for all analyses began at two years post-evaluation to restrict analyses to patients who were presumed to not have had SC present at evaluation.
Results
Nineteen patients were diagnosed with NMSC and 19 with cancers other than NMSC with 10-year cumulative incidences of 15.5% (95% CI: 9.0–27.6) and 13.8% (95% CI: 8.2–20.8), respectively. Age at transplant (HR=1.94; 95% CI: 1.23–3.06) and higher CRP at evaluation (HR=9.49; 95% CI: 1.26–71.58) were jointly associated with NMSC, while and gastrointestinal cGVHD at evaluation (HR=0.26, 95% CI: 0.09–0.78) was associated with reduced risk of NMSC. T-cell depletion at transplant (HR=3.09; 95% CI: 1.17–8.20), lymphoma as transplant indication (HR=3.96; 95% CI: 1.56–10.05), and oral cGVHD severity at evaluation (HR=4.36; 95% CI: 1.52–12.46) were jointly associated with cancers other than NMSC.
Conclusion
This study estimates incidence of SC in a population of severely-affected cGVHD patients and identifies correlations with subsequent development of SC. These factors seem to be different for NMSC and cancers other than NMSC. Further longitudinal investigations accounting for dynamic and cumulative processes are needed to improve understanding and management of SC.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative immunotherapy for many patients with malignant and nonmalignant diseases.1 Advances in allo-HSCT have resulted in reduction of early mortality, giving rise to a growing population of long-term survivors at risk for developing late transplant-related complications, including onset of subsequent cancers (SC). Chronic graft-versus-host disease (cGVHD) is an immunological complication that affects approximately half of patients who survive two years after allo-HSCT.2,3 Most patients with cGVHD require prolonged systemic treatment with immunosuppressive agents and are subjected to GVHD-related tissue damage and immune dysregulation, all of which may promote the development of SC.4,5
Allo-HSCT recipients are at a higher risk of developing new cancers than the general population, with risk particularly elevated for oral, bone, soft tissue, and liver cancers, and greatest risk extending beyond ten years post-transplant.6,7 Additionally, multiple studies have reported elevated risk of non-melanoma skin cancers (NMSC), namely basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), after allo-HSCT.8,9 All of these cancers manifest in organs and tissues typically affected by cGVHD.
The study of SC in cGVHD population is complicated by numerous logistical challenges, including need for data on pre-, peri-, and post-transplant exposures including details of cGVHD manifestations and therapy. Estimating risk of SC is further complicated by malignant disease relapse and its associated treatments, which may alter this risk. Factors found to be associated with increased risk of SC in patients after allo-HSCT include both pre- and post-transplant therapies. Pre-transplant radiation and chemotherapy, transplant conditioning, or both may increase risk of developing cancer after HSCT whereas findings regarding total body irradiation (TBI) conditioning are inconclusive.5,10–12 Older age at transplant may also increase risk of SC.10,11,13 Several factors related to cGVHD and its therapies have also been associated with risk of SC.5,15–21 Acute GVHD (aGVHD) has been associated with an increase in SCC risk, although its role in the development of subsequent cancers is not understood.22 Additionally, azathioprine, a formerly common cGVHD treatment, has been associated with a 2.5-fold increased risk of developing a subsequent cancer, whereas sirolimus may reduce subsequent cancer risk.17 Patients with cGVHD have been shown to be at particularly high risk for non-melanoma skin, oral, and esophageal cancer, with risk of NMSC increasing with duration of immunosuppression; however, the roles of cGVHD severity, clinical presentations, and its treatments remain unclear.5,22,23 This report describes incidence of NMSC and cancers other than NMSC and assesses impact of factors related to cGVHD and its treatments in a prospective cohort of cGVHD patients.
Patients and Methods
Patients
Patients (n=437) were enrolled on the National Institutes of Health (NIH) Chronic Graft-Versus-Host Disease Natural History Protocol (NCT00092235) between October 2004 and April 2019 and underwent one-week multidisciplinary evaluation by subspecialists in dentistry, dermatology, gynecology, ophthalmology, pain and palliative care, rehabilitation medicine, and hematology/oncology. This is an NIH institutional review board-approved study and patients provided informed consent. Chronic GVHD was scored in accordance with 2005 NIH cGVHD diagnostic and staging criteria.24 Follow-up data were collected by annual telephone survey. Patients must have had at least two years of follow-up and must have been free of post-transplant relapse of primary malignancy and free of outcomes of interest at evaluation to be included in analysis. Relapse was treated as a competing risk as subsequent treatment may alter risk of development of a second cancer, thereby violating assumptions inherent to censoring patients at relapse. Patients were excluded from all analyses for the following reasons: found not to have cGVHD at evaluation or unable to complete study (n=22), missing data (n=1), relapse of underlying malignancy following allo-HSCT and preceding evaluation (n=48), diagnosis of cancers other than NMSC following allo-HSCT and preceding evaluation (n=16), insufficient or no follow-up (n=87), and death within two years of evaluation (n=59). The resultant study population included 204 patients.
Ascertainment of Subsequent Cancers
Diagnoses of SC were collected through annual follow-up conducted by telephone survey. Patients were asked if they had developed a new cancer since transplant other than the malignancy they received transplant for. Patients reported SC diagnoses, approximate dates of diagnoses, and provided consent to contact and request records from diagnosing physicians. Patients who did not self-report a new cancer diagnosis were assumed to not have had SC in cumulative incidence calculations. BCC and SCC were categorized as NMSC and all other diagnoses were considered cancers other than NMSC. This study estimated cumulative incidence of and assessed factors for association with NMSC and cancers other than NMSC separately.
Potential Predictors
Potential predictors included pre-transplant factors and transplant characteristics as well as measures of cGVHD severity and treatments collected at evaluation. Pre-transplant factors considered were time from diagnosis to transplant and prior autologous or allogeneic transplants. Transplant characteristics included indication for transplant, type of conditioning regimen, receipt of TBI, and receipt of T-cell depletion for cGVHD prophylaxis. Factors related to cGVHD reflected patients’ courses prior to evaluation (prior aGVHD, number of systemic therapies received for cGVHD, among others) as well as measures of cGVHD activity and severity at evaluation, including NIH organ scores and laboratory measures of inflammation.24
Statistical Analysis
Cumulative incidence was estimated for NMSC competing with death, malignancy relapse, or cancers other than NMSC, and for cancers other than NMSC competing with death or relapse using the method of Gooley.25 Potential predictors of subsequent cancers were assessed using Gray’s test in univariable analyses. The evaluation of factors of associated with the development of these subsequent cancers was done in a hypothesis generating manner, and the results are presented without adjustment for multiple comparisons for that reason. Diagnosis of cancers other than NMSC was a competing event for NMSC analysis, and relapse was a competing event for both analyses because these patients likely received additional treatment that fundamentally altered risk of the event of interest. Patients diagnosed with post-transplant NMSC up to two years post-evaluation were excluded from analyses of NMSC. As noted above, patients who died within two years of enrollment were excluded from these analyses as were any patients with less than two years follow-up to restrict analyses to patients who were presumed to not have had SC present at evaluation. As such, this is a landmark analysis, with time for all analyses began at two years post-evaluation. Cumulative incidence estimates at specific timepoints reported herein are relative to the two-year post-evaluation time point at which follow-up began and are conditional on meeting inclusion criteria for this analysis.
Potential predictors of subsequent cancers were assessed using Gray’s test in univariable analysis.26 Factors initially categorized using three or four groups were subsequently combined into two groups for evaluation of association with cumulative subsequent cancer incidence, with corresponding p-values adjusted by multiplying unadjusted p-values by the number of implicit tests that would be performed to arrive at the grouping. Multivariable Cox proportional hazards model analyses were performed to estimate the independent effects of factors on development of NMSC, censoring for death, relapse of underlying malignancy, diagnosis of cancers other than NMSC, and remaining alive without NMSC at last follow-up. A multivariable Cox model was also developed to estimate independent associations of factors with subsequent cancer other than NMSC, censoring for death, relapse of underlying malignancy, or remaining alive without diagnosis of cancer other than NMSC at last follow-up.
Results
Patient characteristics
Two hundred and four patients with a median follow-up of 84 months were included in analysis. Patients were diagnosed with cGVHD a median of 7 months (IQR, 4.6–12.0) post-transplant and were evaluated a median of 36 (IQR, 21.9–62.6) and 26 (IQR, 12.5–54.7) months post-transplant and post-cGVHD diagnosis, respectively. Median age at evaluation was 47 years (IQR, 31–56). The most common indications for transplant were acute leukemia and lymphoma. Most patients received a related donor transplant (57%) and more patients received stem cells from the peripheral blood (74%) than other sources. Fewer patients received reduced-intensity (38%) than myeloablative conditioning, and a minority of patients (15%) received HLA-mismatched grafts. Thirty-three patients (16%) received some form of T-cell depletion for GVHD prophylaxis. Almost two thirds of patients (n=127, 62%) did not receive TBI as part of conditioning.
Patients had cGVHD involvement in a median of 5 (IQR, 3–6) organs at evaluation. Most patients presented with severe (69%) or moderate (29%) cGVHD per NIH global score.24 The most commonly affected organs were the skin (78%) and eyes (75%). Most (68%) patients had experienced aGVHD prior to cGVHD. Patients had received a median of four systemic immunosuppressive therapies (IQR, 3–5) for cGVHD prior to NIH evaluation. Most patients were receiving moderate- (34%) or high-intensity (41%) immunosuppressive therapy at evaluation.27 Demographic and cGVHD characteristics are shown in Tables I and II.
Table I:
Patient Characteristics
| All Patients N (%) or median (IQR) | |
|---|---|
| All | 204 |
| Sex | |
| Male | 121 (59) |
| Female | 83 (41) |
| Body Mass Index (kg/m2) | 24.0 (21.0–27.7) |
| Underweight/Normal | 118 (58) |
| Overweight | 61 (30) |
| Obese | 23 (11) |
| Unknown | 2 (1) |
| Age at Transplant | 43.5 (25.5–51.5) |
| Age at cGVHD Evaluation | 46.8 (31.3–55.9) |
| Main Disease | |
| ALL, AML, MDS | 112 (55) |
| CML, IMF, MPD | 24 (12) |
| CLL | 12 (6) |
| HL, NHL | 35 (17) |
| MM | 5 (2) |
| Non-Malignant | 16 (8) |
| Number of Prior Auto-HSCT | |
| 0 | 192 (94) |
| 1 | 11 (5) |
| 2 | 1 (<1) |
| Number of Prior Allo-HSCT | |
| 0 | 200 (98) |
| 1 | 4 (2) |
| Disease Diagnosis to Allo-HSCT (months) | 13.5 (5.7–27.9) |
| Disease Status at Transplant | |
| Complete Remission | 90 (44) |
| Other | 103 (51) |
| Unknown | 11 (5) |
| Conditioning Regimen | |
| Myeloablative | 77 (38) |
| Non-Myeloablative | 125 (61) |
| Unknown | 2 (1) |
| Total Body Irradiation | |
| No | 127 (62) |
| Yes | 75 (37) |
| Unknown | 2 (1) |
| Donor Relationship | |
| Related | 117 (57) |
| Unrelated | 87 (43) |
| Stem Cell Source | |
| Bone Marrow | 46 (23) |
| Peripheral Blood | 151 (74) |
| Cord Blood | 7 (3) |
| T-Cell Depletion | |
| No | 159 (78) |
| Yes | 32 (16) |
| Unknown | 13 (6) |
| HLA Match | |
| Mismatch | 31 (15) |
| Match | 166 (81) |
| Unknown | 7 (3) |
ALL indicates acute lymphoblastic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; CML, chronic myelogenous leukemia; IMF, idiopathic myelofibrosis; MPD, myeloproliferative disease; CLL, chronic lymphocytic leukemia; HL, Hodgkin lymphoma; NHL, Non-Hodgkin lymphoma; MM, multiple myeloma; auto-HSCT, autologous hematopoietic stem cell transplant; allo-HSCT, allogeneic hematopoietic stem cell transplant; HLA, human leukocyte antigen.
Table II:
Chronic Graft-versus-Host Disease Characteristics
| All Patients N (%) or median (IQR) | |
|---|---|
| All | 204 |
| Transplant to Consent (months) | 36.2 (21.9–62.6) |
| Transplant to cGVHD Diagnosis (months) | 6.9 (4.6–12.0) |
| cGVHD Diagnosis to Consent (months) | 26.1 (12.5–54.7) |
| cGVHD Organ Involvement | |
| Eyes | 152 (75) |
| Gastrointestinal Tract | 95 (47) |
| Joints and Fascia | 122 (60) |
| Liver | 91 (45) |
| Lungs | 145 (71) |
| Mouth | 134 (66) |
| Skin | 159 (78) |
| Genital (female patients only) | 46 (55) |
| Number of Organs Affected by cGVHD | |
| 1–2 | 13 (6) |
| 3–4 | 62 (30) |
| 5–6 | 73 (36) |
| 7–8 | 56 (27) |
| Average NIH Organ Score | 1.00 (0.71–1.38) |
| NIH Global Score | |
| Mild | 4 (2) |
| Moderate | 60 (29) |
| Severe | 140 (69) |
| Prior Acute GVHD | |
| No | 65 (32) |
| Yes | 138 (68) |
| Unknown | 1 (<1) |
| Number of Prior cGVHD Systemic Treatment Regiments | |
| <2 | 14 (7) |
| 2–3 | 69 (34) |
| 4–5 | 70 (34) |
| >5 | 50 (25) |
| Unknown | 1 (<1) |
| Ever Treatment with cGVHD Systemic Treatments | |
| Steroid | 189 (93) |
| CNI | 173 (85) |
| Sirolimus | 70 (34) |
| MMF | 100 (49) |
| ECP/PUVA | 88 (43) |
| Rituximab | 51 (25) |
| Intensity of Immunosuppression at Evaluation | |
| None/Mild | 49 (24) |
| Moderate | 70 (34) |
| High | 84 (41) |
| Unknown | 1 (<1) |
Abbreviations: CNI–calcineurin inhibitor; MMF–mycophenolate mofetil; ECP–extracorporeal photopheresis; PUVA–psoralen and ultraviolet A therapy.
Intensity of current immunosuppression is defined as mild (single-agent prednisone 0.5 mg/kg/d), moderate (single-agent prednisone 0.5 mg/kg/d and/or any single agent/modality), high (2 or more agents/modalities +/− prednisone 0.5 mg/kg/d)27
NMSC
Nineteen cases of NMSC were observed among 174 patients included in this analysis. Cumulative incidence of NMSC was 15.5% (95% CI: 9.0–27.6) at seven and ten years from initiation of follow-up. Older age at transplant was associated with increased risk of NMSC in univariable analysis. Patients who were older age at evaluation, had received sirolimus or phototherapy for treatment of cGVHD, and higher C-reactive protein (CRP) or natural killer cell count at evaluation, or did not have gastrointestinal (GI) cGVHD at evaluation were found to be at higher risk of NMSC, but these associations were not statistically significant (Figure 1, Supplemental Figures 1-2). Increasing age at transplant (by quartile, HR=1.94; 95% CI: 1.23–3.06), higher CRP at evaluation (HR=9.49; 95% CI: 1.26–71.58) were found to be jointly associated with NMSC in the multivariable Cox model. In contrast, presence of GI cGVHD—meaning an NIH score of 2, 3, or 3—at evaluation (HR=0.26; 95% CI: 0.09–0.78) was found to be associated with reduced risk of NMSC (Table III).
Figure 1:
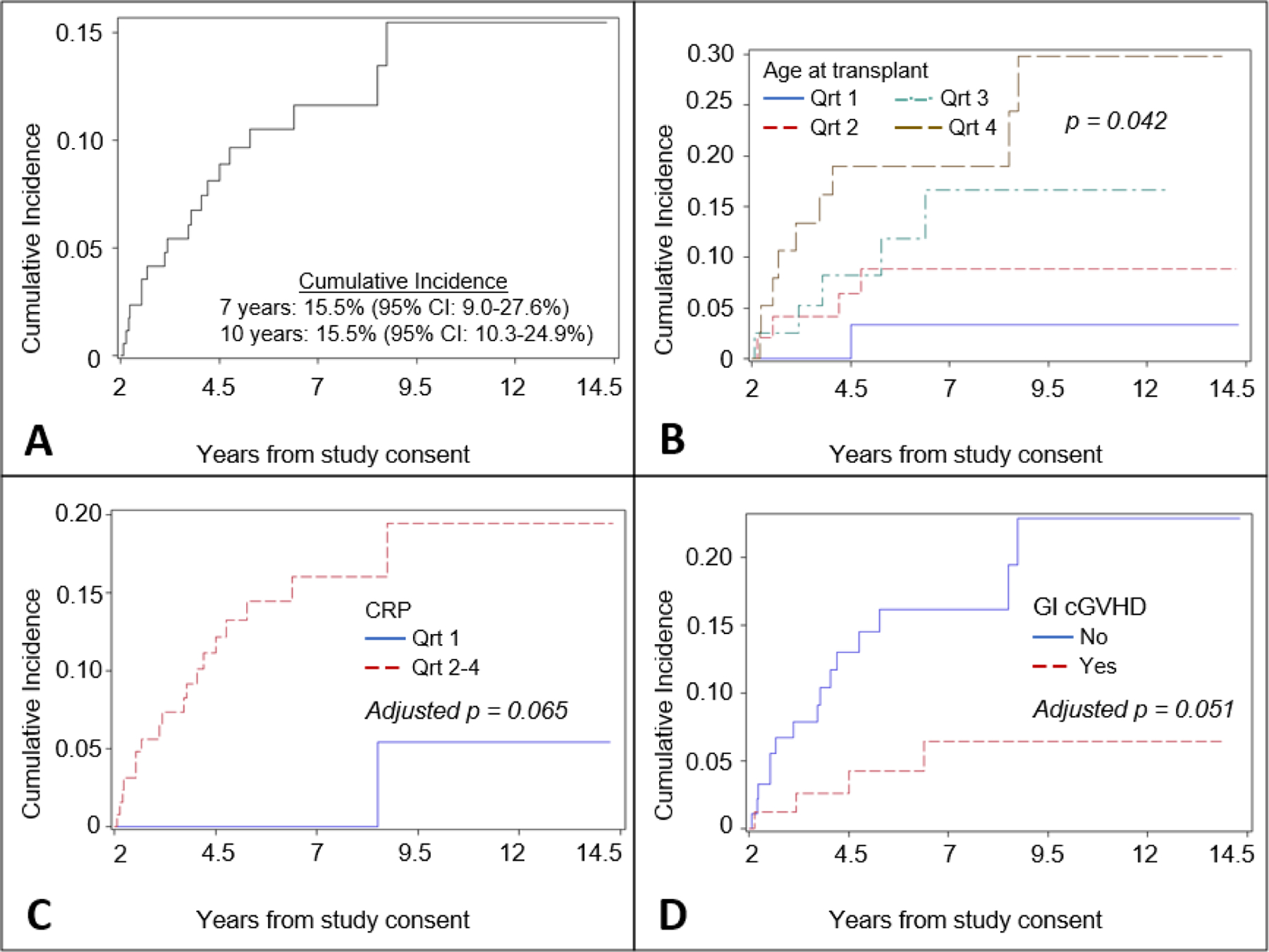
A Cumulative incidence of NMSC competing with death, relapse, or cancers other than NMSC; B Cumulative incidence of NMSC competing with death, relapse, or cancers other than NMSC stratified by age at transplant (Q1: 25, Q2: 43, Q3: 51); C Cumulative incidence of NMSC competing with death, relapse, or cancers other than NMSC stratified by C-reactive protein at evaluation (Q1: 0.55 mg/L)–p-value adjusted for multiple implicit comparisons conducted following combining groups; D Cumulative incidence of NMSC competing with death, relapse, or cancers other than NMSC stratified by gastrointestinal cGVHD at evaluation. Notes: Cumulative incidence estimates in panel A are with respect to initiation of follow-up at two years post-evaluation; Vertical axes have different scales. Abbreviations: CRP–C-reactive protein, GI – gastrointestinal, Qrt–Quartile
Table III:
Multivariable Cox Proportional Hazards Models
| Model | Predictor | HR (95% CI) | p-value |
|---|---|---|---|
| NMSC | |||
| Age at Transplant | |||
| By Quartile | 1.94 (1.23, 3.06) | 0.0047 | |
| C-Reactive Protein | |||
| Quartile 1 | 1.00 (REF) | 0.029 | |
| Quartiles 2–4 | 9.49 (1.26, 71.58) | ||
| GI cGVHD | |||
| No | 1.00 (REF) | 0.017 | |
| Yes | 0.26 (0.09, 0.78) | ||
|
| |||
| Cancer other than NMSC | |||
| T-Cell Depletion | |||
| No | 1.00 (REF) | 0.023 | |
| Yes | 3.09 (1.17, 8.20) | ||
| Lymphoma Indication | |||
| No | 1.00 (REF) | 0.0038 | |
| Yes | 0.25 (0.10, 0.64) | ||
| Oral cGVHD | |||
| None/Mild | 1.00 (REF) | 0.0061 | |
| Moderate/Severe | 4.36 (1.52, 12.46) | ||
Quartiles of age: Q1: 25, Q2: 43, Q3: 51; Quartile of CRP used in the model: Q1: 0.55 mg/L
Cancers other than NMSC
Nineteen cases of cancers other than NMSC were observed among 204 patients included in this analysis. Cancers observed were oral SCC (n=6), melanoma (n=6), prostate (n=2), esophageal (n=2), bladder (n=1), thyroid (n=1), and colon (n=1). Cumulative incidence of cancers other than NMSC was 12.1% (95% CI: 7.2–18.3%) and 13.8% (95% CI 8.2–20.8%) at seven and ten years from initiation of follow-up, respectively.
Factors associated with increased risk of subsequent cancers other than NMSC in univariable analysis were T-cell depletion at transplant, lymphoma as indication for transplant, and increasing oral cGVHD severity. Patients not in complete remission at transplant also had higher risk of cancers other than NMSC, but this association was not statistically significant (Figure 2, Supplemental Figure 3). T-cell depletion (HR=3.09; 95% CI: 1.17–8.20), lymphoma as indication for transplant (HR=3.96; 95% CI: 1.56–10.05), and oral cGVHD severity (HR=4.36; 95% CI: 1.52–12.46) were jointly associated with cancers other than NMSC in the multivariable Cox model (Table III).
Figure 2:
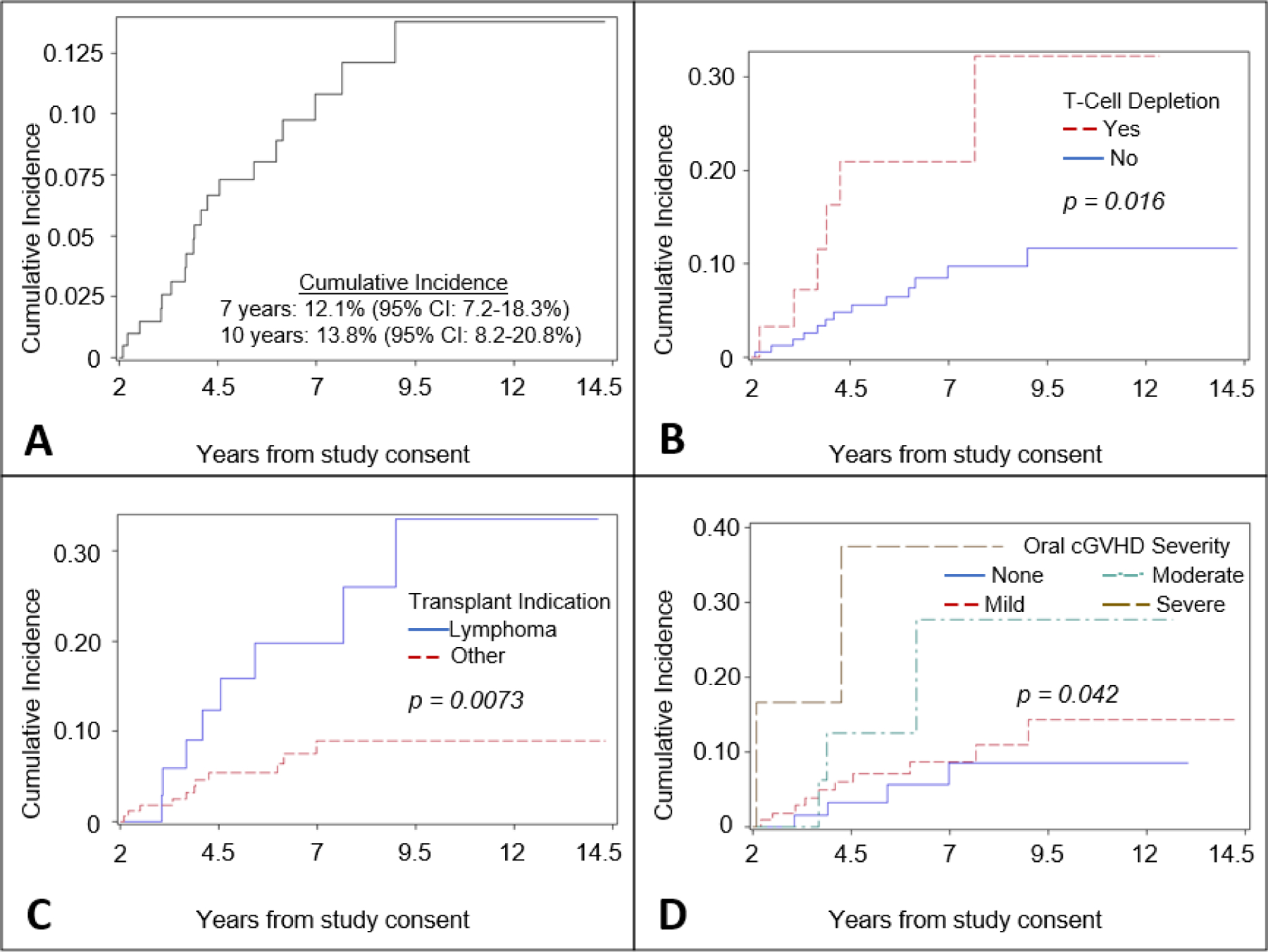
A Cumulative incidence of cancers other than NMSC competing with death or relapse; B Cumulative incidence of cancers other than NMSC competing with death or relapse stratified by receipt of T-cell depletion at transplant; C Cumulative incidence of cancers other than NMSC competing with death or relapse stratified by transplant indication; D Cumulative incidence of cancers other than NMSC competing with death or relapse stratified by NIH oral score at evaluation. Note: cumulative incidence estimates in panel A are with respect to initiation of follow-up at two years post-evaluation. Notes: Cumulative incidence estimates in panel A are with respect to initiation of follow-up at two years post-evaluation; Vertical axes have different scales.
Discussion
In this study of patients with moderate or severe cGVHD, the ten-year cumulative incidences of NMSC and all other subsequent cancers were 15.5% and 13.8%, respectively. These estimates exceed those reported in prior studies; however, this analysis began follow-up two years following evaluation of therapy-refractory cGVHD whereas prior studies began follow-up at transplant. Moreover, prior studies were not conducted solely among patients with cGVHD.7,22
Older age at transplant, higher CRP at evaluation, and presence of GI cGVHD were independently associated with NMSC in this study in multivariable analysis. C-reactive protein, a common biomarker of inflammation, has also previously been associated with cGVHD activity and may be a marker cGVHD-related immune dysregulation and inflammation.28 The significance of the association of GI cGVHD is unclear and it is possible that this association captures some other factors related to development of NMSC. Interestingly, patients with history of treatment of cGVHD with sirolimus were found to be at increased risk of NMSC in univariable analysis despite its purported antineoplastic effects and prior reports of reduced risk among patients treated with this agent.29–32 In this study population, sirolimus was often prescribed later in patients already with refractory cGVHD, and adjustment for measures of disease severity attenuated this association in multivariable modeling.
Greater severity of oral cGVHD per NIH scoring was associated with increased risk of cancers other than NMSC in multivariable analysis. This finding may be attributable to the fact that oral SCC accounted for ~30% of cancers other than NMSC reported in this study. T-cell depletion was also associated with increased cancers other than NMSC risk, which may be explained by altered immune reconstitution among patients in whom this approach is taken for cGVHD prophylaxis. Lastly, lymphoma as indication for transplant was associated with cancers other than NMSC. Lymphoma patients tend to be older, however, age did not remain in the multivariable model and this association may be explained by other differences by indication, such as pre-transplant treatments. Primary therapy for Hodgkin and non-Hodgkin lymphoma involves regimens with multiple chemotherapeutic agents such as alkylating agents and often the incorporation of ionizing radiation, which are known risk factors for the development of therapy-associated myeloid neoplasms.33 In contrast, treatment regimens for myeloid malignancies34 and other transplant indications do not rely so heavily on these agents.
There are limitations to this study related to the population under study. This study did not include patients without cGVHD and was enriched for patients with severe, therapy-refractory cGVHD. Associations of previously reported risk factors for SC were also not observed, suggesting that these factors may not be useful for risk stratification within this subgroup of heavily pre-treated cGVHD patients. Furthermore, patients were only evaluated at a single point in time and dynamic processes related to disease course and treatments as well as cumulative exposures could not be assessed. Moreover, we acknowledge that both analyses had a small sample size, but this decision was made in an effort to study incidence of second cancers among potentially high-risk patients with moderate-severe cGVHD. There are also limitations to this study common to studies of SC among cancer survivors. Additionally, due to the rarity of events, this study grouped all NMSC together as well as all cancers other than NMSC together for analysis, potentially combining cancers of very different risk profiles as one outcome and potentially diluting associations. Finally, this study was unable to estimate baseline or background risks for malignancies under study, complicating interpretations of the contributions of transplant- and cGVHD-related factors to incidence of cancers.
The burden of SC in long-term survivors of allo-HSCT will likely increase as this population continues to grow and age. Significant attention has been paid to estimating risk of SC and identifying risk factors among long-term survivors; however, this is the first study to specifically assess risk among heavily pre-treated patients with moderate and severe cGVHD, who may represent a high-risk subgroup. These factors seem to be different for NMSC and cancers other than NMSC and may represent subsets of patients who may benefit from heightened surveillance for SC. The emergence of an increasing number of prospective long-term follow-up programs for allo-HSCT recipients will enable more comprehensive study of the effects of pre-, peri-, and post-transplant exposures as well as dynamic and cumulative processes throughout these periods on risk of SC. As such studies become possible, it would be prudent to revisit associations reported in this study with more robust investigations.
Supplementary Material
Highlights.
10-year cumulative incidence of non-melanoma skin cancers was 15.5% (95% CI: 9.0–27.6)
10-year cumulative incidence of cancers other than non-melanoma skin cancer was 13.8% (95% CI: 8.2–20.8)
Age at transplant (HR=1.94; 95% CI: 1.23–3.06) and higher CRP at evaluation (HR=9.49; 95% CI: 1.26–71.58) were jointly associated with non-melanoma skin cancer
Gastrointestinal cGVHD at evaluation (HR=0.26, 95% CI: 0.09–0.78) was associated with reduced risk of NMSC
T-cell depletion at transplant (HR=3.09; 95% CI: 1.17–8.20), lymphoma as transplant indication (HR=3.96; 95% CI: 1.56–10.05), and oral cGVHD severity at evaluation (HR=4.36; 95% CI: 1.52–12.46) were jointly associated with cancers other than NMSC
Acknowledgements
The authors have no conflicts of interest to disclose. The authors would like to thank all patients and their families for participating in the NIH cGVHD natural history study. This work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute. The views expressed in this work do not represent the official views of the National Institutes of Health or the United States Government. Author contributions were as follows: participated in data collection (DAS, FP, NH, JN, CR, EWC, JWM, SM, AO, GJ, LC, SZP), designed the research study (DAS, FP, SMS, SZP), provided critical input to design and conduct of the research (NH, AO, PNM, LM), wrote the manuscript (DAS, FP, NH, SZP), and provided critical review of and approved the manuscript (all authors).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Singh AK, McGuirk JP (2016) Allogeneic stem cell transplantation: a historical and scientific overview. Cancer Res 76(22):6445–6451. [DOI] [PubMed] [Google Scholar]
- 2.Zeiser R, & Blazar BR (2017). Pathophysiology of chronic graft-versus-host disease and therapeutic targets. New England Journal of Medicine, 377(26), 2565–2579. [DOI] [PubMed] [Google Scholar]
- 3.Atilla E, Atilla PA, Toprak SK, & Demirer T. (2017). A review of late complications of allogeneic hematopoietic stem cell transplantations. Clinical transplantation, 31(10), e13062. [DOI] [PubMed] [Google Scholar]
- 4.Arai S, Arora M, Wang T, et al. Increasing Incidence of Chronic Graft-versus-Host Disease in Allogeneic Transplantation: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015; 21: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton LM, Saber W, Baker KS, et al. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: The Subsequent Neoplasms Working Group Report. Biol Blood Marrow Transplant. 2017; 23: 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajdic CM, Mayson E, Dodds AJ, et al. Second cancer risk and late mortality in adult Australians receiving allogeneic hematopoietic stem cell transplantation: a population-based cohort study. Biol Blood Marrow Transplant. 2016;22:949–956. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omland SH, Gniadecki R, Haedersdal M, Helweg-Larsen J, Omland LH. Skin cancer risk in hematopoietic stem-cell transplant recipients compared with background population and renal transplant recipients: a population-based cohort study. JAMA Dermatol 2016;152:177–183. [DOI] [PubMed] [Google Scholar]
- 9.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. [DOI] [PubMed] [Google Scholar]
- 10.Czyz A, Łojko-Dankowska A, Matuszak M, et al. Second malignancies after autologous hematopoietic stem cell transplantation following a uniform conditioning regimen in patients with lymphoma characteristics and risk factors analysis. Acta Haematol Pol. 2013;44:6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler C, Khurshid A, Ibrahim J, et al. Incidence of post transplant myelodysplasia/acute leukemia in non-Hodgkin’s lymphoma patients compared with Hodgkin’s disease patients undergoing autologous transplantation following cyclophosphamide, carmustine, and etoposide (CBV). Leuk Lymphoma. 2001;40:499–509. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia andmyelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 14.Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin’s disease: results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395–1404. [DOI] [PubMed] [Google Scholar]
- 15.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneichematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokota A, Ozawa S, Masanori T, et al. Secondary solid tumors after allogeneichematopoietic SCT in Japan. Bone Marrow Transplant. 2012;47:95–100. [DOI] [PubMed] [Google Scholar]
- 17.Chien SH, Liu CJ, Hong YC, et al. Use of azathioprine for graft-vs-host disease is themajor risk for development of secondary malignancies after haematopoietic stem cell transplantation: a nationwide population-based study. Br J Cancer. 2015;112:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimada K, Yokozawa T, Atsuta Y, et al. Solid tumors after hematopoietic stem celltransplantation in Japan: incidence, risk factors and prognosis. Bone Marrow Transplant. 2005;36:115–121. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Chung MJ, Lee JH, Choi BO, Chung JS. Second cancers after hematopoietic stem-cell transplantation with total body irradiation conditioning regimen in hematologic disorders. J Clin Oncol. 2011;29. [Google Scholar]
- 20.Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophagealcancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25:435–441. [DOI] [PubMed] [Google Scholar]
- 21.Shimoni A, Shem-Tov N, Chetrit A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013;27:829–835. [DOI] [PubMed] [Google Scholar]
- 22.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on thedevelopment of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skinand mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. [DOI] [PubMed] [Google Scholar]
- 24.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. NationalInstitutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 25.Gooley TA, Leisinring W, Crowley J, and Storer BE. Estimation of failure probabilitiesin the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18: 695–706. [DOI] [PubMed] [Google Scholar]
- 26.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of acompeting risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27.Mitchell SA, Leidy NK, Mooney KH, et al. Determinants of functional performance inlong-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease. Bone Marrow Transplant. 2010;45:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grkovic L, Baird K, Steinberg SM, et al. Clinical laboratory markers ofinflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26(4):633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geissler EK, Schlitt HJ. Transplantation: Can sirolimus prevent skin cancer in transplantrecipients?. Nature Reviews Nephrology. 2010;6(11):639. [DOI] [PubMed] [Google Scholar]
- 30.Dantal J, Morelon E, Rostaing L, et al. Sirolimus for secondary prevention of skin cancerin kidney transplant recipients: 5-year results. J. Clin. Oncol 2018;36(25):2612–2620. [DOI] [PubMed] [Google Scholar]
- 31.Asleh R, Clavell AL, Pereira NL, et al. Incidence of Malignancies in Patients TreatedWith Sirolimus Following Heart Transplantation. J Am Coll Cardiol. 2019;73(21):2676–88. [DOI] [PubMed] [Google Scholar]
- 32.Yanik EL, Siddiqui K, & Engels EA Sirolimus effects on cancer incidence afterkidney transplantation: a meta-analysis. Cancer medicine, 2015;4(9):1448–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Juhaishi T, Khurana A, & Shafer D. (2019). Therapy-related myeloid neoplasms in lymphoma survivors: reducing risks. Best Practice & Research Clinical Haematology, 32(1), 47–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


