Abstract
Severe traumatic skeletal muscle injuries, such as volumetric muscle loss (VML), result in the obliteration of large amounts of skeletal muscle and lead to permanent functional impairment. Current clinical treatments are limited in their capacity to regenerate damaged muscle and restore tissue function, promoting the need for novel muscle regeneration strategies. Advances in tissue engineering, including cell therapy, scaffold design, and bioactive factor delivery, are promising solutions for VML therapy. Herein, we review tissue engineering strategies for regeneration of skeletal muscle, development of vasculature and nerve within the damaged muscle, and achievements in immunomodulation following VML. In addition, we discuss the limitations of current state of the art technologies and perspectives of tissue-engineered bioconstructs for muscle regeneration and functional recovery following VML.
Keywords: Volumetric muscle loss, tissue engineering, skeletal muscle regeneration, biomaterials, vascularization, innervation, immunomodulation
1. Introduction
Skeletal muscle injury is common following excessive exercise and traumatic incidents, such as motor vehicle accidents, orthopedic surgeries, or combat-related fractures [1–4]. Following minor injuries, skeletal muscle has a remarkable ability to regenerate, which relies on muscle stem cells (MuSCs), also referred to as “satellite cells”, and their interaction with the surrounding microenvironment [3,5]. However, volumetric muscle loss (VML) injury overwhelms the endogenous repair capacity and leads to an irrecoverable functional impairment [6–10]. Following VML, substantial loss of MuSCs diminishes the innate regenerative ability of the injured muscle. Complete removal of the extracellular matrix results in the disappearance of a scaffolding to support cell attachment and guide tissue reconstruction [11]. Moreover, VML induces chronic inflammation that causes continued tissue damage and massive fibrosis, impeding muscle regeneration [12,13]. In this context, VML leads to permanent loss of muscle and life-long disability [14].
Current clinical strategies for VML treatment are limited to free functional muscle transfer, an autologous muscle transplanted to the defect area for functional restoration [6,7]. However, autografting is often associated with donor-site morbidity, limited tissue availability, and complications such as infection and necrosis [7,14,15]. Therefore, the development of regenerative technologies, which can address current challenges and provide substitutions for autologous muscle for transplantation, is vital for VML treatment. The therapeutic success requires a large amount of muscle formation to replace the lost tissue, sufficient vascularization to supply blood flow, functional innervation to generate action potentials, and effective immunomodulation to reduce fibrosis and support tissue regeneration [16–19]. Tissue engineering approaches have tremendous potential for muscle regeneration and functional recovery (Figure 1). In this review, we will summarize current engineering strategies for VML treatment that promote myogenesis, vascularization, innervation, and immunomodulation. The advances and limitations of tissue engineering technologies, including cell therapy, scaffold design, and bioactive factor delivery will be discussed in each category.
Figure 1.
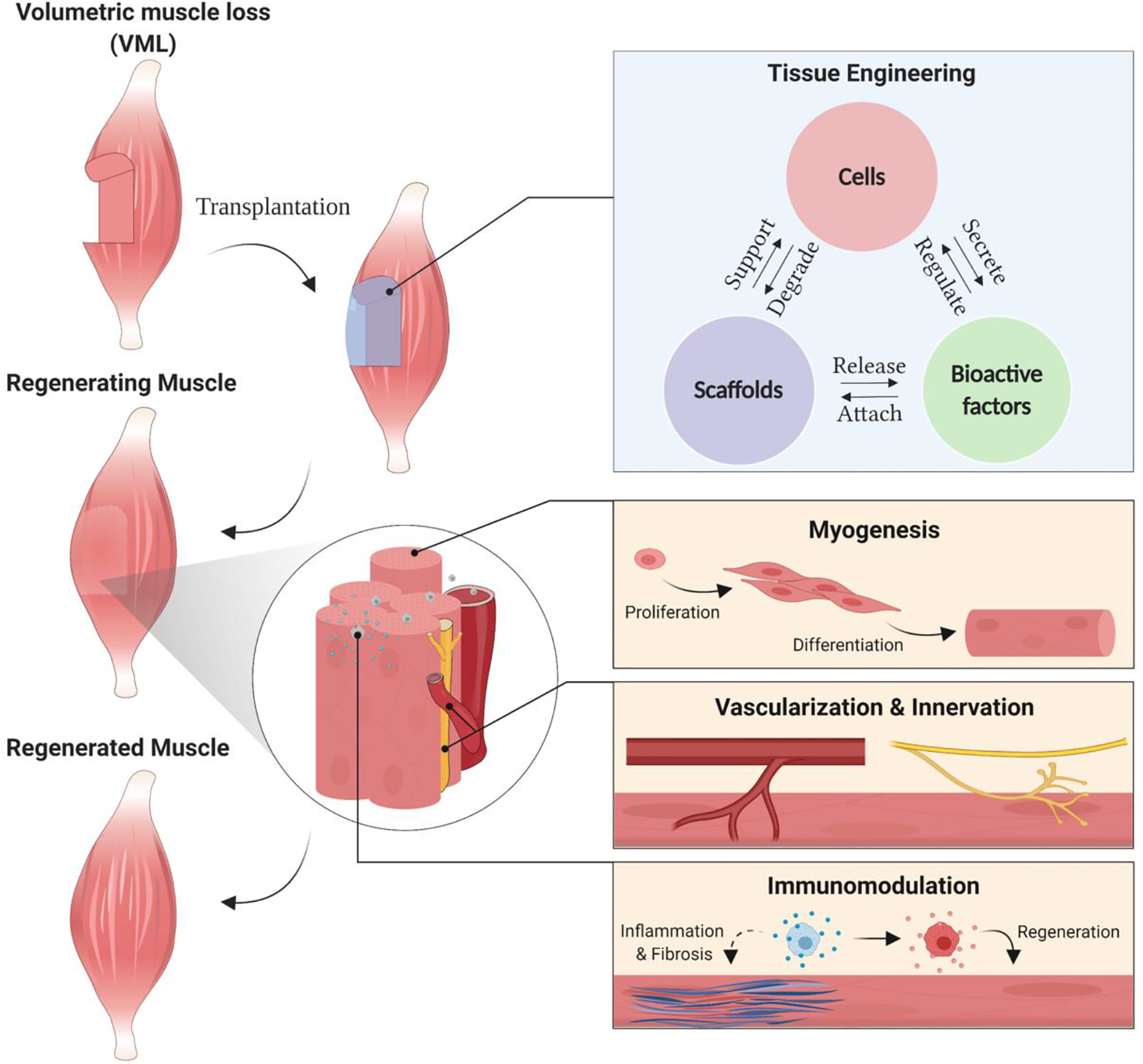
Volumetric Muscle Loss (VML) results in a significant ablation of skeletal muscle. Tissue engineering strategies combining cells, scaffolds, and bioactive factors, present promising solutions for VML therapy. Muscle regeneration and functional recovery following VML requires sophisticated approaches to promote myogenesis, vascularization and innervation, and immunomodulation.
2. Myogenesis
2.1. Cells
Cell-based therapies have great potential for treating VML injuries. Transplanted myogenic cells can form new myofibers as well as repair damaged existing fibers, thereby restoring muscle mass and function. Several studies have investigated acellular approaches for VML treatment, demonstrating improved muscle function but yielding inadequate muscle regeneration [20–22]. Systematic evaluations of muscle biomechanics and histology show that decellularized scaffolds alone are insufficient for promoting de novo muscle fiber formation [23–25]. Therefore, an exogenous stem cell source is likely to be necessary for effective treatment of VML injuries. Cell types that participate in normal skeletal muscle regeneration have been explored as candidates for regenerative medicine and previously reviewed in detail [26,27]. Here, we will highlight the cell sources that have shown potential, specifically for VML therapies, based on their ability to generate new muscle (Figure 2A).
Figure 2.
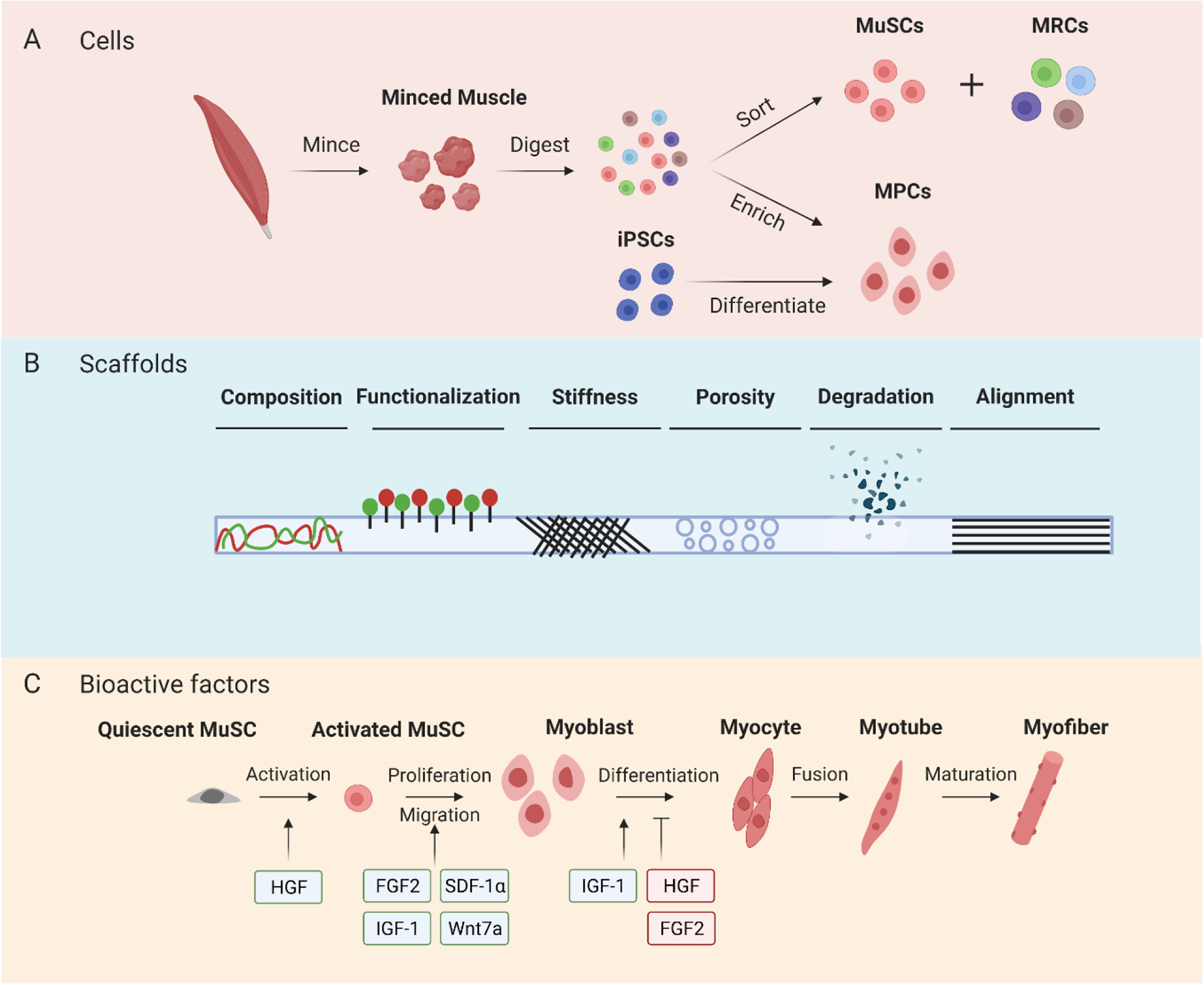
Advances in tissue engineering for enhancing myogenesis following VML. (A) Myogenic cells used for VML bioconstructs are harvested from skeletal muscle or differentiated from iPSCs. Following muscle digestion, MuSCs are isolated by sorting methods whereas MPCs are purified and expanded in vitro. (B) Scaffold physiochemical properties play an important role in skeletal muscle regeneration following VML. (C) Bioactive factors can be incorporated into bioconstructs to modulate different stages of myogenesis.
MuSCs are the predominant muscle fiber forming cells in skeletal muscle tissue. Following injury, MuSCs activate, proliferate, differentiate, and fuse to repair damaged myofibers, as well as form new myofibers. As a result, MuSCs have been extensively investigated as cell sources for regenerative medicine [28,29]. Despite their scarcity in skeletal muscle, MuSCs demonstrate a dramatic proliferation potential in vivo [30]. Freshly isolated MuSCs transplanted into a mouse VML model yielded de novo muscle fiber formation and a 40% recovery of muscle mass after one month [31]. Despite their proliferative potential, it is likely that a large number of MuSCs would need to be transplanted for an improved therapeutic benefit. However, MuSCs expanded in vitro have a drastic reduction in engraftment and regenerative capacity when transplanted back in vivo, compared to freshly isolated MuSCs [32,33]. Several groups have developed in vitro culture conditions that enable MuSC expansion, while maintaining their therapeutic potential [34,35], but cells produced by these methods have not been transplanted into a VML model. On the other hand, various proliferating populations of myogenic progenitors and myogenic cells lines, such as L8 and C2C12 myoblasts, have been investigated for VML applications because they can be easily expanded in vitro [36–38]. In this review, we will refer to myogenic populations that have not been prospectively derived from MuSCs as myogenic progenitor cells (MPCs), even if poorly characterized.
In vivo, MuSCs are supported by other cells in the tissue [39,40]. A mixed cell population termed “muscle resident cells” (MRCs), made up of endothelial cells, hematopoietic cells, fibro-adipogenic progenitors, and fibroblast-like cells, has been shown to enhance the survival of MuSCs both from studies in vitro and in a mouse VML model in vivo [31]. A combination of MuSCs and MRCs doubled the recovery of muscle mass, reduced fibrosis, increased myogenesis, and significantly improved ex vivo muscle function compared to MuSC-only treatment. In the same study, combinatorial co-culture experiments of MuSCs with endothelial cells, MRCs, or MRCs without endothelial cells, revealed that endothelial cells were both necessary and sufficient to sustain MuSC viability, expansion, and engraftment in the VML model [31]. Minced muscle grafts, which include MuSCs and other cells residing in muscle tissue, have also been explored as cell sources for VML therapy. Minced muscle grafts transplanted into a rat VML defect yielded a 55% recovery of functional deficit measured by net torque production [41]. In a larger, porcine model of VML, autologous minced muscle grafts produced a 32% increase in strength after 12 weeks [42]. However, few areas of de novo muscle regeneration and significant fibrosis were observed within the defect area. Moreover, clinical limitations, including inadequate tissue availability and donor site morbidity, must be addressed as a significant volume of autologous minced muscle would be required to repair a defect at the scale of VML injuries.
Induced pluripotent stem cells (iPSCs) are attractive cell candidates for a variety of tissue engineering applications because they are easily obtained, capable of robust proliferation in vitro, and can be differentiated into multiple cell types [43]. iPSC-based strategies investigated for skeletal muscle regeneration include direct transplantation of iPSC-derived MPCs and in vitro engineered skeletal muscle constructs, where iPSCs are differentiated into myotubes in vitro prior to implantation in vivo. In a recent study, human iPSC-derived MPCs transplanted in a mouse VML model yielded an improvement in muscle contractility and donor-derived muscle fiber formation [44]. Moreover, in vitro iPSC-derived functional skeletal muscle constructs have been shown to fuse with endogenous myofibers when implanted in vivo [45–47]. A recent study demonstrated the therapeutic potential of using multilineage engineering to create artificial skeletal muscles containing iPSC-derived myofibers, endothelial cells, pericytes, and motor neurons [48]. Although generating iPSC-derived skeletal muscle constructs, such as induced skeletal muscle (iSKM) bundles or other artificial muscles [45,48], in vitro and then implanting them in vivo is a promising approach for skeletal muscle regeneration, further investigation is needed to determine the efficacy of these therapies in a VML model. In vivo, iSKM bundles have been shown to progressively vascularize when implanted subcutaneously and to successfully engraft in a TA muscle, while maintaining functionality one-week post implantation. Nonetheless, clinical limitations, including potential for tumorigenicity and immunogenicity [49–51], must be addressed to fully realize an iPSC-based therapy for VML.
2.2. Scaffolds
Since VML injury also results in the removal of native ECM, endogenous and transplanted cells lack a scaffold that is necessary to regenerate organized skeletal muscle [15]. A recent study found that acellular muscle architecture is required to guide MuSCs during regeneration [52], underscoring the need for scaffolds that support myogenesis in VML therapies. Scaffolds used for enhancing skeletal muscle regeneration have been reviewed extensively [15,53–55]. Biomaterials can provide essential biochemical and biophysical cues to support cell attachment, survival, proliferation, and myogenic differentiation [53,56]. For example, MPCs encapsulated in an in situ casted fibrin hydrogel engrafted and formed new muscle fibers in a murine VML defect [57]. Similarly, human MPCs micropatterned on poly lactic-co-glycolic acid (PLGA) sheets, stacked to form a 3D construct, and transplanted into a murine VML defect also showed improved engraftment compared to direct cell injection [58]. Here, we will highlight some of the biomaterial properties, including composition, stiffness, porosity, and degradation, that have been shown to improve the regeneration of skeletal muscle following VML (Figure 2B).
Scaffolds for regenerative medicine can generally be characterized as natural or synthetic based on their composition [15,59]. As implied by their name, natural biomaterials are polymers derived from natural sources, such as proteins, polysaccharides, and DNA, and are often biocompatible, biodegradable, and have low cytotoxicity. Despite their biocompatibility, natural biomaterials generally have weak mechanical strength and batch-to-batch variability. Common natural biomaterials that have been used in skeletal muscle tissue engineering include collagen, fibrin, alginate, and decellularized ECM. Synthetic biomaterials, on the other hand, are more scalable and their physicochemical properties are easily tailored for their application. Unlike their natural counterparts, synthetic biomaterials must be functionalized with bioactive cues to make them biocompatible. Common synthetic biomaterials that have been used for skeletal muscle tissue engineering include polyethylene glycol (PEG), polyglycolic acid (PGA), polycaprolactone (PCL), and poly lactic-co-glycolic acid (PLGA). Although natural and synthetic biomaterials are generally associated with the respective properties and limitations mentioned above, scaffold characteristics can be altered by modifying the polymer, introducing new crosslinking chemistries, or mixing biomaterials to produce hybrid scaffolds.
Decellularized ECM (dECM) is the most widely used natural scaffold for VML applications due to the abundance of biochemical cues that are critical for tissue regeneration [60–62]. dECM scaffolds sourced from muscle, small intestine, and urinary bladder have demonstrated variable success in VML models [20,24,63,64]. Differences in ECM composition, decellularization protocols, and scaffold processing are all contributing factors to dECM variability. Other natural materials, such as keratin derived from human hair, have also demonstrated potential as scaffolds for VML therapy [65,66]. Compared to bladder dECM, acellular keratin hydrogels yielded increased muscle regeneration, decreased fibrosis, and improved functional recovery following 12 weeks post-implantation in a rat VML model [65]. Another study explored the ability of a hyaluronic acid hydrogel supplemented with laminin-111, an embryonic isoform of laminin, to support minced muscle grafts in a rat VML model [67]. Hyaluronic acid, a polysaccharide known for its anti-adhesive and anti-inflammatory properties [68], was chosen as a scaffold to effectively shield transplanted cells from the harsh immune environment. Additionally, laminin was incorporated to selectively promote the adhesion, migration, and proliferation of transplanted cells. Consistent with previous minced muscle and scaffold strategies [67,69], co-delivery of hyaluronic acid, laminin, and minced muscle yielded a functional improvement compared to an acellular hydrogel control. However, functional recovery was comparable to that achieved with minced muscle grafts without any scaffolding [67], indicating that the scaffold did not enhance the effect of minced muscle grafts alone.
Scaffold functionalization with bioactive peptides has gained significant attention for improving regeneration in both soft (e.g. skin, muscle, brain) and hard (e.g. bone, cartilage) tissue applications [70,71]. To our knowledge, peptide functionalization has not yet been explored in VML applications but has great potential for enhancing scaffold bioactivity and muscle regeneration. Unlike most natural materials, synthetic materials must be modified to promote the attachment of transplanted and endogenous cells. Synthetic biomaterials for skeletal muscle applications are generally coated, or mixed, with natural materials such as fibrin, laminin, or Matrigel, to promote cell attachment [72–74]. However, incorporating full-length ECM proteins into synthetic scaffolds can elicit an inflammatory immune response and result in undesirable changes to scaffold properties, such as surface charge and topography [75]. Functionalizing scaffolds with small peptides is an attractive alternative to improve bioactivity and overcome the aforementioned limitations associated with full-length proteins. Peptides are able to mimic the bioactivity of their full-length protein counterparts while also being easier to produce and modify under well-defined conditions. Arginine-glycine-aspartate (RGD) peptides tethered to an alginate scaffold have been shown to enhance MPC viability following transplantation into skeletal muscle [76]. In addition to improving cell attachment, peptides have also been incorporated into natural and synthetic scaffolds to promote a variety of cellular responses, including survival, proliferation, angiogenesis, and anti-inflammation [70]. RGD and IKVAV, an isoleucine-lysine-valine-alanine-valine peptide derived from laminin, have been investigated for controlling MPC behavior in vitro [77]. When cultured on top of hyaluronic acid hydrogels functionalized with IKVAV, MPCs demonstrated increased migration and expression of Pax7, a transcription factor involved in MuSC proliferation and differentiation, compared to cells presented with RGD.
Scaffold properties, such as stiffness, degradation, and porosity, also play critical roles in mediating tissue regeneration. Fabrication methods, such as electrospinning, pore generation, and 3D printing, as well as crosslinking techniques can be used to develop scaffolds with physical properties that closely mimic the tissue of interest. An ideal scaffold for VML therapy will likely match the stiffness of skeletal muscle with a Young’s modulus of approximately 12 kPa [78]. Scaffold degradation rate is another key factor that determines the success of a regenerative medicine therapy. Ideally, the rate at which a material degrades should be proportional to the rate of new tissue formation [79]. Relative to the rate of new tissue formation, scaffolds with too slow of a degradation are prone to fibrosis and encapsulation, whereas too fast of a degradation will not provide enough physical support for cells to regenerate the injured tissue [55]. Introducing porosity into scaffolds can allow endogenous cells to participate in the regeneration process and vascularization to take place more easily [80]. Porosity becomes increasingly important for larger tissue defects, such as VML, which require rapid ingrowth of vasculature to deliver essential nutrients and remove waste. A recent study investigating the therapeutic potential of a porous scaffold in a mouse VML model found that a collagen-GAG sponge promoted a functional improvement and upregulation of factors involved in wound healing [81]. Moreover, a collagen-gelatin-laminin sponge implanted in another hindlimb VML model supported the recruitment of endogenous cells, including MuSCs, but yielded limited myofiber regeneration after 2 weeks [82].
In addition to stiffness and composition, the anisotropic, or aligned, organization of skeletal muscle can also be mimicked using scaffold fabrication techniques. Aligned scaffolds more closely resemble the architecture of skeletal muscle and have also been shown to mediate myofiber maturation in vitro [83]. Anisotropic materials for skeletal muscle regeneration have been investigated further and reviewed in detail [84,85]. A recent study demonstrated the importance of alignment in the regenerative response following VML in a rat model by implanting autografts in misaligned orientations [86]. Misaligned autografts, rotated at 45- or 90-degree angles, were shown to have significantly less functional recovery, fewer myofibers, and considerably more fibrosis compared to aligned autografts. Scaffolds with aligned architecture for VML applications have generally been fabricated by electrospinning and 3D printing. Aligned electrospun PCL/dECM scaffolds demonstrated increased myofiber regeneration compared to the PCL only group but did not yield significant improvements in muscle weights or functional recovery in a mouse VML model [87]. Aligned nanofibrillar collagen scaffolds implanted into a mouse VML model yielded de novo myofibers with 60% larger cross-sectional areas compared to dECM controls [88]. Recent advances in 3D bioprinting have drastically enhanced the fabrication of aligned skeletal muscle fibers [89,90]. Human MPCs in a 3D printed scaffold with aligned architecture showed increased muscle regeneration, improved functional recovery, and decreased fibrosis compared to non-printed groups following 8 weeks post implantation in a rat TA VML model [91]. The same bioconstruct, implanted into a pelvic floor VML model, maintained muscle volume over 8 weeks and significantly improved muscle function compared to acellular bioconstructs [92]. 3D printed bioconstructs composed of human MPCs in photo-crosslinkable dECM also demonstrated increased muscle regeneration and functional recovery in a rat TA VML model [93]. Incorporation of non-myogenic cell types into 3D printed bioconstructs have further improved myogenesis in VML lesions [94,95]. Moreover, novel skeletal muscle 3D printing deposition strategies, including in situ and intravital bioprinting, have recently been explored and show great promise for VML therapy [96,97].
2.3. Bioactive Factors
Besides therapeutic cells and scaffolds, bioactive factors, such as growth factors, cytokines, and signaling molecules, are other key mediators of skeletal muscle regeneration [98]. The native regeneration process, including cell recruitment, proliferation, and differentiation, is orchestrated by different biochemical cues [99]. For instance, HGF has been found to be a critical factor for triggering the activation of quiescent MuSCs in vivo [100]. In addition, FGF-2 has been shown to be a potent inducer of MuSC proliferation [101]. Owing to their pivotal role in controlling cell behavior, bioactive factors are of great interest in regenerative medicine. Although the delivery of bioactive factors can improve myogenesis, their dosage must be tightly controlled since suboptimal dose and improper timing may result in adverse outcomes such as increased fibrosis, hypertension, and edema [102,103]. Biomaterials can enable the spatiotemporal control of bioactive factors to guide the regenerative process in vivo. Here, we will summarize the factors that have been used in VML therapies and their delivery strategies (Figure 2C). Moreover, additional factors, which have been shown to have therapeutic potential in the other muscle injury models besides VML, will be covered for future exploration.
2.3.1. Factors for Cell Activation and Recruitment
Owing to the remarkable capabilities of MuSCs for muscle regeneration [104], recruitment of endogenous MuSCs is an attractive approach for in situ muscle regeneration. Bioactive factors have been delivered to recruit host MuSCs and provide proper guidance for muscle regeneration following VML [105,106]. For example, poly(L-lactic acid) (PLLA) scaffolds releasing bioactive factors, including SDF-1α, HGF, IGF-1, and FGF-2, were investigated for their ability to improve MuSC recruitment and myogenesis following VML [105]. Only the scaffolds releasing IGF-1 or FGF-2 demonstrated a significant MuSC enrichment compared to the control scaffold without growth factors. In addition, delivery of IGF-1, FGF2, or HGF yielded more newly formed muscle fibers than control scaffold. The therapeutic effects of HGF on muscle functional recovery following VML was further investigated [106]. A sustained delivery of HGF for 48 hours to mimic its native release kinetics after injury was achieved by adsorbing HGF onto fibrin microthreads. About 91% of isometric twitch force was recovered compared with the contralateral uninjured muscle after 60 days post-injury, while the no intervention group recovered 70%. In addition, although the HGF group recovered 99% force at tetanic contraction, there was no significance between the treatment groups with and without HGF.
Besides recruiting endogenous cells, bioactive factors can be used to induce activation and promote mobilization of transplanted cells. While the therapeutic effects of such a strategy have not yet been explored in VML injury, application to other injury models demonstrate potential. For example, Wnt7a is a potent signaling protein that can promote symmetric MuSC expansion and directional migration of both MuSCs and MPCs [107–109]. In a cryo-injured muscle model, co-delivery of Wnt7a and MuSCs in a hydrogel on top of the injury area significantly increased the migration distance of transplanted MuSCs towards the injury site to form new muscle fibers [110]. Another study that promoted the survival of transplanted MPCs and induced their migration from a scaffold placed on top of the injured tissue was investigated in a muscle laceration model [111]. HGF and FGF2, potent growth factors known to increase proliferation and prevent premature differentiation of myoblasts [112,113], were co-delivered with myoblasts [111]. In response to HGF and FGF2, myoblasts demonstrated increased migration outward from the scaffold and incorporation with host tissues, resulting in a significant reduction of defect area at 30 days post-injury. However, functional recovery was not characterized in this study [111].
2.3.2. Factors for Cell Proliferation
Achieving a large enough number of myogenic cells for regeneration is critical for VML therapy [15,56]. FGF-2 was utilized to promote the in vivo proliferation of L8 myoblasts [114]. In a crush injury model, L8 myoblasts, which were genetically modified to overexpress FGF-2, demonstrated a 53% reduction in apoptotic cell death and a 1.8-fold increase in proliferation compared with control myoblasts [114]. Besides promoting the proliferation of transplanted myoblasts, bioactive factors have also been used to induce endogenous cell proliferation [65,66,115]. However, direct injection of FGF-2, with or without heparin to provide a sustained release, to a crush-injured muscle showed no measurable effect on the number of proliferating cells [115]. A negligible effect of FGF-2 on cell proliferation was also found in VML treatment by delivering FGF-2 in a keratin hydrogel [65,66]. Nevertheless, co-delivery of FGF-2 and IGF-1 demonstrated a synergistic effect on improving functional recovery following VML [66]. Therefore, the delivery of inductive factors can promote myogenic cell proliferation in vivo and improve muscle functional recovery following VML injury.
2.3.4. Factors for Cell Differentiation and Fusion
To achieve functional muscle tissue, myogenic progenitors need to differentiate and fuse together to form mature myofibers. In the native repair process, IGF-1 plays a critical role in regulating myogenic cell differentiation and fusion [116,117]. C2C12 myoblasts cultured on a collagen scaffold with IGF-1 in vitro formed myotubes exhibiting a 1.5-fold increase in length and 2-fold greater nuclei per myotube compared with the myoblasts without IGF-1 [118]. The therapeutic potential of IGF-1 was further explored by transplanting the acellular IGF-1 loaded scaffold into a mouse VML model. Although the density of regenerating fibers in the IGF group increased, there was no statistical significance found compared with scaffold only group [118]. The capability of IGF-1 to induce myogenic differentiation was also investigated in a rabbit VML model [119]. IGF-1 was sustained released to the injury site by dECM scaffolds following VML. The immunohistochemical staining for myosin heavy chain (MHC) illustrated that the dECM scaffold with IGF-1 yielded a significantly increased number of newly formed myofibers and a reduction in collagen deposition compared to a scaffold only group.
3. Vascularization and Innervation
Vascularization and innervation are essential processes for muscle regeneration and functional recovery following VML injury. Vasculature, which is highly dense and organized, provides oxygen and nutrients for skeletal muscle. Although spontaneous revascularization is triggered in response to muscle injury without any treatment, the ingrowth of blood vessels is too slow to meet the high metabolic demand of muscle regeneration in large size of defect like VML [16,120,121]. Bioconstruct vascularization is particularly challenging for VML therapy due to the large defect size and fibrosis at the injury site [122]. In addition, VML injury also results in significant axotomy of motoneurons and removal of the neuromuscular junctions (NMJs) [123]. Denervation of injured muscle results in functional impairment and muscle fiber atrophy [124]. Therefore, emerging studies have focused on developing novel approaches to promote vascularization and innervation for muscle regeneration. In this section, we will summarize current strategies to improve vascularization and innervation of skeletal muscle by cell delivery, scaffold design, and bioactive factor delivery (Figure 3).
Figure 3.
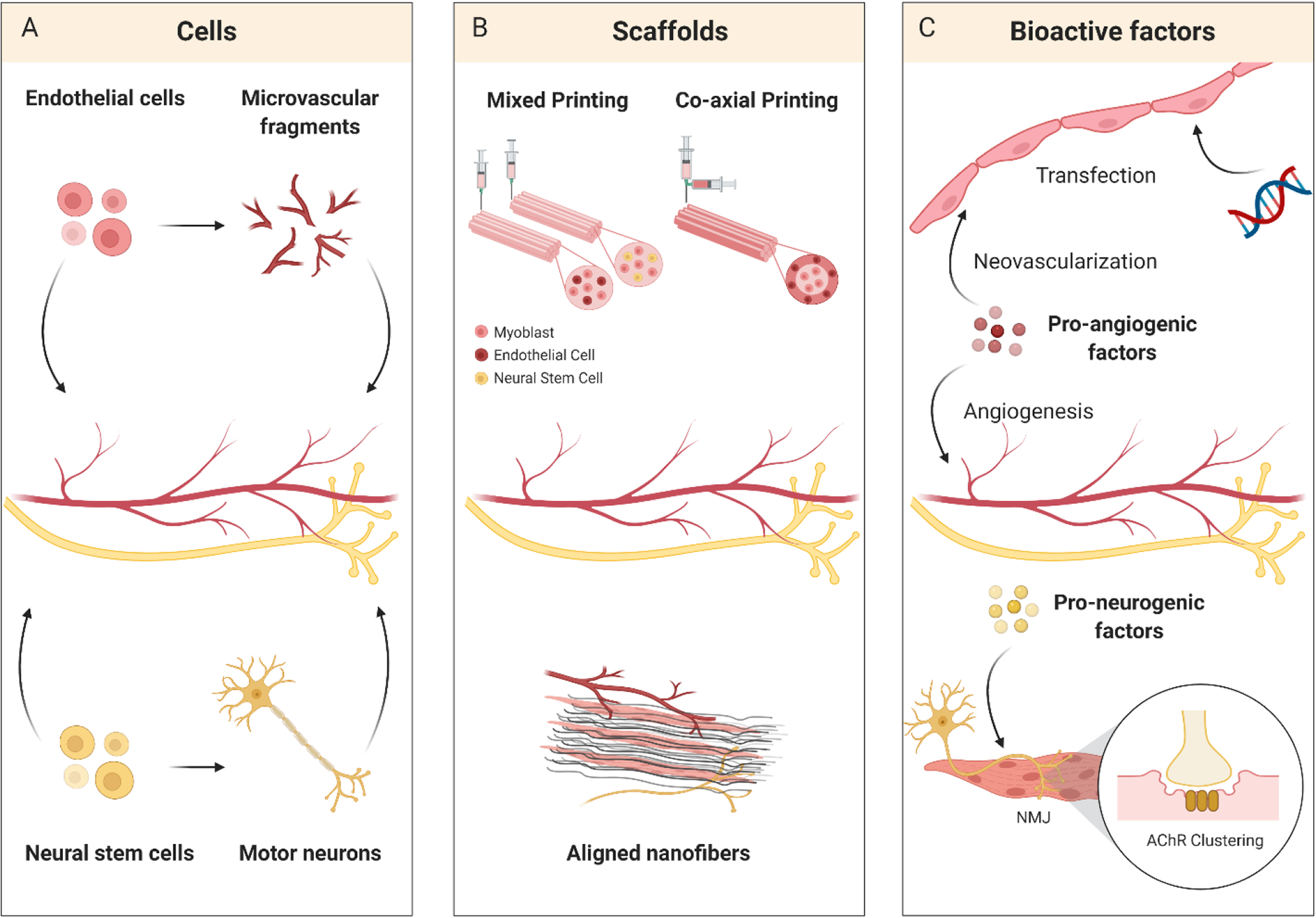
Enhancing bioconstruct vascularization and innervation for VML therapy. (A) Vascular and neural cells can be incorporated into VML bioconstructs to support regenerating muscle. (B) Scaffold fabrication and patterning techniques, including 3D bioprinting and aligning nanofibers, recreate complex tissue architecture and organization. (C) Pro-angiogenic and pro-neurogenic factor delivery can recruit vasculature and nerves to improve skeletal muscle regeneration and function.
3.1. Cells
Endothelial cells are known to play an important role in muscle regeneration [125]. Therefore, endothelial cells have been studied extensively for creating vascular networks to enhance VML therapies. In vitro and in vivo tissue engineering strategies, where endothelial cells are used to form vasculature prior to implantation or are transplanted directly, have both been investigated. Pre-vascularization of bioconstructs with human umbilical vein endothelial cells (HUVECs) was investigated in a mouse VML model [126]. Prior to implantation, bioconstructs were cultured in vitro to allow co-cultured HUVECs and C2C12s to form aligned vessels and myotubes, respectively. Ten days after implantation in vivo, pre-vascularized bioconstructs successfully anastomosed with host vasculature and were perfused with host red blood cells. However, pre-vascularized bioconstructs were surrounded by fibrosis and yielded minimal myofiber formation, unlike their non-vascularized counterparts. In another study, endothelial cells and MuSCs on a dECM scaffold were directly transplanted, without a pre-vascularization culture period, into a similar VML model [31]. Endothelial cells proliferated and contributed to vasculogenesis within the bioconstructs 21 days post implantation. Currently, both in vitro and in vivo tissue engineering approaches using endothelial cells show promise for enhancing vascularization of bioconstructs for VML therapy.
Using endothelial cells to develop functional vascular networks, whether pre-formed in vitro or via direct cell transplantation in vivo, is often a lengthy process. Microvessel fragments (MVFs) are attractive bioconstruct pre-vascularization units because they already have developed microvessel morphology, rapidly assemble into networks when transplanted in vivo, and can be easily isolated in large quantities from adipose tissue [127]. MVFs are inherently more complex than endothelial cells and have been shown to contain mesenchymal stem cells, which contribute to their regenerative potential [128]. Collagen hydrogels containing MVFs yielded a higher vessel density within a VML defect compared to hydrogels with adipose derived stem cells after 7 days [129]. At 14 days post injury, MVF bioconstructs contained 75% of the vessel density of uninjured controls. Moreover, perfusion of the MVF network was observed in just 7 days and was maintained at 14 days post injury. Despite evidence of anastomosis with host vasculature, bioconstruct perfusion was still low and heterogeneously distributed within the defect. More recent work investigated the vascularization potential of MVFs with or without the addition of myoblasts in a rat VML model [130]. Moreover, more small-diameter vessels were observed in both of the pre-vascularized groups than in acellular hydrogel and autograft controls.
Similar to the aforementioned pre-vascularization strategies, using progenitor or differentiated cell populations, pre-innervation approaches for VML applications have entailed the incorporation of neurons or neural stem cells (NSCs) into bioconstructs. Addition of motor neurons to bioconstructs containing differentiated C2C12s demonstrated improved myocyte maturation in vitro [131]. Following implantation into a rat VML model, these pre-innervated bioconstructs maintained muscle cross-sectional area after 3 weeks, unlike the non-innervated, acellular, or non-treated control groups. In addition, pre-innervated bioconstructs enhanced the recruitment of endogenous MuSCs and the formation of NMJs near the injury area. Compared to non-innervated and acellular groups, pre-innervated bioconstructs also promoted vascularization within and outside the injury area. Another promising strategy for pre-innervating bioconstructs that has been tested in a VML model involves the transplantation of NSCs instead of differentiated neurons. A recent study demonstrated that the addition of human NSCs to human MPCs yielded improved myofiber and NMJ formation after 8 weeks in a rat VML model, compared to bioconstructs containing only MPCs [95]. The addition of NSCs to bioconstructs also yielded a decrease in fibrosis relative to MPC-only and non-treated controls. In contrast to pre-innervated bioconstructs [131], which demonstrated increased vascularization compared with their non-innervated counterparts, no significant improvements in vascularization were observed upon the addition of NSCs to MPC bioconstructs [95].
3.2. Scaffolds
The arrangement of myofibers, blood vessels, and nerves in native skeletal muscle is highly organized and essential for proper muscle function. 3D bioprinting has emerged as a promising tool for fabricating complex bioconstructs that mimic the highly organized, aligned structure of native skeletal muscle tissue [91,132]. Bioconstructs can be pre-vascularized and pre-innervated by spatially patterning a combination of multiple cell types, biomaterials, and bioactive factors. Techniques for 3D bioprinting vasculature and nerve have both been extensively reviewed for other tissue applications [121,133]. The role of spatial patterning in pre-vascularization was specifically evaluated in a rat VML model [94]. Bioconstructs composed of human MPCs and HUVECs were 3D printed in two distinct conformations; MPC-HUVEC hybrid fibers (mixed printing) and MPC fibers, each surrounded by a layer of HUVECs (coaxial printing). Coaxially printed bioconstructs yielded a significant increase in TA muscle weight compared to mixed printed bioconstructs. In addition, coaxially printed bioconstructs vascularized and anastomosed with host vasculature more than mixed printed and MPC-only groups. Muscles treated with coaxially printed bioconstructs also achieved approximately an 85% recovery of the force production of uninjured muscle. 3D printed bioconstructs, which have been pre-innervated by the incorporation of human NSCs, have also shown promise for enhancing skeletal muscle regeneration following VML [95]. Bioconstructs containing NSCs yielded a significantly higher number of NMJs at 8 weeks post implantation in a rat VML model compared to MPC-only bioconstructs. Moreover, 3D printed bioconstructs, with and without NSCs, became highly vascularized within 4 weeks and contained more blood vessels than uninjured muscle.
In addition to their role in myofiber maturation discussed previously, aligned fibrillar scaffolds have also been investigated for their ability to enhance vascularization and innervation during skeletal muscle regeneration following VML. Compared to 3D bioprinting, where feature sizes below several microns are generally unable to be fabricated, electrospinning and shear-based extrusion methods can produce biomaterial fibers at the micro- and nano-scale [134,135]. Moreover, electrospun and shear-based extruded fibers can be aggregated in parallel to generate scaffolds with aligned architecture, more closely mimicking the architecture of native ECM [136]. Acellular, aligned nanofibrillar collagen scaffolds transplanted into a VML defect yielded improved density of perfused microvessels compared to non-aligned scaffolds [88]. C2C12s and human endothelial cells co-cultured on the same aligned scaffold also demonstrated a significant increase in perfused vessel density compared to non-aligned scaffolds in a VML model [137]. In addition, alignment was also shown to support the formation of organized vascular networks with greater anisotropy than non-alignment.
3.3. Bioactive Factors
3.3.1. Bioactive Factors for Vascularization
Angiogenic factors are well-established therapeutics for improving tissue vascularization [120,138]. Current strategies of angiogenic factor delivery in VML therapy have focused on promoting and accelerating the ingrowth of pre-existing vessels toward the injury site by recruiting endogenous endothelial or progenitor cells. For instance, IGF-1 loaded scaffolds can significantly increase the density of perfused microvessels compared to empty scaffolds following VML injury [118]. Improving muscle angiogenesis using other angiogenic factors has also been investigated in muscle ischemic injury. Sustained release of factors, including vascular endothelial growth factor (VEGF), SDF-1, FGF-2, and FGF-9, to ischemic muscle was shown to promote vascular density and improve angiogenesis at the injury site [139–144]. These factors can serve as potential candidates for VML therapy. Besides delivering angiogenic proteins, delivery of genes encoding for angiogenic factors is another interesting approach for improving vascularization during muscle regeneration. For example, mRNA encoding HGF was loaded into scaffolds along with Lipofectamine transfection agent, which enabled the intracellular delivery of the mRNA [145]. Two weeks after VML injury, the HGF encoded mRNA resulted in a 1.3-fold increase in capillary density compared with luciferase encoded mRNA [145].
In addition to inducing endogenous vessel infiltration, other applications that have not been explored in VML model, such as improving transplanted cell engraftment and stabilizing newly formed vessels, also have great therapeutic potential for VML. For example, angiogenic factors have been integrated into endothelial cell-laden bioconstructs to improve the survival of transplanted cells and stimulate de novo vascularization. HUVECs co-delivered with VEGF exhibited increased survival after transplantation into ischemic muscle, leading to improved tissue perfusion and muscle regeneration [146]. In addition to inducing the formation of new vessels, delivery of angiogenic factors can also promote maturation to develop functional vascular networks [120]. The combination of factors associated with both vessel formation and maturation has been found to be a more promising approach than the delivery of single factors [147]. Nevertheless, different factors have their own time window of action during vascularization [148], suggesting that distinct release kinetics are required for each of the factors. Simultaneous delivery of VEGF and PDGF-BB did not induce stable increase in blood vessel density [148,149]. In contrast, a sequential delivery of VEGF and PDGF-BB to ischemic muscle yielded increased vessel density at 2 weeks and maturity at 4 weeks compared with single factor delivery [149]. This result suggests that delivery of bioactive factor combinations in their physiological sequences may greatly improve the regeneration process. Biomaterials, in this context, serve as a powerful technology for controlling the release kinetics of different bioactive factors to mimic the native regeneration process.
3.3.2. Bioactive Factors for Innervation
Although bioactive factor delivery is extensively explored in nerve regeneration [150], using bioactive factors to improve innervation for VML treatment is still a nascent field. A recent study investigated the therapeutic potential of IGF-1 for the innervation of VML injured muscle [118]. Scaffolds loaded with IGF-1 yielded a greater number of mature NMJs compared with empty scaffolds. Another strategy to achieve innervation for VML therapy is to induce acetylcholine receptor (AChR) clustering in bioconstructs by delivering agrin, a large proteoglycan for inducing AChR clustering [151,152]. Muscle-derived cells were seeded on scaffolds coated with agrin and cultured for 6 days in vitro [151]. A dramatic enhancement of AChR clustering has been found in the combination of agrin presence and cyclic stretch, providing a potential approach for VML treatment. The in vivo therapeutic effect for VML was further explored by physically adsorbing or chemically tethering agrin to the scaffold prior to implantation [152]. A significant increase of neuromuscular junctions and neural infiltration was found in both groups containing agrin 4 weeks after VML injury. In addition, the scaffolds that were chemically tethered with agrin achieved a further improvement of muscle regeneration compared with the physically adsorbed group due to a prolonged release profile.
4. Immunomodulation
The immune system plays a critical role in tissue regeneration. The innate and adaptive immune systems are both key mediators of the injury site microenvironment and are known to influence progenitor cell expansion, survival, and integration [153]. Immediately following injury, the innate immune response results in a cascade of inflammatory signals, infiltration of immune cells, and deposition of ECM. During normal regeneration, the inflammatory response generally resolves quickly as tissues begin to repair. However, a prolonged inflammatory response leads to pathological fibrosis, insufficient regeneration, and impairment of normal tissue function [154]. Immunomodulation is therefore critical for promoting tissue regeneration and has been reviewed extensively [153,155–157].
VML injuries result in a dysregulated immune response that causes sustained tissue inflammation, pathological fibrosis, and impaired muscle regeneration [12,17,18]. In skeletal muscle, the inflammatory response is temporally coupled with myogenesis during regeneration [158]. Macrophages, which polarize between pro-inflammatory (M1) and pro-regenerative (M2) phenotypes during regeneration, play an important role in regulating myogenesis, inflammation, and fibrosis [159,160]. Specifically, macrophages modulate fibro-adipogenic progenitor (FAP) proliferation and differentiation into fibroblasts or adipocytes [161,162]. A prolonged inflammatory response leads to an increase in the number of FAPs and the production of extracellular matrix. Regulatory T-cells (Treg) have been shown to regulate macrophage polarization towards the pro-regenerative phenotype and drive the later stages of muscle regeneration [158]. In this section, we will highlight the immunomodulatory cells, scaffolds, and bioactive factors that have been used to enhance skeletal muscle regeneration following VML.
4.1. Cells
Mesenchymal stem cells (MSCs) exhibit anti-inflammatory and immunomodulatory effects on both innate and adaptive immune cells [153]. For example, transplanted MSCs promote the transition of macrophages from the M1 to M2 phenotype, inhibit immune cell proliferation, and induce the formation of Treg cells in vivo [163]. Therefore, MSCs have been used as an immunomodulatory cell source for enhancing regeneration in many tissue engineering applications [164]. MSCs transplanted into a rat VML defect significantly increased macrophage polarization to the M2 phenotype after 2 weeks compared to the untreated control [165]. In addition, MSC treatment yielded significantly increased muscle fiber regeneration and decreased fibrosis after 8 weeks compared to the untreated control. Transplanting MSCs with a dECM scaffold demonstrated a synergistic effect, further improving macrophage polarization and muscle regeneration [165]. In a similar VML model, MSCs derived from adipose tissue (adipose stem cells (ASCs)) in a collagen hydrogel also promoted an M2 macrophage transition and decreased inflammatory cytokines [166]. In addition, ASC treatment demonstrated improved myogenesis, increased blood flow restoration, and decreased fibrosis compared to the acellular hydrogel control. Improved myogenesis and vascularization were also observed in muscles treated with ASCs in a collagen hydrogel following a full thickness VML injury [129]. In a more severe VML mouse model, where TA and EDL muscles were removed, ASCs seeded on electrospun fibrin scaffolds yielded improved muscle regeneration and decreased fibrosis compared to acellular controls [167]. However, muscle fiber regeneration after 3 months of implantation was limited. Although few transplanted ASCs were found to fuse with host myofibers, mesenchymal stem cells are unlikely to replace a myogenic cell source.
4.2. Scaffolds
Biomaterials generally trigger an immune response in vivo [168,169]. Scaffold physicochemical properties play a critical role in the host immune reaction and can be altered to modulate the immune microenvironment and promote tissue regeneration. The interactions between biomaterials and the immune system have been reviewed in detail [170–173]. Immunomodulatory properties of scaffolds used for VML therapy have also been investigated. Small intestinal submucosa dECM, cardiac dECM, and muscle dECM/PCL blended scaffolds yielded an increased M2-macrophage polarization in VML models compared to untreated controls [87,165,174]. Although urinary bladder dECM implanted into a rat VML model did not recapitulate autograft macrophage polarization dynamics over 8 weeks, M2-macrophage polarization was comparable at the final time point [175]. Mechanistic studies of the adaptive immune response in skeletal muscle regeneration following VML revealed that dECM scaffolds induce a pro-regenerative response via T helper 2 cells, which in turn guide macrophage polarization [176]. A direct comparison of natural and synthetic scaffolds implanted into a VML defect revealed divergent immune responses [177]. Specifically, M2-macrophage markers were up-regulated in dECM treated groups and down-regulated in PEG and poly(ethylene) scaffolds after 3 weeks. Synthetic scaffold groups induced a chronic neutrophil infiltrate, which was dependent on material stiffness and size. In addition to increasing neutrophil number, increases in scaffold stiffness caused decreased M2-macrophage polarization in synthetic scaffolds [177]. Similar to dECM scaffolds, natural biomaterial hydrogels, including elastin and a fibrin/laminin-111 blend, also demonstrated increased M2 macrophage polarization in VML models [178,179]. However, muscle regeneration was minimal as both approaches lacked a myogenic cell source.
4.3. Bioactive Factors
Local delivery of immunomodulatory drugs in conjunction with bioconstructs is another promising approach for enhancing tissue regeneration. FDA approved immunosuppressants have been investigated in regenerative therapies for artery, bone, and nerve tissue [180–182]. FTY70 and tacrolimus, immunosuppressants used to treat multiple sclerosis and organ rejection, respectively, have also been used to enhance regeneration following VML injury [183,184]. FTY720 loaded PLGA films implanted into a VML mouse model yielded an increased number of anti-inflammatory monocytes and M2 macrophages at the injury site, compared to PLGA films alone [183]. In addition, FTY720 treatment accelerated vascularization and yielded increased muscle regeneration. A combination therapy of minced muscle grafts and systemic delivery of tacrolimus demonstrated increased muscle regeneration and decreased fibrosis in a porcine VML model, compared to minced muscle grafts alone [184]. In addition, systemic delivery of tacrolimus moderately improved muscle function in muscles treated with minced muscle grafts. Although delivery of immunomodulatory drugs in VML therapies is still nascent, local and systemic delivery methods are both viable approaches to improve regeneration.
5. Summary and Future Perspectives
The ultimate goals of VML treatment are to fully regenerate the lost muscle and recover tissue function. To achieve these goals, a variety of tissue engineering approaches, including cell therapy, scaffold design, and bioactive factor delivery, have been developed. Although a significant improvement has been made in the regeneration of vascularized and innervated muscle with reduced scar formation, challenges still persist in this field. To investigate therapeutic efficacy, multiple animal models of VML have been developed across species and evaluated in different muscles [64]. The critical size of defects among different models ranges from 15% to 40% of muscle mass [8–10]. Besides the size of defects, there is still a lack of standard criteria in dimensions, location, and muscle type. In addition to the characterization of pre-clinical models, strategies for scale-up to yield clinical-grade bioconstructs also need to be developed. Resources of therapeutic cells with preferred properties, such as potential for self-renewal, robust regenerative capability, and easy accessibility, limit the clinical translation of cell therapy. Future materials need to be developed with consistent composition and highly tunable properties as synthetic scaffolds and favorable biological properties as natural materials. A more comprehensive understanding of the concentrations and combinations of cellular and molecular components will facilitate the rational design of the bioconstructs.
In addition to the strategies mentioned above, exercise is an emerging approach for improving muscle regeneration. Evidence indicates that exercise, either as voluntary running or high-intensity interval training, can promote muscle formation with vascularization and innervation as well as reduced fibrosis following the transplantation of bioconstructs [31,118,185]. An immediate rehabilitation regimen post bioconstruct transplantation has been shown to promote scaffold remodeling, but may hinder regeneration and increase fibrosis [20,31,186]. Beginning exercise 1 to 2 weeks after transplantation, on the other hand, can enhance the muscle regeneration potential of the cell-seeded or growth factor-laden scaffold [118,185]. Another important goal is to restore the integration of muscle and its neighboring tendon tissues. Creation of the interface between muscle and tendon tissues requires precise imitation of the different biological and mechanical properties between distinct tissues. The development of the 3D bioprinting technologies makes it possible to have precise spatial control of bioconstruct architecture at the microscale and build up a complex myotendinous junction structure [187,188].
In summary, tissue engineering is a promising field that applies multidisciplinary strategies to develop substitutes for lost muscle tissue and restore its function. While a number of achievements have been made in skeletal muscle tissue engineering, VML therapy as a complex process is still challenging and requires systematic and sophisticated approaches. The research in biology, material science, engineering, and medicine need to converge for future clinical success.
Acknowledgements
This work was supported by grants from the NIH (AG036695, AR073248) and the Department of Veterans Affairs (I01RX001222, I01BX002324) to T.A.R. All figures created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Credit
This manuscript was written by IE, DW, and TAR.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Thomas A. Rando reports financial support was provided by Stanford University. Thomas A. Rando reports financial support was provided by National Institutes of Health. Thomas A. Rando reports financial support was provided by US Department of Veterans Affairs.
Bibliography
- [1].Ebbeling CB, Clarkson PM. Exercise-induced muscle damage and adaptation. Sports Med 1989;7:207–34. doi: 10.2165/00007256-198907040-00001. [DOI] [PubMed] [Google Scholar]
- [2].Cross JD, Ficke JR, Hsu JR, Masini BD, Wenke JC. Battlefield orthopaedic injuries cause the majority of long-term disabilities. J Am Acad Orthop Surg 2011;19 Suppl 1:S1–7. doi: 10.5435/00124635201102001-00002. [DOI] [PubMed] [Google Scholar]
- [3].Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res 2012;347:759–74. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- [4].Järvinen TAH, Järvinen TLN, Kääriäinen M, Aärimaa V, Vaittinen S, Kalimo H, et al. Muscle injuries: optimising recovery. Best Pract Res Clin Rheumatol 2007;21:317–31. doi: 10.1016/j.berh.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [5].Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 2012;139:2845–56. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- [6].Grogan BF, Hsu JR, Skeletal Trauma Research Consortium. Volumetric muscle loss. J Am Acad Orthop Surg 2011;19 Suppl 1:S35–7. doi: 10.5435/00124635-201102001-00007. [DOI] [PubMed] [Google Scholar]
- [7].Garg K, Ward CL, Hurtgen BJ, Wilken JM, Stinner DJ, Wenke JC, et al. Volumetric muscle loss: persistent functional deficits beyond frank loss of tissue. J Orthop Res 2015;33:40–6. doi: 10.1002/jor.22730. [DOI] [PubMed] [Google Scholar]
- [8].Anderson SE, Han WM, Srinivasa V, Mohiuddin M, Ruehle MA, Moon JY, et al. Determination of a critical size threshold for volumetric muscle loss in the mouse quadriceps. Tissue Eng Part C Methods 2019;25:59–70. doi: 10.1089/ten.TEC.2018.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu X, Corona BT, Chen X, Walters TJ. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access 2012;1:280–90. doi: 10.1089/biores.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vega-Soto EE, Rodriguez BL, Armstrong RE, Larkin LM. A 30% Volumetric Muscle Loss Does Not Result in Sustained Functional Deficits after a 90-Day Recovery in Rats. Regen Eng Transl Med 2020;6:62–8. doi: 10.1007/s40883-019-00117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Caldwell CJ, Mattey DL, Weller RO. Role of the basement membrane in the regeneration of skeletal muscle. Neuropathol Appl Neurobiol 1990;16:225–38. doi: 10.1111/j.1365-2990.1990.tb01159.x. [DOI] [PubMed] [Google Scholar]
- [12].Larouche J, Greising SM, Corona BT, Aguilar CA. Robust inflammatory and fibrotic signaling following volumetric muscle loss: a barrier to muscle regeneration. Cell Death Dis 2018;9:409. doi: 10.1038/s41419-018-0455-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Garg K, Corona BT, Walters TJ. Therapeutic strategies for preventing skeletal muscle fibrosis after injury. Front Pharmacol 2015;6:87. doi: 10.3389/fphar.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Corona BT, Rivera JC, Owens JG, Wenke JC, Rathbone CR. Volumetric muscle loss leads to permanent disability following extremity trauma. J Rehabil Res Dev 2015;52:785–92. doi: 10.1682/JRRD.2014.07.0165. [DOI] [PubMed] [Google Scholar]
- [15].Grasman JM, Zayas MJ, Page RL, Pins GD. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater 2015;25:2–15. doi: 10.1016/j.actbio.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gilbert-Honick J, Grayson W. Vascularized and innervated skeletal muscle tissue engineering. Adv Healthc Mater 2020;9:e1900626. doi: 10.1002/adhm.201900626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aguilar CA, Greising SM, Watts A, Goldman SM, Peragallo C, Zook C, et al. Multiscale analysis of a regenerative therapy for treatment of volumetric muscle loss injury. Cell Death Discov 2018;4:33. doi: 10.1038/s41420-018-0027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Greising SM, Rivera JC, Goldman SM, Watts A, Aguilar CA, Corona BT. Unwavering pathobiology of volumetric muscle loss injury. Sci Rep 2017;7:13179. doi: 10.1038/s41598-017-13306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Greising SM, Corona BT, McGann C, Frankum JK, Warren GL. Therapeutic Approaches for Volumetric Muscle Loss Injury: A Systematic Review and Meta-Analysis. Tissue Eng Part B Rev 2019;25:510–25. doi: 10.1089/ten.TEB.2019.0207. [DOI] [PubMed] [Google Scholar]
- [20].Dziki J, Badylak S, Yabroudi M, Sicari B, Ambrosio F, Stearns K, et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Npj Regen Med 2016;1:16008. doi: 10.1038/npjregenmed.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen XK, Walters TJ. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J Plast Reconstr Aesthet Surg 2013;66:1750–8. doi: 10.1016/j.bjps.2013.07.037. [DOI] [PubMed] [Google Scholar]
- [22].Turner NJ, Yates AJ, Weber DJ, Qureshi IR, Stolz DB, Gilbert TW, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A 2010;16:3309–17. doi: 10.1089/ten.TEA.2010.0169. [DOI] [PubMed] [Google Scholar]
- [23].Quarta M, Cromie Lear MJ, Blonigan J, Paine P, Chacon R, Rando TA. Biomechanics show stem cell necessity for effective treatment of volumetric muscle loss using bioengineered constructs. Npj Regen Med 2018;3:18. doi: 10.1038/s41536-018-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Aurora A, Roe JL, Corona BT, Walters TJ. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials 2015;67:393–407. doi: 10.1016/j.biomaterials.2015.07.040. [DOI] [PubMed] [Google Scholar]
- [25].Garg K, Ward CL, Rathbone CR, Corona BT. Transplantation of devitalized muscle scaffolds is insufficient for appreciable de novo muscle fiber regeneration after volumetric muscle loss injury. Cell Tissue Res 2014;358:857–73. doi: 10.1007/s00441-014-2006-6. [DOI] [PubMed] [Google Scholar]
- [26].Pantelic MN, Larkin LM. Stem cells for skeletal muscle tissue engineering. Tissue Eng Part B Rev 2018;24:373–91. doi: 10.1089/ten.TEB.2017.0451. [DOI] [PubMed] [Google Scholar]
- [27].Sicari BM, Dearth CL, Badylak SF. Tissue engineering and regenerative medicine approaches to enhance the functional response to skeletal muscle injury. Anat Rec (Hoboken) 2014;297:51–64. doi: 10.1002/ar.22794. [DOI] [PubMed] [Google Scholar]
- [28].Judson RN, Rossi FMV. Towards stem cell therapies for skeletal muscle repair. Npj Regen Med 2020;5:10. doi: 10.1038/s41536-020-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shayan M, Huang NF. Pre-Clinical Cell Therapeutic Approaches for Repair of Volumetric Muscle Loss. Bioengineering (Basel) 2020;7. doi: 10.3390/bioengineering7030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- [31].Quarta M, Cromie M, Chacon R, Blonigan J, Garcia V, Akimenko I, et al. Bioengineered constructs combined with exercise enhance stem cell-mediated treatment of volumetric muscle loss. Nat Commun 2017;8:15613. doi: 10.1038/ncomms15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 2014;20:255–64. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 2005;309:2064–7. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- [34].Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Charville GW, Cheung TH, Yoo B, Santos PJ, Lee GK, Shrager JB, et al. Ex Vivo Expansion and In Vivo Self-Renewal of Human Muscle Stem Cells. Stem Cell Rep 2015;5:621–32. doi: 10.1016/j.stemcr.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc 2008;3:1501–9. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- [37].Rodriguez BL, Vega-Soto EE, Kennedy CS, Nguyen MH, Cederna PS, Larkin LM. A tissue engineering approach for repairing craniofacial volumetric muscle loss in a sheep following a 2, 4, and 6-month recovery. PLoS One 2020;15:e0239152. doi: 10.1371/journal.pone.0239152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].VanDusen KW, Syverud BC, Williams ML, Lee JD, Larkin LM. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A 2014;20:2920–30. doi: 10.1089/ten.TEA.2014.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep 2013;14:1062–72. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- [41].Corona BT, Garg K, Ward CL, McDaniel JS, Walters TJ, Rathbone CR. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. Am J Physiol Cell Physiol 2013;305:C761–75. doi: 10.1152/ajpcell.00189.2013. [DOI] [PubMed] [Google Scholar]
- [42].Ward CL, Pollot BE, Goldman SM, Greising SM, Wenke JC, Corona BT. Autologous minced muscle grafts improve muscle strength in a porcine model of volumetric muscle loss injury. J Orthop Trauma 2016;30:e396–403. doi: 10.1097/BOT.0000000000000673. [DOI] [PubMed] [Google Scholar]
- [43].Chen KG, Mallon BS, McKay RDG, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014;14:13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wu J, Matthias N, Bhalla S, Darabi R. Evaluation of the Therapeutic Potential of Human iPSCs in a Murine Model of VML. Mol Ther 2020. doi: 10.1016/j.ymthe.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rao L, Qian Y, Khodabukus A, Ribar T, Bursac N. Engineering human pluripotent stem cells into a functional skeletal muscle tissue. Nat Commun 2018;9:126. doi: 10.1038/s41467-017-02636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, et al. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J 2010;24:2245–53. doi: 10.1096/fj.09-137174. [DOI] [PubMed] [Google Scholar]
- [47].van der Wal E, Herrero-Hernandez P, Wan R, Broeders M, In ‘t Groen SLM, van Gestel TJM, et al. Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Rep 2018;10:1975–90. doi: 10.1016/j.stemcr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Maffioletti SM, Sarcar S, Henderson ABH, Mannhardt I, Pinton L, Moyle LA, et al. Three-Dimensional Human iPSC-Derived Artificial Skeletal Muscles Model Muscular Dystrophies and Enable Multilineage Tissue Engineering. Cell Rep 2018;23:899–908. doi: 10.1016/j.celrep.2018.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real PJ, Mácia A, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010;28:1568–70. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Miyagoe-Suzuki Y, Takeda S. Skeletal muscle generated from induced pluripotent stem cells - induction and application. World J Stem Cells 2017;9:89–97. doi: 10.4252/wjsc.v9.i6.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhao T, Zhang Z-N, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature 2011;474:212–5. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- [52].Webster MT, Manor U, Lippincott-Schwartz J, Fan C-M. Intravital Imaging Reveals Ghost Fibers as Architectural Units Guiding Myogenic Progenitors during Regeneration. Cell Stem Cell 2016;18:243–52. doi: 10.1016/j.stem.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Smoak MM, Mikos AG. Advances in biomaterials for skeletal muscle engineering and obstacles still to overcome. Mater Today Bio 2020;7:100069. doi: 10.1016/j.mtbio.2020.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Dunn A, Talovic M, Patel K, Patel A, Marcinczyk M, Garg K. Biomaterial and stem cell-based strategies for skeletal muscle regeneration. J Orthop Res 2019;37:1246–62. doi: 10.1002/jor.24212. [DOI] [PubMed] [Google Scholar]
- [55].Cezar CA, Mooney DJ. Biomaterial-based delivery for skeletal muscle repair. Adv Drug Deliv Rev 2015;84:188–97. doi: 10.1016/j.addr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Liu J, Saul D, Böker KO, Ernst J, Lehman W, Schilling AF. Current methods for skeletal muscle tissue repair and regeneration. Biomed Res Int 2018;2018:1984879. doi: 10.1155/2018/1984879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Matthias N, Hunt SD, Wu J, Lo J, Smith Callahan LA, Li Y, et al. Volumetric muscle loss injury repair using in situ fibrin gel cast seeded with muscle-derived stem cells (MDSCs). Stem Cell Res 2018;27:65–73. doi: 10.1016/j.scr.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Boldrin L, Malerba A, Vitiello L, Cimetta E, Piccoli M, Messina C, et al. Efficient delivery of human single fiber-derived muscle precursor cells via biocompatible scaffold. Cell Transplant 2008;17:577–84. doi: 10.3727/096368908785095980. [DOI] [PubMed] [Google Scholar]
- [59].Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices 2011;8:607–26. doi: 10.1586/erd.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Csapo R, Gumpenberger M, Wessner B. Skeletal Muscle Extracellular Matrix - What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front Physiol 2020;11:253. doi: 10.3389/fphys.2020.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res 2014;163:268–85. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Badylak SF, Dziki JL, Sicari BM, Ambrosio F, Boninger ML. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials 2016;103:128–36. doi: 10.1016/j.biomaterials.2016.06.047. [DOI] [PubMed] [Google Scholar]
- [63].Urciuolo A, Urbani L, Perin S, Maghsoudlou P, Scottoni F, Gjinovci A, et al. Decellularised skeletal muscles allow functional muscle regeneration by promoting host cell migration. Sci Rep 2018;8:8398. doi: 10.1038/s41598-018-26371-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sarrafian TL, Bodine SC, Murphy B, Grayson JK, Stover SM. Extracellular matrix scaffolds for treatment of large volume muscle injuries: A review. Vet Surg 2018;47:524–35. doi: 10.1111/vsu.12787. [DOI] [PubMed] [Google Scholar]
- [65].Passipieri JA, Baker HB, Siriwardane M, Ellenburg MD, Vadhavkar M, Saul JM, et al. Keratin hydrogel enhances in vivo skeletal muscle function in a rat model of volumetric muscle loss. Tissue Eng Part A 2017;23:556–71. doi: 10.1089/ten.TEA.2016.0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Baker HB, Passipieri JA, Siriwardane M, Ellenburg MD, Vadhavkar M, Bergman CR, et al. Cell and Growth Factor-Loaded Keratin Hydrogels for Treatment of Volumetric Muscle Loss in a Mouse Model. Tissue Eng Part A 2017;23:572–84. doi: 10.1089/ten.TEA.2016.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Goldman SM, Corona BT. Co-delivery of micronized urinary bladder matrix damps regenerative capacity of minced muscle grafts in the treatment of volumetric muscle loss injuries. PLoS One 2017;12:e0186593. doi: 10.1371/journal.pone.0186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater Weinheim 2011;23:H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kasukonis B, Kim J, Brown L, Jones J, Ahmadi S, Washington T, et al. Codelivery of Infusion Decellularized Skeletal Muscle with Minced Muscle Autografts Improved Recovery from Volumetric Muscle Loss Injury in a Rat Model. Tissue Eng Part A 2016;22:1151–63. doi: 10.1089/ten.TEA.2016.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hosoyama K, Lazurko C, Muñoz M, McTiernan CD, Alarcon EI. Peptide-Based Functional Biomaterials for Soft-Tissue Repair. Front Bioeng Biotechnol 2019;7:205. doi: 10.3389/fbioe.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev 2015;94:53–62. doi: 10.1016/j.addr.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Perry L, Landau S, Flugelman MY, Levenberg S. Genetically engineered human muscle transplant enhances murine host neovascularization and myogenesis. Commun Biol 2018;1:161. doi: 10.1038/s42003-018-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Ziemkiewicz N, Talovic M, Madsen J, Hill L, Scheidt R, Patel A, et al. Laminin-111 functionalized polyethylene glycol hydrogels support myogenic activity in vitro. Biomed Mater 2018;13:065007. doi: 10.1088/1748-605X/aad915. [DOI] [PubMed] [Google Scholar]
- [74].Shandalov Y, Egozi D, Koffler J, Dado-Rosenfeld D, Ben-Shimol D, Freiman A, et al. An engineered muscle flap for reconstruction of large soft tissue defects. Proc Natl Acad Sci USA 2014;111:6010–5. doi: 10.1073/pnas.1402679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003;24:4385–415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [76].Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials 2011;32:8905–14. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Silva Garcia JM, Panitch A, Calve S. Functionalization of hyaluronic acid hydrogels with ECM-derived peptides to control myoblast behavior. Acta Biomater 2019;84:169–79. doi: 10.1016/j.actbio.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Zhang H, Zhou L, Zhang W. Control of scaffold degradation in tissue engineering: a review. Tissue Eng Part B Rev 2014;20:492–502. doi: 10.1089/ten.TEB.2013.0452. [DOI] [PubMed] [Google Scholar]
- [80].Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev 2013;19:485–502. doi: 10.1089/ten.TEB.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Panayi AC, Smit L, Hays N, Udeh K, Endo Y, Li B, et al. A porous collagen-GAG scaffold promotes muscle regeneration following volumetric muscle loss injury. Wound Repair Regen 2020;28:61–74. doi: 10.1111/wrr.12768. [DOI] [PubMed] [Google Scholar]
- [82].Haas GJ, Dunn AJ, Marcinczyk M, Talovic M, Schwartz M, Scheidt R, et al. Biomimetic sponges for regeneration of skeletal muscle following trauma. J Biomed Mater Res A 2019;107:92–103. doi: 10.1002/jbm.a.36535. [DOI] [PubMed] [Google Scholar]
- [83].Liao I-C, Liu JB, Bursac N, Leong KW. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell Mol Bioeng 2008;1:133–45. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Jana S, Levengood SKL, Zhang M. Anisotropic Materials for Skeletal-Muscle-Tissue Engineering. Adv Mater Weinheim 2016;28:10588–612. doi: 10.1002/adma.201600240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Politi S, Carotenuto F, Rinaldi A, Di Nardo P, Manzari V, Albertini MC, et al. Smart ECM-Based Electrospun Biomaterials for Skeletal Muscle Regeneration. Nanomaterials (Basel) 2020;10. doi: 10.3390/nano10091781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kim J, Kasukonis B, Roberts K, Dunlap G, Brown L, Washington T, et al. Graft alignment impacts the regenerative response of skeletal muscle after volumetric muscle loss in a rat model. Acta Biomater 2020;105:191–202. doi: 10.1016/j.actbio.2020.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Patel KH, Talovic M, Dunn AJ, Patel A, Vendrell S, Schwartz M, et al. Aligned nanofibers of decellularized muscle extracellular matrix for volumetric muscle loss. J Biomed Mater Res Part B Appl Biomater 2020;108:2528–37. doi: 10.1002/jbm.b.34584. [DOI] [PubMed] [Google Scholar]
- [88].Nakayama KH, Alcazar C, Yang G, Quarta M, Paine P, Doan L, et al. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. Npj Regen Med 2018;3:16. doi: 10.1038/s41536-018-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Blake C, Massey O, Boyd-Moss M, Firipis K, Rifai A, Franks S, et al. Replace and repair: Biomimetic bioprinting for effective muscle engineering. APL Bioengineering 2021;5:031502. doi: 10.1063/5.0040764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhuang P, An J, Chua CK, Tan LP. Bioprinting of 3D in vitro skeletal muscle models: A review. Mater Des 2020;193:108794. doi: 10.1016/j.matdes.2020.108794. [DOI] [Google Scholar]
- [91].Kim JH, Seol Y-J, Ko IK, Kang H-W, Lee YK, Yoo JJ, et al. 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci Rep 2018;8:12307. doi: 10.1038/s41598-018-29968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kim JH, Ko IK, Jeon MJ, Kim I, Vanschaayk MM, Atala A, et al. Pelvic floor muscle function recovery using biofabricated tissue constructs with neuromuscular junctions. Acta Biomater 2021;121:237–49. doi: 10.1016/j.actbio.2020.12.012. [DOI] [PubMed] [Google Scholar]
- [93].Lee H, Kim W, Lee J, Park KS, Yoo JJ, Atala A, et al. Self-aligned myofibers in 3D bioprinted extracellular matrix-based construct accelerate skeletal muscle function restoration. Appl Phys Rev 2021;8:021405. doi: 10.1063/5.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Choi Y-J, Jun Y-J, Kim DY, Yi H-G, Chae S-H, Kang J, et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019;206:160–9. doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- [95].Kim JH, Kim I, Seol Y-J, Ko IK, Yoo JJ, Atala A, et al. Neural cell integration into 3D bioprinted skeletal muscle constructs accelerates restoration of muscle function. Nat Commun 2020;11:1025. doi: 10.1038/s41467-020-14930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Russell CS, Mostafavi A, Quint JP, Panayi AC, Baldino K, Williams TJ, et al. in situ printing of adhesive hydrogel scaffolds for the treatment of skeletal muscle injuries. ACS Appl Bio Mater 2020;3:1568–79. doi: 10.1021/acsabm.9b01176. [DOI] [PubMed] [Google Scholar]
- [97].Urciuolo A, Poli I, Brandolino L, Raffa P, Scattolini V, Laterza C, et al. Intravital three-dimensional bioprinting. Nat Biomed Eng 2020;4:901–15. doi: 10.1038/s41551-020-0568-z. [DOI] [PubMed] [Google Scholar]
- [98].Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science 2009;324:1673–7. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Syverud BC, VanDusen KW, Larkin LM. Growth factors for skeletal muscle tissue engineering. Cells Tissues Organs (Print) 2016;202:169–79. doi: 10.1159/000444671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 1998;194:114–28. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- [101].Pawlikowski B, Vogler TO, Gadek K, Olwin BB. Regulation of skeletal muscle stem cells by fibroblast growth factors. Dev Dyn 2017;246:359–67. doi: 10.1002/dvdy.24495. [DOI] [PubMed] [Google Scholar]
- [102].Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Baoge L, Van Den Steen E, Rimbaut S, Philips N, Witvrouw E, Almqvist KF, et al. Treatment of skeletal muscle injury: a review. ISRN Orthop 2012;2012:689012. doi: 10.5402/2012/689012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Le Grand F, Rudnicki MA. Skeletal muscle satellite cells and adult myogenesis. Curr Opin Cell Biol 2007;19:628–33. doi: 10.1016/j.ceb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ju YM, Atala A, Yoo JJ, Lee SJ. In situ regeneration of skeletal muscle tissue through host cell recruitment. Acta Biomater 2014;10:4332–9. doi: 10.1016/j.actbio.2014.06.022. [DOI] [PubMed] [Google Scholar]
- [106].Grasman JM, Do DM, Page RL, Pins GD. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials 2015;72:49–60. doi: 10.1016/j.biomaterials.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Le Grand F, Jones AE, Seale V, Scimè A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 2009;4:535–47. doi: 10.1016/j.stem.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].von Maltzahn J, Bentzinger CF, Rudnicki MA. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat Cell Biol 2011;14:186–91. doi: 10.1038/ncb2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bentzinger CF, von Maltzahn J, Dumont NA, Stark DA, Wang YX, Nhan K, et al. Wnt7a stimulates myogenic stem cell motility and engraftment resulting in improved muscle strength. J Cell Biol 2014;205:97–111. doi: 10.1083/jcb.201310035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Han WM, Mohiuddin M, Anderson SE, García AJ, Jang YC. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater 2019;94:243–52. doi: 10.1016/j.actbio.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA 2006;103:2494–9. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Tortorella LL, Milasincic DJ, Pilch PF. Critical proliferation-independent window for basic fibroblast growth factor repression of myogenesis via the p42/p44 MAPK signaling pathway. J Biol Chem 2001;276:13709–17. doi: 10.1074/jbc.M100091200. [DOI] [PubMed] [Google Scholar]
- [113].Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol 2000;278:C174–81. doi: 10.1152/ajpcell.2000.278.1.C174. [DOI] [PubMed] [Google Scholar]
- [114].Stratos I, Madry H, Rotter R, Weimer A, Graff J, Cucchiarini M, et al. Fibroblast growth factor-2-overexpressing myoblasts encapsulated in alginate spheres increase proliferation, reduce apoptosis, induce adipogenesis, and enhance regeneration following skeletal muscle injury in rats. Tissue Eng Part A 2011;17:2867–77. doi: 10.1089/ten.tea.2011.0239. [DOI] [PubMed] [Google Scholar]
- [115].Mitchell CA, McGeachie JK, Grounds MD. The exogenous administration of basic fibroblast growth factor to regenerating skeletal muscle in mice does not enhance the process of regeneration. Growth Factors 1996;13:37–55. doi: 10.3109/08977199609034565. [DOI] [PubMed] [Google Scholar]
- [116].Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Interact 2007;7:208–18. [PubMed] [Google Scholar]
- [117].Chargé SBP, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- [118].Alcazar CA, Hu C, Rando TA, Huang NF, Nakayama KH. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater Sci 2020;8:5376–89. doi: 10.1039/d0bm00990c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lee H, Ju YM, Kim I, Elsangeedy E, Lee JH, Yoo JJ, et al. A novel decellularized skeletal muscle-derived ECM scaffolding system for in situ muscle regeneration. Methods 2020;171:77–85. doi: 10.1016/j.ymeth.2019.06.027. [DOI] [PubMed] [Google Scholar]
- [120].Rouwkema J, Rivron NC, van Blitterswijk CA. Vascularization in tissue engineering. Trends Biotechnol 2008;26:434–41. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- [121].Tomasina C, Bodet T, Mota C, Moroni L, Camarero-Espinosa S. Bioprinting vasculature: materials, cells and emergent techniques. Materials (Basel) 2019;12. doi: 10.3390/ma12172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carnes ME, Pins GD. Skeletal Muscle Tissue Engineering: Biomaterials-Based Strategies for the Treatment of Volumetric Muscle Loss. Bioengineering (Basel) 2020;7. doi: 10.3390/bioengineering7030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Corona BT, Flanagan KE, Brininger CM, Goldman SM, Call JA, Greising SM. Impact of volumetric muscle loss injury on persistent motoneuron axotomy. Muscle Nerve 2018;57:799–807. doi: 10.1002/mus.26016. [DOI] [PubMed] [Google Scholar]
- [124].Gutmann E, Zelená J. Morphological changes in the denervated muscle. In: Gutmann E, editor. The Denervated Muscle, Boston, MA: Springer US; 1962, p. 57–102. doi: 10.1007/978-1-4899-4854-0_3. [DOI] [Google Scholar]
- [125].Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Gilbert-Honick J, Iyer SR, Somers SM, Lovering RM, Wagner K, Mao H-Q, et al. Engineering functional and histological regeneration of vascularized skeletal muscle. Biomaterials 2018;164:70–9. doi: 10.1016/j.biomaterials.2018.02.006. [DOI] [PubMed] [Google Scholar]
- [127].Frueh FS, Später T, Scheuer C, Menger MD, Laschke MW. Isolation of Murine Adipose Tissue-derived Microvascular Fragments as Vascularization Units for Tissue Engineering. J Vis Exp 2017. doi: 10.3791/55721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].McDaniel JS, Pilia M, Ward CL, Pollot BE, Rathbone CR. Characterization and multilineage potential of cells derived from isolated microvascular fragments. J Surg Res 2014;192:214–22. doi: 10.1016/j.jss.2014.05.047. [DOI] [PubMed] [Google Scholar]
- [129].Pilia M, McDaniel JS, Guda T, Chen XK, Rhoads RP, Allen RE, et al. Transplantation and perfusion of microvascular fragments in a rodent model of volumetric muscle loss injury. Eur Cell Mater 2014;28:11–23; discussion 23. doi: 10.22203/ecm.v028a02. [DOI] [PubMed] [Google Scholar]
- [130].Li M-T, Ruehle MA, Stevens HY, Servies N, Willett NJ, Karthikeyakannan S, et al. * Skeletal Myoblast-Seeded Vascularized Tissue Scaffolds in the Treatment of a Large Volumetric Muscle Defect in the Rat Biceps Femoris Muscle. Tissue Eng Part A 2017;23:989–1000. doi: 10.1089/ten.TEA.2016.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Das S, Browne KD, Laimo FA, Maggiore JC, Hilman MC, Kaisaier H, et al. Pre-innervated tissue-engineered muscle promotes a pro-regenerative microenvironment following volumetric muscle loss. Commun Biol 2020;3:330. doi: 10.1038/s42003-020-1056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol 2016;34:312–9. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- [133].Yu X, Zhang T, Li Y. 3D printing and bioprinting nerve conduits for neural tissue engineering. Polymers (Basel) 2020;12. doi: 10.3390/polym12081637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv 2010;28:325–47. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- [135].Pedde RD, Mirani B, Navaei A, Styan T, Wong S, Mehrali M, et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv Mater Weinheim 2017;29. doi: 10.1002/adma.201606061. [DOI] [PubMed] [Google Scholar]
- [136].Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006;17:R89–106. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- [137].Nakayama KH, Quarta M, Paine P, Alcazar C, Karakikes I, Garcia V, et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun Biol 2019;2:170. doi: 10.1038/s42003-019-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Li J, Zhang Y-P, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003;60:107–14. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- [139].Doi K, Ikeda T, Marui A, Kushibiki T, Arai Y, Hirose K, et al. Enhanced angiogenesis by gelatin hydrogels incorporating basic fibroblast growth factor in rabbit model of hind limb ischemia. Heart Vessels 2007;22:104–8. doi: 10.1007/s00380-006-0934-0. [DOI] [PubMed] [Google Scholar]
- [140].Layman H, Spiga M-G, Brooks T, Pham S, Webster KA, Andreopoulos FM. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemic model. Biomaterials 2007;28:2646–54. doi: 10.1016/j.biomaterials.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Wang L, Cao L, Shansky J, Wang Z, Mooney D, Vandenburgh H. Minimally invasive approach to the repair of injured skeletal muscle with a shape-memory scaffold. Mol Ther 2014;22:1441–9. doi: 10.1038/mt.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost 2007;5:590–8. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- [143].Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- [144].Said SS, Yin H, Elfarnawany M, Nong Z, O’Neil C, Leong H, et al. Fortifying Angiogenesis in Ischemic Muscle with FGF9-Loaded Electrospun Poly(Ester Amide) Fibers. Adv Healthc Mater 2019;8:e1801294. doi: 10.1002/adhm.201801294. [DOI] [PubMed] [Google Scholar]
- [145].Zaitseva TS, Alcazar C, Zamani M, Hou L, Sawamura S, Yakubov E, et al. Aligned Nanofibrillar Scaffolds for Controlled Delivery of Modified mRNA. Tissue Eng Part A 2019;25:121–30. doi: 10.1089/ten.TEA.2017.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Jay SM, Shepherd BR, Bertram JP, Pober JS, Saltzman WM. Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J 2008;22:2949–56. doi: 10.1096/fj.08-108803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Li J, Wei Y, Liu K, Yuan C, Tang Y, Quan Q, et al. Synergistic effects of FGF-2 and PDGF-BB on angiogenesis and muscle regeneration in rabbit hindlimb ischemia model. Microvasc Res 2010;80:10–7. doi: 10.1016/j.mvr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [148].Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 1998;125:1591–8. [DOI] [PubMed] [Google Scholar]
- [149].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [150].Kingham PJ, Terenghi G. Bioengineered nerve regeneration and muscle reinnervation. J Anat 2006;209:511–26. doi: 10.1111/j.1469-7580.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Scott JB, Ward CL, Corona BT, Deschenes MR, Harrison BS, Saul JM, et al. Achieving Acetylcholine Receptor Clustering in Tissue-Engineered Skeletal Muscle Constructs In vitro through a Materials-Directed Agrin Delivery Approach. Front Pharmacol 2016;7:508. doi: 10.3389/fphar.2016.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Gilbert-Honick J, Iyer SR, Somers SM, Takasuka H, Lovering RM, Wagner KR, et al. Engineering 3D skeletal muscle primed for neuromuscular regeneration following volumetric muscle loss. Biomaterials 2020;255:120154. doi: 10.1016/j.biomaterials.2020.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Godwin JW, Pinto AR, Rosenthal NA. Chasing the recipe for a pro-regenerative immune system. Semin Cell Dev Biol 2017;61:71–9. doi: 10.1016/j.semcdb.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014;15:14–25. doi: 10.1016/j.stem.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Julier Z, Park AJ, Briquez PS, Martino MM. Promoting tissue regeneration by modulating the immune system. Acta Biomater 2017;53:13–28. doi: 10.1016/j.actbio.2017.01.056. [DOI] [PubMed] [Google Scholar]
- [157].Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat Med 2014;20:857–69. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- [158].Tidball JG. Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 2017;17:165–78. doi: 10.1038/nri.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–62. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Wosczyna MN, Rando TA. A muscle stem cell support group: coordinated cellular responses in muscle regeneration. Dev Cell 2018;46:135–43. doi: 10.1016/j.devcel.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lemos DR, Babaeijandaghi F, Low M, Chang C-K, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med 2015;21:786–94. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- [162].Moratal C, Raffort J, Arrighi N, Rekima S, Schaub S, Dechesne CA, et al. IL-1β- and IL-4-polarized macrophages have opposite effects on adipogenesis of intramuscular fibro-adipogenic progenitors in humans. Sci Rep 2018;8:17005. doi: 10.1038/s41598-018-35429-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. Journal of Cellular Immunotherapy 2016;2:3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- [164].Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. Npj Regen Med 2019;4:22. doi: 10.1038/s41536-019-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Qiu X, Liu S, Zhang H, Zhu B, Su Y, Zheng C, et al. Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Res Ther 2018;9:88. doi: 10.1186/s13287-018-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Huang H, Liu J, Hao H, Chen D, Zhizhong L, Li M, et al. Preferred M2 Polarization by ASC-Based Hydrogel Accelerated Angiogenesis and Myogenesis in Volumetric Muscle Loss Rats. Stem Cells Int 2017;2017:2896874. doi: 10.1155/2017/2896874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Gilbert-Honick J, Ginn B, Zhang Y, Salehi S, Wagner KR, Mao H-Q, et al. Adipose-derived Stem/Stromal Cells on Electrospun Fibrin Microfiber Bundles Enable Moderate Muscle Reconstruction in a Volumetric Muscle Loss Model. Cell Transplant 2018;27:1644–56. doi: 10.1177/0963689718805370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011;32:6692–709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- [169].Vasconcelos DP, Águas AP, Barbosa MA, Pelegrín P, Barbosa JN. The inflammasome in host response to biomaterials: Bridging inflammation and tissue regeneration. Acta Biomater 2019;83:1–12. doi: 10.1016/j.actbio.2018.09.056. [DOI] [PubMed] [Google Scholar]
- [170].Chung L, Maestas DR, Housseau F, Elisseeff JH. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev 2017;114:184–92. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- [171].Andorko JI, Jewell CM. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng Transl Med 2017;2:139–55. doi: 10.1002/btm2.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sadtler K, Singh A, Wolf MT, Wang X, Pardoll DM, Elisseeff JH. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat Rev Mater 2016;1:16040. doi: 10.1038/natrevmats.2016.40. [DOI] [Google Scholar]
- [173].Sadtler K, Allen BW, Estrellas K, Housseau F, Pardoll DM, Elisseeff JH. The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Nontraumatic Applications. Tissue Eng Part A 2017;23:1044–53. doi: 10.1089/ten.TEA.2016.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Dziki JL, Sicari BM, Wolf MT, Cramer MC, Badylak SF. Immunomodulation and mobilization of progenitor cells by extracellular matrix bioscaffolds for volumetric muscle loss treatment. Tissue Eng Part A 2016;22:1129–39. doi: 10.1089/ten.TEA.2016.0340. [DOI] [PubMed] [Google Scholar]
- [175].Aurora A, Corona BT, Walters TJ. A Porcine Urinary Bladder Matrix Does Not Recapitulate the Spatiotemporal Macrophage Response of Muscle Regeneration after Volumetric Muscle Loss Injury. Cells Tissues Organs (Print) 2016;202:189–201. doi: 10.1159/000447582. [DOI] [PubMed] [Google Scholar]
- [176].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016;352:366–70. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Sadtler K, Wolf MT, Ganguly S, Moad CA, Chung L, Majumdar S, et al. Divergent immune responses to synthetic and biological scaffolds. Biomaterials 2019;192:405–15. doi: 10.1016/j.biomaterials.2018.11.002. [DOI] [PubMed] [Google Scholar]
- [178].Ibáñez-Fonseca A, Santiago Maniega S, Gorbenko Del Blanco D, Catalán Bernardos B, Vega Castrillo A, Álvarez Barcia ÁJ, et al. Elastin-Like Recombinamer Hydrogels for Improved Skeletal Muscle Healing Through Modulation of Macrophage Polarization. Front Bioeng Biotechnol 2020;8:413. doi: 10.3389/fbioe.2020.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Marcinczyk M, Dunn A, Haas G, Madsen J, Scheidt R, Patel K, et al. The Effect of Laminin-111 Hydrogels on Muscle Regeneration in a Murine Model of Injury. Tissue Eng Part A 2019;25:1001–12. doi: 10.1089/ten.TEA.2018.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Awojoodu AO, Ogle ME, Sefcik LS, Bowers DT, Martin K, Brayman KL, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci USA 2013;110:13785–90. doi: 10.1073/pnas.1221309110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].O’Neill E, Rajpura K, Carbone EJ, Awale G, Kan H-M, Lo KW-H. Repositioning Tacrolimus: Evaluation of the Effect of Short-Term Tacrolimus Treatment on Osteoprogenitor Cells and Primary Cells for Bone Regenerative Engineering. Assay Drug Dev Technol 2019;17:77–88. doi: 10.1089/adt.2018.876. [DOI] [PubMed] [Google Scholar]
- [182].Yin Y, Xiao G, Zhang K, Ying G, Xu H, De Melo BAG, et al. Tacrolimus- and Nerve Growth Factor-Treated Allografts for Neural Tissue Regeneration. ACS Chem Neurosci 2019;10:1411–9. doi: 10.1021/acschemneuro.8b00452. [DOI] [PubMed] [Google Scholar]
- [183].San Emeterio CL, Olingy CE, Chu Y, Botchwey EA. Selective recruitment of non-classical monocytes promotes skeletal muscle repair. Biomaterials 2017;117:32–43. doi: 10.1016/j.biomaterials.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Corona BT, Rivera JC, Wenke JC, Greising SM. Tacrolimus as an adjunct to autologous minced muscle grafts for the repair of a volumetric muscle loss injury. J EXP ORTOP 2017;4:36. doi: 10.1186/s40634-017-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Izadi MR, Habibi A, Khodabandeh Z, Nikbakht M. Synergistic effect of high-intensity interval training and stem cell transplantation with amniotic membrane scaffold on repair and rehabilitation after volumetric muscle loss injury. Cell Tissue Res 2020. doi: 10.1007/s00441-020-03304-8. [DOI] [PubMed] [Google Scholar]
- [186].Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Merceron TK, Burt M, Seol Y-J, Kang H-W, Lee SJ, Yoo JJ, et al. A 3D bioprinted complex structure for engineering the muscle-tendon unit. Biofabrication 2015;7:035003. doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- [188].Baldino L, Cardea S, Maffulli N, Reverchon E. Regeneration techniques for bone-to-tendon and muscle-to-tendon interfaces reconstruction. Br Med Bull 2016;117:25–37. doi: 10.1093/bmb/ldv056. [DOI] [PubMed] [Google Scholar]


