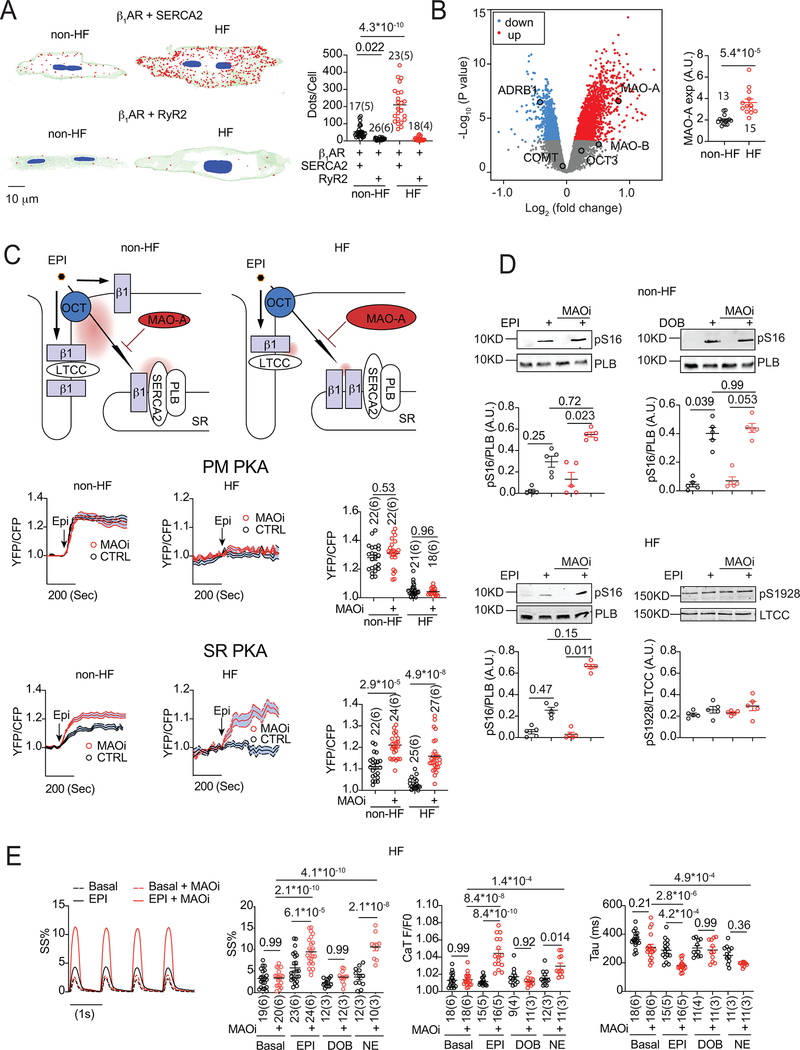
Desensitization of β1 adrenergic receptor (β1AR) and depressed cardiac contractility are hallmarks of heart failure (HF). Therefore, clinical drugs have been primarily aimed at rescuing β1ARs at the plasma membrane (PM) in therapy. This paradigm has been challenged by emerging evidence of functioning intracellular β1ARs at the sarcoplasmic reticulum (SR). 1 The SR-β1AR regulates local protein kinase A (PKA) phosphorylation of phospholamban (PLB) and excitation-contraction (EC) coupling. Thus, enhancing β1AR signaling at the SR represents an appealing approach for effectively improving contractility in HF. We found that elevation of monoamine oxidase A (MAO-A) in HF prevents local β1AR-PKA-PLB signaling at the SR. Inhibition of MAO-A rescues local β1AR signaling, phosphorylation of PLB, and EC-coupling in HF.
We applied chronic intraperitoneal injection of β-agonist isoproterenol to induce HF. We examined SR-β1AR association with sarcoplasmic/ endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) using proximity ligation assay (PLA, Figure 1A). HF adult ventricular myocytes (AVMs) displayed more PLA signals between β1AR and SERCA2a than non-HF cells, suggesting an increased β1AR association with SERCA2a in failing hearts. 1 There was minimal PLA signal between β1AR and ryanodine receptor 2 in non-HF and HF AVMs (Figure 1A). Failing hearts usually have low catecholamine contents associated with contractile dysfunction. 2 Catecholamines are imported via organic cation transporter (OCT) 1 and degraded by MAOs in hearts. 1, 2 Transcriptomic analysis of patients with dilated cardiomyopathy 4 revealed significant down-regulation of β1AR (ADRB1) and up-regulation of MAO-A, but no change in MAO-B, OCT3, and catechol-O-methyltransferase (COMT, another catecholamine degradation enzyme; Figure 1B).
FIGURE.
A). PLA assay of AVMs co-stained with anti-β11AR/SERCA2a or anti-β1AR/RyR2 antibodies. Representative 3D images were randomly selected and quantified with Image J.
B). Volcano plot of transcriptome mRNA from human HF patients with dilated cardiomyopathy relative to non-HF patients (GSE3586). Dot-plot shows the mRNA expression of MAO-A from non-HF and HF patients.
C). Schematic of intracellular SR-β1AR signaling in non-HF and HF AVMs. AVMs expressing PKA biosensors anchored on the PM and SR were pretreated with MAOi (clorgyline, 5 μM, 5 minutes) before stimulation with EPI (1 μM). Traces show time courses of the changes in FRET YFP/CFP ratio. Dot-plots show maximal increases in FRET ratio.
D). Detection of PKA phosphorylation of PLB at serine 16 (pS16) and LTCC Cav1.2 at serine 1928 (pS1928) after stimulation with EPI (1 μM) or DOB (1 μM) in the presence of MAOi. A.U., arbitrary unit.
E). HF AVMs were preloaded with Ca2+ indicator Fluo-4 (2 μM), paced at 1Hz, and pretreated with MAOi. Ca2+ transient (CaT) and sarcomere shortening (SS) were recorded in response to EPI, NE, or DOB. Dot-plots show maximal changes in SS, CaT amplitude (F/F0), and Ca2+ decay (Tau).
Dot-plots show mean ± SEM of the number of AVMs from mice (indicated). For Panel D, p values were obtained in paired comparisons only after a significance found in a non-parametric Kruskal-Wallis test. All other data passed Shapiro-Wilk normality test. p values were obtained after two-way ANOVA analysis followed by Tukey’s test (A, C, and E) or Student’s t-test (B).
We employed fluorescence resonance energy transfer (FRET)-based A kinase activity reporter 3 (AKAR3) 1 to assess the impacts of MAO-A on local PKA activity at the PM or SR (Figure 1C). In non-HF AVMs, epinephrine (EPI) induced robust increases in PKA activity at the PM and SR. MAO-A inhibitor clorgyline (MAOi) enhanced PKA activity only at the SR. In HF AVMs, EPI induced negligible PKA activation, and MAOi selectively enhanced PKA activation at the SR. These observations imply that the upregulated MAO-A in HF limits local β1AR-PKA activation. Inhibition of MAO-A rescues intracellular β1AR-PKA signaling at the SR.
Activation of SR-β1AR promotes PKA phosphorylation of PLB, a SERCA2a regulator, to enhance Ca2+ transients (CaTs). MAOi selectively enhanced PKA phosphorylation of PLB in non-HF AVMs after stimulation with EPI but not dobutamine (DOB), a non-substrate for MAO (Figure 1D). In HF AVMs, MAOi enhanced EPI-induced increases in PKA phosphorylation of PLB at the SR but not L-type Ca2+ channel at the PM (Figure 1D).
Norepinephrine (NE), EPI, and DOB promoted little contractile response in HF AVMs. MAOi significantly rescued NE and EPI but not DOB-induced E-C coupling (Figure 1E). Collectively, our results indicate inhibition of MAO-A restores SR-localized β1AR-PKA-PLB signaling and EC-coupling in HF.
The expression of MAO-A is increased by HF-associated pathological stresses including inflammation, aging, and reactive oxidative species. The expression of MAO-A is also regulated by hormones such as thyroid and estrogen, which affects SR calcium handling and cardiac contractility. 4 Our data indicate that MAO-A inhibitors may hold promise in rescuing SR-β1AR signaling and enhancing cardiac contractility while reducing oxidative stress in HF.2
Data Availability.
The methods, data, and materials are available upon request.
C57BL/6J male mice (2–4-month-old) were randomly assigned for intraperitoneal injection of saline or ISO (30 mg/kg/day, 14 days) and blinded for data analysis. Animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH) and the protocols approved by the University of California Davis IACUC.
Acknowledgments
SOURCES OF FUNDING
This work was supported by NIH-HL147263 and Veteran affair BX005100 (YKX) and AHA postdoctoral fellowships (YW, QS).
Footnotes
DISCLOSURE
None.
Publisher's Disclaimer: This article is published in its accepted form. It has not been copyedited and has not appeared in an issue of the journal. Preparation for inclusion in an issue of Circulation Research involves copyediting, typesetting, proofreading, and author review, which may lead to differences between this accepted version of the manuscript and the final, published version.
REFERENCES
- 1.Wang Y, Shi Q, Li M, Zhao M, Reddy Gopireddy R, Teoh J-P, Xu B, Zhu C, Ireton KE, et al. Intracellular β1-adrenergic receptors and organic cation transporter 3 mediate phospholamban phosphorylation to enhance cardiac contractility. Circ Res. 2021;128:246–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res. 2010. January 8;106(1):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kääb S, Kreuzer E, Steinbeck G, Mansmann U, et al. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J Am Coll Cardiol. 2006;48:1610–1617. [DOI] [PubMed] [Google Scholar]
- 4.Trivieri MG, Oudit GY, Rajan Sah R, Benoit-Gilles Kerfant B, Hui Sun H, Anthony O Gramolini AO, Yan Pan Y, Alan D Wickenden AD, Walburga Croteau W,et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc Natl Acad Sci U S A. 2006. April 11;103(15):6043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The methods, data, and materials are available upon request.
C57BL/6J male mice (2–4-month-old) were randomly assigned for intraperitoneal injection of saline or ISO (30 mg/kg/day, 14 days) and blinded for data analysis. Animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH) and the protocols approved by the University of California Davis IACUC.