Abstract
Due to the ease of use and excellent safety profile, ultrasound is a promising technique for both diagnosis and site-specific therapy. Ultrasound-based techniques have been developed to enhance the pharmacokinetics and efficacy of therapeutic agents in cancer treatment. In particular, transfection with exogenous nucleic acids has the potential to stimulate an immune response in the tumor microenvironment. Ultrasound-mediated gene transfection is a growing field, and recent work has incorporated this technique into cancer immunotherapy. Compared with other gene transfection methods, ultrasound-mediated gene transfection has a unique opportunity to augment the intracellular uptake of nucleic acids while safely and stably modulating the expression of immunostimulatory cytokines. The development and commercialization of therapeutic ultrasound systems further enhance the potential translation. In this Review, we introduce the underlying mechanisms and ongoing preclinical studies of ultrasound-based techniques in gene transfection for cancer immunotherapy. Furthermore, we expand on aspects of therapeutic ultrasound that impact gene therapy and immunotherapy, including tumor debulking, enhancing cytokines and chemokines and altering nanoparticle pharmacokinetics as these effects of ultrasound cannot be fully dissected from targeted gene therapy. We finally explore the outlook for this rapidly developing field.
Keywords: Ultrasound, Cancer, Gene therapy, Immunotherapy
I. Introduction
Ultrasound has been used as a common diagnostic tool in medicine for more than fifty years. Recently, ultrasound therapy has expanded in clinical applications. Ultrasound can be combined with other therapies to treat various diseases, including cancer [1–4]. The ability of ultrasound to simultaneously image and generate local therapeutic effects improves spatial selectivity and therefore can reduce systemic toxicity [5]. Here, we will examine the application of therapeutic ultrasound in cancer immunotherapy, with a particular focus on the incorporation of ultrasound-mediated transfection to alter tumor and immune cell phenotypes.
The effect of ultrasound on tissue varies with the acoustic frequency (Figure 1), and therapeutic ultrasound typically employs a center frequency ranging from 20 kHz to 5 MHz. Within the lower frequency range (20–100 kHz) sound waves can destroy kidney stones or coronary artery calcifications [6, 7] and enhance transdermal drug delivery [8, 9], allowing lidocaine, insulin or vaccines to diffuse through the permeabilized skin. With frequencies ranging from 500 kHz to 2 MHz, focused ultrasound enhances drug transport through the cornea [10] and enhances fracture healing [11]. Frequencies above 1 MHz have also been applied in mechanical and thermal high-intensity focused ultrasound (HIFU) for tumor ablation [12, 13]. Some of these treatments have been approved for clinical use, whereas most are in pre-clinical development.
Figure 1.
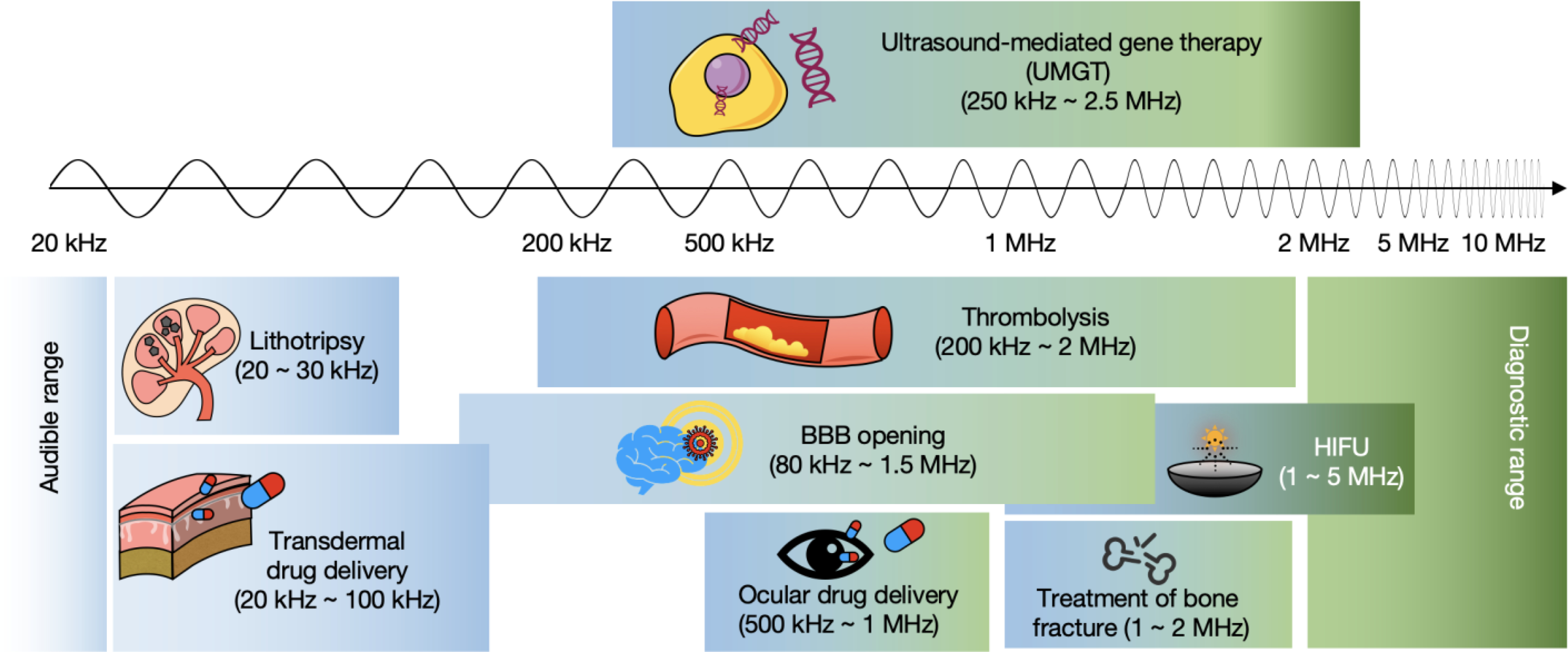
Schematic of current research related to therapeutic ultrasound and UMGT with the corresponding frequency ranges.
Microbubbles (MBs) are used as contrast agents in diagnostic applications, and MBs have been explored recently in therapeutic protocols [4]. The combination of ultrasound and MBs has been applied for transient blood-brain barrier (BBB) opening [3, 14], thrombolysis [15], and gene transfection [16, 17]. Particularly in the presence of MBs, ultrasound can achieve localized transfection [18]. Ultrasound-mediated gene therapy (UMGT) is minimally invasive, and has been safely applied over a range of frequency (250 kHz - 2.25 MHz) and intensity (0.1 – 4 W/cm2).
UMGT is typically performed in vivo after systemic or local administration of MBs and nucleic acids. While in vivo UMGT is reported to achieve a 10 to 30-fold increase in transfection efficiency over the application of nucleic acids alone, in vitro results have shown up to several thousand-fold increase in efficacy, motivating numerous studies to further optimize preclinical ultrasound transfection parameters [19]. In comparison to other transfection approaches, UMGT may improve spatial and temporal control, since transfection can be localized, monitored by imaging and titrated as to the dose [20]. In addition, molecularly-targeted MBs can be employed to carry nucleic acids. Molecular targeting enhances UMGT by bringing MBs and genetic materials close to the tumor cells [21].
Importantly, UMGT does not require dedicated therapeutic ultrasound devices. Fully featured, affordable and practical theragnostic devices can be applied in combination with specific ultrasound intensities and protocols [22]. Still, there are limitations of diagnostic equipment in theragnostics. The center frequency, acoustic intensity and pulse repetition frequency operate within limits that were optimized for imaging rather than therapy. Further, the sequencing of pulses between lines-of-sight within an image frame is often difficult to discern and therefore quantification of the acoustic dose is challenging.
Immunotherapy has shifted the paradigm for cancer treatment, and can induce long-lasting responses in patients with a subset of cancers. Immunotherapeutic agents are often used to activate the immune system, or to block immunosuppressive checkpoints in the tumor microenvironment (TME) [23, 24]. To date, the benefit of immunotherapy has been limited to a minority of patients with specific cancer types, with particular limitations in the treatment of solid tumors. The ability of ultrasound to debulk the TME while enhancing beneficial immune cell populations is particularly attractive. Herein, this review begins with a concise background on gene therapy, the underlying mechanisms of therapeutic ultrasound and the related bioeffects, followed by a summary of the opportunities to integrate ultrasound within gene therapy and immunotherapy protocols. We particularly focus on the intersection of ultrasound, gene therapy and immunotherapy.
II. Gene delivery
Currently, there are various methods for the delivery of therapeutic genetic materials, including viral and non-viral delivery systems, each with advantages and limitations discussed below (Table 1).
Table 1.
Summary of the advantages and limitations of gene transfection methods.
| Methods | Advantages | Limitations | Ref. | |
|---|---|---|---|---|
| Viral delivery | Adenovirus Retrovirus Lentiviruses AAVs |
High transfer efficiency Clinically approved Varied duration of expression |
Innate immune response Insertional mutagenesis Complexity in production and scaleup Small payload in AAVs |
[25–29] |
| Non-viral vectors | Liposomes Nanoparticles Polymers Dendrimers |
Easy to prepare Inexpensive to produce Targeted delivery Enhanced circulation time for some constructs |
Relatively low transfer efficiency Burden on the liver to metabolize Toxicity of cationic lipids |
[30–35] |
| Physical methods | Microinjection Biolistic particle delivery Electroporation Phototransfection |
Relatively high transfer efficiency Less dependent on cell types and conditions No need for vectors and carriers |
Invasive Requires expensive and dedicated instrumentation Low penetration of light in tissues |
[36–41] |
| UMGT | Noninvasive Site-specific Viral vectors and carriers enhance efficacy but are not required Temporal control Available devices |
Insufficient preclinical and clinical research Technically demanding |
[21, 42–46] |
2.1. Viral vectors
Viral vectors have been widely applied in clinical transfection protocols due to the fact that viruses efficiently transfer DNA or RNA into host cells. Recently, gene therapy clinical trials have relied on adenoviruses [25], lentiviruses [25, 47] and adeno-associated viruses (AAVs) [48]. Although viral vectors have substantially advanced the field of gene-based immunotherapy, disadvantages and safety issues include potential toxic immunostimulatory effects, varied tropism and difficulties in targeting tissues of interest [28]. Other concerns include limited gene payload capacity, induction of a potentially fatal inflammatory response, carcinogenesis, and insertional mutagenesis [29, 49].
2.2. Non-viral delivery systems
Non-viral delivery vehicles and other physical methods have been investigated to provide an alternative to viral systems. Chemical approaches include modified delivery systems that can bind or electrostatically-encapsulate condensed gene constructs to facilitate endocytosis [50]. Delivery systems include liposomes, nanoparticles, polymers and dendrimers [30], and typically incorporate cationic or ionizable lipids, peptides, or polymers to enhance uptake and nuclear localization [4, 51]. Nanoparticles can be designed with a high loading capacity, extended pharmacokinetic profile and controlled release properties to increase the therapeutic index of immunotherapeutic genes and protect nucleic acids from degradation [32]. By modifying surface ligands, nanoparticles can specifically target host cells and can be engineered to release their cargo in response to biochemical changes in the TME or external stimuli [33]. In addition, nanoparticles can be tuned to stimulate immune cells [34]. Furthermore, nanoparticle delivery systems can prevent off-target effects and systemic toxicity, when compared to viruses, reducing dose-limiting toxicity. Molecularly-targeted non-viral delivery systems can selectively activate immunotherapies at the target site [50]. Nevertheless, the transfection efficiency of nanoparticle delivery systems is less than that obtained with viruses [30, 51].
Physical methods, including microinjection [36], biolistic particle bombardment [37], phototransfection [38], electroporation [40, 41] and ultrasound-mediated transfection [22] induce pores in cell membranes to introduce their cargo into cells. These methods often lead to transient therapeutic gene expression [36–38, 52]. Electroporation can deliver genetic materials by applying short electric pulses that can transiently generate perforations in the cell membrane [40, 41, 52]. This technology was used with cDNA in natural killer (NK) cells, to engineer primary NK cells to express chimeric antigen receptors (CARs) [39] or generate immune cytokines [53]. Stable transgene expression has been achieved with electroporation-based regimens; however, the efficacy is typically lower compared to viral transfection. In some cases, such physical methods are invasive and limited to superficial tissues; this reduces the potential applications [50]. This drawback can potentially be circumvented with ultrasound techniques.
2.3. UMGT
UMGT has shown the capacity for delivering genes into cells of interest and includes a variety of applications [43, 54–56]. For example, 1-MHz focused ultrasound enhanced DNA plasmid transfer with polyethyleneimine (PEI) MBs in xenograft sarcoma models [57]. Systemic administration of DNA-cationic lipid complexes followed by the localized application of ultrasound in mice increased transfection, and consequently led to a significant tumor growth reduction [45]. Similarly, in hepatocellular carcinoma models, following the transfection of pre-miR-139 and -378a plasmids, phosphoinositide 3-kinase catalytic subunit alpha (PI3K CA) expression was inhibited, resulting in tumor suppression [58].
Advantages for those entering the field include the site specificity; disadvantages include the currently limited number of preclinical studies and the technical challenges for those trained in the biological sciences. Many parameters impact the resulting efficacy of ultrasound techniques, and the nonlinear oscillation and nonlinear response to input parameters can be difficult for those lacking training in physics to appreciate. For example, while most studies have used low ultrasound frequencies and a relatively small range of parameters, transfection can also be accomplished with higher frequencies. Yoon et al. used 150-MHz (high-frequency) ultrasound to accomplish precise targeting and size-dependent macromolecular delivery with low cytotoxicity [59]. The acoustic pulses perturbed the lipid bilayer of the cell membrane of a targeted cell to induce intracellular delivery of exogenous molecules. Clustered regularly interspaced short palindromic repeats-associated protein-9 nuclease (CRISPR-Cas9) was then used for gene editing [60].
In the following section, as summarized in Figure 2, we explore the mechanisms of therapeutic ultrasound in gene therapy.
Figure 2.
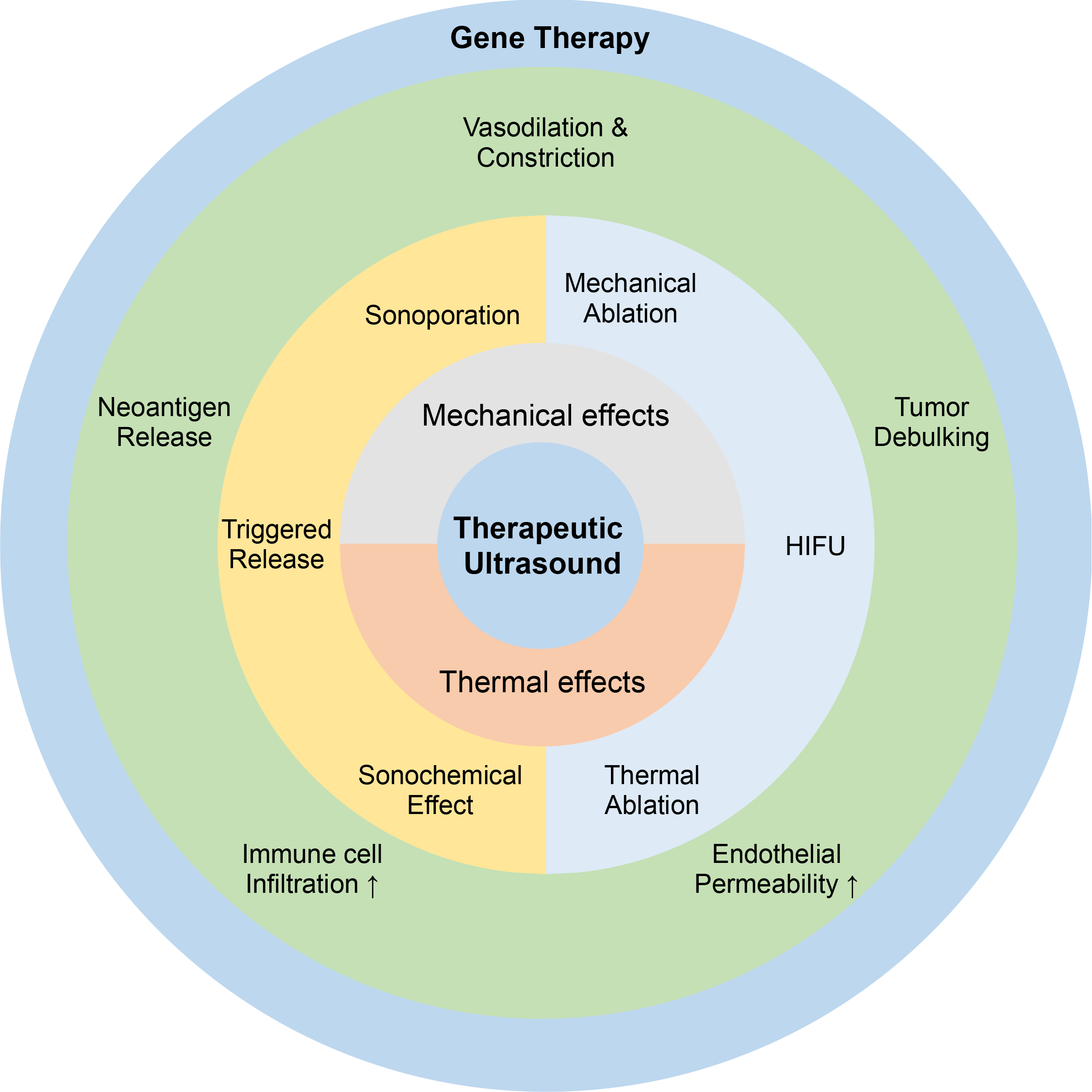
The mechanisms of therapeutic ultrasound in gene therapy, including mechanical and thermal effects with the induced biological effect in various aspects.
III. Therapeutic ultrasound and its applications in gene therapy and immunotherapy
The key to the versatility of therapeutic ultrasound results from the range of interactions and the titrated and controlled intensity of these interactions. Further development of ultrasound in gene therapy requires a better understanding of these underlying mechanisms in order to plan for future clinical applications. The TME, which not only includes tumor cells, but also stromal fibroblasts, infiltrating immune cells, blood vessels and the extracellular matrix [61], plays an important role in cancer growth, development and progression [62]. Ultrasound-induced effects spanning each TME component are the foundation for ultrasound-mediated gene transfer. Therefore, here we begin with a brief discussion of the underlying physical mechanisms by which ultrasound results in transfection or alters the biodistribution of therapeutics. Such mechanisms include thermal and mechanical effects. Although pressure oscillations directly affect cells and tissues, secondary effects of insonation can also play an important role and are also described here.
3.1. Thermal effects
Ultrasound has the potential to rapidly increase the local temperature due to energy absorption. The impact of ultrasound-induced heating varies with the amplitude and duration of the temperature change. Thermal effects directly alter gene and protein expression [63–65], e.g., heat shock proteins in the TME can influence immunotherapy and cancer therapeutic efficacy [66, 67]. A commonly utilized metric to gauge these effects is the cumulative equivalent minutes at 43°C (CEM43).
where t represents the treatment time, T is the applied temperature at the target site during the treatment, and R is a constant. The CEM43 reflects the impact of the heat exposure [68]. For a low CEM43 as encountered in hyperthermia, temporary and reversible effects, such as vasodilation or vasoconstriction or a change in pH enhanced immune response [69, 70]. Higher CEM43 can result in permanent changes such as tissue coagulation and cellular effects such as apoptosis, necrosis, immunogenic cell death and cytokine release. A CEM43 thermal dose of 240 or above results in tissue ablation [71]. The presence of MBs or nanodroplets can significantly enhance the energy absorption and consequently the temperature elevation [72, 73]. Clinical adaptation of local hyperthermia has been limited due to the challenge of non-invasive temperature and CEM43 control. Magnetic Resonance Imaging (MRI) is frequently applied for real-time and noninvasive thermometry but the cost limits its usage.
The acoustic intensity in tissue is determined by the pulse duration, instantaneous intensity (directly related to the acoustic pressure), and pulse repetition frequency (PRF) [74]. Intensity can be reported in terms of spatial peak (SP, referring to the region in space where the intensity is maximum) or spatial average (SA, integrating the intensity field and its variations in space) and temporal peak (TP, referring to the maximum instantaneous intensity during the pulse) or temporal average (TA, integrating the intensity in time). For example, the spatial-peak temporal-average acoustic intensity (ISPTA, commonly expressed in W/cm2) is defined as the maximum intensity measured at any point in the ultrasound beam averaged over the pulse repetition period and is a good indicator of the magnitude of thermal bioeffects (e.g. a higher ISPTA will result in a greater temperature change). A given temporal-average intensity (ITA) can be achieved both with a low amplitude and long pulse with a high PRF (high duty cycle) or a short and high amplitude pulse with a low PRF (low duty cycle) (Figure 3). In many therapeutic ultrasound papers, the specific intensity measure (e.g. ISPTA vs ISATA) is not clearly described and in those cases is described here as intensity or is interpreted from the referenced papers. This is a limitation of current therapeutic ultrasound research.
Figure 3.
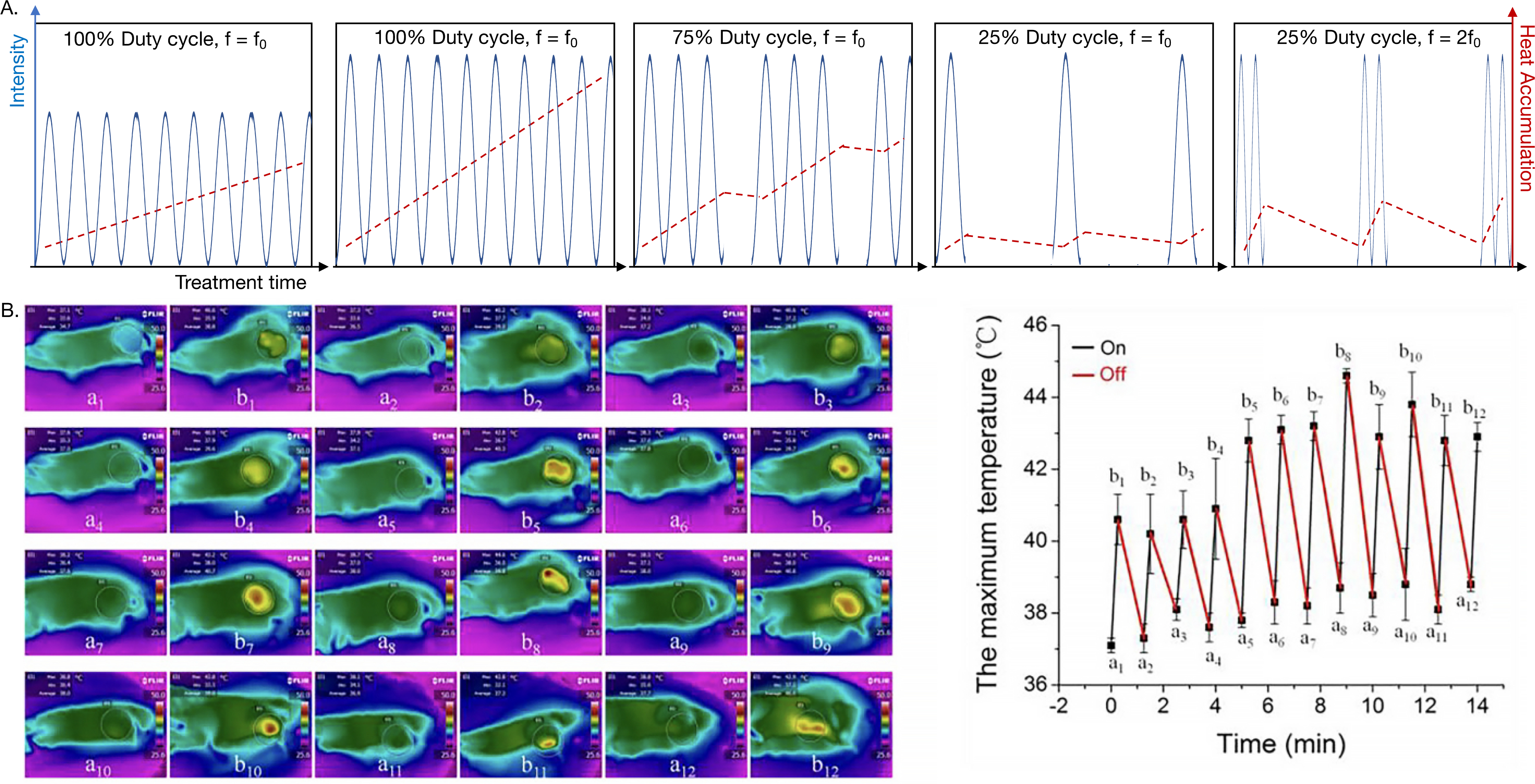
A) Schematic of thermal effects produced by ultrasound. The temperature elevation varies with acoustic intensity, duty cycle, treatment time and ultrasound frequency. At a given duty cycle, higher intensity leads to greater heat accumulation. B) Infrared (IR) temperature maps at the corresponding time points before and after ultrasound irradiation cycles, and the measured maximum temperature at each point [76]. Adapted from [76]. Copyright from Ivyspring International Publisher.
Energy absorption in tissue is also proportional to the ultrasound frequency, meaning that at a given ITA, a higher frequency will yield a greater temperature elevation. The spatial characteristics of the acoustic beam (focal depth and focus size) are set by the transducer dimensions and shape (flat or focused). Focused transducers are generally employed in UMGT to limit exposure to the surrounding healthy tissue. The use of phased arrays adds the ability to move the focus in space under imaging guidance with electronic steering but requires dedicated hardware [75].
3.2. Mechanical force and cavitation
Mechanical effects of insonation also play an important role in ultrasound therapy. Cavitation results from the nucleation, growth and collapse of gaseous cavities induced by pressure changes from ultrasound waves [77]. Such cavities can be generated from dissolved gas in the bloodstream. The acoustic pressure induced by ultrasound waves oscillates between compression and rarefaction phases; during rarefaction phases of high amplitude, bubble nucleation can occur (Figure 4A). While high amplitude, short ultrasound bursts propagate through tissue and interact with bubbles, cavitation can be accomplished with minimal thermal elevation. A parameter used to determine the likelihood of cavitation is the mechanical index (MI) [78]. The MI is defined as the maximum value of the peak rarefactional pressure (or peak negative pressure) divided by the square root of the acoustic center frequency. In the presence of MBs, this index has been updated due to the greater effect of center frequency [79]. Injected MBs enhance and localize cavitation activity with the oscillation characterized by high-speed photography of MB oscillation (Figure 4B) [80, 81].
Figure 4.
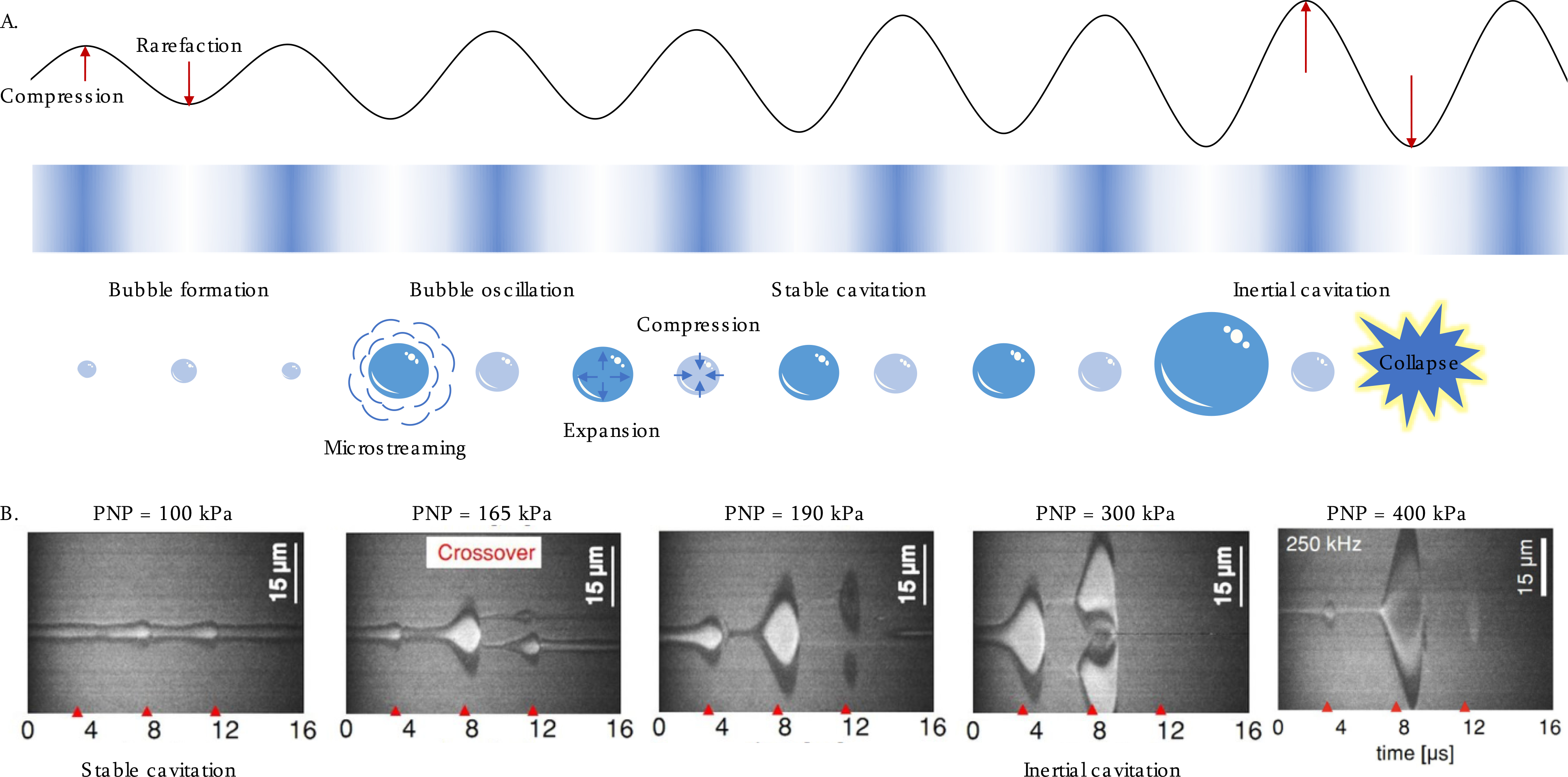
A) Schematic of non-thermal mechanical force, and the induced acoustic cavitation effect. In an oscillating pressure field, MBs expand and contract in response to the compression and rarefaction phases of the pressure wave. Stable cavitation occurs at low pressures resulting in microstreaming around the MBs. At higher pressures, this regime transforms into inertial cavitation where the increased expansion is followed by a violent collapse generating shock waves and the MBs destruction. B) Images of oscillating MBs (initial radius = 1.5 μm) captured by ultra-high-speed camera showing the transition from stable to inertial cavitation at ultrasound frequency of 250 kHz when increasing the acoustic pressure [86].
Cavitation regimes include stable and inertial cavitation [77]. Stable cavitation is the sustained oscillation of gas bubbles as the pressure fluctuates, which can result in adiabatic heat generation, microstreaming of surrounding fluid and localized shear stresses [82]. Such cavitation can induce cellular changes such as membrane permeabilization, referred to as sonoporation [83].
When the rate of MB collapse is enhanced by fluid inertia, the collapse velocity is enhanced and the phenomenon is termed inertial cavitation. The energy released during bubble collapse can generate violent shock waves with pressure up to 10,000 atm during a single ultrasound exposure within a duration of <1 μs [84]. Inertial cavitation can cause irreversible tissue lesions, depending on the bubble size range. Both cavitation regimes can lead to substantial physical, chemical and biological effects in surrounding tissues [85].
3.3. Endocytosis and therapeutic delivery via sonoporation
A common mechanism behind ultrasonic enhancement of drug or gene uptake involves cavitation-induced transient membrane perforation of target cells, e.g. endothelial cells on the BBB or tumor cells in the TME [87, 88] (Figure 5A). For example, cellular uptake of hypoxia-inducible factor 1 alpha (HIF-1α) siRNA was increased by 90% in MDA-MB-231 human breast cancer cells following insonation [89]. The concentration of pDNA encoding for luciferase was enhanced significantly in the cytoplasm and not in endosomes as a result of ultrasound, indicating that the internalization can be diffusive and not endocytosis-based.
Figure 5.
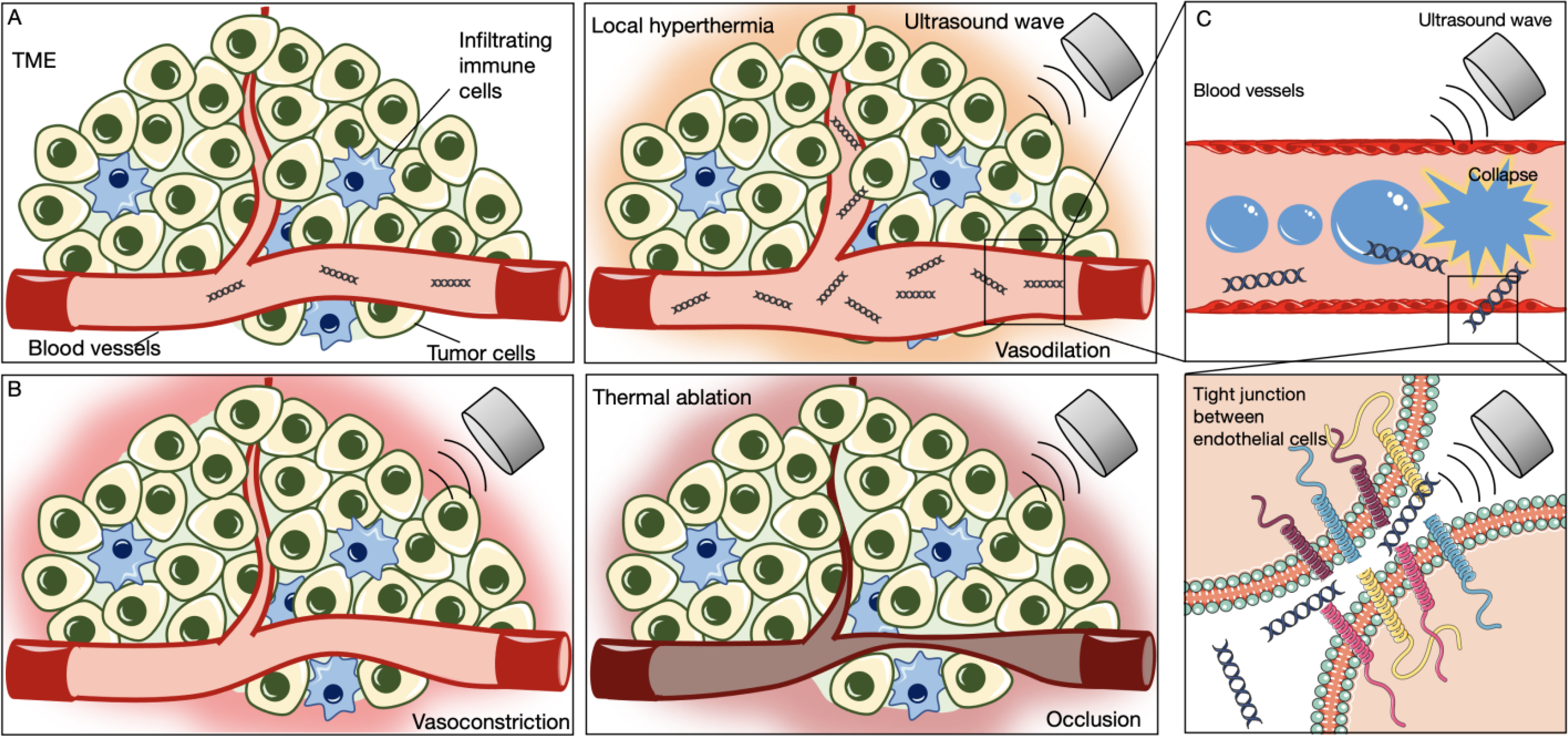
Schematics of A) vasodilation caused by minimal local hyperthermia, B) vasoconstriction and thermal ablation with the increased energy absorption, and C) temporary enhancement of vascular permeability by reversible inhibition of tight junction between endothelial cells.
The MBs used in transfection protocols are commercially available and are approved for use in clinical imaging protocols. Importantly, since MBs act both as contrast agents and transfection potentiators, it is possible to increase the spatial localization of gene delivery by monitoring MB oscillation in tumors through ultrasound imaging guidance [21, 86]. MB-mediated gene delivery achieved localized transfection within the area of ultrasound exposure, and can further refine site-specificity on the cellular or even molecular level. MBs can also be functionalized by conjugating peptide ligands or antibodies on the surface [90]. MB accumulation in tumors was enhanced by surface grafting of ligands such as vascular endothelial growth factor receptor 2 (VEGFR2) or αvβ3 integrins [91–93].
The ultrasound intensity required for sonoporation typically ranges between 0.5 and 3 W/cm2. The peak negative pressure (PNP) required for inertial cavitation in the presence of MBs is frequency dependent and is typically in the range of 0.2 to 1.5 MPa. For example, at a PNP of 200 kPa and frequency of 2.25 MHz, transfection was obtained in 5–10% of total cells with cell viability of 80–90% [94]. When the acoustic pressure was increased above 300 kPa, MB destruction increased, 15–25% expressed the reporter gene, and the cell viability was ~70%. At a frequency near 1.3 MHz, sonoporation has been accomplished with diagnostic ultrasound systems and intensities (0.1 to 100 mW/cm2) [16, 95]. The duty cycle is an additional important parameter in UMGT [96], and typically ranges from 20 to 50% in gene therapy studies. The reported ultrasound exposure duration ranges from a few seconds to 30 minutes. The thermal effect also depends on ultrasound exposure parameters, tissue properties and beam configuration [97]. Tran et al. investigated lower-pressure conditions close to the inertial cavitation threshold of MBs, and discovered that prolonging pulse duration time enhanced gene transfer efficiency without cell damage [98].
3.4. Effect of ultrasound on vasculature
3.4.1. Vasodilation and constriction
Due to the direct reflective activation of vascular smooth muscles via temperature receptors, when the CEM43 is low, thermal effects can induce vasodilation [99], increasing blood flow in a local region. The enhanced blood volume can enhance drug or gene delivery to the target site (Figure 5A). In the context of mild hyperthermia, intratumoral pressure can be reduced and nanoparticle accumulation increased [100]. In the context of thermal ablation, nanoparticle accumulation can be enhanced in the ablated rim [13].
For mild hyperthermia, the process is largely reversible, and vessels can respond without tissue damage. However, a higher CEM43 can induce temporary or permanent vasoconstriction within the tumor core while also creating a hyperemic rim. As shown in Figure 5B, when exposed to high intensity ultrasonic pulses, blood vessels can undergo a temporary or even permanent reduction in diameter. HIFU can also be combined with MBs to achieve an anti-vascular effect to deprive nutrients to the TME [101]. Antivascular treatments result in hypoxia and enhanced immune cell recruitment [102].
3.4.2. Endothelial permeability enhancement
In addition to cell membrane permeability enhancement, cavitation can enhance the effective endothelial permeability to further enhance therapeutic delivery (Figure 5C). Physiological barriers exist between the vessel lumen and surrounding tissue, including tight junctions that limit drug delivery to the target sites [103]. In addition to the thermal effects on the vasculature described above, focused ultrasound can transiently increase vascular permeability through mechanical effects, thereby temporarily allowing therapeutic agents to diffuse into the surrounding tissue with higher efficiency [104, 105]. The systemic administration of MBs acting as cavitation nuclei particularly enhances permeability at the site of ultrasound application [80]. This phenomenon has been studied in opening the blood-brain barrier (BBB), a particularly challenging tight obstruction of capillary endothelial cells that can inhibit the invasion of exotoxins and therapeutics [106]. Using cytokine analysis and enzyme-linked immuno sorbent assay (ELISA), ultrasound-mediated BBB opening produced an immediate sterile inflammatory response (SIR) in the parenchyma including increases in heat-shock protein (HSP) 70, interleukin-1 (IL-1), IL-18, and tumor necrosis factor-alpha (TNFα) [107]. Within limited ultrasound parameter sets, the BBB opening has been shown to be reversible after several hours via MRI and histological analysis [108]. Mechanical BBB opening has been applied in UMGT for the treatment of brain tumors, such as glioma [109] or glioblastoma [110]. In a preclinical dose escalation study, increasing focused ultrasound intensity within safety limits in glioblastoma tumors induced therapeutic benefits including increasing tumor infiltrating lymphocytes (TILs) and creating an immunostimulatory TME [111]. We find that ultrasound-mediated BBB opening has been combined with either gene therapy [109, 110, 112] or immune therapy [113, 114] but not yet reported with the combination of the two.
3.5. Triggered release from vehicles
Ultrasound can also trigger therapeutic agent release from delivery systems (Figure 6A) such as MBs [115], nanoparticles [116], liposomes [117] and polymeric constructs [118]. For example, in [93], a plasmid encoding for herpes simplex virus thymidine kinase (pHSV-TK) was bound to VEGFR2-targeted cationic MBs to reduce the required systemic dose. The plasmids were released at the tumor site by applying ultrasound. In vivo studies with brain tumor-bearing rats showed that the tumor volume was reduced significantly after systemic administration of MBs-encapsulated pHSV-TK combined with focused ultrasound [93].
Figure 6.
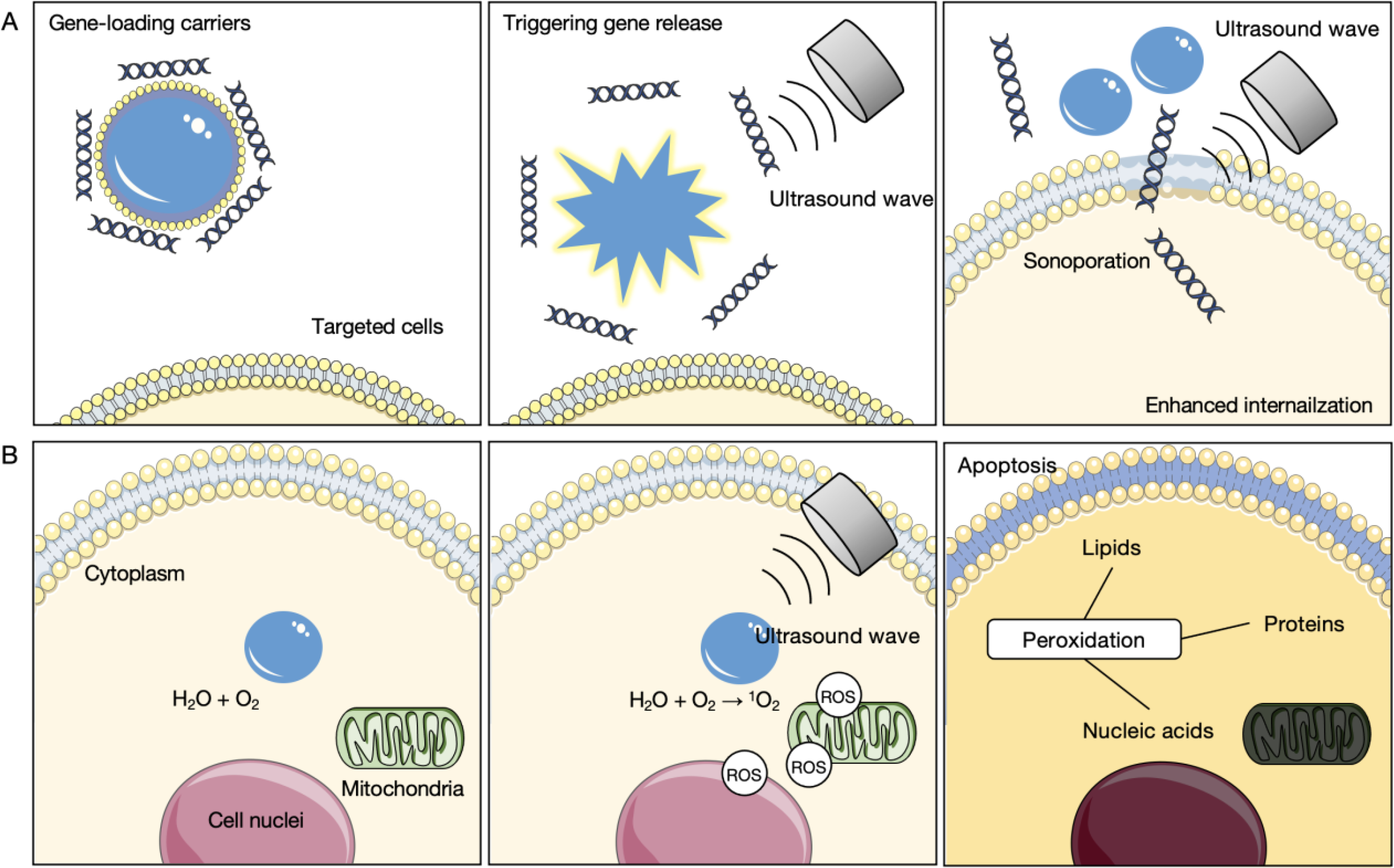
Schematic of the effect on cells for transfection, including A) triggering gene release from gene-loading carriers and enhanced gene internalization by sonoporation, and B) generation of cytotoxic radicals by the sonochemical effect.
3.6. Reactive oxygen species (ROS) and sonochemical effects
Particularly when MBs are used to deliver gene therapy through enhanced membrane permeability, focused ultrasound can produce free radicals that result in chemical transformations in the surrounding environment [119]. During the cavitation of gaseous bubbles, the core temperature can instantaneously increase by ~1000 K, leading to sonochemical effects in the medium. Such effects induce the reaction of water molecules and dissolved oxygen, resulting in a higher local concentration of free radicals, such as reactive oxygen species (ROS), to enhance cytotoxicity (Figure 6B). Additionally, ultrasound can be combined with sonosensitive compounds to facilitate the generation of ROS that induces peroxidation to cell membranes and mitochondria, referred to as the sonodynamic effect [120]. Sonosensitive compounds are usually photosensitizers owning to their aromatic ring structure that facilitates effective photon energy transfer, including hematoporphyrin, phthalocyanine, pheophorbide or erythrosine [121]. The incorporation of sonodynamic effects has been proposed as a synergistic technique for cancer immunotherapy, leading to calreticulin expression on the cell surface, an antitumor vaccination and abscopal effects [122].
3.7. Release of antigen
The thermal and mechanical effects generated by focused ultrasound can facilitate the release of tumor antigen into the bloodstream and the draining lymphatics [123–126]. Tumor antigen released into circulation can be used as a biomarker for cancer progression or recurrence [127]. These biomarkers are typically too dilute to be detected in the blood. However, after insonation, increased levels may be more easily detected. After tumor treatment, focused ultrasound is proposed to track therapeutic efficacy by monitoring circulating biomolecules [128]. Several groups have focused on the detection of circulating protein and nucleic acid biomarkers that are enhanced after insonation. For example, preclinically, carcinoembryonic antigen (CEA), cancer antigen 19–9 (CA19–9), miR-141 and miR-200c were enhanced by insonation of liver tumors [125]. Detection of enhanced protein markers was also established after uterine ablation in human studies [125]. Antigenic debris can recruit antigen-presenting cells (APCs), such as DCs and macrophages which prime CD8+ T cells for an antigen-specific cellular response [129]. Tumor-specific antigen [130] has been detected on macrophages or DCs in the blood and spleen following immunotherapy protocols that incorporate ultrasound [123].
3.8. Impact of therapeutic ultrasound on tumor burden, cytokines and chemokines
In UMGT, therapeutic ultrasound can also debulk tumors [21]. The T-cell invigoration-to-tumor burden ratio has been shown to be associated with anti-PD-1 response [131]. Therefore, reducing tumor burden through UMGT may impact treatment efficacy even in the absence of an effective transfection.
Following specific focused ultrasound protocols, wound healing and inflammation in the TME upregulate danger signals, such as HSPs, damage-associated molecular patterns (DAMPs) [97] and ATP [132, 133]. In [134], a non-ablative pulsed ultrasound protocol (1.15 MHz, PNP of 6 MPa, intensity spatial average temporal peak (ISATP) of 2683 W/cm2, 10% duty cycle, 5 Hz PRF, 100 pulses per site), altered the expression of cytokines, chemokines, and cell adhesion molecules, and immune cell phenotypes shifted to an inflamed TME. Melanoma (B16 cell line) and breast cancer (4T1 cell line) growth rates decreased as a result of the protocol. Proteomic changes were observed within 24 h, and the tumor growth rate slowed over 5 days after pulsed ultrasound. However, the immune cell trafficking and activation of signaling pathways between the two tumor types differed [134].
As a result of thermal and mechanical ablative ultrasound alone or in combination with immune agonists, RNA sequencing (RNAseq) and T cell receptor sequencing (TCRseq) have been applied to define the changes in the tumor and TME [80, 135]. MRI was used to monitor the tumor temperature in each study. For thermal ablation, the goal was to achieve a local temperature of 65°C or slightly greater within the central ½ fraction of the murine tumor. For mechanical ablation, a PNP of 16.9 MPa at 3 MHz was used to mechanically disrupt the tumor [135]. Based on this work, both mechanical HIFU and thermal ablation induced a potent inflammatory response and changed macrophage polarization compared to a no-treatment control (NTC). HIFU also upregulated innate immune receptors and related pathways. Priming with an immune agonist (CpG) attenuated the increase in inflammatory cytokines (e.g. IL-6) and further increased expression of innate immune receptors. Intra-tumoral antigen cross-presentation reached ~8% of CD45+ cells with ablation alone. Ablation combined with CpG amplified cross-presentation in the same-site draining lymph node (~16% of CD45+ cells) compared to ablation only (~0.1% of CD45+ cells). Type I interferon (IFN) release also increased with the ablation-immunotherapy treatment as compared with ablation or immunotherapy alone. Expression of T-cell activation genes increased up to 89-fold with ablation-immunotherapy treatment as compared to the NTC. Similar CDR3 sequences arose between mice after immunotherapy, whereas TCR overlap between mice was minimal before treatment. The number of unique CDR3 rearrangements in the distant tumor increased when ablation was combined with the immunotherapy protocol.
Non-thermal HIFU ablation, specifically histotripsy [136, 137] and boiling histotripsy also induce bioeffects that complement immunotherapy. Histotripsy is defined as short (<50 msec) very high intensity ultrasound pulses that create controlled cavitation and mechanically homogenize targeted tissues. Histotripsy (50 pulses at 100 Hz PRF, estimated ~30 MPa PNP, duration from 4 to 15 min) increased both innate and adaptive immune cell populations in treated and distant tumors [136]. Boiling histotripsy is defined as a shock front with amplitude sufficient to induce vapor bubble formation in less than 20 msec, pulse lengths of 2 to 4 times longer than the time to reach boiling, and duty factors of less than 2%. In [138], the time to reach boiling was estimated as 2.7 msec (shock amplitude of 70 MPa, pulse duration of 10 msec and PRF of 1 Hz) and the protocol released 3 – 32-fold more tumor-derived miRNA than ultrasound permeabilization or mild heating. Similarly, in a renal cell carcinoma rat model, boiling histotripsy enhanced CD8+ cytotoxic T cell infiltration in both treated and abscopal tumors [139]. Combining boiling histotripsy (shockwave amplitude of 80 MPa, peak positive pressure of 85 MPa, peak negative pressure of 14 MPa) with checkpoint inhibitors such as anti-PD-1 and anti-CTLA-4 resulted in an increase of systemic TILs that translated to improved survival benefits [140].
In breast cancer patients, HSP-70 expression increased in cancer cells within the central necrotic zone after insonation, while only a few positively-stained cells were observed in the periphery [141, 142]. Further, focused ultrasound can decrease the level of immunosuppressive cytokines, such as VEGF and transforming growth factor-beta (TGF- β), to alleviate tumor-induced immunosuppression and renew antitumor immunity [142, 143]. TGF-β can reduce antigen presentation to DCs, T cell differentiation, macrophage and NK cell proliferation, and immunostimulatory cytokine secretion [144]. Consequently, therapeutic ultrasound application can result in tumor regression and a reduction in metastasis [145]. These effects must also be considered when dissecting the impact of UMGT on the immune system.
IV. Current immunotherapy approaches
New immunotherapy protocols aim to improve efficacy and safety. To date, the benefit of immunotherapy has been limited to a minority of patients with specific cancer types. Further, a considerable subset of patients who initially respond eventually relapse [146]. Conventional vaccination methods for immunotherapy can induce tumor-associated antigen (TAA)-specific cytotoxic T lymphocytes (CTLs), but have typically shown limited clinical efficacy [147].
Recombinant cytokines for immune cell activation were the first immunotherapies approved by the US Food and Drug Administration (FDA), including interferon-alpha (INF-α) for hairy cell leukemia [148] and IL-2 for metastatic renal cancer and metastatic melanoma [149]. However, unfavorable pharmacokinetics of these recombinant cytokines can cause severe adverse effects such as cytokine release syndrome [150]. Over the past several years, ipilimumab, a checkpoint inhibitor that can specifically target cytotoxic T lymphocyte associated protein 4 (CTLA4) [151] was approved for clinical treatment of advanced melanoma [152, 153]. Shortly thereafter, mAbs targeting programmed cell death 1 (PD-1) or its ligand, PD-1 ligand 1 (PD-L1) were developed for clinical use [154]. While these mAbs showed high efficacy in certain groups of patients, efficacy is greatest in cancers with elevated expression of checkpoint receptors [155, 156].
Recently, CAR-T cell therapy was developed as an alternative approach to small molecule and biologic-based therapies. Similar to adoptive cell transfer commonly seen in preclinical models, T cells isolated from the host were modified with gamma-retroviral or lentiviral vectors to express a CAR and then re-injected. CAR-T therapy has been approved to treat hematological malignancies with trials in other tumors underway [157]. Despite the remarkable efficacy in hematological cancer, it remains challenging to promote infiltration of CAR-T cells into solid tumors. Major hurdles of current immunotherapeutic interventions include inefficient tumor infiltration and changes in the T cell phenotype after migration [158]. Overcoming the aforementioned impediments will likely require innate immune activation. To introduce nucleic acids, including plasmids, small interfering RNA (siRNA), messenger RNA (mRNA) or microRNA (miRNA) [159] into target cells, the fundamental engineering challenge is to develop safe and effective techniques to deliver those nucleic acids [160]. In the next section, we describe the combination of UMGT and immunotherapy protocols.
V. Ultrasound, gene delivery and immunotherapy
In combination with immunotherapy, UMGT can be used to treat cancer and produce an efficacious immune response in preclinical models. UMGT can be subdivided to include four approaches: tumor transfection with ultrasound only, MB-based transfection, ultrasound-sensitive delivery systems and modulation of immune cell functionality. As summarized in Table 2, the parameters in UMGT vary and the corresponding approaches are explained in the following sections.
Table 2.
Summary of preclinical studies of UMGT for cancer immunotherapy.
| UMGT Approaches | Tumor models | Gene constructs | Ultrasound parameters | Target cell | Ref. |
|---|---|---|---|---|---|
| 5.1 Transfection with ultrasound | Fibrosarcoma, murine models | GM-CSF pDNA | f = 1 MHz, ISATA = 1.0 W/cm2, Duty cycle = 20%, t = 5 min | Tumor cells | [159] |
| Melanoma and renal carcinoma murine models | IL-12 pDNA | f = 200 kHz (laboratory lithotripter similar to Dornier HM-3 lithotripter), PNP = 7.4 MPa, PRF = 2 Hz, Shock Waves = 500 (Duty cycle: 0.5%) + intratumoral air bubbles | Tumor cells | [160] | |
| 5.2 MB-based transfection | Prostate cancer murine models, human prostate tumor xenografts | IL-27 pDNA | f = 1 MHz, PNP = 0.12 MPa, ISATA = 1 W/cm2, PRF = 2 Hz, Duty cycle = 50%, t = 2 min, + SonoVue MBs | Tumor cells | [161] |
| Melanoma murine models | IFN-β pDNA | f = 1.011 MHz, ISATA = 0.22 W/cm2, Duty cycle = 50%, PRF = 0.5 Hz, t = 3 min, + Sonazoid MBs | Tumor cells | [162] | |
| Cervical cancer murine models | miR-34a | f = 1 MHz, I = 1.5 W/cm2, Duty cycle = 50%, t = 90 s, + cationic lipid MBs | Tumor cells | [163] | |
| Breast cancer, murine models | IFN-β pDNA | f = 250 kHz, PNP = 500 kPa, ISPTA = 1 W/cm2, PRF = 30 Hz, t = 3 min, Duty Cycle = 12%, + anti-EpCAM conjugated MBs | Stromal cells, tumor cells | [21] | |
| 5.3 Ultrasound-responsive delivery system | Ovarian carcinoma, murine models | IL-12 pDNA | f = 1 MHz, I = 0.7 W/cm2, t = 60 s, + liposomal bubbles | Cells in TME | [164] |
| Lymphoma murine models | OVA pDNA | f = 2.062 MHz, I = 4 W/cm2, Duty cycle = 50%, t = 20 s, +mannose-modified liposomes | APCs | [165] | |
| Melanoma murine models | pUb-M cDNA | Duty cycle = 50%, PRF = 10 Hz, t = 2 min, +mannose-modified liposomes f = 1.045 MHz, I = 1 W/cm2 (for intravenous injection) f = 2.062 MHz, I = 4 W/cm2 (for intradermal and intrasplenic injection) |
APCs | [166] | |
| 5.4 Modulation of immune cell functionality | Lymphoma and leukemia cell models | Anti-CD19 CAR gene loaded lentiviruses | f = 2 MHz, ISPTA = 0.6 W/cm2, PNP = 0.6 MPa, PRF = 5 Hz, Duty cycle = 5%, t = 10 min, +MBs | Jurkat T cells and PBMCs | [167] |
| Lymphoma and prostate cancer models | Cre-lox gene with HSP promotor | f = 1.5 MHz | T cells | [168] | |
| DCs from bone marrow of C57Bl/6 mice | mRNA encoding luciferase or GFP | f = 1 MHz, I = 2 W/cm2, Duty cycle = 50% t = 30 s, + lipoplexes + MBs | DCs | [169] | |
| Melanoma lung metastasis murine models | Antigen mRNA and TriMix mRNA | f = 1 MHz, ISPTA = 2 W/cm2, PNP = 800 kPa, Duty cycle = 20%, PRF = 100 Hz, t = 30 s, lipid MBs | DCs | [170] | |
| Hepatocellular carcinoma human cells | Foxp3 siRNA | f = 2.5 MHz, MI = 1.4, t = 150 s, + SonoVue MBs | Tregs | [171] |
Abbreviation: f: frequency, I: acoustic intensity (this notation is used when the specific intensity metric cannot be determined), PNP: peak negative pressure, PRF: pulse repetition frequency, MI: mechanical index, EpCAM: Epithelial cell adhesion molecule, IFN-β: Interferon-beta, IL-27: interleukin 27, IL-12: interleukin 12, GM-CSF: granulocyte-macrophage colony-stimulating factor, OVA: ovalbumin, HSP: heat shock protein, Foxp3: Forkhead box P3, APC: antigen-presenting cells, DCs: dendritic cells, PMBCs: peripheral blood mononuclear cells, ISPTA: Spatial peak temporal average intensity, ISATA: Spatial average temporal average intensity
5.1. Transfection with ultrasound without other components
Ultrasound alone has been observed to deliver DNA plasmids efficiently, accurately and safely without transfection reagents. In one study, an unfocused therapeutic ultrasound system (Sonitron 2000, Rich-Mar Corp., Inola, OK, USA) with an unfocused 1.13-cm2 1-MHz applicator was used to insonify a relatively large region. ISATA of 1.0 W/cm2 at 20% duty cycle for 5 min (60 J/cm2) was found to optimize transfection of reporter genes and was applied in an immunotherapy regimen [159]. Higher intensities reduced the effective transfection. In this work, naked plasmids coding for granulocyte-macrophage colony-stimulating factor (GM-CSF) were used to treat fibrosarcoma-bearing mice (Figure 7A). The efficiency was comparable to electroporation-based gene transfer. Ultrasonic shock-wave treatment combined with IL-12 plasmids has been investigated in both mouse melanoma and renal carcinoma models. A laboratory lithotripter system applied 500 shock waves at a 2-Hz rate to enhance IL-12 expression, resulting in significant tumor reduction as compared to the corresponding monotherapies [160].
Figure 7.
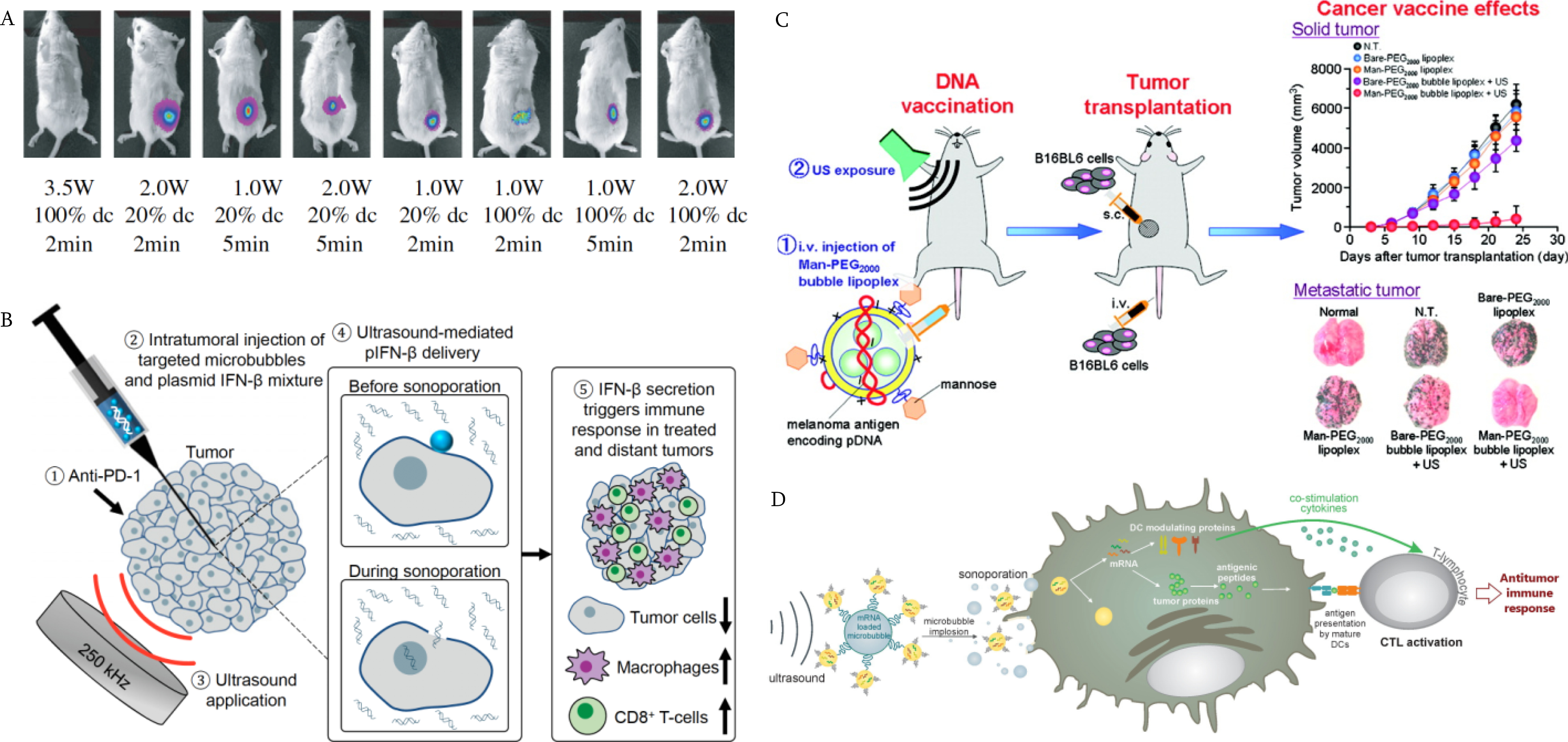
Approaches of UMGT for cancer immunotherapy. A) Transfection with plasmids and ultrasound. The ultrasound parameters were optimized to administer an immune gene construct in murine fibrosarcoma tumor models [159]. Copyright (2010) Elsevier; B) Transfection mediated by MB oscillation. Low frequency ultrasound was applied with the mixture of IFN-β plasmids and targeted MBs, and the immune cells were recruited at both sites of treated and distant tumors [21]. Copyright (2020) National Academy of Sciences; C) Transfection by ultrasound-sensitive delivery system. The mannose-modified lipoplexes were developed as ultrasound-responsive and APC-selective carriers for tumor antigen gene delivery. Reprinted with permission from [166]. Copyright (2011) American Chemical Society; D) Modulation of immune cells using UMGT. TriMix mRNA-loaded MBs were used to transfect DCs by sonoporation to activate T cells [170] Copyright (2014) Elsevier.
5.2. MB-based transfection
MB-based gene delivery is performed by administration of MBs and immunotherapeutic materials prior to insonation. Many studies have utilized a transmission center frequency of 1 MHz, and the ultrasound intensity ranged from 0.15 to 2 W/cm2. Lower insonation pressures can prolong pulse duration time and enhance gene transfer efficiency without cell damage [162]. For example, SonoVue MBs were used to transfer an IL-27 plasmid with a 1 MHz transducer at an ISATA of 1 W/cm2 (SonoPore KTAC-4000, Protech International) [161]. The sonoporation strategy was studied in both fully murine models and human prostate tumor xenografts, resulting in over 60-fold increase in expression as compared with either MBs alone or naked DNA. Similarly, Sonazoid MBs combined with 1.011 MHz oscillation frequency at an ISATA of 0.22 W/cm2 (SonoPore KTAC-4000) facilitated IFN-β plasmid delivery to treat melanoma [162]. In the above studies, MBs were simply mixed with genes, while in [35] cationic lipid MBs were used as carriers for miR34a, and were designed to treat cervical cancer by improving the efficacy in anti-PD-1 therapy.
Additionally, a protocol with a lower transmission frequency (250 kHz) and PNP of 500 kPa efficiently transfected tumor and stromal cells with DNA plasmid encoding IFN-β [21] (Figure 7B). MB wall collapse velocity was more rapid with the lower ultrasound frequency. The MB oscillation reached an expansion ratio of 35, resulting in a 150-fold increase of transfected cells observed after the 250 kHz insonation (H115, Sonic Concepts, Bothell, Washington). As reported in this study, the tumors were debulked, the plasmid transfection rates were increased, and more immune cells infiltrated the TME.
5.3. Ultrasound-responsive delivery systems
With systemic injection of plasmids, in vivo MB-based gene transfer is inefficient due to the different pharmacokinetic profiles of the nucleic acids and MBs. MBs have a limited circulation time (typically a few minutes), while nucleic acids can be loaded by alternative carriers for prolonged half-life and have been reported to circulate for extended periods after cutaneous injection [165]. Therefore, it is challenging to efficiently deliver both MBs and nucleic acids to the target sites simultaneously. Ultrasound-responsive liposomes with diameters ranging from 150 to 200 nm have been used to improve delivery. In one study, liposomes encapsulating perfluoropropane gas were used to deliver the IL-12 gene with a 1-MHz center frequency and intensity of 0.7 W/cm2 [164]. This approach reduced systemic adverse effects and resulted in a tumor regression rate of 80%. In other studies, mannosylated lipoplexes encapsulating perfluoropropane gas were developed to selectively target and transfect APCs to treat cancer [166]. With ultrasound exposure (f = 2.062 MHz, intensity 4.0 W/cm2), the transfection efficiency of pCMV-OVA was significantly increased. Mannose-modified lipoplexes encapsulating pCMV-OVA [165] or pUb-M encoding ubiquitylated melanoma-specific antigens, gp100 and tyrosinase-related protein (TRP-2) [166] were then applied in lymphoma or melanoma therapy, respectively (Figure 7C). As a result, the immune response was enhanced and sustained after transfection.
5.4. Modulation of immune cell functionality
Adoptive cell therapy uses cells from the patients’ own immune system to eliminate cancer [172] after isolation and expansion in vitro. Ultrasound is an ideal tool to precisely control genes to enhance immune response. Chimeric antigen receptor-expressing T (CAR-T) cells are commonly used as immune cell vaccines [173]. Piezo-1 in T cells was integrated with engineered genetic circuits in live HEK293T cells to convert ultrasound-activated Piezo1 into transcriptional activity. MB oscillation using ultrasound at a center frequency of 2 MHz and PNP of 0.6 MPa stimulated the expression of the anti-CD19-CAR, enabling T cells to recognize and kill CD19+ cancer cells [167].
Heat generated by an MRI-guided focused ultrasound system composed of a 1.5 MHz 8-element annular array transducer has also been used for CAR-T control through a heat shock protein driven Cre-lox gene switch [168]. By generating heat (43°C), ultrasound induced the activation of the Cre-lox gene, and elevated the level of anti-CD19-CAR or prostate-specific membrane antigen (PSMA)-CAR expression. In both lymphoma and prostate cancer mouse models, CAR-T cells were injected locally at tumor sites and led to a reduction in tumor growth.
For DC-based vaccines, the feasibility and safety were tested with in vitro DCs harvested from murine bone marrow. Messenger RNA encoding GFP or luciferase was mixed with cationic lipoplexes, and MBs loaded with mRNA-lipoplexes were incubated with DCs. As a result of insonation of the complex, transfection efficiency was enhanced, with limited impact on DC viability and maturation [170]. In another study, MBs loaded with both TAA-encoding mRNA as well as immunomodulating TriMix mRNA were utilized to sonoporate using ultrasound at a frequency of 1 MHz and ISPTA of 2 W/cm2 to load the mRNA [170]. The resulting DCs activated CD8+ T cells produce antigen-specific lysis of target cells (Figure 7D), resulting in significant tumor regression. 30% of the vaccinated animals maintained long-term antigen-specific immunological memory.
Lastly, MB-based techniques have been applied to transfect regulatory T cells (Tregs) with Forkhead box P3 (Foxp3) [171]. Tregs are responsible for reducing immune response to maintain homeostasis and self-tolerance, but also inhibit anti-cancer immunity. The ultrasound parameters were optimized to result in 50% in Treg transfection without an effect on proliferation. Once transfected with Foxp3 siRNA using ultrasound with 2.5-MHz center frequency and MI of 1.4, the Tregs were deactivated, and the immunosuppressive TME was reversed [171].
5.5. Integration of UMGT in combinational regimens
UMGT has been combined with other immunotherapies, such as immune checkpoint blockade, to improve the recruitment of tumor-specific T cells and their functionality [21, 174, 175]. UMGT-based immunotherapy can further modulate TME and prime immune cells by combining the technique with other non-immunological approaches, such as radiotherapy, chemotherapy [176], ablation therapy [135], photothermal therapy (PTT) [177], and photodynamic therapy (PDT) [89]. By exploiting the immunogenic cell death-inducing properties of conventional therapies, UMGT-based strategies can not only kill cancer cells but also stimulate in situ vaccination. Moreover, the gene transfection process can also be enhanced by combinatorial methods. A challenge of adenoviral methods is their high immunogenicity and host-specificity during systemic circulation. Therefore, a method using MBs and ultrasound was developed to protect human adenoviruses and deliver them to the target tumor site [178]. Transfection efficiency was enhanced without reducing activation of innate or acquired immunity.
VI. Limitations, challenges and future application of ultrasound in immunotherapy
The combination of ultrasound with drug and gene therapy is attractive due to the unique capabilities of therapeutic ultrasound to reduce major physiological barriers for delivery. In addition, the rapid growth of our understanding of immune cell receptors and signaling has provided a wealth of opportunities to transfect tumor, immune and stromal cells to treat cancer. Thus, the convergence of therapeutic ultrasound, gene therapy and immunotherapy is a particularly timely area of study. Further, the number of clinical studies that incorporate therapeutic ultrasound is rapidly increasing. At Stanford University, successful clinical trials have now led to the incorporation of therapeutic ultrasound in clinical care. Still, the safety of therapeutic ultrasound in particular tissues and organs must be established before gene therapy or immunotherapy can be incorporated. Such safety studies are underway around the world, and we are optimistic that combinatorial studies will follow soon. A study of the combination of focused ultrasound ablation and aPD-1 is underway (NCT04116320), and it is likely that other studies combining therapeutic ultrasound with approved agonist or checkpoint antibodies will emerge soon. Ablative protocols do induce a vaccination effect and this is exploited in such studies. Similarly, the use of ultrasound and MBs to open the BBB is the focus of multiple planned and recruiting human trials [179], and therefore the opportunity to pair this work with approved immunotherapies is likely imminent. The incorporation of UMGT with nanodelivery of nucleic acids in human immunotherapy protocols will require additional study. Most optimization studies for such UMGT techniques have been limited to pre-clinical models, and have not yet been tested in primates nor humans. Studies in large models (particularly porcine and canine models) will be required steps for translation.
Importantly, we emphasize that the many biological effects of therapeutic ultrasound can be synergistic with gene and immune therapy. Therapeutic ultrasound has the potential to selectively ablate tissue deep within the body without impacting the surrounding tissue, and can be repeated as needed. This debulking effect, together with the ability to release tumor-specific protein and nucleic acids are likely the greatest strengths of the technique. However, many therapeutic ultrasound protocols do induce additional biological changes. Therefore, combinatorial protocols involving ultrasound and gene therapy or immunotherapy must carefully account for individual effects through adequate controls. In this review, we have included considerations of vasoconstriction or dilation, cell membrane perforation, triggered cargo release, ROS generation, antigen release, and cytokine and chemokine production, as each will impact the ultimate therapeutic result.
6.1. Parameter space
A subset of current studies has shown different outcomes and effects depending on ultrasound parameters, MB, drug and gene dosage, and administration routes of MBs and genes. It will be important for prospective studies to systematically assess the parameter space of UMGT. Consistent reporting of all parameters is also key to the repeatability and assessment of protocols. In many cases, ISPTA is reported; however, PNP and duty cycle or equivalent parameters must also be provided in order to replicate the study. The development of standardized methods to measure and report pressure, pulse length and associated parameters is needed. Although the American Institute of Ultrasound in Medicine (AIUM) established guidelines for safe use of ultrasound [180], there are still few standards for therapeutic ultrasound and UMGT applications.
6.2. Drug carriers and targeted MBs
New delivery systems to carry and release nucleic acids are needed. Many recent studies involve loading nucleic acids onto cationic MBs; however, cationic materials suffer from limited circulation time and potential toxicity [181]. Additionally, improving MB binding specificity could enhance transfection efficiency and therapeutic outcome. Strategies include the development of alternate bioconjugation methods, and the identification of additional disease-specific ligands [182].
Further, much of the previous work has emphasized DNA as the nucleic acid cargo due to its stability. However, transport across the nuclear membrane is challenging and relatively few studies have documented how this transport changes with ultrasound or MB parameters. The emergence of RNA therapeutics provides an attractive alternative for UMGT with both silencing and transfection options increasing.
6.3. Molecular imaging
Molecular Imaging is an evolving discipline that enables the noninvasive visualization, evaluation and quantification of specific biologic processes at the cellular levels in living subjects [183, 184] and provides a potential strategy to characterize transfection efficiency and immunotherapeutic efficacy during and after UMGT [185]. For example, CAR-T cells designed to express both herpes simplex virus type 1 thymidine kinase (HSV1-TK) as a reporter gene and IL-13 as a therapeutic gene have been reported [186]. This concept could be applied to further improve the UMGT development in immunotherapy.
Final summary
Strategies to combine therapeutic ultrasound with gene and immune therapies are developing to treat cancer, and the convergence of the three fields is particularly exciting. We look forward to further advances in this promising field.
Acknowledgements
The authors acknowledge the support of this work by the National Institutes of Health (R01CA112356, R01CA211602, R01CA210553, R01CA227687, R01CA253316, R01CA250557 and R01EB02609), and China Scholarship Council (No. 201906010082).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Mitragotri S, Healing sound: the use of ultrasound in drug delivery and other therapeutic applications, Nature Reviews Drug Discovery, 4 (2005) 255–260. [DOI] [PubMed] [Google Scholar]
- [2].Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IRS, Overview of Therapeutic Ultrasound Applications and Safety Considerations, J Ultrasound Med, 31 (2012) 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McMahon D, O’Reilly MA, Hynynen K, Therapeutic Agent Delivery Across the Blood-Brain Barrier Using Focused Ultrasound, Annu Rev Biomed Eng, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Deprez J, Lajoinie G, Engelen Y, De Smedt SC, Lentacker I, Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery, Adv Drug Deliv Rev, 172 (2021) 9–36. [DOI] [PubMed] [Google Scholar]
- [5].Panje CM, Wang DS, Willmann JK, Ultrasound and Microbubble-Mediated Gene Delivery in Cancer: Progress and Perspectives, Investigative radiology, 48 (2013) 755–769. [DOI] [PubMed] [Google Scholar]
- [6].Karimi Galougahi K, Shlofmitz E, Jeremias A, Gogia S, Kirtane AJ, Hill JM, Karmpaliotis D, Mintz GS, Maehara A, Stone GW, Shlofmitz RA, Ali ZA, Therapeutic Approach to Calcified Coronary Lesions: Disruptive Technologies, Current Cardiology Reports, 23 (2021) 33. [DOI] [PubMed] [Google Scholar]
- [7].Li M, Sankin G, Vu T, Yao J, Zhong P, Tri-modality cavitation mapping in shock wave lithotripsy, J Acoust Soc Am, 149 (2021) 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seah BC, Teo BM, Recent advances in ultrasound-based transdermal drug delivery, Int J Nanomedicine, 13 (2018) 7749–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang R, Bian Q, Xu Y, Xu D, Gao J, Recent advances in mechanical force-assisted transdermal delivery of macromolecular drugs, Int J Pharm, 602 (2021) 120598. [DOI] [PubMed] [Google Scholar]
- [10].Lafond M, Aptel F, Mestas JL, Lafon C, Ultrasound-mediated ocular delivery of therapeutic agents: a review, Expert Opin Drug Deliv, 14 (2017) 539–550. [DOI] [PubMed] [Google Scholar]
- [11].Jiriys George Ginini DS, Emodi Omri, Rachmiel Adi, Low Intensity Pulsed Ultrasound for Accelerating Distraction Osteogenesis: A Systematic Review of Experimental Animal Studies, Journal of Molecular and Clinical Medicine, 1 (2018). [Google Scholar]
- [12].Huang X, Yuan F, Liang M, Lo HW, Shinohara ML, Robertson C, Zhong P, M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer, PLoS One, 7 (2012) e41632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wong AW, Fite BZ, Liu Y, Kheirolomoom A, Seo JW, Watson KD, Mahakian LM, Tam SM, Zhang H, Foiret J, Borowsky AD, Ferrara KW, Ultrasound ablation enhances drug accumulation and survival in mammary carcinoma models, J Clin Invest, 126 (2016) 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, Asquier N, Law-Ye B, Leclercq D, Bissery A, De Rycke Y, Trosch C, Capelle L, Sanson M, Hoang-Xuan K, Dehais C, Houillier C, Laigle-Donadey F, Mathon B, Andre A, Lafon C, Chapelon JY, Delattre JY, Carpentier A, Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma, Clin Cancer Res, 25 (2019) 3793–3801. [DOI] [PubMed] [Google Scholar]
- [15].Goel L, Jiang X, Advances in Sonothrombolysis Techniques Using Piezoelectric Transducers, Sensors (Basel), 20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bez M, Sheyn D, Tawackoli W, Avalos P, Shapiro G, Giaconi JC, Da X, David SB, Gavrity J, Awad HA, Bae HW, Ley EJ, Kremen TJ, Gazit Z, Ferrara KW, Pelled G, Gazi D, In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs, Science translational medicine, 9 (2017) eaal3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H, Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle, Mol Ther, 19 (2011) 895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Carson AR, McTiernan CF, Lavery L, Hodnick A, Grata M, Leng X, Wang J, Chen X, Modzelewski RA, Villanueva FS, Gene therapy of carcinoma using ultrasound-targeted microbubble destruction, Ultrasound Med Biol, 37 (2011) 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shapiro G, Wong AW, Bez M, Yang F, Tam S, Even L, Sheyn D, Ben-David S, Tawackoli W, Pelled G, Ferrara KW, Gazit D, Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation, J Control Release, 223 (2016) 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Newman CM, Bettinger T, Gene therapy progress and prospects: ultrasound for gene transfer, Gene Ther, 14 (2007) 465–475. [DOI] [PubMed] [Google Scholar]
- [21].Ilovitsh T, Feng Y, Foiret J, Kheirolomoom A, Zhang H, Ingham ES, Ilovitsh A, Tumbale SK, Fite BZ, Wu B, Raie MN, Zhang N, Kare AJ, Chavez M, Qi LS, Pelled G, Gazit D, Vermesh O, Steinberg I, Gambhir SS, Ferrara KW, Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites, Proc Natl Acad Sci U S A, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bez M, Foiret J, Shapiro G, Pelled G, Ferrara KW, Gazit D, Nonviral ultrasound-mediated gene delivery in small and large animal models, Nat Protoc, 14 (2019) 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schreiber RD, Old LJ, Smyth MJ, Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion, Science, 331 (2011) 1565–1570. [DOI] [PubMed] [Google Scholar]
- [24].He X, Xu C, Immune checkpoint signaling and cancer immunotherapy, Cell Res, 30 (2020) 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G, Viral vector platforms within the gene therapy landscape, Signal Transduct Target Ther, 6 (2021) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Peters C, Grandi P, Nigim F, Updates on Oncolytic Virus Immunotherapy for Cancers, Molecular Therapy - Oncolytics, 12 (2019) 259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kallert SM, Darbre S, Bonilla WV, Kreutzfeldt M, Page N, Muller P, Kreuzaler M, Lu M, Favre S, Kreppel F, Lohning M, Luther SA, Zippelius A, Merkler D, Pinschewer DD, Replicating viral vector platform exploits alarmin signals for potent CD8(+) T cell-mediated tumour immunotherapy, Nat Commun, 8 (2017) 15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arbuthnot P, Viral Vectors for Delivery of Antiviral Sequences, Gene Therapy for Viral Infections 2015, pp. 95–126. [Google Scholar]
- [29].Bushman FD, Retroviral Insertional Mutagenesis in Humans: Evidence for Four Genetic Mechanisms Promoting Expansion of Cell Clones, Mol Ther, 28 (2020) 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Torres-Vanegas JD, Cruz JC, Reyes LH, Delivery Systems for Nucleic Acids and Proteins: Barriers, Cell Capture Pathways and Nanocarriers, Pharmaceutics, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Juliano RL, The delivery of therapeutic oligonucleotides, Nucleic Acids Res, 44 (2016) 6518–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kirtane AR, Panyam J, Polymer nanoparticles: Weighing up gene delivery, Nat Nanotechnol, 8 (2013) 805–806. [DOI] [PubMed] [Google Scholar]
- [33].Wei L, Shi J, Jagoda E, Wong K, Opina C, Strauss J, Szajek L, Choyke P, Jacobs P, Hill GC, Kamble R, <strong>Development of 89Zr-Avelumab for Clinical Studies</strong>, Journal of Nuclear Medicine, 60 (2019) 1060. [Google Scholar]
- [34].Kim EY, Schulz R, Swantek P, Kunstman K, Malim MH, Wolinsky SM, Gold nanoparticle-mediated gene delivery induces widespread changes in the expression of innate immunity genes, Gene Ther, 19 (2012) 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhu X, Xu Y, Solis LM, Tao W, Wang L, Behrens C, Xu X, Zhao L, Liu D, Wu J, Zhang N, Wistuba II, Farokhzad OC, Zetter BR, Shi J, Long-circulating siRNA nanoparticles for validating Prohibitin1-targeted non-small cell lung cancer treatment, Proc Natl Acad Sci U S A, 112 (2015) 7779–7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chow YT, Chen S, Wang R, Liu C, Kong CW, Li RA, Cheng SH, Sun D, Single Cell Transfection through Precise Microinjection with Quantitatively Controlled Injection Volumes, Sci Rep, 6 (2016) 24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].O’Brien JA, Lummis SC, Nano-biolistics: a method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles, BMC Biotechnology, 11 (2011) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mthunzi P, Dholakia K, Gunn-Moore F, Phototransfection of mammalian cells using femtosecond laser pulses: optimization and applicability to stem cell differentiation, J Biomed Opt, 15 (2010) 041507. [DOI] [PubMed] [Google Scholar]
- [39].Carlsten M, Childs RW, Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications, Front Immunol, 6 (2015) 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Seyed Jafari SM, Blank F, Ramser HE, Woessner AE, Shafighi M, Geiser T, Quinn KP, Hunger RE, Gazdhar A, Efficacy of Combined in-vivo Electroporation-Mediated Gene Transfer of VEGF, HGF, and IL-10 on Skin Flap Survival, Monitored by Label-Free Optical Imaging: A Feasibility Study, Front Surg, 8 (2021) 639661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Campillo-Davo D, De Laere M, Roex G, Versteven M, Flumens D, Berneman ZN, Van Tendeloo VFI, Anguille S, Lion E, The Ins and Outs of Messenger RNA Electroporation for Physical Gene Delivery in Immune Cell-Based Therapy, Pharmaceutics, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Escoffre J-M, Zeghimi A, Novell A, Bouakaz A, In-vivo gene delivery by sonoporation: recent progress and prospects, Current Gene Therapy, 13 (2013) 2–14. [DOI] [PubMed] [Google Scholar]
- [43].Xenariou S, Liang HD, Griesenbach U, Zhu J, Farley R, Somerton L, Singh C, Jeffery PK, Scheule RK, Cheng SH, Geddes DM, Blomley M, Alton EW, Low-frequency ultrasound increases non-viral gene transfer to the mouse lung, Acta Biochim Biophys Sin, 42 (2010) 45–51. [DOI] [PubMed] [Google Scholar]
- [44].Huber PE, Mann MJ, Melo LG, Ehsan A, Kong D, Zhang L, Rezvani M, Peschke P, Jolesz F, Dzau VJ, Hynynen K, Focused ultrasound (HIFU) induces localized enhancement of reporter gene expression in rabbit carotid artery, Gene Ther, 10 (2003) 1600–1607. [DOI] [PubMed] [Google Scholar]
- [45].Anwer K, Kao G, Proctor B, Anscombe I, Florack V, Earls R, Wilson E, McCreery T, Unger E, Rolland A, Sullivan S, Ultrasound enhancement of cationic lipid-mediated gene transfer to primary tumors following systemic administration, Gene Ther, 7 (2000) 1833–1839. [DOI] [PubMed] [Google Scholar]
- [46].Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R, Local delivery of plasmid DNA into rat carotid artery using ultrasound, Circulation, 105 (2002) 1233–1239. [DOI] [PubMed] [Google Scholar]
- [47].Page A, Fusil F, Cosset FL, Toward Tightly Tuned Gene Expression Following Lentiviral Vector Transduction, Viruses, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Riyad JM, Weber T, Intracellular trafficking of adeno-associated virus (AAV) vectors: challenges and future directions, Gene Ther, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Dave UP, Jenkins NA, Copeland NG, Gene Therapy Insertional Mutagenesis Insights, Science, 303 (2004) 333. [DOI] [PubMed] [Google Scholar]
- [50].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Non-viral vectors for gene-based therapy, Nat Rev Genet, 15 (2014) 541–555. [DOI] [PubMed] [Google Scholar]
- [51].Schlich M, Palomba R, Costabile G, Mizrahy S, Pannuzzo M, Peer D, Decuzzi P, Cytosolic delivery of nucleic acids: The case of ionizable lipid nanoparticles, Bioeng Transl Med, (2021) e10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Potter H, Transfection by electroporation, Curr Protoc Mol Biol, Chapter 9 (2003) Unit 9 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bhatia S, Longino NV, Miller NJ, Kulikauskas R, Iyer JG, Ibrani D, Blom A, Byrd DR, Parvathaneni U, Twitty CG, Campbell JS, Le MH, Gargosky S, Pierce RH, Heller R, Daud AI, Nghiem P, Intratumoral Delivery of Plasmid IL12 Via Electroporation Leads to Regression of Injected and Noninjected Tumors in Merkel Cell Carcinoma, Clin Cancer Res, 26 (2020) 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Xie A, Belcik T, Qi Y, Morgan TK, Champaneri SA, Taylor S, Davidson BP, Zhao Y, Klibanov AL, Kuliszewski MA, Leong-Poi H, Ammi A, Lindner JR, Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles, JACC Cardiovasc Imaging, 5 (2012) 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Fujii H, Sun Z, Li SH, Wu J, Fazel S, Weisel RD, Rakowski H, Lindner J, Li RK, Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice, JACC Cardiovasc Imaging, 2 (2009) 869–879. [DOI] [PubMed] [Google Scholar]
- [56].Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR, Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle, Circ Res, 101 (2007) 295–303. [DOI] [PubMed] [Google Scholar]
- [57].Sirsi SR, Hernandez SL, Zielinski L, Blomback H, Koubaa A, Synder M, Homma S, Kandel JJ, Yamashiro DJ, Borden MA, Polyplex-microbubble hybrids for ultrasound-guided plasmid DNA delivery to solid tumors, J Control Release, 157 (2012) 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dong W, Wu PY, Zhou D, Huang JX, Qin MF, Yang XX, Wan MX, Zong YJ, Ultrasound-Mediated Gene Therapy of Hepatocellular Carcinoma Using Pre-Microrna Plasmid-Loaded Nanodroplets, Ultrasound in Medicine and Biology, 46 (2020) 90–107. [DOI] [PubMed] [Google Scholar]
- [59].Yoon S, Wang P, Peng Q, Wang Y, Shung KK, Acoustic-transfection for genomic manipulation of single-cells using high frequency ultrasound, Sci Rep, 7 (2017) 5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yoon S, Kim MG, Chiu CT, Hwang JY, Kim HH, Wang Y, Shung KK, Direct and sustained intracellular delivery of exogenous molecules using acoustic-transfection with high frequency ultrasound, Sci Rep, 6 (2016) 20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang J, Zhang L, Wan D, Zhou L, Zheng S, Lin S, Qiao Y, Extracellular matrix and its therapeutic potential for cancer treatment, Signal Transduct Target Ther, 6 (2021) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR, Targeting Tumor Microenvironment for Cancer Therapy, Int J Mol Sci, 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Evans SS, Repasky EA, Fisher DT, Fever and the thermal regulation of immunity: the immune system feels the heat, Nat Rev Immunol, 15 (2015) 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wu F, Wang ZB, Cao YD, Zhou Q, Zhang Y, Xu ZL, Zhu XQ, Expression of tumor antigens and heat-shock protein 70 in breast cancer cells after high-intensity focused ultrasound ablation, Ann Surg Oncol, 14 (2007) 1237–1242. [DOI] [PubMed] [Google Scholar]
- [65].Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, Moonen CTW, Image-guided, noninvasive, spatiotemporal control of gene expression, Proceedings of the National Academy of Sciences, 106 (2009) 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Pockley AG, Henderson B, Extracellular cell stress (heat shock) proteins-immune responses and disease: an overview, Philos Trans R Soc Lond B Biol Sci, 373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shevtsov M, Huile G, Multhoff G, Membrane heat shock protein 70: a theranostic target for cancer therapy, Philosophical Transactions of the Royal Society B: Biological Sciences, 373 (2018) 20160526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jones EL, Oleson JR, Prosnitz LR, Samulski TV, Vujaskovic Z, Yu D, Sanders LL, Dewhirst MW, Randomized trial of hyperthermia and radiation for superficial tumors, J Clin Oncol, 23 (2005) 3079–3085. [DOI] [PubMed] [Google Scholar]
- [69].Calderwood SK, Hyperthermia, the Tumor Microenvironment and Immunity, Tumor Ablation 2013, pp. 29–37. [Google Scholar]
- [70].Dunne M, Regenold M, Allen C, Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy, Adv Drug Deliv Rev, 163–164 (2020) 98–124. [DOI] [PubMed] [Google Scholar]
- [71].Fite BZ, Wong A, Liu Y, Mahakian LM, Tam SM, Aina O, Hubbard NE, Borowsky A, Cardiff RD, Dumont E, Ferrara KW, Magnetic resonance imaging assessment of effective ablated volume following high intensity focused ultrasound, PLoS One, 10 (2015) e0120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Phillips LC, Puett C, Sheeran PS, Wilson Miller G, Matsunaga TO, Dayton PA, Phase-shift perfluorocarbon agents enhance high intensity focused ultrasound thermal delivery with reduced near-field heating, J Acoust Soc Am, 134 (2013) 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gnanaskandan A, Hsiao CT, Chahine G, Contrast agent shell properties effects on heat deposition in bubble enhanced high intensity focused ultrasound, J Acoust Soc Am, 149 (2021) 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Nelson TR, Fowlkes JB, Abramowicz JS, Church CC, Ultrasound biosafety considerations for the practicing sonographer and sonologist, J Ultrasound Med, 28 (2009) 139–150. [DOI] [PubMed] [Google Scholar]
- [75].Liu J, Foiret J, Stephens DN, Le Baron O, Ferrara KW, Development of a spherically focused phased array transducer for ultrasonic image-guided hyperthermia, Phys Med Biol, 61 (2016) 5275–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang K, Xu H, Chen H, Jia X, Zheng S, Cai X, Wang R, Mou J, Zheng Y, Shi J, CO2 bubbling-based ‘Nanobomb’ System for Targetedly Suppressing Panc-1 Pancreatic Tumor via Low Intensity Ultrasound-activated Inertial Cavitation, Theranostics, 5 (2015) 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dalecki D, Mechanical bioeffects of ultrasound, Annu Rev Biomed Eng, 6 (2004) 229–248. [DOI] [PubMed] [Google Scholar]
- [78].Apfel RE, Holland CK, Gauging the likelihood of cavitation from short-pulse, low-duty cycle diagnostic ultrasound, Ultrasound Med Biol, 17 (1991) 179–185. [DOI] [PubMed] [Google Scholar]
- [79].Bader KB, Holland CK, Gauging the likelihood of stable cavitation from ultrasound contrast agents, Phys Med Biol, 58 (2013) 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Caskey CF, Stieger SM, Qin S, Dayton PA, Ferrara KW, Direct observations of ultrasound microbubble contrast agent interaction with the microvessel wall, J Acoust Soc Am, 122 (2007) 1191–1200. [DOI] [PubMed] [Google Scholar]
- [81].Chomas JE, Dayton P, May D, Ferrara K, Threshold of fragmentation for ultrasonic contrast agents, J Biomed Opt, 6 (2001) 141–150. [DOI] [PubMed] [Google Scholar]
- [82].Wan M, Feng Y, Cavitation In Biomedicine: Principles and Techniques, Springer; 2015. [Google Scholar]
- [83].Lentacker I, De Cock I, Deckers R, De Smedt SC, Moonen CT, Understanding ultrasound induced sonoporation: definitions and underlying mechanisms, Adv Drug Deliv Rev, 72 (2014) 49–64. [DOI] [PubMed] [Google Scholar]
- [84].Nagatomo D, Horie T, Hongo C, Ohmura N, Effect of ultrasonic pretreatment on emulsion polymerization of styrene, Ultrason Sonochem, 31 (2016) 337–341. [DOI] [PubMed] [Google Scholar]
- [85].O’Brien WD Jr., Ultrasound-biophysics mechanisms, Prog Biophys Mol Biol, 93 (2007) 212–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ilovitsh T, Ilovitsh A, Foiret J, Caskey CF, Kusunose J, Fite BZ, Zhang H, Mahakian LM, Tam S, Butts-Pauly K, Qin S, Ferrara KW, Enhanced microbubble contrast agent oscillation following 250 kHz insonation, Sci Rep, 8 (2018) 16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Qin P, Han T, Yu ACH, Xu L, Mechanistic understanding the bioeffects of ultrasound-driven microbubbles to enhance macromolecule delivery, J Control Release, 272 (2018) 169–181. [DOI] [PubMed] [Google Scholar]
- [88].Mehier-Humbert S, Bettinger T, Yan F, Guy RH, Ultrasound-mediated gene delivery: kinetics of plasmid internalization and gene expression, J Control Release, 104 (2005) 203–211. [DOI] [PubMed] [Google Scholar]
- [89].Sun S, Xu Y, Fu P, Chen M, Sun S, Zhao R, Wang J, Liang X, Wang S, Ultrasound-targeted photodynamic and gene dual therapy for effectively inhibiting triple negative breast cancer by cationic porphyrin lipid microbubbles loaded with HIF1alpha-siRNA, Nanoscale, 10 (2018) 19945–19956. [DOI] [PubMed] [Google Scholar]
- [90].Chang S, Guo J, Sun J, Zhu S, Yan Y, Zhu Y, Li M, Wang Z, Xu RX, Targeted microbubbles for ultrasound mediated gene transfection and apoptosis induction in ovarian cancer cells, Ultrason Sonochem, 20 (2013) 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yuan HX, Wang WP, Wen JX, Lin LW, Exner AA, Guan PS, Chen XJ, Dual-Targeted Microbubbles Specific to Integrin alphaVbeta3 and Vascular Endothelial Growth Factor Receptor 2 for Ultrasonography Evaluation of Tumor Angiogenesis, Ultrasound Med Biol, 44 (2018) 1460–1467. [DOI] [PubMed] [Google Scholar]
- [92].Hu C, Jiang D, Wu M, Wang J, Zhang R, Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of VEGFR2-targeted CD-TK-loaded cationic nanobubbles in the treatment of bladder cancer, J Cancer Res Clin Oncol, 146 (2020) 1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chang EL, Ting CY, Hsu PH, Lin YC, Liao EC, Huang CY, Chang YC, Chan HL, Chiang CS, Liu HL, Wei KC, Fan CH, Yeh CK, Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors, J Control Release, 255 (2017) 164–175. [DOI] [PubMed] [Google Scholar]
- [94].Mehier-Humbert S, Yan F, Frinking P, Schneider M, Guy RH, Bettinger T, Ultrasound-Mediated Gene Delivery: Influence of Contrast Agent on Transfection, Bioconjugate chemistry, 18 (2007) 652–662. [DOI] [PubMed] [Google Scholar]
- [95].Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA, Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology, Proc Natl Acad Sci U S A, 103 (2006) 8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Ferrara K, Pollard R, Borden M, Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery, Annu Rev Biomed Eng, 9 (2007) 415–447. [DOI] [PubMed] [Google Scholar]
- [97].van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Futterer JJ, den Brok MH, Adema GJ, Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies, Cancer Immunol Immunother, 66 (2017) 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tran DM, Harrang J, Song S, Chen J, Smith BM, Miao CH, Prolonging pulse duration in ultrasoundmediated gene delivery lowers acoustic pressure threshold for efficient gene transfer to cells and small animals, J Control Release, 279 (2018) 345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Maruo A, Hamner CE, Rodrigues AJ, Higami T, Greenleaf JF, Schaff HV, Nitric oxide and prostacyclin in ultrasonic vasodilatation of the canine internal mammary artery, The Annals of Thoracic Surgery, 77 (2004) 126–132. [DOI] [PubMed] [Google Scholar]
- [100].Watson KD, Lai CY, Qin S, Kruse DE, Lin YC, Seo JW, Cardiff RD, Mahakian LM, Beegle J, Ingham ES, Curry FR, Reed RK, Ferrara KW, Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors, Cancer Res, 72 (2012) 1485–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu Z, Gao S, Zhao Y, Li P, Liu J, Li P, Tan K, Xie F, Disruption of tumor neovasculature by microbubble enhanced ultrasound: a potential new physical therapy of anti-angiogenesis, Ultrasound Med Biol, 38 (2012) 253–261. [DOI] [PubMed] [Google Scholar]
- [102].Hunt SJ, Gade T, Soulen MC, Pickup S, Sehgal CM, Antivascular ultrasound therapy: magnetic resonance imaging validation and activation of the immune response in murine melanoma, J Ultrasound Med, 34 (2015) 275–287. [DOI] [PubMed] [Google Scholar]
- [103].Izadifar Z, Babyn P, Chapman D, Mechanical and Biological Effects of Ultrasound: A Review of Present Knowledge, Ultrasound Med Biol, 43 (2017) 1085–1104. [DOI] [PubMed] [Google Scholar]
- [104].Sheikov N, McDannold N, Sharma S, Hynynen K, Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium, Ultrasound Med Biol, 34 (2008) 1093–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, Bernabeu MO, Fukumura D, McDannold N, Jain RK, Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption, Proc Natl Acad Sci U S A, 115 (2018) E8717–E8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang N, Yan F, Liang X, Wu M, Shen Y, Chen M, Xu Y, Zou G, Jiang P, Tang C, Zheng H, Dai Z, Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy, Theranostics, 8 (2018) 2264–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kovacs ZI, Kim S, Jikaria N, Qureshi F, Milo B, Lewis BK, Bresler M, Burks SR, Frank JA, Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation, Proceedings of the National Academy of Sciences, 114 (2017) E75–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Kinoshita M, McDannold N, Jolesz FA, Hynynen K, Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption, Proc Natl Acad Sci U S A, 103 (2006) 11719–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Zhao G, Huang Q, Wang F, Zhang X, Hu J, Tan Y, Huang N, Wang Z, Wang Z, Cheng Y, Targeted shRNA-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth, Cancer Lett, 418 (2018) 147–158. [DOI] [PubMed] [Google Scholar]
- [110].Yang Q, Zhou Y, Chen J, Huang N, Wang Z, Cheng Y, Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound, Int J Nanomedicine, 16 (2021) 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Chen K-T, Chai Wen-Yen, Lin Ya-Jui, Lin Chia-Jung, Chen Pin-Yuan, Tsai Hong-Chieh, Huang Chiung-Yin, Kuo John S., Liu Hao-Li, Wei Kuo-Chen., Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors, Science Advances, 7 (2021) eabd0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Curley CT MB, Negron K, Kim N, Garrison WJ, Miller GW, Kingsmore KM, Thim EA, Song J, Munson JM, Klibanov AL, Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection, Science Advances, 6 (2020) eaay1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chen PY, Hsieh HY, Huang CY, Lin CY, Wei KC, Liu HL, Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study, J Transl Med, 13 (2015) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, Hynynen K, Focused ultrasound delivers targeted immune cells to metastatic brain tumors, Cancer Res, 73 (2013) 1892–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Xie A, Wu MD, Cigarroa G, Belcik JT, Ammi A, Moccetti F, Lindner JR, Influence of DNA-Microbubble Coupling on Contrast Ultrasound-Mediated Gene Transfection in Muscle and Liver, J Am Soc Echocardiogr, 29 (2016) 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Husseini GA, Pitt WG, Micelles and nanoparticles for ultrasonic drug and gene delivery, Adv Drug Deliv Rev, 60 (2008) 1137–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Suzuki R, Takizawa T, Negishi Y, Hagisawa K, Tanaka K, Sawamura K, Utoguchi N, Nishioka T, Maruyama K, Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound, J Control Release, 117 (2007) 130–136. [DOI] [PubMed] [Google Scholar]
- [118].Rapoport NY, Christensen DA, Fain HD, Barrows L, Gao Z, Ultrasound-triggered drug targeting of tumors in vitro and in vivo, Ultrasonics, 42 (2004) 943–950. [DOI] [PubMed] [Google Scholar]
- [119].Mason TJ, Cobley AJ, Graves JE, Morgan D, New evidence for the inverse dependence of mechanical and chemical effects on the frequency of ultrasound, Ultrason Sonochem, 18 (2011) 226–230. [DOI] [PubMed] [Google Scholar]
- [120].Tachibana K, Feril LB Jr., Ikeda-Dantsuji Y, Sonodynamic therapy, Ultrasonics, 48 (2008) 253–259. [DOI] [PubMed] [Google Scholar]
- [121].Hiraoka W, Honda H, Feril LB Jr., Kudo N, Kondo T, Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing, Ultrason Sonochem, 13 (2006) 535–542. [DOI] [PubMed] [Google Scholar]
- [122].Zhang Q, Bao C, Cai X, Jin L, Sun L, Lang Y, Li L, Sonodynamic therapy-assisted immunotherapy: A novel modality for cancer treatment, Cancer Sci, 109 (2018) 1330–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chavez M, Silvestrini MT, Ingham ES, Fite BZ, Mahakian LM, Tam SM, Ilovitsh A, Monjazeb AM, Murphy WJ, Hubbard NE, Davis RR, Tepper CG, Borowsky AD, Ferrara KW, Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation, Theranostics, 8 (2018) 3611–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].D’Souza AL, Tseng JR, Pauly KB, Guccione S, Rosenberg J, Gambhir SS, Glazer GM, A strategy for blood biomarker amplification and localization using ultrasound, Proceedings of the National Academy of Sciences, 106 (2009) 17152–17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].D’Souza AL, Chevillet JR, Ghanouni P, Yan X, Tewari M, Gambhir SS, Tumor characterization by ultrasound-release of multiple protein and microRNA biomarkers, preclinical and clinical evidence, PLoS One, 13 (2018) e0194268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Pacia CP, Zhu L, Yang Y, Yue Y, Nazeri A, Michael Gach H, Talcott MR, Leuthardt EC, Chen H, Feasibility and safety of focused ultrasound-enabled liquid biopsy in the brain of a porcine model, Sci Rep, 10 (2020) 7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hristova VA, Chan DW, Cancer biomarker discovery and translation: proteomics and beyond, Expert Rev Proteomics, 16 (2019) 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Peng D, Xu T, Mason TJ, Wu W, A study of ovarian cancer biomarker amplification using ultrasound for early stage detection, Ultrasonics, 54 (2014) 451–454. [DOI] [PubMed] [Google Scholar]
- [129].Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF, Understanding the tumor immune microenvironment (TIME) for effective therapy, Nat Med, 24 (2018) 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Leko V, Rosenberg SA, Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors, Cancer Cell, 38 (2020) 454–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, Adamow M, Kuk D, Panageas KS, Carrera C, Wong P, Quagliarello F, Wubbenhorst B, D’Andrea K, Pauken KE, Herati RS, Staupe RP, Schenkel JM, McGettigan S, Kothari S, George SM, Vonderheide RH, Amaravadi RK, Karakousis GC, Schuchter LM, Xu X, Nathanson KL, Wolchok JD, Gangadhar TC, Wherry EJ, T-cell invigoration to tumour burden ratio associated with anti-PD-1 response, Nature, 545 (2017) 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hu Z, Yang XY, Liu Y, Morse MA, Lyerly HK, Clay TM, Zhong P, Release of endogenous danger signals from HIFU-treated tumor cells and their stimulatory effects on APCs, Biochem Biophys Res Commun, 335 (2005) 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Deng J, Zhang Y, Feng J, Wu F, Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses, Ultrasound Med Biol, 36 (2010) 441–448. [DOI] [PubMed] [Google Scholar]
- [134].Cohen G, Chandran P, Lorsung RM, Aydin O, Tomlinson LE, Rosenblatt RB, Burks SR, Frank JA, Pulsed-Focused Ultrasound Slows B16 Melanoma and 4T1 Breast Tumor Growth through Differential Tumor Microenvironmental Changes, Cancers (Basel), 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Fite BZ, Wang J, Kare AJ, Ilovitsh A, Chavez M, Ilovitsh T, Zhang N, Chen W, Robinson E, Zhang H, Kheirolomoom A, Silvestrini MT, Ingham ES, Mahakian LM, Tam SM, Davis RR, Tepper CG, Borowsky AD, Ferrara KW, Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer, Sci Rep, 11 (2021) 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Qu S, Worlikar T, Felsted AE, Ganguly A, Beems MV, Hubbard R, Pepple AL, Kevelin AA, Garavaglia H, Dib J, Toma M, Huang H, Tsung A, Xu Z, Cho CS, Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy, J Immunother Cancer, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xu Z, Hall TL, Vlaisavljevich E, Lee FT Jr., Histotripsy: the first noninvasive, non-ionizing, non-thermal ablation technique based on ultrasound, Int J Hyperthermia, 38 (2021) 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Chevillet JR, Khokhlova TD, Giraldez MD, Schade GR, Starr F, Wang YN, Gallichotte EN, Wang K, Hwang JH, Tewari M, Release of Cell-free MicroRNA Tumor Biomarkers into the Blood Circulation with Pulsed Focused Ultrasound: A Noninvasive, Anatomically Localized, Molecular Liquid Biopsy, Radiology, 283 (2017) 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Schade GR, Wang Y-N, D’Andrea S, Hwang JH, Liles WC, Khokhlova TD, Boiling Histotripsy Ablation of Renal Cell Carcinoma in the Eker Rat Promotes a Systemic Inflammatory Response, Ultrasound in Medicine & Biology, 45 (2019) 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Eranki A, Srinivasan P, Ries M, Kim A, Lazarski CA, Rossi CT, Khokhlova TD, Wilson E, Knoblach SM, Sharma KV, Wood BJ, Moonen C, Sandler AD, Kim PCW, High-Intensity Focused Ultrasound (HIFU) Triggers Immune Sensitization of Refractory Murine Neuroblastoma to Checkpoint Inhibitor Therapy, Clinical Cancer Research, 26 (2020) 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wu F, Wang ZB, Lu P, Xu ZL, Chen WZ, Zhu H, Jin CB, Activated anti-tumor immunity in cancer patients after high intensity focused ultrasound ablation, Ultrasound Med Biol, 30 (2004) 1217–1222. [DOI] [PubMed] [Google Scholar]
- [142].Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P, Wu F, Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment, Ultrasound Med Biol, 34 (2008) 81–87. [DOI] [PubMed] [Google Scholar]
- [143].Budhu S, Schaer DA, Li Y, Toledo-Crow R, Panageas K, Yang X, Zhong H, Houghton AN, Silverstein SC, Merghoub T, Wolchok JD , Blockade of surface-bound TGF-beta on regulatory T cells abrogates suppression of effector T cell function in the tumor microenvironment, Sci Signal, 10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong BH, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW, Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates, Nature, 557 (2018) 575–579. [DOI] [PubMed] [Google Scholar]
- [145].Huang Y, Chen Y, Zhou S, Chen L, Wang J, Pei Y, Xu M, Feng J, Jiang T, Liang K, Liu S, Song Q, Jiang G, Gu X, Zhang Q, Gao X, Chen J, Dual-mechanism based CTLs infiltration enhancement initiated by Nanosapper potentiates immunotherapy against immune-excluded tumors, Nat Commun, 11 (2020) 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kirkwood J, Cancer immunotherapy: the interferon-alpha experience, Semin Oncol, 29 (2002) 18–26. [DOI] [PubMed] [Google Scholar]
- [147].Rosenberg SA, IL-2: the first effective immunotherapy for human cancer, J Immunol, 192 (2014) 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Naing A, Najjar J, Immunotherapy, Springer; 2017. [Google Scholar]
- [149].Leach DR, Krummel MF, Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade, Science, 271 (1996) 1734–1736. [DOI] [PubMed] [Google Scholar]
- [150].Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, Improved survival with ipilimumab in patients with metastatic melanoma, N Engl J Med, 363 (2010) 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA, Genetic basis for clinical response to CTLA-4 blockade in melanoma, N Engl J Med, 371 (2014) 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, Safety, activity, and immune correlates of anti-PD-1 antibody in cancer, N Engl J Med, 366 (2012) 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sharma P, Allison JP, The future of immune checkpoint therapy, Science, 348 (2015) 56–61. [DOI] [PubMed] [Google Scholar]
- [154].Wei SC, Levine JH, Cogdill AP, Zhao Y, Anang NAS, Andrews MC, Sharma P, Wang J, Wargo JA, Pe’er D, Allison JP, Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade, Cell, 170 (2017) 1120–1133 e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gill S, June CH, Going viral: chimeric antigen receptor T-cell therapy for hematological malignancies, Immunol Rev, 263 (2015) 68–89. [DOI] [PubMed] [Google Scholar]
- [156].Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S, Cold Tumors: A Therapeutic Challenge for Immunotherapy, Front Immunol, 10 (2019) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Anguela XM, High KA, Entering the Modern Era of Gene Therapy, Annual review of medicine, 70 (2019) 273–288. [DOI] [PubMed] [Google Scholar]
- [158].Banchereau J, Palucka K, Immunotherapy: Cancer vaccines on the move, Nat Rev Clin Oncol, 15 (2018) 9–10. [DOI] [PubMed] [Google Scholar]
- [159].Casey G, Cashman JP, Morrissey D, Whelan MC, Larkin JO, Soden DM, Tangney M, O’Sullivan GC, Sonoporation mediated immunogene therapy of solid tumors, Ultrasound Med Biol, 36 (2010) 430–440. [DOI] [PubMed] [Google Scholar]
- [160].Song J, Tata D, Li L, Taylor J, Bao S, Miller DL, Combined shock-wave and immunogene therapy of mouse melanoma and renal carcinoma tumors, Ultrasound Med Biol, 28 (2002) 957–964. [DOI] [PubMed] [Google Scholar]
- [161].Zolochevska O, Xia X, Williams BJ, Ramsay A, Li S, Figueiredo ML, Sonoporation delivery of interleukin-27 gene therapy efficiently reduces prostate tumor cell growth in vivo, Hum Gene Ther, 22 (2011) 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Yamaguchi K, Feril LB Jr., Tachibana K, Takahashi A, Matsuo M, Endo H, Harada Y, Nakayama J, Ultrasound-mediated interferon beta gene transfection inhibits growth of malignant melanoma, Biochem Biophys Res Commun, 411 (2011) 137–142. [DOI] [PubMed] [Google Scholar]
- [163].Qin YE, Tang WF, Xu Y, Wan FR, Chen AH, Ultrasound-Mediated Co-Delivery of miR-34a and sPD-1 Complexed with Microbubbles for Synergistic Cancer Therapy, Cancer Manag Res, 12 (2020) 2459–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Suzuki R, Namai E, Oda Y, Nishiie N, Otake S, Koshima R, Hirata K, Taira Y, Utoguchi N, Negishi Y, Nakagawa S, Maruyama K, Cancer gene therapy by IL-12 gene delivery using liposomal bubbles and tumoral ultrasound exposure, J Control Release, 142 (2010) 245–250. [DOI] [PubMed] [Google Scholar]
- [165].Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M, Development of an ultrasound-responsive and mannose-modified gene carrier for DNA vaccine therapy, Biomaterials, 31 (2010) 7813–7826. [DOI] [PubMed] [Google Scholar]
- [166].Un K, Kawakami S, Suzuki R, Maruyama K, Yamashita F, Hashida M, Suppression of melanoma growth and metastasis by DNA vaccination using an ultrasound-responsive and mannose-modified gene carrier, Mol Pharm, 8 (2011) 543–554. [DOI] [PubMed] [Google Scholar]
- [167].Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang YJ, Sadelain M, Shung KK, Chien S, Wang Y, Mechanogenetics for the remote and noninvasive control of cancer immunotherapy, Proc Natl Acad Sci U S A, 115 (2018) 992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Wu Y, Liu Y, Huang Z, Wang X, Jin Z, Li J, Limsakul P, Zhu L, Allen M, Pan Y, Bussell R, Jacobson A, Liu T, Chien S, Wang Y, Acoustogenetic Control of CAR T Cells via Focused Ultrasound, BioRxiv, (2020). [Google Scholar]
- [169].De Temmerman ML, Dewitte H, Vandenbroucke RE, Lucas B, Libert C, Demeester J, De Smedt SC, Lentacker I, Rejman J, mRNA-Lipoplex loaded microbubble contrast agents for ultrasound-assisted transfection of dendritic cells, Biomaterials, 32 (2011) 9128–9135. [DOI] [PubMed] [Google Scholar]
- [170].Dewitte H, Van Lint S, Heirman C, Thielemans K, De Smedt SC, Breckpot K, Lentacker I, The potential of antigen and TriMix sonoporation using mRNA-loaded microbubbles for ultrasound-triggered cancer immunotherapy, J Control Release, 194 (2014) 28–36. [DOI] [PubMed] [Google Scholar]
- [171].Shi C, Zhang Y, Yang H, Dong T, Chen Y, Xu Y, Yang X, Combined effect of ultrasound/SonoVue microbubble on CD4(+)CD25(+) regulatory T cells viability and optimized parameters for its transfection, Ultrasonics, 62 (2015) 97–102. [DOI] [PubMed] [Google Scholar]
- [172].Hawkins RE, Gilham DE, Debets R, Eshhar Z, Taylor N, Abken H, Schumacher TN, Consortium A, Development of adoptive cell therapy for cancer: a clinical perspective, Hum Gene Ther, 21 (2010) 665–672. [DOI] [PubMed] [Google Scholar]
- [173].Gianpietro Dotti SG, Savoldo Barbara, Brenner Malcolm K., Design and development of therapies using chimeric antigen receptor-expressing T cells, Immunological Reviews, 257 (2014) 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Wang J, Huang CH, Echeagaray OH, Amirfakhri S, Blair SL, Trogler WC, Kummel AC, Chen CC, Microshell Enhanced Acoustic Adjuvants for Immunotherapy in Glioblastoma, Advanced Therapeutics, 2 (2019). [Google Scholar]
- [175].Zhang FJN, Kheirolomoom A, Liu P, Feng Y, Tumbale S, Raie M, Wu B, Wang J, Fite BZ, Dai Z, Ferrara KW, Optimization of Microbubble-Based DNA Vaccination with Low-Frequency Ultrasound for Enhanced Cancer Immunotherapy, Advanced Therapeutics, In press (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Yoshida M, Kawakami S, Kono Y, Un K, Higuchi Y, Maruyama K, Yamashita F, Hashida M, Enhancement of the anti-tumor effect of DNA vaccination using an ultrasound-responsive mannose-modified gene carrier in combination with doxorubicin-encapsulated PEGylated liposomes, Int J Pharm, 475 (2014) 401–407. [DOI] [PubMed] [Google Scholar]
- [177].Li X, Yue X, Wang J, Liang X, Jing L, Lin L, Yang Y, Feng S, Qian Y, Dai Z, Prussian blue nanoparticle-loaded microbubbles for photothermally enhanced gene delivery through ultrasound-targeted microbubble destruction, Science Bulletin, 61 (2016) 148–156. [Google Scholar]
- [178].De Carlo F, Thomas L, Brooke B, Varney ET, Nande R, Boskovic O, Marshall GD, Claudio PP, Howard CM, Microbubble-mediated delivery of human adenoviruses does not elicit innate and adaptive immunity response in an immunocompetent mouse model of prostate cancer, J Transl Med, 17 (2019) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound, Nat Commun, 10 (2019) 4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Nyborg WL, Biological effects of Ultrasound: Development of Safety Guidelines. Part II: General Review, Ultrasound in Med. & Biol, 27 (2001) 301–303. [DOI] [PubMed] [Google Scholar]
- [181].Knudsen KB, Northeved H, Kumar PE, Permin A, Gjetting T, Andresen TL, Larsen S, Wegener KM, Lykkesfeldt J, Jantzen K, Loft S, Moller P, Roursgaard M, In vivo toxicity of cationic micelles and liposomes, Nanomedicine, 11 (2015) 467–477. [DOI] [PubMed] [Google Scholar]
- [182].Staquicini FI, Smith TL, Tang FHF, Gelovani JG, Giordano RJ, Libutti SK, Sidman RL, Cavenee WK, Arap W, Pasqualini R, Targeted AAVP-based therapy in a mouse model of human glioblastoma: a comparison of cytotoxic versus suicide gene delivery strategies, Cancer Gene Ther, 27 (2020) 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Xiao Z, Puré E, Imaging of T-cell Responses in the Context of Cancer Immunotherapy, Cancer Immunol Res, 9 (2021) 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Mayer AT, Gambhir SS, The Immunoimaging Toolbox, J Nucl Med, 59 (2018) 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Foley JL, Eames M, Snell J, Hananel A, Kassell N, Aubry J-F, Image-guided focused ultrasound: state of the technology and the challenges that lie ahead, Imaging Med, 5 (2013) 357–370. [Google Scholar]
- [186].Keu KV, Witney TH, Yaghoubi S, Rosenberg J, Kurien A, Magnusson R, Williams J, Habte F, Wagner JR, Forman S, Brown C, Allen-Auerbach M, Czernin J, Tang W, Jensen MC, Badie B, Gambhir SS, Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma, Science translational medicine, 9 (2017) eaag2196. [DOI] [PMC free article] [PubMed] [Google Scholar]


