Abstract
Drug delivery research pursues many types of carriers including proteins and other macromolecules, natural and synthetic polymeric structures, nanocarriers of diverse compositions and cells. In particular, liposomes and lipid nanoparticles represent arguably the most advanced and popular human-made nanocarriers, already in multiple clinical applications. On the other hand, red blood cells (RBCs) represent attractive natural carriers for the vascular route, featuring at least two distinct compartments for loading pharmacological cargoes, namely inner space enclosed by the plasma membrane and the outer surface of this membrane. Historically, studies of liposomal drug delivery systems (DDS) astronomically outnumbered and surpassed the RBC-based DDS. Nevertheless, these two types of carriers have different profile of advantages and disadvantages. Recent studies showed that RBC-based drug carriers indeed may feature unique pharmacokinetic and biodistribution characteristics favorably changing benefit/risk ratio of some cargo agents. Furthermore, RBC carriage cardinally alters behavior and effect of nanocarriers in the bloodstream, so called RBC hitchhiking (RBC-HH). This article represents an attempt for the comparative analysis of liposomal vs RBC drug delivery, culminating with design of hybrid DDSs enabling mutual collaborative advantages such as RBC-HH and camouflaging nanoparticles by RBC membrane. Finally, we discuss the key current challenges faced by these and other RBC-based DDSs including the issue of potential unintended and adverse effect and contingency measures to ameliorate this and other concerns.
Visual Abstarct
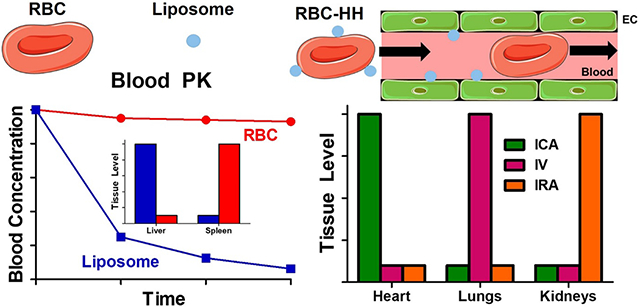
Section 1. Introduction. RBC and liposomes: a historical overview.
Mephistopheles, seeking Dr. Faust’s signature on the devil’s contract, coined an aphorism “Blood is a juice of a very special kind,” which could characterize today’s field of hematology. Indeed, the mysterious ever-changing liquid is enormously important and complex. Take, for example, the ubiquitous corpuscles that paint blood red. Their narrative speaks of a dualism of many sorts including changing their very color from crimson in arteries to purple in veins.
Two individuals discovered erythrocytes independently. In 1658, Jan Swammerdam, a Dutch biologist, described miniature corpuscles in frog blood. In 1674, erythrocytes were redescribed by his famous compatriot, inventor of advanced microscopes and amateur-scientist Antonie van Leeuwenhoek. Royalties paid visits to him in the city of Delft to honor his discoveries. During a European tour in 1698, Peter the Great met Leeuwenhoek, who presented to the young Russian Emperor an optical device to observe blood in the capillaries.
Both the Latin and English terms, erythrocytes and red blood cells (RBCs) are misnomers, as RBCs are not complete cells. Cells, especially blood cells – leukocytes, lymphocytes, monocytes and even cell remnants platelets, are more complex structurally and functionally, diverse and heterogenous, delicate and capricious, reactive and dangerous than RBCs. Remnants of reticulocytes, RBCs lack nuclei and organelles, and are in essence membrane vesicles filled with hemoglobin. Yet, of course these refined biconcave discs are not that simple.
Indeed, in the three centuries that ensued since their discovery, RBCs have become the subject of many revelations. Accolades for RBC studies include Nobel Prizes to Ronald Ross for studies of the pathogenesis of malaria (1902), Jules Bordet for the discovery of the complement system (1919), Karl Landsteiner for the discovery of blood groups that enabled blood transfusion (1930), George H. Whipple for studies of anemia (1934), Max Perutz for studies of hemoglobin (1959), Peter Agre and Roderick McKinnon for discovery of aquaporins in RBC membranes (2003), Tu Youyou for new therapy against malaria (2015), and William Kaelin, Peter Ratcliffe, and Gregg Semenza for studies of biomedical features of oxygen and erythropoietin (2019).
RBCs are abundant, docile, and incredibly stable. As a blood transporting agent, these features lend themselves to carry drugs. Indeed, ideas to encapsulate pharmacological agents into isolated RBC to prolong circulation evolved nearly half a century ago [1, 2]. Alas, soon after these promising initial studies, the pandemics of HIV, hepatitis, and other infections transmitted with blood products all but decimated RBC-based drug delivery research for several decades.
Liposomes, artificial multimolecular assemblies made of phospholipids, cholesterol and other components, which form membranous structures with sizes ranging from <100 nm to ~500 nm, were discovered, or rather, invented in the early 1960s in Cambridge by Alec D. Bangham [3–5]. Curiously, he was a hematologist studying blood coagulation. Liposomes initially attracted the attention of basic researchers as models of biological membranes. This aspect remains an important area of liposome research. However, within a decade of their discovery, liposomes became a cornerstone in drug delivery research, giving rise to multiple formulations of synthetic nanocarriers. These advanced means for delivery of drugs in the body have yet to be the focus of a coveted Nobel Prize (which actually might happen in a few months this year, taking into account the global impact of BioNTech/Pfizer and Moderna COVID19 vaccine designed by the team led by Drew Weissman and Kati Kariko, based on modified mRNA packed into lipid nanoparticles).
Today, the internet search terms “erythrocyte” and “RBC” yield several orders of magnitude more hits than “liposome”, reflecting the relative biomedical significance of these subjects studied for three centuries vs. half of a century, respectively (Table 1). However, a similar search on drug delivery, carriers, and targeting yields more hits for liposomes than erythrocytes and RBC combined. This is especially obvious in scientific databases such as PubMed: a search for liposome in the context of drug delivery and targeting yields about an order of magnitude more hits than a search for erythrocyte and two orders of magnitude more hits than a search for RBC (Figure 1). Furthermore, the liposome search hits deal with drug delivery, whereas most of the RBC and erythrocyte hits are irrelevant.
Table 1.
Liposomes and RBC/erythrocytes: history and citations.
| Category | Parameters | Liposomes | RBC |
|---|---|---|---|
| General search | Internet (Google) | 18 million | Erythrocytes: ~18 million |
| Red blood cells: ~435 million | |||
| PubMed | 70,000 | Erythrocytes: ~250,000 | |
| RBC: ~250,000 | |||
| Drug delivery | Internet (Google) | 3.8 million | Erythrocytes: 1.5 million |
| RBC: 2.7 million | |||
| PubMed | 18,300 | Erythrocytes: 2,500 | |
| RBC: 520 | |||
| Drug carriers | Internet (Google) | 7.6 million | Erythrocytes: 1.5 million |
| RBC: 5.4 million | |||
| PubMed | 47,800 | Erythrocytes: 2,800 | |
| RBC: 360 | |||
| Drug targeting | Internet (Google) | 14.9 million | Erythrocytes: 3.5 million |
| RBC: 5.4 million | |||
| PubMed | 15,700 | Erythrocytes: 2,600 | |
| RBC: 390 |
Figure 1.
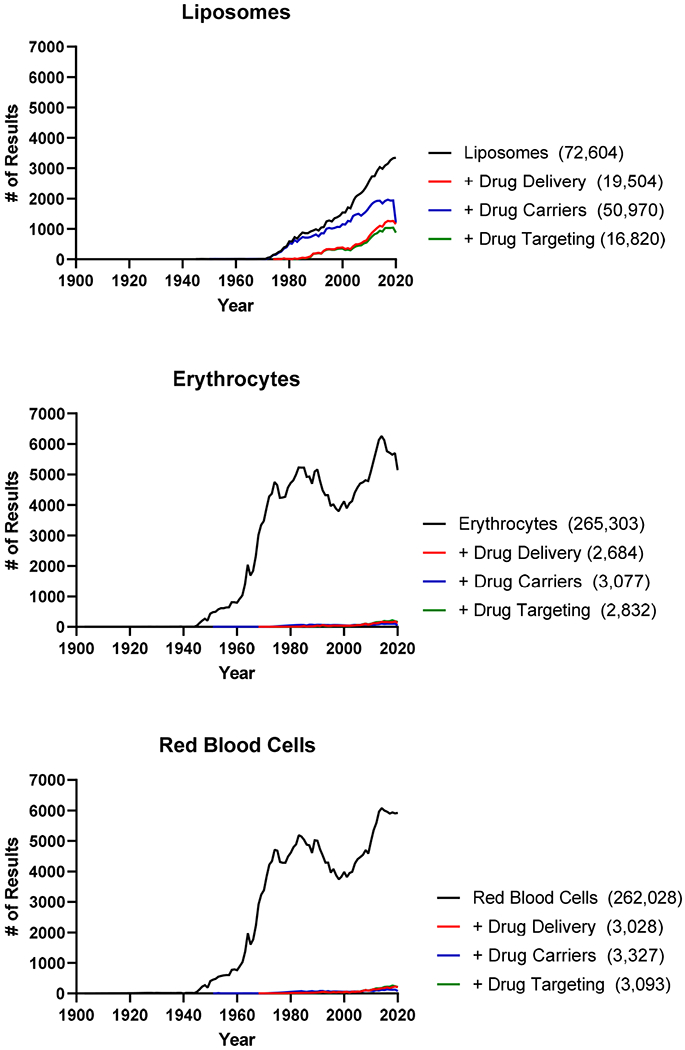
Number of publications returned annually from 1903-2020 via a PubMed search for liposomes, erythrocytes, and red blood cells.
Section 2. Translational aspects of artificial and natural carriers.
Since the mid-20th century, scores of researchers have worked on liposomes and other artificial carriers for drug delivery and targeting. These synthetic drug delivery systems (DDS) are amenable to controlled synthesis, purification, and characterization at the molecular and atomic levels, allowing rational and modular design, theoretical analysis and modeling, reiterative re-engineering, and modifications yielding structural and functional diversity of versatile pharmaceutical formulations. Artificial DDSs seem to have features that help meet challenges of industrial translation (stability, scaling up), commercialization and medical use (storage, distribution, dosing, timing, regulatory affairs).
Infusion of natural carriers, such as RBCs, has been used in clinical practice for centuries, dating back to the first xenotransfusion of sheep blood into humans by Richard Lower and Edmund King in 1667. The success of blood transfusions was dramatically increased by Landsteiner’s discovery of the main blood groups in the early 20th century. It is, in part, the presence of blood groups and unwanted immune responses against incompatible blood types, that limits ‘off-the-shelf’ applications of cell-based DDSs in clinical medicine. As a result, the use of RBCs as a DDS is currently limited to individualized therapy, wherein autologous, ex vivo-modified RBCs are reinfused into patients. This has limited the development of natural carriers for drug delivery applications and has made the ability to directly load circulating RBCs in vivo a critical direction of research for the field.
The roster of artificial carriers for drug delivery encompasses a multitude of classes and types of compounds including linear and branched polymers, polymersomes, and diverse micelles formed by amphiphilic di-block co-polymers, nanogels, lipid nanoparticles (LNP), dendrimers, protein conjugates, fusions, and multimolecular assemblies, etc. It seems safe to proclaim; however, that liposomes represent arguably one of the most studied and clinically relevant DDS. In many cases, comparison of drug delivery by RBC vs. liposomes would be similar to other nanocarriers; hence this paper focuses on liposomes, quite appropriately for celebration of the career and legacy of Frank Szoka [6–15].
Section 3. Overview of RBC and liposomes as drug delivery systems.
Section 3.1. RBC and liposomes: general, membrane and biomechanical features.
Scores of different garden varieties of liposomes have been described since Bangham. These multitudes feature distinct sizes, charges, and structure of the membranes (Figure 2). For example, large multilamellar vesicles (MLV) have found use for dermal drug delivery and cosmetics. In contrast, small unilamellar vesicles (SUV) represent arguably the most useful formulation for drug delivery via the bloodstream.
Figure 2:
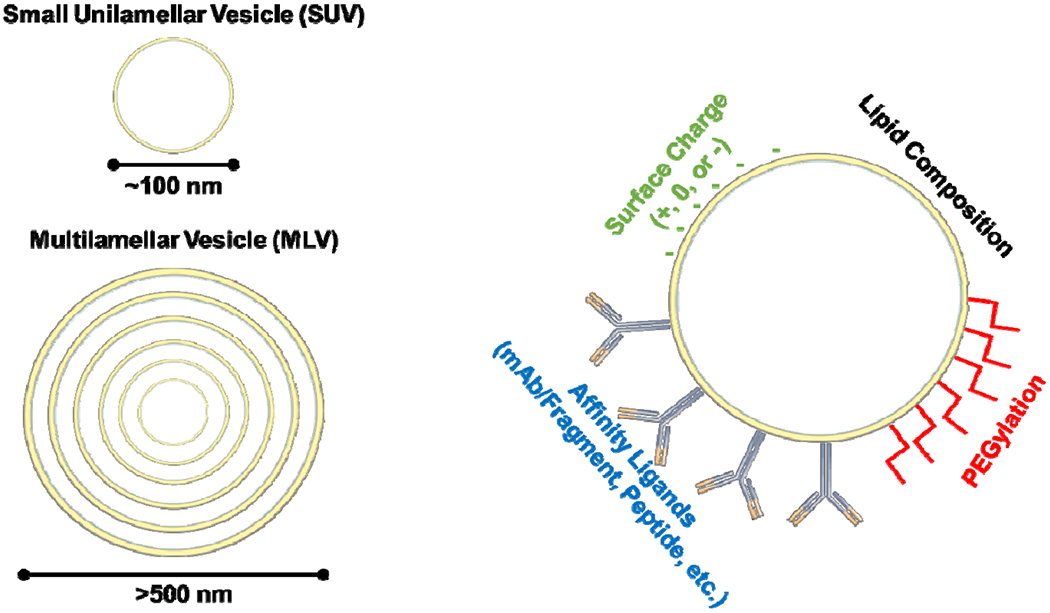
Liposome engineering strategies. Liposomes can be produced with different numbers of lipid bilayers (lamellarity) and the lipid composition and surface coating can be engineered based on intended use.
Liposomes and RBC are very different from almost every aspect except that both represent in essence vesicles formed by a membrane bilayer composed of phospholipids, cholesterol and other components. RBC membranes contain scores of glycoproteins, which either span the membrane or are anchored in one of its leaflets. The inner surface of RBC membrane is reinforced by the cytoskeleton containing tens of proteins including spectrin forming the lattice underlying the membrane and connected to its integral and associated glycoproteins (Table 3).
Table 3.
Structural and biomechanical features of liposomes vs RBCs. Calculations of the liposome surface area and volume assumes 5 nm thick bilayer and range of particle diameters from 50-250 nm [18].
| Features | Parameters | Liposomes | RBC |
|---|---|---|---|
| Particle | Character and source | Artificial, synthesis | Natural, blood |
| General structure | Vesicle | Vesicle | |
| Size | 50-250 nm | 5,000 nm | |
| Shape | Spheres and elongated | Bi-concave disks | |
| Surface charge | Variable | Negative | |
| Homogeneity | Variable | Exceptionally high | |
| Inner volume | 3.3x10−5-7.2x10−3 μm3 | 94 μm3 [16] | |
| Surface area | 7.85x10−3- 0.20 μm2 | 136 μm2 | |
| Membrane | General structure | Phospholipid bilayer | Similar to liposomes |
| Leaflet asymmetry | Can be engineered | Yes (PS inside) | |
| Common lipid additions | Cholesterol | Diverse [17] | |
| Exotic lipid additions | Sphingomyelin, steroids | Diverse | |
| Main non-lipid components | None | Glycoproteins | |
| Outer surface additions | PEG, targeting moieties | Glycocalyx, sialic acids | |
| Inner surface additions | May contain some above | Membrane cytoskeleton | |
| General features | Relatively simple | Complex | |
| Biomechanics | Deformability | Variable | High |
| Mechanical sturdiness | Limited | Exceptionally high | |
| Role in the longevity | Unknown | Very important | |
| Distribution in flow | Marginal zone | Main stream | |
| “Collective behavior” | Not known | Form stacks | |
Abbreviations: PS - phosphatidylserine
These structural elements provide RBCs with exceptional biomechanical features, including their ability to change shape, permitting squeezing through capillaries smaller than the RBC and maintain their structural integrity under such harsh hydrodynamic conditions as the heart chambers and the aorta. This is one of the reasons why liposomes and RBC have dramatically different behaviors in the body.
Section 3.2. RBCs and liposomes in circulation.
From the first studies of liposome properties in vivo, it was apparent that, without additional engineering, liposomes injected directly into the bloodstream are cleared within minutes [19, 20]. These early studies not only revealed the poor circulation time of liposomes, but also their dose-dependence in pharmacokinetics (PK), primary clearance organ (liver), and main cells responsible for elimination (Kupffer cells and hepatocytes) [20]. Reasons for this rapid elimination from the circulation include: A) formation of a protein corona that includes molecules promoting recognition by phagocytes (e.g., complement and immunoglobulins) [21–24]; B) subsequent uptake by macrophages of the reticuloendothelial system (e.g., hepatic Kupffer cells); and C) extravasation into tissues with sufficiently ‘leaky’ vasculature (e.g., liver, spleen, tumor, sites of inflammation).
The poor circulation time of unmodified liposomes is a substantial barrier to liposome delivery to the desired organs, particularly if extravasation is required to reach the intended site, as in solid tumors. For the past 40 years, there has been significant investment in approaches to prolong the circulation time of liposomes, providing them with sufficient time to capitalize on enhanced vascular permeability in pathologically-altered tissues such as tumors and sites of inflammation. Early studies focused on introducing lipids into the bilayer that would either alter physicochemical properties, such as charge, thereby reducing interactions with endothelial cells and opsonins [25–28]. The most well-established technique used to prolong the circulation of liposomes is to coat the particle surface with polyethyleneglycol (PEG), which was first reported in the early 1990s to improve the circulatory half-life of liposomes from <30 minutes to 5 hours [29, 30]. PEGylated liposomes, also referred to as stealth liposomes, are widely used by groups pursuing liposomal drug delivery. Mechanistically, the flexible, hydrophilic PEG chains promote retention of liposomes in the bloodstream by repelling opsonins [31], inhibiting cellular interactions [32], and improving particle stability [33].
A more elaborate strategy that has been utilized to avoid clearance by the immune system is to mimic natural pathways that are employed by host cells and infectious agents (e.g., bacteria and viruses) to evade the host defense system. For example, when compared to spherical particles, elongated, flexible nanoparticles (filomicelles) that are able to align with blood flow were shown to have prolonged PK and improved targeting due to evasion of immune clearance [34–36].
In contrast to rapidly cleared liposomes, the typical lifespan of RBCs in circulation is on the order of 4 months, which is controlled by the interplay of several key factors. In circulation, the RBC population is, in essence, a distribution of cells ranging from freshly matured RBCs to aged, rigid RBCs about to be cleared by the spleen. First, RBCs are highly deformable, due to their lack of a nucleus. It has been appreciated for over 50 years that deformability of RBCs is critical to their in vivo lifespan [37, 38]. In fact, the spleen has evolved to sequester RBCs that have become more rigid as part of its natural function in clearing senescent and damaged RBCs [39, 40]. Second, many membrane components of the RBC protect them from opsonization and lysis by complement, including decay accelerating factor (DAF, CD55) [41–43], CD59 [44, 45], and Complement Receptor 1 (CR1, CD35) [46], blockade from recognition by host defense via terminal sialic acids of the glycocalyx [47–49], and specific ligands inhibiting uptake by phagocytes, such as CD47 [50–52]. Loss of any or all of these factors protect RBCs as a result of pathologies or normal cellular aging/senescence will accelerate RBC clearance, largely by red pulp macrophages in the spleen.
In recent years, there has been interest in harnessing the molecular mechanisms utilized by RBCs to protect themselves from elimination as tools to improve the circulation time of artificial nanoparticles. These approaches include: A) engineering biomechanical properties of nanoparticles to mimic those of RBCs [53, 54], B) conjugation of peptides derived from the “don’t eat me” marker, CD47, to the surface of nanoparticles in order to inhibit phagocytosis [55–58], C) inhibition of complement binding to nanoparticles by co-administering peptides derived from DAF [59], and D) camouflaging nanoparticles by wrapping them with fragments of the RBC membrane [60–63].
Of these approaches, there has been perhaps the most significant investment into coating nanoparticles with fragments of the RBC membrane. While this strategy does lead to substantial improvements in nanoparticle PK [62, 63], it does not lead to circulation times even remotely approaching that of pristine RBCs. This is likely due to factors including: A) damage to the RBC membrane during processing likely leads to loss of key structural features, such as shielding of phosphatidylserine on the inner leaflet of the membrane bilayer, and potential membrane inversion [64], and B) the biomechanical features of RBC-camouflaged nanoparticles do not match those of the natural RBC.
Section 4: Liposomal drug delivery: a bird’s eye view glancing over the huge continent.
The literature on liposomal drug delivery is immense (Figure 1). It encompasses tens of thousands of publications presenting hundreds of formulations of liposomes and other lipid-based DDS formulations including lipid nanoparticles (LNPs) that recently advanced to the use in patients for delivery of siRNA and mRNA-vaccines.
Any attempt to overview this field in the space of this paper would be overtly superficial and fragmentary. Here we just breeze over this field touching upon a few aspects most relevant in the context of comparison of liposomes and RBCs, while addressing interested readers to landmark reviews on liposome drug delivery, including the contributions of the esteemed addressee of this dedicated volume, one of the leading liposome pioneers and experts, Frank Szoka. Dr. Szoka’s work spanned over 40 years including the seminal PNAS manuscript in 1987 on reverse phase evaporation formulation of liposomes cited over 2300 times [65], among other foundational works [66–68], to the significant contributions in gene delivery [69–72], liposome formulation [73–75], targeting anti-microbial delivery [76], and cancer [66, 68–90].
Section 4.1. Loading liposomes: inner, surface and membrane.
In five decades of exploration of liposomal DDSs, these nanocarriers have been loaded with a wide variety of pharmacological agents. Insofar, the majority of agents have been encapsulated into the inner volume encircled by the liposome bilayer and several formulations employ intercalation of hydrophobic agents into the membrane proper, whereas coupling cargoes onto liposome surfaces represents a more exotic loading approach.
A diverse array of cargo drugs have been loaded into liposomes, ranging from small molecules to proteins and nucleic acids. A classic example of a small molecule drug that is efficiently loaded into liposomes is doxorubicin, which is able to achieve such high concentrations inside the particle that it forms crystals that are enveloped by the membrane. Loading of biotherapeutics into the aqueous core of liposomes provides protection from extracellular proteases/nucleases and permits intracellular delivery of the encapsulated therapeutic. Successful loading and delivery of any therapeutic using liposomes requires careful particle formulation.
Loading of small molecule drugs, for which there is the most experience in development of liposomal formulations, is dependent on the physicochemical properties of the drug itself, namely hydrophilicity/lipophilicity (Log(P)), solubility, pKa, etc. Considering one of these properties – hydrophilicity – as an example, a hydrophilic drug is defined as highly soluble (>1000 mg/ml), soluble (33-100 mg/ml) and sparingly soluble (10-33 mg/ml) [91]. Notable among the latter category is doxorubicin, which was the first FDA-approved liposomal formulation, Doxil [92, 93]. Essential considerations for the design of liposomes containing small molecule hydrophilic drug include composition of the lipid membrane and its permeability, driven in part by phospholipid tail length and charge [91], the size of the vesicles, and the approach to drug loading (see Table 5). As lipophilicity of drugs increases, the localization of drugs within the liposome shifts from the aqueous core (hydrophilic) to the lipid bilayer (hydrophobic), with amphililic molecules either associating with the membrane/core interface or forming micelles within the aqueous core.
Table 5.
Liposomal drug loading.
| Loading Method | Localization | Drug Properties | %EE | Pros | Cons | References [91, 97–104] | |
|---|---|---|---|---|---|---|---|
| Passive | Thin film hydration | Aqueous core | Hydrophilic small molecules Proteins |
<5% | Small batch Good for lab scale |
Low encapsulation Small internal volume Not scalable High drug leak |
[67, 105] |
| Lipid bilayer | Lipophilic small molecules | <5% | |||||
| Freeze/thaw-extension of TFH | As above | As above | 5-20% | Increased internal volume Improved PDI |
Not scalable High drug leak |
[91, 106–108] | |
| Reverse phase evaporation | Aqueous core | Hydrophilic drugs | ≤50% | Increased loading Useful for protein drugs |
Solvent contamination | [65, 91, 107] [109] | |
| Active | pH/salt gradient | Aqueous core | Weak base small molecules | ≤100% | Increased loading | Limited to weak bases Potential resistance to drug release at target site |
[91, 110, 111] |
| Ionic gradient | Aqueous core | Weak acid small molecules | ≤100% | Increased loading and retention | Limited to weak acids | [100, 112, 113] | |
| Cyclodextrin pre-encapsulation | Aqueous core | Hydrophobic or poorly soluble/non-ionizable drugs | ≤95% | Enables active loading of non-ionizable drugs | Limited utility | [114–116] | |
| Dehydration-rehydration | Aqueous core | Proteins, other large molecules | 20-52% | Increased loading of large molecules | Potential protein denaturation | [91, 108, 117, 118] | |
Abbreviations: EE-encapsulation efficiency; TFH- thin film hydration, PDI-polydispersity index
Computational modeling of drug properties has been developed enabling the identification of viable drug candidates for remote loading into liposomes describing advantageous loading and leak parameters [94–96]. Good candidate drugs, or “active pharmaceutical ingredients” (API) were defined as loading at a drug to lipid mole ratio of >0.2-0.3 and load with 70-90% efficiency [96].
Section 4.2. Modification of liposomes by PEG, ligands, membrane permeating and responsive elements.
In all cases, the surface features of liposomes mediate their interactions with desirable targets and undesirable off-target destinations, which can be modulated by, among other methods, conjugation of affinity ligands or PEG (5-10 mol%) to the surface, respectively. By attaching affinity ligands (e.g. antibodies and fragments [Fab, scFv]) specific for target determinants to the surface of liposomes, both tissue and cellular selectivity of delivery can be enhanced. Beyond antibody-derived molecules, other affinity ligands that have been used include, carbohydrates, peptides, endogenous molecules (e.g. transferrin, growth factors, etc.), and aptamers.
In the early 1990s, it was described that by adding PEG to the surface of a liposome, the blood half-life could be improved from minutes to hours, likely by reducing opsonization and RES clearance [29, 30]. This engineering strategy was critical in development of liposomal doxorubicin (Doxil) and other FDA-approved formulations [93, 119]. The molecular features of PEG (e.g. hydrophilicity, hydration capacity, etc.) sterically impedes opsonization of liposomes [120]. Kristensen et al demonstrated that when stealth and antibody-stealth liposomes were probed in vivo, surface bound protein was found in greater amounts when compared to in vitro incubation with fetal bovine albumin serum. However, the protein coating was described as sparse, and in both cases, the liposome’s size and targeting properties were retained [121]. PEGylated lipids were originally thought to be immunologically inert, but immune responses to PEG-conjugated nanocarriers have been revealed to result in rapid clearance upon repeated administration, the production of antibodies against carrier components, and infusion reactions such as complement (C) activation-related pseudoallergy (CARPA) [122]. Correspondingly, PEG liposomes have resulted in cases of allergenic responses even in the treatment of naive patients [123].
Active, ligand-mediated liposome targeting was developed to provide specificity and focus to liposomal drug delivery. Early studies of monoclonal antibodies and scFv attached to stealth liposomes showed enhanced targeting including significant tumor accumulation with as few as 10s of ligands per particle attached to a PEGylated liposome [124, 125]. Expansive research has emerged since then into active targeting of liposomes using a variety of ligand types [103, 104, 126–129], and for myriad applications and therapeutic focus [97, 103, 129–131]. Ligand conjugation to formed liposomes is commonly facilitated by binding the targeting moiety to a functionalized phospholipid headgroup anchor exposed and somewhat distal to the vesicle surface [74]. Derivatization of lipids for this purpose is a wide field [132, 133] and the chemistries vary [102, 126, 134–141].
Coupling affinity ligands and other functional groups to the liposome surface provides benefits by attaching to the end groups of flexible spacers (e.g. PEG). However, this revealed a mechanistic conundrum. By ‘fouling’ PEG molecules with affinity ligands, the stealthiness (long circulation time, evasion of phagocytic clearance) provided by PEG was abrogated, resulting in more rapid clearance from the circulation. On the other hand, attaching ligands directly to the surface of the liposome (and between PEG molecules) impaired affinity-based interactions with the target, while maintaining stealthiness. Engineering of PEG (and other spacers) can help restore the balance between stealthiness and targeting. For example, by coupling PEG to the liposome surface with labile linkers sensitive to pathological changes in pH, temperature, enzymatic activity (e.g. thrombin, matrix metalloproteinases), etc., particles will remain stealthy (and thereby avoid clearance) until they reach the desired site. Upon exposure to pathophysiological cues, the PEG coating will be shed, exposing affinity ligands that can be directly coupled to the surface of the liposome, permitting specific recognition of target cells.
Once the target cell is engaged, internalization becomes the priority. Two major approaches to use for targeting are cellular determinants involved in vesicular uptake (e.g., endocytosis, phagocytosis or pinocytosis) and using membrane-permeating peptides inserting amphiphilic sequences into the plasmalemma and forming transmembrane channels. The specificity and safety of membrane permeabilizing proteins (MPPs) is a somewhat contentious issue. Indeed, making holes in the plasmalemma may be dangerous for the cell; after all, hemolysis caused by complement poking the RBC membrane is the natural way of interaction of MPP with cellular membranes.
Section 4.3. Clinically used formulations.
Today, liposomes are widely used clinically for a variety of indications by multiple routes of delivery. The first liposomal nanomedicine to be FDA approved was Doxil (doxorubicin) in 1995 for the treatment of Kaposi’s sarcoma. Doxil was soon followed by approval of two liposomal formulations of amphotericin B, a potent but toxic anti-fungal used to treat invasive fungal infections. It is telling that the first approved medications were against cancer and infection, respectively, as the bulk of approved liposomes have remained in these two therapeutic categories (Figure 3). Most striking is the use of liposomal chemotherapies to treat cancer, whereby the liposome allows both for protection of the drug from host defenses and protection of off-target host cells from drug toxicities. Currently approved liposomal nanomedicines are used to treat solid tumors such as breast, ovarian, and pancreatic cancer as well as liquid tumors such as leukemia and lymphoma. While most liposomal formulations have been designed to be intravenously injected, there are several notable exceptions. Curosurf (poractant alpha) is delivered directly to the trachea where its site of action is in the distal lung alveoli. Visudyne (verteporfin) is delivered ocularly. Newest to the market, Arikayce (amikacin), is delivered via inhalation to treat nontuberculous mycobacterial pulmonary infection. Finally, two mRNA Sars-CoV-2 vaccines were granted emergency use authorization by the FDA in 2020. While these formulations are lipid nanoparticles not liposomes, their approval and use are noteworthy due to widespread use and acceptance worldwide [149–154].
Figure 3.
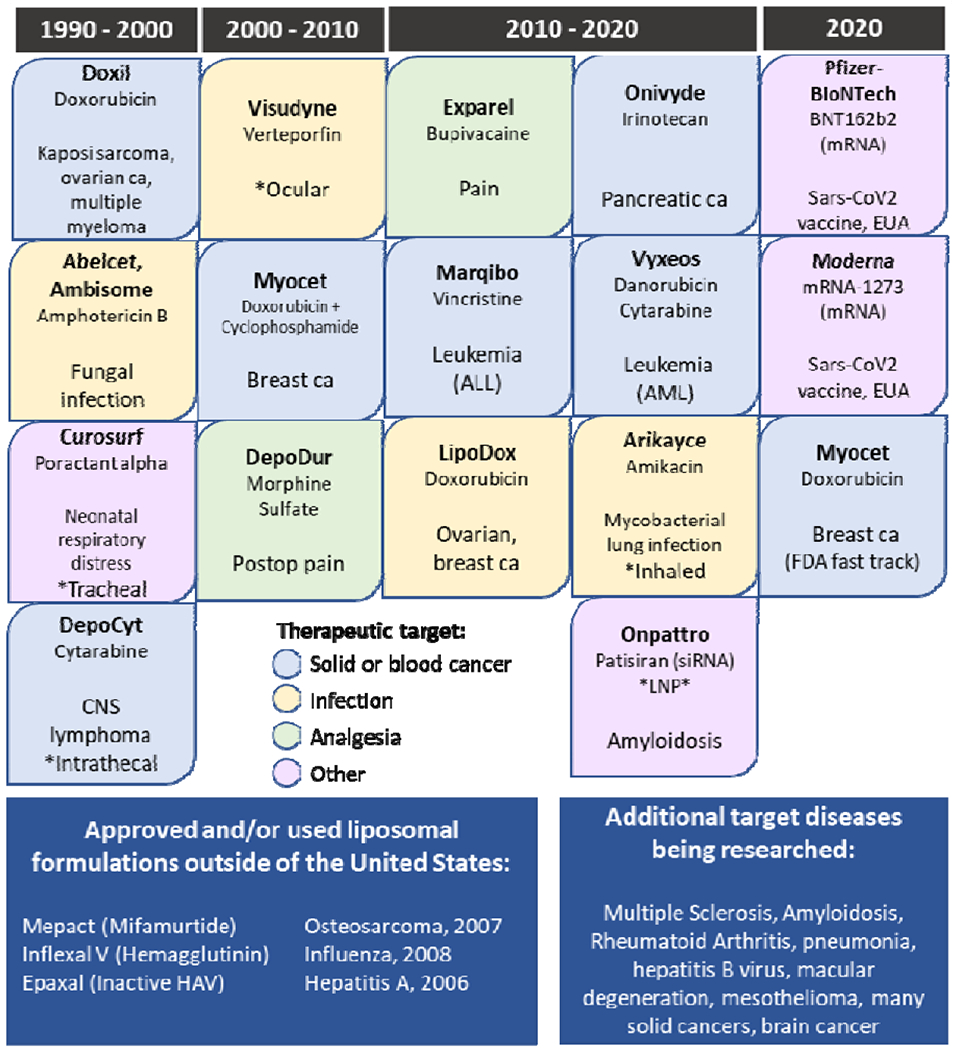
FDA-approved liposomal nanomedicines, including select lipid-based, non-liposome formulations
Section 4.4. Persisting challenges of liposomal DDS: limited bioavailability and efficacy of drug delivery.
Liposomes, LNPs and other lipid-based DDS provide remarkable improvements of the PK/BD, targeting and intracellular delivery of pharmacological agents. Indeed, the rapidly growing roster of these formulations approved for clinical use and in the human studies serves as convincing testimony of the general success of this DDS platform. However, persisting challenges remain, especially when we assess DDS performance in absolute rather than relative units. Analysis of two delivery parameters, namely blood level and uptake by the target, illustrates the point.
First, blood PK remains limited even for the most long-circulating formulations of PEG-stealth and camouflaged nanoparticles. It is true that they may afford orders of magnitude prolongation of the blood PK. However, their comparison with PK of basal formulations, featuring dismal t1/2 as short as few minutes, makes an impressive relative but not so impressive absolute gains. In general, blood PK has a lot of room for improvement.
Second, accumulation in the target tissues in most cases remains at levels of a percent of injected dose (%ID), even for DDS targeted by affinity ligands or equipped by sensors of the local microenvironment. The lion’s share of injected DDS goes elsewhere and misses the target.
New approaches are needed to resolve these formidable challenges. It appears that one such approach is to converge advantages of liposomes and other nanocarriers with advantages offered by the natural blood carriers, red blood cells.
Section 5: RBC drug delivery.
The general notion of using RBC as a carrier for drug delivery has been conceptualized and preliminary characterized in experimental model systems in the 1970s [2]. Initial animal studies provided rather mixed results, likely due to loss of RBC biocompatibility [155, 156]. In the 1980s, RBC drug delivery had been surpassed by liposomes and other synthetic carriers. Nevertheless, several labs persisted and devised biomedically useful approaches for RBC loading by drugs progressing from in vitro proof of principle [1, 157–159] and studies in animal models [160, 161], to clinical trials in human patients [162–165]. At the present time, there are two modes of drug loading to RBC: encapsulation into the inner volume vs. surface loading.
Section 5.1. Loading cargoes to carrier RBC.
From the somewhat oversimplified drug delivery standpoint, an RBC, like a liposome, has just the same three compartments that can be used for loading of pharmacological cargoes: A) aqueous inner volume, B) plasma membrane surface; and, C) membrane bilayer proper (Table 7). The loading into the RBC membrane itself is yet to be to explored. This proposition might seem strange on the first glance, but one must remember that a significant (in some cases major) fraction of some drugs injected in the bloodstream seems to naturally partition rapidly exactly into this underused compartment [166].
Table 7.
Methods of RBC drug loading.
| Drug loading | Approaches | |
|---|---|---|
| Inner | Via pores induced by hypotonic swelling | Established |
| Using MPP | Exploratory | |
| Genetic modification of reticulocytes | In progress | |
| Surface | Chemical conjugation to surface proteins | Exploratory |
| Insertion into membrane phospholipids | As above | |
| Physical absorption | As above | |
| Binding mediated by affinity ligand | As above | |
| Membrane | ||
Abbreviations: MPP – membrane permeabilizing protein
To our knowledge, most drug delivery applications of RBCs that have reached the clinical phase of development employ the first compartment, i.e., encapsulation of cargoes into RBC. Surface loading approaches, which in theory are arguably more diversified in terms of the loading mechanisms and features of amenable cargoes, are rapidly picking up the developmental race. Below we briefly outline inner and surface loading.
Section 5.1.2. Encapsulation of cargoes into RBC.
RBC represent, in essence, membrane vesicles filled with hemoglobin that renders this corpuscle with an ability to bind, transport, and release oxygen - arguably, the most important natural cargo of RBC. From the initial experimental attempts to use RBC for drug delivery, most researchers loaded pharmacological cargoes - small drugs and biological macromolecules alike – into the inner space of RBC [2]. Such loading usually occurs at the expense of loss of at least fraction of hemoglobin (and many other RBC proteins, which normally reside within the aqueous inner volume). Some drugs and radioisotopes (e.g., 51Cr) may have affinity for hemoglobin that can be harnessed to achieve high internal cargo loading.
In a way, placement of the cargo inside RBC is similar to encapsulation into liposome. However, in contrast with liposomes, which can be loaded during their formation with minimal interference in the integrity and features of the membrane, RBC is already pre-formed by Mother Nature and, therefore, cargoes must be smuggled across the RBC membrane. This is not a trivial task taking into consideration the key role of this unique structure in RBC biocompatibility and longevity.
There are several approaches to achieve this goal including use of membrane-permeating peptides (MPP) and amphiphilic agents, such as TAT-peptide from HIV, which has been employed for delivery of a plethora of agents including diverse nanocarriers into the cytosol of variety of cells. TAT is positively charged and likely its cationic nature plays a role in universality of its binding to the plasmalemma of diverse cell types, usually negatively charged. The mechanism, specificity, safety and limitations of TAT-mediated delivery into cells apparently involves macropinocytosis and perhaps alternative mechanisms of active vesicular transport, which do not exist in RBC. Most likely, TAT and other MPPs form mono- and multimolecular insertions into RBC membrane bilayer, just as melittin, the hemolytic agent from bee venom. The downsides of MPP-mediated drug loading into RBC include obvious risk of hemolysis, limited dosing, technical difficulties, lack of specificity, and translational issues, e.g., QC, scale up, cost.
Alternative approaches are based on formation of transient openings in the RBC membrane. Some relatively exotic methods use physical force such as ultrasound, but so far, the most popular and clinically advanced method is based on formation of transient openings in the RBC membrane via osmotic swelling in hypotonic solution. Cargo enters the RBC in exchange for hemoglobin leaving the cell, both driven by a concentration gradient, and post-loading the RBC (or, more precisely, its ghost) gets resealed by return to isotonic buffer. There are several European models of semi-automatic machines designed for this procedure, which takes about half an hour and can be applied to either autologous blood taken from the patient before a procedure or allogeneic donor blood.
RBC-encapsulated pharmacological agents including drugs, nanocarriers, biotherapeutics, probes and magnetic compounds have been devised and studied in vitro, and, to a lesser extent, in vivo – both in laboratory animals and in patients. The advantages of having drugs inside RBC, just like in liposomes, include reduced unintended interactions of the cargo with the body and, in many cases, deceleration of clearance from blood of agents that have short life span in circulation. Thus, enhancement of bioavailability of a circulating pool of RBC-encapsulated drugs can potentiate and prolong effects of drugs that act upon target molecules diffusing via the RBC membrane.
Alternatively, “silent” RBC-encapsulated agents may be viewed as pro-drugs, which act on their therapeutic targets upon release from the RBC carrier. However, the controlled release of the cargoes from RBC carriers is a fairly challenging and as of today not achieved goal. Prototype studies imply that natural mechanisms of RBC lysis by complement might be co-opted for this purpose, but translation of this idea to the practical use has yet to be achieved [167].
Finally, RBC-encapsulated agents may exert their effects upon uptake by phagocytic cells. In this context, the more damaged the carrier RBC is due to loading, the more effective is the “passive targeting” to host defense cells. This paradigm offers an advantage of co-opting natural biological mechanisms for delivery agents modulating host defense in many possible ways. First, RBC-loaded with anti-inflammatory drugs such as dexamethasone or other steroids or NSAIDs can help to minimize systemic adverse effects of these drugs. On the contrary, delivery of agents inducing activity or transformation of macrophages from M1 to M2 phenotypes and vice versa may help modulate these components of anti-tumor defense. Further, delivery of antigens loaded into RBC to the macrophages including dendritic cells may help modulate presentation to lymphocytes leading to cellular and humoral immunity or/and immunological memory.
Section 5.1.3. Coupling therapeutics to the RBC surface.
Surface RBC loading has also been explored for decades using diverse methods and cargoes. The most advanced and widely explored approaches include non-specific adsorption, insertion into phospholipid bilayer, chemical conjugation to RBC reactive groups (e.g., on membrane glycoproteins), genetic modification of RBC precursor cells, and affinity targeting to RBC surface determinants. These approaches include non-covalent attachment of agents via streptavidin to RBC covalently conjugated with biotin derivatives. Of note, such biotinylated RBCs are widely employed in clinical investigations to trace circulation of RBC in the bloodstream after injection in human subjects. Detection of biotinylated erythrocytes provides more accurate measurements than can be afforded by optical tracings, while avoiding use of radioactive isotopes undesirable for studies in humans.
Coupling drugs to the RBC surface avoids membrane damage inflicted by creation of pores or transmembrane permeation needed for encapsulation in the inner space. It also bypasses the need for release of drugs encapsulated in RBCs which is yet to be developed into a practically applicable approach [168]. Further, surface loading can be performed in one step, either in vitro or in vivo.
Section 5.1.4. Chemical conjugation of drugs to RBC.
Chemical conjugation of antigens and immunoglobulins to RBCs (devised a century ago for agglutination-based immunological tests) has been adopted to conjugate cargoes to carrier RBCs [169–171]. However, non-specific cross-linking agents such as glutaraldehyde and tannic acid provided no control over the attachment site and profoundly altered RBC membranes.
These methods were surpassed by conjugation to RBC amino acids [172–174], sulfhydryl groups [175], sugars [176] and lipids [177, 178]. However, biocompatibility of modified RBCs remained an issue [179–183]. Early RBC carriers activated complement [177, 184], leading to hemolysis [185] and rapid elimination of RBCs [184, 186–189].
Development of more precisely controlled conjugation methods [171, 177, 190–192], preserving endogenous inhibitors of complement (DAF and CD59) in the RBC membrane [192–194] permitted coupling of drugs to RBCs with less damage. The circulation of RBC/drug complexes approximated those of control RBCs for at least several days after injection in rats and mice [190, 195].
Of note, DAF and CD59 are anchored onto the luminal surface of RBC membranes via a glycophosphatidyl inositol (GPI) anchor [196]. Methods to insert DAF, CD59 and other GPI-linked proteins into the RBC plasmalemma using lipid anchors have been developed [197, 198]. Insertion of GPI-anchored CD59 into the RBC plasmalemma protects against complement [199]. Insertion of GPI-linked cargoes into RBCs might be employed for drug delivery [200], in particularly for transfer cargoes to vascular cells [201] and to host defense cells [202].
Further, non-covalent attachment via streptavidin provides a modular approach for RBC surface loading of diverse biotinylated agents. Biotin groups for this purpose can be inserted non-covalently via phospholipid biotin derivatives, or covalently conjugated to selected amino acids including tyrosine and glycine and sugar moieties [167, 177, 187, 189–191, 194, 195]. Genetic modification of reticulocytes allowing site-specific conjugation of biotin groups offers a new approach for this purpose [203]. Of note, biotinylated RBCs are widely employed in clinical investigations to trace circulation of RBC in the bloodstream after injection in human subjects. Detection of biotinylated erythrocytes provides more accurate measurements than can be afforded by optical tracings, while avoiding use of radioactive isotopes undesirable for studies in humans [204–206].
Section 5.1.5. Targeting cargoes to RBC surface determinants.
As Karl Landsteiner discovered a century ago, RBCs express on their surface scores of epitopes recognizable by immunoglobulins that can be found in blood of animals injected with RBC - heterologous and homologous (i.e., of different and the same animal species) [207, 208]. This discovery -- defining ABO blood groups on human RBC -- provided the basis for safe, effective worldwide practice of blood transfusion beyond the realm of autologous RBC i.e., auto-donated by the same patient (as it turned out, autologous RBC may inflict immune reactions when injected after inadequate storage leading to RBC damage).
Taking the paramount importance of safe blood transfusion and management of natural blood group conflict between the fetus and mother, the multitudes of studies identified the specific RBC proteins and glycoproteins defining the immunological properties of RBCs. In addition to ABO and Rhesus families of RBC antigens, these efforts yielded tens of other epitopes exposed normally or upon RBC senescence and their mapping on RBC proteomics. Also, clinical and animal studies characterized the consequences and management of the hemolysis ensuing from the RBC immunological conflict. These studies defined the toxic features of free hemoglobin and mechanisms of acute damage in diverse organs notably the kidney. Pathological pro-inflammatory adhesion of RBC to endothelium and host defense cells plays important roles in malaria, PNH, SCD and other RBC-related conditions.
This wealth of knowledge of “what can go wrong” in relationships of RBCs with the rest of the body provides a priceless inheritance for the RBC drug delivery field. In particular, the notion of immunological recognition of RBC antigens and epitopes provided the basis for the RBC drug delivery strategies using affinity ligands for non-covalent loading of drugs and their carriers to RBC surface. This is a highly modular and versatile approach.
Varieties of ligands binding to RBC surface determinants and used for affinity loading of the cargoes include antibodies, their fragments and recombinant derivatives including single chain variable region fragments (scFvs) and heavy chain-only camelid antibody fragments (nanobodies), as well as affinity peptides isolated by screening of phage display libraries. With respect to IgG-based ligands, the above sequence represents the line of progression that started with use of antibodies - polyclonal in the early studies and predominantly monoclonal counterparts for the last quarter of century. Yet, all antibody-based ligands have translational challenges and, more importantly, exert unintended effects on the RBC including but not limited to epitope clustering, cross-linking, agglutination, rigidification and Fc-fragment phagocytosis and complement activation. Using scFv and nanobodies and other recombinant derivatives featuring monovalency and absence of an Fc domain solves these issues.
Varieties of the RBC determinants used for binding of these ligands and their conjugates include epitopes of major RBC proteins such as glycophorin A (GPA), band 3, and Rh system antigens, as well as relatively scarce RBC sites such as CR1. The latter determinant is fairly unique in that it allows non-damaging binding to RBC of not just CR1 antibody, but multimolecular conjugates consisting of anti-CR1 and antibodies to pathological mediators, pathogens and cytokines. RBC carrying such multimolecular complexes bind these objects circulating in the bloodstream and deliver them to macrophages in the RES where this blood waste gets captured from the RBC surface without damaging the RBC. It may be that in addition to some not yet understood features of CR1, its low density on the RBC is important (<1,000 copies per RBC). High-density RBC glycoproteins such as GPA and Band 3 enable much heavier loading of ligand-coupled drugs and nanocarriers to RBC, but exceeding 10,000-20,000 molecules per RBC impairs carrier biocompatibility. More recent studies addressed this issue using human RBC and found that epitopes of Rh system seem more amenably undamaging binding of ligands than GPA and Band 3 [209].
The varieties of cargoes conjugated with these ligands using chemical or recombinant techniques include model drugs and drug delivery systems including antibody-coated liposomes and agents for management of thrombosis, bleeding, inflammation, stroke, blood clearance of pathogens, immune response and other conditions. In general, RBC surface loading offers unique features and opportunities including: A) Unprecedented level of control of specific sites on the RBC used for binding of the cargo and dosing of the load, B) Cognizant modulation of the strength of binding, allowing control of the rate of loading and its reversibility, and C) the affinity-mediated binding of drugs to the RBC surface allows unique possibilities to load drugs and nanocarriers directly on RBC circulating in bloodstream, avoiding the necessity of ex vivo loading and transfusion.
Section 5.1.6. Functional features of RBC-conjugated drugs.
The roster of pharmacological agents surface-loaded onto RBCs is extended, diverse and grows rapidly. It features proteins including ligands of all sorts (antibodies, immunoglobulins and their fragments, peptides, modulators such as cytokines and molecules capturing these mediators), enzymes (e.g., fibrinolytics), and non-enzymatic modulators (e.g., thrombomodulin). Chemical conjugation of diverse polymers has been designed to mask blood group determinants in the quest for artificial universal donor blood as well as to improve the hydrodynamic properties of modified RBC and provide the flexible extended linkers for coupling of other cargoes.
Candidate cargoes for surface loading RBCs include antigens and cytokines to modulate host defense [210], present ligands for vascular targeting of RBC cargoes [170, 211, 212], capture circulating pathological agents [213–215], or provide complement inhibitors to protect RBCs against pathological hemolysis, e.g., in the syndrome paroxysmal nocturnal hematuria (PNH) [216].
Due to relatively limited accessibility of extravascular compartments to the 5-micron RBC, this carrier seems most attractive for the agents whose targets are localized within the blood. For example, considerable attention is devoted to RBC surface loading and delivery of drugs that help control blood fluidity, coagulation, bleeding and thrombosis included conjugating to RBCs fibrinolytics [211] and heparin [217]. Anti-thrombotic agents used in emergent settings [218, 219] but requiring rapid elimination dictates use of high doses [220] posing risk of bleeding [221–224] and other side effects [225].
Coupling anti-thrombotic drugs to the RBC [211, 217, 226] prolongs their circulation and minimizes diffusion into the CNS and pre-existing hemostatic plugs. RBC/PA complexes injected in mice and rats circulate orders of magnitude longer than PA, providing prophylactic delivery of PA to the interior of nascent thrombi while a 10-fold higher dose of soluble PA is ineffective [227–229]. RBC/PA trapped within growing clots rapidly form patent channels that permit perfusion of oxygen-carrying host RBCs prior to complete clot disintegration [227, 228]. Delivery of PAs to the interior of nascent thrombi, while sparing preformed clots, make them suitable for thromboprophylaxis in settings where the risk of thrombosis and bleeding are both high [229, 230].
RBC carriage favorably alters several important PA features: i) it switches PA from activating pro-inflammatory receptors in the CNS parenchyma to protective signaling via intravascular receptors [231–234]; ii) the RBC glycocalyx attenuates inactivation of coupled PA by plasma inhibitors [235] and its adverse interactions with vascular cells [236]. RBC/PA: i) lyse cerebral thrombi in mice and rats, providing reperfusion, brain protection and improving survival; ii) alleviates brain injury in rats with intracranial hemorrhage and blunt trauma; and iii) protects brain in pigs with cerebral thrombosis [233, 234, 237–239]. In stark contrast, free PA causes CNS bleeding, neurotoxicity and lethality in these settings.
RBC may also be utilized to modulate the immune response – skewing it towards either immunogenic or tolerogenic phenotypes. One such approach involves loading RBCs with either steroids or protein antigens [240, 241]. Several studies have highlighted the ability of RBC-based drug delivery to bias the immune response [242–245]; however, the mechanisms underlying the directionality of immune modulation (activation vs. suppression) are not fully elucidated at this point [246, 247]. It is likely that this response is controlled by a myriad of factors, including, but not limited to: loading approach, extent of loading, changes in RBC biocompatibility, surface patterning of antigen, dose amount, and route of administration [248, 249]. This nascent and rapidly evolving field of RBC-based immune modulation has potential applications in a wide range of therapeutic areas.
Section 5.2. Clinical testing and applications of RBC-encapsulated drugs.
RBC-encapsulated drugs are currently used therapeutically in clinical trials and several formulations have reached clinical trials (Table 9) [250–252]. Two RBC-encapsulated enzymes, asparaginase and thymidine phosphorylase, have been used in humans. Asparaginase has been encapsulated into RBCs and studied for therapeutic use in mammals since 1976 [253] and in humans since 2011 [254]. Known to be effective in acute lymphoblastic leukemia (ALL), the enzyme asparaginase has been a mainstay in the treatment of ALL since the 1970s. However, it exhibits significant toxicity to non-cancer cells. By encapsulating asparaginase into autologous RBCs in a study of 13 patients, half-life was increased from 10 to 50 hours and no clinical host toxicity was observed [164, 165]. Further developed by Erytech Pharma and now called GRASPA®, RBC-encapsulated asparaginase causes fewer and less severe allergic reactions compared to the usual asparaginase formulation used in pediatric ALL [254]. Currently, GRASPA is in a Phase 3 trials for pancreatic duct adenocarcinoma and Phase 2 for refractory acute lymphoblastic leukemia.
Table 9.
Examples of DDS based on encapsulation of cargoes into RBC.
| Cargo | Disease | Principle | Testing Phase |
|---|---|---|---|
| L-Asparaginase | Acute lymphoblastic leukemia Pancreatic cancer Ulcerative Colitis |
RBC are loaded with asparaginase to act as drug depot that increases enzyme half-life and reduces toxicity to host | Phase 2 Phase 3 Phase 2 |
| Phenylalanine ammonia lyase (PAL) | Phenylketonuria | RBC are modified to produce PAL, an enzyme that breaks down phenylalanine, with increased half life and reduced immune-mediated side effects | Phase 1 (active, not recruiting) |
| Thymidine phosphorylase | Mitochondrial neurogastrointestinal encephalomyopathy | RBC are loaded with thymidine phosphorylase, replacing the enzyme missing in this autosomal recessive condition | Phase 2 |
| Adenosine deaminase | ADA | Progenitor cells are transduced with lentiviral vector- cDNA for human ADA | Phase 2 |
| Dexamethasone | Ataxia telangectasia Breast cancer |
Delivery to host defense cells *only non-enzyme drug* |
Phase 3 Phase 3 |
Thymidine phosphorylase, another enzyme that has been encapsulated by RBC, has also seen early progress in humans. A Phase 1 trial in which 3 subjects with mitochondrial neurogastrointestinal encephalomyopathy were given erythrocyte-encapsulated thymidine phosphorylase, two patients were noted to improve clinically, and none had serious adverse events [255, 256]. This therapy has been approved for Phase 2 testing although is not yet recruiting.
The last RBC-carried enzyme, phenylalanine ammonia lyase (PAL) is not encapsulated, but is expressed by genetically engineered transfused RBC and used to treat phenylketonuria, an inborn defect in metabolism. The investigational drug, RTX-134, is composed of allogeneic RBC that are engineered to express Anabaena variabilis phenylalanine ammonia lyase [257]. Recruiting for the Phase 1b trial is currently on hold.
One non-enzyme small molecule drug, dexamethasone, has been encapsulated into RBC as dexamethasone-21-phosphate (dex-21-P) [181]. Several studies have demonstrated clinical improvement in patients with irritable bowel disease who were treated with autologous dex-21-P loaded RBC [258].
Section 5.3. Genetically Modified RBC.
In recent years, there have been concerted efforts made to genetically engineer RBC precursors to provide mature RBCs with desirable features. One such example that is relevant for drug delivery is the introduction of functional groups permitting site-specific conjugation of molecules to the RBC surface. By introducing an amino acid sequence recognized by bacterial Sortase A (LPETG) into select membrane proteins, specific conjugation of biotin [259] and antigenic peptides [260] was achieved without substantial adverse effects on the RBC. More recently, direct fusions of RBC surface proteins to antibody fragments have been introduced to generate RBCs with endogenous affinity to select target molecules [261], In addition to drug delivery applications, genetic engineering of RBC precursors has been used to improve outcomes in transfusion medicine by generating RBCs that have the appropriate surface markers for a given patient [262]. This approach has great potential in improving outcomes in patient populations requiring chronic blood transfusions (e.g. sickle cell disease, β-thalassemia, etc.).
Section 5.4. Applications of RBCs as Sensors
Beyond the use of RBCs as ‘natural’ DDSs, there has been significant interest in converting RBCs into biosensors and diagnostic agents [263]. Encapsulation of magnetic nanoparticles, namely superparamagnetic iron oxide nanoparticles (SPIO), into RBCs has been shown to improve the circulation time of these particles [264]. This approach has been applied in animal models to use SPIO-loaded RBCs as MRI contrast agents that can be used for long-term monitoring [265–267]. This provides utility in monitoring disease progression in chronic diseases without the need for frequent administration of contrast agents.
RBCs have also been loaded with molecules that change their optical activity in response to a given stimulus, termed erythrosensors, providing a non-invasive approach to detect concentrations of certain molecules in the bloodstream [268]. A fairly straightforward example that was developed for in vitro use was encapsulation of a pH-sensitive fluorophore (FITC) into erythrocytes to monitor pH changes. Briefly, as the internal pH of the erythrosensor equilibrated to the bulk solution pH, fluorescence changed in a quantifiable manner, providing a highly sensitive readout of pH [269]. An ideal erythrosensor would be able to detect changes in blood markers in vivo (e.g. blood glucose levels) with high sensitivity. There have been several publications describing the use of red blood cell-derived vesicles as biosensors [270, 271]; however, to date, there are no descriptions of in vivo erythrosensor applications using intact RBCs or RBC ghosts.
Section 5.5. Comparison of RBC vs liposomal DDS.
As highlighted above (Section 3.2), the behavior of RBC and liposomes in the circulation is drastically different, with RBCs circulating for months and liposomes circulating for no more than a few days, despite substantial efforts being devoted to engineering their PK. However, beyond simple consideration of circulation time, their capacity for achieving drug delivery to desired sites must be considered when devising a drug delivery strategy (Table 10).
Table 10.
Comparison of drug delivery features of liposomes and RBC.
| Features | Parameters | Liposomes | RBC |
|---|---|---|---|
| Carrier | Target access | Relatively high | High in blood |
| Extravasation | Yes | Normally none | |
| Effect of pathology | Varies from EPR to blocking access | Massive uptake in clots and bleeding sites | |
| Circulation time | Minutes to days | Months | |
| Majority of injected dose | Rapidly taken by RES | Remains in blood cells | |
| Clearance organs | Liver | Spleen | |
| DDS utility | Improving PK of insoluble drugs | Long-term pool in blood | |
| Targets | Blood, RES and tissue components | Blood, vascular cells and RES | |
| Intracellular delivery | Yes, in many cell types via many mechanisms | To macrophages in RES | |
| Advances and issues | Versatility | High | Limited |
| Control of structure | Considerable | ||
| Challenges | Heterogeneity | ||
| Immunogenicity | Relatively low | Self-tolerance to autologous RBC |
A key component to drug delivery is access of the DDS to the target site, which is controlled by many carrier (size, charge, flexibility, targeting ligand, etc.) and pathology (perfusion, edema, cellular infiltration) -dependent properties. For a target that resides within the vasculature, both liposome- and RBC-based DDS will face no accessibility barriers following intravascular administration, barring complete loss of blood flow to the pathologically altered tissue. On the other hand, micron-sized RBCs have effectively no access to extravascular sites under normal conditions, save for the splenic red pulp. Sites of bleeding would provide a rare example where RBCs could be used for drug delivery to an extravascular site. On the other hand, liposomes are generally an order of magnitude smaller than RBCs, with typical diameters of 50-300 nm. This smaller size may allow extravasation of liposomal DDS into select tissues with ‘leaky’ vasculature, such as liver, spleen, bone marrow, tumors, and sites of inflammation [272].
Beyond achieving delivery on a tissue-level, drugs must reach the desired cells and sub-cellular destinations in order to elicit a beneficial effect. Internalization of RBCs is only performed by phagocytes that recognize damaged or aged cells; therefore, achieving intracellular drug delivery with drugs encapsulated within the RBC is likely only to provide high drug load in splenic macrophages. Intracellular delivery of liposomes can be achieved not only non-specifically by phagocytes, but also through affinity targeting to internalizable membrane receptors. This can provide delivery of liposomes and their encapsulated cargoes to specific cells and endosomal sorting pathways.
By considering circulation time (3.2), clearance organs (3.2), and target accessibility, potential biomedical applications of RBC- and liposome-based DDS can be identified. RBCs, which circulate for months and are confined to the bloodstream, are able to serve as long-term depots of drug in the bloodstream. Small molecule drugs will utilize the RBC carrier as a slow-release depot, with the drug leaking from the long-circulating RBCs into the plasma. This would be analogous to either a slow infusion, but with much greater capacity for providing sustained delivery over prolonged periods of time. On the other hand, liposomes, while useful for chronic applications (e.g. repeated doses of Doxil in oncology), are not able to match the 4 month lifespan of RBCs. This suggests that RBC carriage could be amenable to reducing the dosing frequency needed compared to liposomal drugs. However, these carriers are excellent for enabling use of insoluble drugs that cannot be otherwise administered parenterally. Liposomes are also useful for directing increased fractions of the dose relative to free drug to desired sites, either passively (EPR, edema) or via active targeting, sparing organs that may be susceptible to adverse effects. For example, liposomal doxorubicin reduces drug uptake into the heart, thereby reducing the severe, long-term cardiotoxicities associated with free doxorubicin administration [273, 274].
In addition to considerations of PK and biodistribution, utility of any DDS can be limited by recognition by host defense systems, particularly with chronic dosing. While lipid vesicles are not generally immunogenic per se, engineering their surface to improve their in vivo behavior may result in unwanted immune system recognition. Following the first injection, the circulation time of PEGylated liposomes is typically significantly longer than their bare counterparts. However, the accelerated blood clearance (ABC) phenomenon, wherein clearance increases upon subsequent doses, was identified within just a few years of PEGylation becoming a popular approach in nanomedicine [275, 276]. The underlying mechanism of this phenomenon was identified as formation of anti-PEG IgM following liposome injection, indicating formation of an adaptive immune response [277, 278]. The use of RBCs as DDS is not anticipated to lead to any adverse immune reactions as current protocols rely on reinfusion of drug-loaded, autologous RBCs, which will be recognized as ‘self’ by the host immune system.
Section 6. Using RBC for vascular delivery of nanocarriers.
The earliest report of loading nanoparticles onto the RBC surface was published 45 years ago by Juliano and Stamp who extracted sialoglycoproteins from erythrocyte membranes and incorporated them into liposomes. These liposomes were then bound to the RBC surface in vitro and the authors suggested that this approach could be useful for the development of targeted liposomes [279]. Antibody-based coupling of liposomes to the RBC surface was first described by the Papahadjopoulos group who used anti-RBC (Fab)2 and Fab fragments to obtain specific binding of liposomes to human RBC [280, 281]. The potential for antibody-directed loading of liposomes onto the erythrocyte surface was demonstrated in blood in the mid-1980s, both in vitro and in vivo, reducing uptake by the RES without impacting RBC circulation [282, 283]. In the late 1980s, a comparison of the effects of liposomes coated with anti-RBC Fab on RBC circulation was performed that demonstrated dose-dependent clearance of RBC induced by liposome injection [284, 285]. These pioneering studies were critical in demonstrating A) specific binding of immunoliposomes to RBCs, B) PK of RBC-coupled liposomes, and C) impact of RBC-coupled liposomes on erythrocyte biocompatibility; however, there were no clear biomedical applications of RBC-directed nanocarriers.
In addition to antibody-based targeting, in recent years several groups have reported alternative affinity ligands for coupling nanoparticles to the surface of RBCs. One of the most widely reported of these is the glycophorin A-binding peptide, ERY1, which has been shown to provide specific binding of nanoparticles to RBCs with minimal effects on RBC biocompatibility [286, 287]. Corn starch nanoparticles were demonstrated to preferentially bind to malaria-infected RBCs vs. naive RBCs due to expression of a hexose transporter in infected cells [288].
Targeting liposomes to the RBC membrane was first utilized for treatment of malaria in preclinical species. Liposomes coated with RBC-binding antibody fragments were used to facilitate the delivery of chloroquine to RBCs in mice infected with Plasmodium berghei [289–292]. Despite promising results of this strategy in the early 1990s, relatively few reports were published describing RBCs as a carrier for nanoparticles over the ensuing decades. This approach was revived in 2015 with a report describing treatment of Plasmodium falciparum infection in mice with glycophorin A-targeted liposomes [293]. Further work improved the specificity of liposomal treatment of malaria by targeting to a parasite-derived RBC surface marker (Plasmodium falciparum erythrocyte membrane protein 1, PfEMP1) to deliver multiple anti-malarial drugs to infected erythrocytes [294–296].
Recently, two forms of targeting to murine glycophorin A, antibody (Ter119) and peptide (ERY1), were compared as approaches to prolong the circulation of tissue plasminogen activator (tPA) encapsulated within nanoparticles. The authors demonstrated that nanoparticles with Ter119 on the surface had markedly prolonged PK and reduced uptake by the RES vs. peptide-coated and uncoated nanoparticles without overt adverse effects [297]. This demonstrates the key role that affinity ligand selection can play in utilizing RBCs to enhance PK of nanoparticles.
The use of RBCs as supercarriers for liposomes and other nanoparticles has significant advantages beyond modulation of pharmacokinetics and immune recognition of cargo nanocarriers. RBCs also have an extraordinary ability to enhance delivery of nanoparticles to specific, desirable sites in the vasculature. This approach, termed RBC hitchhiking, will be discussed in detail below.
Section 6.1. Nanoparticles hitchhiking on RBC (RBC HH): from early prototypes to proof of principle in animal studies.
Like other pharmacological cargoes, nanocarriers including diverse imaging probes and contrast agents have also been loaded both into the RBC inner volume and onto the RBC surface [267, 298]. In fact, binding of immunoliposomes to RBC have been reported in the 1980s [284, 285]. Standard approaches using osmotic swelling permit encapsulation of particles smaller than 50 nm [299, 300], while surface loading to RBC is more size-permissive [301]. Studies of several groups including our laboratories showed that loading onto RBCs cardinally changes the fate of nanoparticles (NP) in vivo.
First, coupling to RBCs prolongs NP circulation [302–304]. After IV injection in rats, polystyrene particles (~200-450 nm in diameter) were cleared in minutes, whereas RBC-bound nanoparticles remained in circulation for hours [305]. Dual labeling showed that while NPs eventually are removed from the blood, the RBCs to which NP were attached remained in circulation [305]. A kinetic model of detachment of nanoparticles from the RBC surface was consistent with the measured pharmacokinetics of RBC-bound nanoparticles [305]. Further, the in vitro rate of shear-induced detachment correlates with the in vivo circulation in rats [306]. Mechanical forces eventually remove nanoparticles from the RBC surface, which led to their clearance by the liver and spleen [307].
After IV injection, RBC-bound NP rapidly accumulate in the lungs, transferring to the vascular cells from RBC squeezing through the pulmonary microvascular bed, the first “capillary trap” after IV injection [307]. This is an example of the classical first pass phenomenon, described for several DDS targeted to endothelial cells [308–311]. First pass transfer varies for RBC-bound liposomes, albumin-NPs, lipid nanoparticles, polystyrene NP, and AAV loaded on RBC. For example, approximately 5 vs 30% of injected dose of RBC-bound PS NP vs. liposomes accumulated in mouse lungs after IV injection [312]. RBC HH improved lung-to-liver ratio for liposomes by almost 2 orders of magnitude.
Double-isotope tracing of cargo nanocarriers (including liposomes) and carrier RBC, flow cytometry and confocal microscopy asserted that A) after unloading of NP in the capillaries RBC continue to circulate as normal RBC, B) NP delivered by RBC to the pulmonary microvasculature transfer to endothelial cells, and C) neither carrier RBC nor cargo nanocarriers clumped or were entrapped in the lung capillary lumens [312]. A moderate fraction of the RBC HH NPs was taken up by marginated leukocytes [312], white blood cells that transiently reside in the capillary lumen, especially in the lungs [313]. Live cell imaging in vitro reveals that stationary phagocytes would grab RBCs flowed past them and remove the adsorbed NPs [312]. Thus, RBC HH with translatable NPs is produced by transfer from RBCs to endothelial cells and marginated leukocytes.
RBC HH of nanocarriers to the lungs might have important diagnostic and therapeutic implications [307, 312, 314, 315]. For example, RBC HH of nanocarriers loaded with fibrinolytic plasminogen activators afforded more effective dissolution of pulmonary thrombi in mouse model of fibrin clots lodged in the lung vasculature [312]. RBC HH of nanoparticles showed promising results in treating lung metastasis in murine models [314, 315]. NP loading has been reproduced using mouse, rat, pig and human RBCs. RBC HH worked in vivo in pigs, and in fresh, ex vivo human lungs that were oxygenated and perfused [312].
RBC HH is not limited to the lungs. It can be directed to other organs by infusion in the feeding arteries using intra-arterial (IA) catheters. Animal studies showed that this administration maneuver (widely used in clinical practice) enables an order of magnitude enhancement of the uptake of RBC-bound NP in the heart, kidneys and brain of mice [312].
The lung uptake of RBC-hitchhiking NPs was comparable with that of NPs targeted to lungs by antibodies to endothelial epitopes. For example, antibodies and single chain variable fragments to PECAM-1 and ICAM-1 are known to target cargoes to the vasculature especially in the lungs after IV injection [316–319]. Adsorption of ICAM-1 antibody on RBC HH NP enhanced residence of NP in the lungs, whereas pulmonary uptake increased to >50% vs 20% of injected dose for RBC-loaded vs. free anti-PECAM-NPs [312]. Combining RBC HH with NP targeting mediated by affinity ligands represents an attractive avenue of further development of this approach. In addition, modulation of NP geometry and mechanical feature allows the possibility to optimize their targeting and uptake by the target cells [320, 321]. In this context it is important that RBC-hitchhiking anti-ICAM-1 targeted rod-shaped nanoparticles exhibited a 6-fold improvement in lung/liver accumulation ratio compared to that by their spherical counterparts [322].
Section 6.2. RBC HH: New developments
Conventionally, RBC HH was devised for delivering nanocarriers to vasculatures immediately downstream the injection sites [307, 323]. New advances are emerging to understand the interplay between RBC HH and host defense system and to exploit the same for modulation of immune responses. In particular, nanocarriers carrying immunomodulatory agents, for example, antigens or adjuvants, can be delivered to target organs (e.g., lungs) by RBC HH and regulate local immune environments [324, 325]. This is exemplified by the recent demonstration that RBC HH could leverage metastases in the lungs and enable the induction of systemic anti-tumor immune responses for tumor eradication [324]. In brief, ImmunoBaits, chemokine loaded PLGA nanocarriers, were dominantly delivered to the lung capillary endothelium by RBC HH where metastasized cancer cells prefer to reside. Co-localization of ImmunoBaits with metastasized tumor cells and subsequent release of immunomodulatory chemokines awakened host defense cells to attack tumor cells that generated a systemic immune response that were able to eradicate local lung metastases while also preventing tumor recurrence [324]. This example highlights the immunological therapeutic potential that the combination of RBC HH with selected immunomodulatory nanocarriers can achieve opening up a window of opportunity to target disease environments for controlled regulation of immune responses.
Biologically, RBCs have close contact with immune cells in immunoactive organs such as the liver and spleen. In fact, immune cells in the spleen and liver continuously screen the status of circulating RBCs [50, 326]. For example, stationary phagocytes in the liver and spleen including Kupffer cells recognize and take up pathogens that bind to RBC surface via the immune adherence mechanism [327, 328]. These immunological attributes open up new possibilities of engineering RBC HH to deliver nanocarriers to these immune cells for immunomodulation purposes. The RBC HH process itself has been demonstrated to be able to modulate RBC biochemical and biophysical properties [329, 330] which in turn alter the location of and responses to the delivered nanocarriers. In particular, attachment of nanocarriers to RBCs induces concentration-dependent membrane alternations to carrier RBCs [325, 330]. It was demonstrated that when the nanocarrier to RBC ratio is increased above a certain threshold, the stiffness of RBC membranes increases due to limited fluidity of lipid domains [330]. Nanocarrier-induced RBC membrane stiffening likely reduces the stretching of RBCs in lung capillaries and thus reduces deposition of nanocarriers in the lungs. In addition, the increase in nanocarrier to RBC ratios also flips phosphatidylserine from the inner membrane to be expressed on to outer membrane of RBCs [325] which contributes to the increased cross-talk between immune cells in the spleen and RBCs. The two effects (RBC membrane stiffening and elevated phosphatidylserine expression), when combined, make a new RBC HH system capable of bypassing lung delivery and depositing nanocarriers to the spleen [325]. By controlling the nanocarrier to RBC ratio, the RBC HH system managed to prevent lung accumulation and caused enough phosphatidylserine expression to facilitate efficient delivery to the antigen-presenting cells in the spleen. This unique hand-off mechanism demonstrated significantly better efficacy for sustained splenic delivery of nanocarriers as compared to unhitchiked counterparts, without the sacrifice of the carrier RBCs. When using ovalbumin-coated nanocarriers as a model, hitchhiked nanocarriers led to an improved humoral and cellular response to the attached antigen. When tested in an ovalbumin-expressing lymphoma model, vaccination by hitchhiked nanocarriers performed as well as the CpG adjuvant without the need for an exogenous adjuvant [325]. This RBC HH approach involving transiently perturbed RBCs opens up opportunities for targeted delivery of nanocarriers to the spleen and for adjuvancy without depending on exogenous adjuvants which expands possibilities in the vaccine design space for speeding the vaccine development cycle to cope with many diseases including pandemic diseases such as the COVID-19.
In the abovementioned examples, RBC HH generally employs native or mildly-disturbed RBCs as a shuttle to deliver nanocarriers/cargos to immune cells with the carrier RBCs being non-sacrificed. However, in a broader context of RBC-mediated immunomodulation, engineered RBCs, if designed in a controlled mechanism, can directly engage with or enhance their uptake by host defense cells [242, 249, 331–337]. For example, RBCs engineered to express immunomodulatory ligands on their surfaces are able to directly activate innate and/or adaptive immune cells via surface ligand binding [334–337]. For instance, RBCs with surface-expressed 41BBL and IL-15TP (RTX-240) could activate and proliferate NK cells and T cells, showing promise in Phase I clinical trials for treating solid tumors and acute myeloid leukemia [335]. Engineered RBCs or antigens that can lead to antigen binding to RBCs have also been shown to be able to engage with the immune system [249, 331–333] most likely attributed to the enhanced uptake of the RBC-antigen system by immune cells in the liver and spleen. It should be noted that these RBC-antigen systems could also induce immune tolerance apart from immune stimulation. For example, engineered antigens that bind to RBCs in vivo demonstrated the induction of an immune tolerance that involves the depletion of effector T cells and activation of T regulatory cells [331, 333]. When using a peptide islet β-cell autoantigen as an antigen, the tolerance response elicited by this system prevented the onset of autoimmune diabetes [333]. The mechanisms controlling the immune stimulation vs. immune tolerance of RBCs carrying antigens are emerging and are not completely clear [338]. The factors affecting this process likely include the nature and loading of antigens onto RBCs, the extent of the changes of carrier RBCs, the immune cell types and immune environment to which the antigens are being delivered, the dose and administration routes, among others [338]. These above examples highlight the opportunities and clinical potential of engineered RBCs for controlled modulation of immunity to develop therapies that could treat many diseases including cancer, autoimmune diseases, and infectious diseases.
Advancing RBC HH for modulation of a multitude of immune responses opens up unique opportunities and has shown promise in the space of vaccine design, adjuvant design, immunotherapy development, among other areas. The versatility of RBC HH, if engineered in a well-controlled manner, could enable fine targeting of certain immunity arms and even specific immune cell types. The diversity of immunomodulatory agents/nanocarriers further expand the potential of RBC HH for immunomodulation. However, the innate and adaptive arms of the immune system are so complex that subtle changes in any one step of the immune cascade can completely change the outcome. More studies are needed to illustrate the interactions of RBC HH with each step of the immune cascade in a more detailed mechanistic manner and to understand factors governing the immunological outcomes that RBC HH can achieve. With these better understandings, it is possible to design tailored RBC hitchhiking systems for specific immunomodulation applications.
Section 6.3. RBC HH: Challenges and perspectives
In order to successfully translate RBC HH from preclinical models into the clinical domain, there are several key hurdles that must be overcome beyond delivery to the target tissue. As RBC HH is a new approach in the field of drug delivery, it is likely that key parameters for optimization of this technology have not even been anticipated to date. Optimization of the safety of this technology is arguably at the top of the list of requirements for clinical translation [339]. Nonetheless, it can be appreciated that attaching pharmacologic cargoes, such as nanoparticles, to RBCs may alter not only the nanoparticle, but also the carrier RBC [340].
Following injection of nanoparticles adsorbed to carrier RBCs (RBC HH), a large fraction of the injected dose will be rapidly delivered to the target vascular bed. However, a non-insignificant fraction of the dose will remain associated with the RBC and, as such, will not behave similarly to a nanoparticle administered in free form. One would expect that RBC-associated nanoparticles would be retained in the circulation longer than free nanoparticles and would reside in the cellular fraction of the bloodstream. Prolonged association with RBCs may result in a shift in the primary route of clearance from hepatic Kupffer cells (free) to a mix of splenic and hepatic macrophages (RBC HH). This shift in clearance pathways is reflective of the route of elimination of modified RBCs in vivo.
Modification of RBCs, particularly ex vivo, will, without fail, lead to alterations in the RBC carrier. In the context of clinical drug development, the billion-dollar question will be if changes in the RBC lead to unacceptable safety risks in the intended patient population. As an example, hypotonic swelling of RBCs to encapsulate small molecule and protein therapeutics results in the RBC losing hemoglobin, having altered biomechanical properties, and having changes in the organization of the plasma membrane [50, 341–345]. While surface loading is unlikely to result in losses of hemoglobin, it does come with its own risks.
If coupling to the surface of the RBC is performed in an uncontrolled manner, it is likely that there will be blockade of the very surface markers that allow RBCs to circulate for months without clearance by the immune system (Section 3.2). The loss of function of these critical markers will cause the RBC to appear as though it is ready to be eliminated, as would be expected for aged, damaged, or infected RBC [171, 187, 191, 193–195]. Not only is this accelerated clearance of RBCs undesirable from a biological perspective, but also it will abrogate the ability of the carrier RBC to serve as a drug delivery system [177, 188]. If a large fraction of RBCs is directed to the RES due to uncontrolled drug coupling, there is the potential for overload of these sentinel macrophages resulting in reduced immune function or hyperactivation of the immune system, resulting in a cytokine storm [346–348]. In addition to clearance by tissue-resident phagocytes, modified RBCs are ripe for attack by circulating components of the immune system, such as complement. This could result in intravascular hemolysis, leading to significant endothelial and renal toxicities, or aggregation of RBCs, resulting in vessel occlusion, ischemia, and damage to the endothelium [195, 349].
A critical, potentially overlooked factor in RBC-based nanoparticle drug delivery is the impact of physicochemical and biomechanical properties of the cargo nanoparticle on the RBC. For example, rigid polystyrene nanoparticle adsorption onto RBCs led to significant reductions in RBC biocompatibility (enhanced sensitivity to stresses, agglutination, hemolysis) [187, 349]. However, low density (20-50 NP/RBC) coupling of other nanocarriers (lysozyme-dextran nanogels, liposomes, etc.) did not adversely affect RBC biocompatibility when assessed both in vitro and in vivo. This highlights the need to investigate the impact of potential nanocarriers on the RBC early on in development to preempt any potential carrier-related toxicities.
Section 7. Conclusions
For the past half-century, liposomes (and other artificial nanocarriers) have been designed with the goal in mind of improving the safety & efficacy of cargo drugs. Despite tens of thousands of publications describing nanoparticle-based drug delivery, there are only a handful of clinically used DDSs. Here, we compare and contrast the features of artificial DDSs with RBCs as a model of a ‘natural’ DDS. Several decades of research have highlighted approaches to both load cargo drugs inside the RBC and couple drugs to the RBC surface in order to take advantage of the features of RBCs that are favorable for drug delivery, namely prolonged circulation time, high abundance, and natural tolerogenicity. Within just the past few years, we have described a new approach for RBC-based drug delivery, RBC HH, which provides high levels of delivery to the vascular bed immediately downstream of the injection site. These advances have allowed RBC-based drug delivery to progress from the halls of academia into industrial drug development pipelines.
Table 2.
Comparison of the translational and practical aspects of artificial DDS such as liposomes, and natural carriers, RBCs. Drugless liposomes and other nanocarriers themselves have limited, if any medical utility, except use as “semi-placebo” control for beneficial and adverse effect of the carriers in normal and diseased organisms. In contrast, RBCs have tremendous medical utility for transfusion, imaging blood perfusion, and diagnostic use, among other applications. However, RBC-based DDSs are inferior to artificial carriers in most aspects of industrial development, commercialization, pharmaceutical administration, and medical utility.
| Liposomes | RBCs | |
|---|---|---|
| Nature | Artificial | Natural |
| Industrialization | ||
| Scaling-up, QC | Workable | Challenging |
| Storage | Amenable | Limited |
| Regulatory affairs | Challenging | Very challenging |
| Commercialization | ||
| Patentability | High | Relatively limited |
| Volume of use | Intermediate | Low |
| Cost | Modest | Moderate to high |
| ROI | Relatively high | Relatively low |
| Administration | ||
| Formulations | Bulk production | Currently ad hoc on site |
| Distribution in population | Doable | Next to impossible |
| Treatment | Standardized | Individualized |
| Dosing | Workable | Complicated |
| Hospital settings | Regular | Advanced |
| Medical utility per se | ||
| Therapeutic | None | Tremendous: transfusion |
| Labeled carrier | Limited | High: measuring perfusion, RBC longevity |
| Diagnostic value | None | High: Detecting anemia, inflammation, hemolysis |
| Drug Delivery Utility | ||
| General | Very significant | Exploratory |
| Disease conditions | Oncology, infections, inflammation, hematology, neurology, metabolic disorders | Oncology, neurology, inflammation, bleeding, coagulation, thrombosis, metabolic disorders |
| Clinical trials | Hundreds | Several |
| Clinical use | Tens of approved formulations | Few entering early stage |
Abbreviations: QC – quality control, ROI – return on investment.
Table 4.
Behavior of liposomes and RBC in the circulation.
| Features | Parameters | Liposomes | RBC |
|---|---|---|---|
| Circulation | Life span in blood | Minutes – Hours | 4 Months |
| Biocompatibility | Limited | Exceptionally high | |
| Distribution in blood | Plasma + Leukocytes | Cellular fraction (pellet) | |
| Access to tissues | Select tissues | Normally none | |
| Protection from clearance | Passive non-specific (PEG) | Active: DAF, CD59, CD47 | |
| Changes with time | Unknown, NSU | Starvation & senescence | |
| Clearance | Prevalent normal pathway | Variable, NSU | Phagocytosis |
| Tissues and compartments | RES, tissues & lymphatics | RES | |
| Clearing organ of RES | Liver | Spleen | |
| Mechanism of clearance | Uptake by phagocytes | Retention in follicles | |
| Recognition mechanisms | APC and other opsonins | Complement | |
| Opsonization pathways | Non-specific | Loss of DAF, CD47, sialic acid | |
| Rate | Fast | ??? | |
| Abnormalities | Premature senescence | Not known, NSU | Rigidification & fragility; congenital cytoskeletal protein defects; cytosolic enzymopathies |
| Vascular adhesion | Similar to above | SCD, sepsis, malaria | |
| Lysis | Similar to above | PNH, snake venom, circulating auto/alloantibodies | |
Abbreviations: NSU: not sufficiently understood, RES – reticuloendothelial system, PEG – polyethylene glycol, DAF – Decay Acceleration Factor, SCD – Sickle Cell Disease, PNH – paroxysmal nocturnal hematuria, APC – alternative pathway of complement.
Table 6.
Surface modifications of liposomes
Table 8.
Impact of RBC loading on cargo drug and carrier erythrocyte.
| Inner loading | Surface loading | ||
|---|---|---|---|
| Effect on cargo | Prolonging PK | Yes | Yes |
| Isolating from blood | Yes | No | |
| Masking by glycocalyx | No | Yes | |
| Altering interaction with tissues | Blocks | Modulates clustering, accessibility, | |
| Effect on RBC | Loss of hemoglobin | Yes | None |
| Membrane damage | Yes | None | |
| Exposure of interior components | Yes PS, etc. | No | |
| Masking of exterior components | Yes | Yes, controllable | |
| Biomechanical alterations | Yes | Rigidification | |
| Biocompatibility | Reduction or loss | ||
| Life span in bloodstream | |||
| Initial reports | in vitro | Late 1970s | Early 1980s |
| in vivo in animals | Early 1980s | Early 1990s | |
| in human patients | First decade 2000s | In progress | |
| Administration | Injection of pre-loaded RBC | Yes | Yes |
| Drug targeting to RBC in vivo | No | Yes | |
| Activity | Drug release | Necessary for some cargoes | Release from RBC benefits some cargoes |
| Target molecules diffusion into RBC | Necessary for many cargoes (enzymes) | Not needed | |
| Types of cargoes | Chemical agents | DEX, DOX | No |
| Enzymes | Multiple | Multiple | |
| Proteins | Yes | Yes | |
| Antigens | Yes | Yes | |
| Imaging probes | Yes | Yes | |
| Nanocarriers | Yes | Multiple |
Abbreviations: PS – phosphatidylserine, DEX – dexamethasone, DOX - doxorubicin
Funding:
This work was supported by the National Institutes of Health [grant numbers R01HL143806 (VRM) and R01HL155106 (VRM)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dale GL, Kuhl W, Beutler E, Incorporation of glucocerebrosidase into Gaucher’s disease monocytes in vitro, Proc Natl Acad Sci U S A, 76 (1979) 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ihler GM, Glew RH, Schnure FW, Enzyme loading of erythrocytes, Proc Natl Acad Sci U S A, 70 (1973) 2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bangham AD, Horne RW, Glauert AM, Dingle JT, Lucy JA, Action of saponin on biological cell membranes, Nature, 196 (1962) 952–955. [DOI] [PubMed] [Google Scholar]
- [4].Horne RW, Bangham AD, Whittaker VP, Negatively Stained Lipoprotein Membranes, Nature, 200 (1963) 1340. [DOI] [PubMed] [Google Scholar]
- [5].Bangham AD, Horne RW, Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents as Observed in the Electron Microscope, J Mol Biol, 8 (1964) 660–668. [DOI] [PubMed] [Google Scholar]
- [6].Legendre JY, Szoka FC Jr., Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: comparison with cationic liposomes, Pharm Res, 9 (1992) 1235–1242. [DOI] [PubMed] [Google Scholar]
- [7].Ellens H, Bentz J, Szoka FC, pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact, Biochemistry, 23 (1984) 1532–1538. [DOI] [PubMed] [Google Scholar]
- [8].Szoka F, Olson F, Heath T, Vail W, Mayhew E, Papahadjopoulos D, Preparation of unilamellar liposomes of intermediate size (0.1-0.2 mumol) by a combination of reverse phase evaporation and extrusion through polycarbonate membranes, Biochim Biophys Acta, 601 (1980) 559–571. [DOI] [PubMed] [Google Scholar]
- [9].Li W, Szoka FC Jr., Lipid-based nanoparticles for nucleic acid delivery, Pharm Res, 24 (2007) 438–449. [DOI] [PubMed] [Google Scholar]
- [10].Plank C, Mechtler K, Szoka FC Jr., Wagner E, Activation of the complement system by synthetic DNA complexes: a potential barrier for intravenous gene delivery, Hum Gene Ther, 7 (1996) 1437–1446. [DOI] [PubMed] [Google Scholar]
- [11].Zelphati O, Szoka FC Jr., Mechanism of oligonucleotide release from cationic liposomes, Proc Natl Acad Sci U S A, 93 (1996) 11493–11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu Y, Szoka FC Jr., Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection, Biochemistry, 35 (1996) 5616–5623. [DOI] [PubMed] [Google Scholar]
- [13].Olson F, Hunt CA, Szoka FC, Vail WJ, Papahadjopoulos D, Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes, Biochim Biophys Acta, 557 (1979) 9–23. [DOI] [PubMed] [Google Scholar]
- [14].Szoka F Jr., Papahadjopoulos D, Comparative properties and methods of preparation of lipid vesicles (liposomes), Annu Rev Biophys Bioeng, 9 (1980) 467–508. [DOI] [PubMed] [Google Scholar]
- [15].Szoka F Jr., Papahadjopoulos D, Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation, Proc Natl Acad Sci U S A, 75 (1978) 4194–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Diez-Silva M, Dao M, Han J, Lim CT, Suresh S, Shape and Biomechanical Characteristics of Human Red Blood Cells in Health and Disease, MRS Bull, 35 (2010) 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Virtanen JA, Cheng KH, Somerharju P, Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model, Proc Natl Acad Sci U S A, 95 (1998) 4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tharad S, Uzulmez O, Promdonkoy B, Toca-Herrera JL, Cholesterol Increases Lipid Binding Rate and Changes Binding Behavior of Bacillus thuringiensis Cytolytic Protein, Int J Mol Sci, 19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gregoriadis G, Ryman BE, Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases, Biochem J, 124 (1971) 58P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gregoriadis G, Ryman BE, Fate of protein-containing liposomes injected into rats. An approach to the treatment of storage diseases, Eur J Biochem, 24 (1972) 485–491. [DOI] [PubMed] [Google Scholar]
- [21].Moghimi SM, Patel HM, Tissue specific opsonins for phagocytic cells and their different affinity for cholesterol-rich liposomes, FEBS Lett, 233 (1988) 143–147. [DOI] [PubMed] [Google Scholar]
- [22].Roerdink F, Wassef NM, Richardson EC, Alving CR, Effects of negatively charged lipids on phagocytosis of liposomes opsonized by complement, Biochim Biophys Acta, 734 (1983) 33–39. [DOI] [PubMed] [Google Scholar]
- [23].Scieszka JF, Maggiora LL, Wright SD, Cho MJ, Role of complements C3 and C5 in the phagocytosis of liposomes by human neutrophils, Pharm Res, 8 (1991) 65–69. [DOI] [PubMed] [Google Scholar]
- [24].Chonn A, Semple SC, Cullis PR, Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes, J Biol Chem, 267 (1992) 18759–18765. [PubMed] [Google Scholar]
- [25].Hnatowich DJ, Clancy B, Investigations of a new, highly negative liposome with improved biodistribution for imaging, J Nucl Med, 21 (1980) 662–669. [PubMed] [Google Scholar]
- [26].Gregoriadis G, Senior J, The phospholipid component of small unilamellar liposomes controls the rate of clearance of entrapped solutes from the circulation, FEBS Lett, 119 (1980) 43–46. [DOI] [PubMed] [Google Scholar]
- [27].Senior J, Crawley JC, Gregoriadis G, Tissue distribution of liposomes exhibiting long half-lives in the circulation after intravenous injection, Biochim Biophys Acta, 839 (1985) 1–8. [DOI] [PubMed] [Google Scholar]
- [28].Gabizon A, Papahadjopoulos D, Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors, Proc Natl Acad Sci U S A, 85 (1988) 6949–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klibanov AL, Maruyama K, Torchilin VP, Huang L, Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes, FEBS Lett, 268 (1990) 235–237. [DOI] [PubMed] [Google Scholar]
- [30].Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A, Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo, Biochim Biophys Acta, 1066 (1991) 29–36. [DOI] [PubMed] [Google Scholar]
- [31].Foteini P, Pippa N, Naziris N, Demetzos C, Physicochemical study of the protein-liposome interactions: influence of liposome composition and concentration on protein binding, J Liposome Res, 29 (2019) 313–321. [DOI] [PubMed] [Google Scholar]
- [32].Yuda T, Maruyama K, Iwatsuru M, Prolongation of liposome circulation time by various derivatives of polyethyleneglycols, Biol Pharm Bull, 19 (1996) 1347–1351. [DOI] [PubMed] [Google Scholar]
- [33].Needham D, McIntosh TJ, Lasic DD, Repulsive interactions and mechanical stability of polymer-grafted lipid membranes, Biochim Biophys Acta, 1108 (1992) 40–48. [DOI] [PubMed] [Google Scholar]
- [34].Christian DA, Cai S, Garbuzenko OB, Harada T, Zajac AL, Minko T, Discher DE, Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage, Mol Pharm, 6 (2009) 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE, Shape effects of filaments versus spherical particles in flow and drug delivery, Nat Nanotechnol, 2 (2007) 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Preissler G, Loehe F, Huff IV, Ebersberger U, Shuvaev VV, Bittmann I, Hermanns I, Kirkpatrick JC, Fischer K, Eichhorn ME, Winter H, Jauch KW, Albelda SM, Muzykantov VR, Wiewrodt R, Targeted endothelial delivery of nanosized catalase immunoconjugates protects lung grafts donated after cardiac death, Transplantation, 92 (2011) 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weed RI, The importance of erythrocyte deformability, Am J Med, 49 (1970) 147–150. [DOI] [PubMed] [Google Scholar]
- [38].Rogausch H, Influence of erythrocyte deformability on circulation, Res Exp Med (Berl), 160 (1973) 89–94. [DOI] [PubMed] [Google Scholar]
- [39].Safeukui I, Buffet PA, Deplaine G, Perrot S, Brousse V, Sauvanet A, Aussilhou B, Dokmak S, Couvelard A, Cazals-Hatem D, Mercereau-Puijalon O, Milon G, David PH, Mohandas N, Sensing of red blood cells with decreased membrane deformability by the human spleen, Blood Adv, 2 (2018) 2581–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Duez J, Holleran JP, Ndour PA, Pionneau C, Diakite S, Roussel C, Dussiot M, Amireault P, Avery VM, Buffet PA, Mechanical clearance of red blood cells by the human spleen: Potential therapeutic applications of a biomimetic RBC filtration method, Transfus Clin Biol, 22 (2015) 151–157. [DOI] [PubMed] [Google Scholar]
- [41].Nicholson-Weller A, Burge J, Austen KF, Purification from guinea pig erythrocyte stroma of a decay-accelerating factor for the classical c3 convertase, C4b,2a, J Immunol, 127 (1981) 2035–2039. [PubMed] [Google Scholar]
- [42].Kinoshita T, Medof ME, Silber R, Nussenzweig V, Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria, J Exp Med, 162 (1985) 75–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nicholson-Weller A, Wang CE, Structure and function of decay accelerating factor CD55, J Lab Clin Med, 123 (1994) 485–491. [PubMed] [Google Scholar]
- [44].Zalman LS, Wood LM, Muller-Eberhard HJ, Isolation of a human erythrocyte membrane protein capable of inhibiting expression of homologous complement transmembrane channels, Proc Natl Acad Sci U S A, 83 (1986) 6975–6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Venneker GT, Asghar SS, CD59: a molecule involved in antigen presentation as well as downregulation of membrane attack complex, Exp Clin Immunogenet, 9 (1992) 33–47. [PubMed] [Google Scholar]
- [46].Freysdottir J, Sigfusson A, A flow cytometric assay for measuring complement receptor 1 (CR1) and the complement fragments C3d and C4d on erythrocytes, J Immunol Methods, 142 (1991) 45–52. [DOI] [PubMed] [Google Scholar]
- [47].Halbhuber KJ, Gliesing M, Stibenz D, Makovitzky J, Topo-optical investigations of the human erythrocyte glycocalyx-age related changes, Histochemistry, 81 (1984) 187–193. [DOI] [PubMed] [Google Scholar]
- [48].Zimmermann N, Halbhuber KJ, Linss W, Feuerstein H, Immunocytochemical investigations of the membrane of experimentally altered and physiologically aged erythrocytes, Acta Histochem Suppl, 33 (1986) 61–67. [PubMed] [Google Scholar]
- [49].Zimmerman N, [Immunocytochemical determination of membrane-bound IgG using physiologically aged and experimentally aged erythrocytes], Folia Haematol Int Mag Klin Morphol Blutforsch, 116 (1989) 683–691. [PubMed] [Google Scholar]
- [50].Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP, Role of CD47 as a marker of self on red blood cells, Science, 288 (2000) 2051–2054. [DOI] [PubMed] [Google Scholar]
- [51].Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE, Species- and cell type-specific interactions between CD47 and human SIRPalpha, Blood, 107 (2006) 2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khandelwal S, van Rooijen N, Saxena RK, Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation, Transfusion, 47 (2007) 1725–1732. [DOI] [PubMed] [Google Scholar]
- [53].Merkel TJ, Jones SW, Herlihy KP, Kersey FR, Shields AR, Napier M, Luft JC, Wu H, Zamboni WC, Wang AZ, Bear JE, DeSimone JM, Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles, Proc Natl Acad Sci U S A, 108 (2011) 586–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S, Red blood cell-mimicking synthetic biomaterial particles, Proc Natl Acad Sci U S A, 106 (2009) 21495–21499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE, Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles, Science, 339 (2013) 971–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hayat SMG, Jaafari MR, Hatamipour M, Penson PE, Sahebkar A, Liposome Circulation Time is Prolonged by CD47 Coating, Protein Pept Lett, 27 (2020) 1029–1037. [DOI] [PubMed] [Google Scholar]
- [57].Gheibi Hayat SM, Jaafari MR, Hatamipour M, Jamialahmadi T, Sahebkar A, Harnessing CD47 mimicry to inhibit phagocytic clearance and enhance anti-tumor efficacy of nanoliposomal doxorubicin, Expert Opin Drug Deliv, 17 (2020) 1049–1058. [DOI] [PubMed] [Google Scholar]
- [58].Gheibihayat SM, Jaafari MR, Hatamipour M, Sahebkar A, Improvement of the pharmacokinetic characteristics of liposomal doxorubicin using CD47 biomimickry, J Pharm Pharmacol, 73 (2021) 169–177. [DOI] [PubMed] [Google Scholar]
- [59].Gifford G, Vu VP, Banda NK, Holers VM, Wang G, Groman EV, Backos D, Scheinman R, Moghimi SM, Simberg D, Complement therapeutics meets nanomedicine: overcoming human complement activation and leukocyte uptake of nanomedicines with soluble domains of CD55, J Control Release, 302 (2019) 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hu CM, Fang RH, Luk BT, Chen KN, Carpenter C, Gao W, Zhang K, Zhang L, ‘Marker-of-self’ functionalization of nanoscale particles through a top-down cellular membrane coating approach, Nanoscale, 5 (2013) 2664–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Piao JG, Wang L, Gao F, You YZ, Xiong Y, Yang L, Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy, ACS Nano, 8 (2014) 10414–10425. [DOI] [PubMed] [Google Scholar]
- [62].Rao L, Xu JH, Cai B, Liu H, Li M, Jia Y, Xiao L, Guo SS, Liu W, Zhao XZ, Synthetic nanoparticles camouflaged with biomimetic erythrocyte membranes for reduced reticuloendothelial system uptake, Nanotechnology, 27 (2016) 085106. [DOI] [PubMed] [Google Scholar]
- [63].Rao L, Bu LL, Xu JH, Cai B, Yu GT, Yu X, He Z, Huang Q, Li A, Guo SS, Zhang WF, Liu W, Sun ZJ, Wang H, Wang TH, Zhao XZ, Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance, Small, 11 (2015) 6225–6236. [DOI] [PubMed] [Google Scholar]
- [64].AlQahtani SA, Harisa GI, Badran MM, AlGhamdi KM, Kumar A, Salem-Bekhit MM, Ahmad SF, Alanazi FK, Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines, Artif Cells Nanomed Biotechnol, 47 (2019) 989–996. [DOI] [PubMed] [Google Scholar]
- [65].Szoka F, Papahadjopoulos D, PROCEDURE FOR PREPARATION OF LIPOSOMES WITH LARGE INTERNAL AQUEOUS SPACE AND HIGH CAPTURE BY REVERSE-PHASE EVAPORATION, Proceedings of the National Academy of Sciences of the United States of America, 75 (1978) 4194–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mayhew E, Rustum Y, Szoka F, EFFICACY OF LIPOSOME-ENTRAPPED CYTOSINE-ARABINOSIDE (ARA-C) COMPARED WITH INFUSED FREE ARA-C AGAINST L1210 TUMOR, Proceedings of the American Association for Cancer Research, 21 (1980) 293–293. [Google Scholar]
- [67].Szoka F, Papahadjopoulos D, COMPARATIVE PROPERTIES AND METHODS OF PREPARATION OF LIPID VESICLES (LIPOSOMES), Annual Review of Biophysics and Bioengineering, 9 (1980) 467–508. [DOI] [PubMed] [Google Scholar]
- [68].Szoka FC, Jones CS, UPTAKE OF LIPOSOME-ENCAPSULATED AGENTS, Methods in Enzymology, 149 (1987) 143–147. [DOI] [PubMed] [Google Scholar]
- [69].Plank C, Mechtler K, Szoka FC, Wagner E, Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery, Human Gene Therapy, 7 (1996) 1437–1446. [DOI] [PubMed] [Google Scholar]
- [70].Xu YH, Szoka FC, Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection, Biochemistry, 35 (1996) 5616–5623. [DOI] [PubMed] [Google Scholar]
- [71].Zelphati O, Szoka FC, Liposomes as a carrier for intracellular delivery of antisense oligonucleotides: A real or magic bullet?, Journal of Controlled Release, 41 (1996) 99–119. [Google Scholar]
- [72].Legendre JY, Szoka FC, DELIVERY OF PLASMID DNA INTO MAMMALIAN-CELL LINES USING PH-SENSITIVE LIPOSOMES - COMPARISON WITH CATIONIC LIPOSOMES, Pharmaceutical Research, 9 (1992) 1235–1242. [DOI] [PubMed] [Google Scholar]
- [73].Kierstead PH, Okochi H, Venditto VJ, Chuong TC, Kivimae S, Frechet JMJ, Szoka FC, The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes, Journal of Controlled Release, 213 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kohli AG, Kierstead PH, Venditto VJ, Walsh CL, Szoka FC, Designer lipids for drug delivery: From heads to tails, Journal of Controlled Release, 190 (2014) 274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Guo X, MacKay JA, Szoka FC, Mechanism of pH-triggered collapse of phosphatidylethanolamine liposomes stabilized by an ortho ester polyethyleneglycol lipid, Biophysical Journal, 84 (2003) 1784–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Iman M, Huang ZH, Alavizadeh SH, Szoka FC, Jaafari MR, Biodistribution and In Vivo Antileishmanial Activity of 1,2-Distigmasterylhemisuccinoyl-sn-Glycero-3-Phosphocholine Liposome-Intercalated Amphotericin B, Antimicrobial Agents and Chemotherapy, 61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dolor A, Kierstead P, Dai ZP, Szoka FC, Sterol-modified PEG lipids: alteration of the bilayer anchoring moiety has an unexpected effect on liposome circulation, Chemical Communications, 54 (2018) 11949–11952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Eliaz RE, Nir S, Marty C, Szoka FC, Determination and modeling of kinetics of cancer cell killing by doxorubicin and doxorubicin encapsulated in targeted liposomes, Cancer Research, 64 (2004) 711–718. [DOI] [PubMed] [Google Scholar]
- [79].Eliaz RE, Szoka FC, Liposome-encapsulated doxorubicin targeted to CD44: A strategy to kill CD44-overexpressing tumor cells, Cancer Research, 61 (2001) 2592–2601. [PubMed] [Google Scholar]
- [80].Kohli AG, Kieler-Ferguson HM, Chan D, Szoka FC, A robust and quantitative method for tracking liposome contents after intravenous administration, Journal of Controlled Release, 176 (2014) 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li WJ, Szoka FC, Lipid-based nanoparticles for nucleic acid delivery, Pharmaceutical Research, 24 (2007) 438–449. [DOI] [PubMed] [Google Scholar]
- [82].MacKay JA, Deen DF, Szoka FC, Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating, Brain Research, 1035 (2005) 139–153. [DOI] [PubMed] [Google Scholar]
- [83].Peschka R, Dennehy C, Szoka FC, A simple in vitro model to study the release kinetics of liposome encapsulated material, Journal of Controlled Release, 56 (1998) 41–51. [DOI] [PubMed] [Google Scholar]
- [84].Platt VM, Szoka FC, Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor, Molecular Pharmaceutics, 5 (2008) 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sockolosky JT, Szoka FC, The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy, Advanced Drug Delivery Reviews, 91 (2015) 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Szoka FC, THE MACROPHAGE AS THE PRINCIPAL ANTIGEN-PRESENTING CELL FOR LIPOSOME-ENCAPSULATED ANTIGEN, Research in Immunology, 143 (1992) 186–188. [DOI] [PubMed] [Google Scholar]
- [87].Tang J, Srinivasan S, Yuan WM, Ming R, Liu YY, Dai ZP, Noble CO, Hayes ME, Zheng N, Jiang WL, Szoka FC, Schwendeman A, Development of a flow-through USP 4 apparatus drug release assay for the evaluation of amphotericin B liposome, European Journal of Pharmaceutics and Biopharmaceutics, 134 (2019) 107–116. [DOI] [PubMed] [Google Scholar]
- [88].Tran DT, Noble CO, Hayes ME, Szoka FC, Intravenous Administration of Liposome Encapsulated DFO Efficiently Removes Iron from the Liver and Spleen of Iron Overloaded Mice, Blood, 124 (2014). [Google Scholar]
- [89].Venditto VJ, Szoka FC, Cancer nanomedicines: So many papers and so few drugs!, Advanced Drug Delivery Reviews, 65 (2013) 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Xu YH, Hui SW, Frederik P, Szoka FC, Physicochemical characterization and purification of cationic lipoplexes, Biophysical Journal, 77 (1999) 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Eloy JO, de Souza MC, Petrilli R, Barcellos JPA, Lee RJ, Marchetti JM, Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery, Colloids and Surfaces B-Biointerfaces, 123 (2014) 345–363. [DOI] [PubMed] [Google Scholar]
- [92].Haran G, Cohen R, Bar LK, Barenholz Y, TRANSMEMBRANE AMMONIUM-SULFATE GRADIENTS IN LIPOSOMES PRODUCE EFFICIENT AND STABLE ENTRAPMENT OF AMPHIPATHIC WEAK BASES, Biochimica Et Biophysica Acta, 1151 (1993) 201–215. [DOI] [PubMed] [Google Scholar]
- [93].Barenholz Y, Doxil (R) - The first FDA-approved nano-drug: Lessons learned, Journal of Controlled Release, 160 (2012) 117–134. [DOI] [PubMed] [Google Scholar]
- [94].Cern A, Golbraikh A, Sedykh A, Tropsha A, Barenholz Y, Goldblum A, Quantitative structure - property relationship modeling of remote liposome loading of drugs, Journal of Controlled Release, 160 (2012) 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cern A, Marcus D, Tropsha A, Barenholz Y, Goldblum A, New drug candidates for liposomal delivery identified by computer modeling of liposomes’ remote loading and leakage, Journal of Controlled Release, 252 (2017) 18–27. [DOI] [PubMed] [Google Scholar]
- [96].Park K, Rational drug loading of liposomes revisited, Journal of Controlled Release, 252 (2017) 125–125. [DOI] [PubMed] [Google Scholar]
- [97].Abu Lila AS, Ishida T, Liposomal Delivery Systems: Design Optimization and Current Applications, Biological & Pharmaceutical Bulletin, 40 (2017) 1–10. [DOI] [PubMed] [Google Scholar]
- [98].Ahmed KS, Hussein SA, Ali AH, Korma SA, Qiu LP, Chen JH, Liposome: composition, characterisation, preparation, and recent innovation in clinical applications, Journal of Drug Targeting, 27 (2019) 742–761. [DOI] [PubMed] [Google Scholar]
- [99].Allen TM, Cullis PR, Liposomal drug delivery systems: From concept to clinical applications, Advanced Drug Delivery Reviews, 65 (2013) 36–48. [DOI] [PubMed] [Google Scholar]
- [100].Gubernator J, Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity, Expert Opinion on Drug Delivery, 8 (2011) 565–580. [DOI] [PubMed] [Google Scholar]
- [101].Has C, Pan SR, Vesicle formation mechanisms: an overview, Journal of Liposome Research, (2020). [DOI] [PubMed] [Google Scholar]
- [102].Patil YP, Jadhav S, Novel methods for liposome preparation, Chem Phys Lipids, 177 (2014) 8–18. [DOI] [PubMed] [Google Scholar]
- [103].Torchilin VP, Multifunctional nanocarriers, Advanced Drug Delivery Reviews, 58 (2006) 1532–1555. [DOI] [PubMed] [Google Scholar]
- [104].Torchilin VP, Multifunctional nanocarriers, Advanced Drug Delivery Reviews, 64 (2012) 302–315. [DOI] [PubMed] [Google Scholar]
- [105].Lasic DD, THE MECHANISM OF VESICLE FORMATION, Biochemical Journal, 256 (1988) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mayer LD, Hope MJ, Cullis PR, Janoff AS, SOLUTE DISTRIBUTIONS AND TRAPPING EFFICIENCIES OBSERVED IN FREEZE-THAWED MULTILAMELLAR VESICLES, Biochimica Et Biophysica Acta, 817 (1985) 193–196. [DOI] [PubMed] [Google Scholar]
- [107].Pattni BS, Chupin VV, Torchilin VP, New Developments in Liposomal Drug Delivery, Chemical Reviews, 115 (2015) 10938–10966. [DOI] [PubMed] [Google Scholar]
- [108].Senior J, Gregoriadis G, Dehydration-rehydration vesicle methodology facilitates a novel approach to antibody binding to liposomes, Biochim Biophys Acta, 1003 (1989) 58–62. [DOI] [PubMed] [Google Scholar]
- [109].Cortesi R, Esposito E, Gambarin S, Telloli P, Menegatti E, Nastruzzi C, Preparation of liposomes by reverse-phase evaporation using alternative organic solvents, J Microencapsul, 16 (1999) 251–256. [DOI] [PubMed] [Google Scholar]
- [110].Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB, Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles, Biochimica Et Biophysica Acta-Reviews on Biomembranes, 1331 (1997) 187–211. [DOI] [PubMed] [Google Scholar]
- [111].Madden TD, Harrigan PR, Tai LC, Bally MB, Mayer LD, Redelmeier TE, Loughrey HC, Tilcock CP, Reinish LW, Cullis PR, The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: a survey, Chem Phys Lipids, 53 (1990) 37–46. [DOI] [PubMed] [Google Scholar]
- [112].Barenholz Y, Relevancy of drug loading to liposomal formulation therapeutic efficacy, Journal of Liposome Research, 13 (2003) 1–8. [DOI] [PubMed] [Google Scholar]
- [113].Avnir Y, Ulmansky R, Wasserman V, Even-Chen S, Broyer M, Barenholz Y, Naparstek Y, Amphipathic weak acid glucocorticoid Prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a beagle dog, Arthritis and Rheumatism, 58 (2008) 119–129. [DOI] [PubMed] [Google Scholar]
- [114].Sur S, Fries AC, Kinzler KW, Zhou SB, Vogelstein B, Remote loading of preencapsulated drugs into stealth liposomes, Proceedings of the National Academy of Sciences of the United States of America, 111 (2014) 2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Aloisio C, Antimisiaris SG, Longhi MR, Liposomes containing cyclodextrins or meglumine to solubilize and improve the bioavailability of poorly soluble drugs, Journal of Molecular Liquids, 229 (2017) 106–113. [Google Scholar]
- [116].Modi S, Xiang TX, Anderson BD, Enhanced active liposomal loading of a poorly soluble ionizable drug using supersaturated drug solutions, Journal of Controlled Release, 162 (2012) 330–339. [DOI] [PubMed] [Google Scholar]
- [117].Zadi B, Gregoriadis G, A Novel Method for High-Yield Entrapment of Solutes into Small Liposomes, Journal of Liposome Research, 10 (2000) 73–80. [Google Scholar]
- [118].Xu X, Costa A, Burgess DJ, Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction, Pharm Res, 29 (2012) 1919–1931. [DOI] [PubMed] [Google Scholar]
- [119].Kraft JC, Freeling JP, Wang ZY, Ho RJY, Emerging Research and Clinical Development Trends of Liposome and Lipid Nanoparticle Drug Delivery Systems, Journal of Pharmaceutical Sciences, 103 (2014) 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Howard M, Jay M, Dziubla T, Lu X, PEGylation of Nanocarrier Drug Delivery Systems: State of the Art, Journal of Biomedical Nanotechnology, 4 (2008) 133–148. [Google Scholar]
- [121].Kristensen K, Engel TB, Stensballe A, Simonsen JB, Andresen TL, The hard protein corona of stealth liposomes is sparse, Journal of Controlled Release, 307 (2019) 1–15. [DOI] [PubMed] [Google Scholar]
- [122].Mohamed M, Abu Lila AS, Shimizu T, Alaaeldin E, Hussein A, Sarhan HA, Szebeni J, Ishida T, PEGylated liposomes: immunological responses, Science and Technology of Advanced Materials, 20 (2019) 710–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Deodhar S, Dash AK, Long circulating liposomes: challenges and opportunities, Therapeutic Delivery, 9 (2018). [DOI] [PubMed] [Google Scholar]
- [124].Allen TM, Brandeis E, Hansen CB, Kao GY, Zalipsky S, A NEW STRATEGY FOR ATTACHMENT OF ANTIBODIES TO STERICALLY STABILIZED LIPOSOMES RESULTING IN EFFICIENT TARGETING TO CANCER-CELLS, Biochimica Et Biophysica Acta-Biomembranes, 1237 (1995) 99–108. [DOI] [PubMed] [Google Scholar]
- [125].Blume G, Cevc G, Crommelin M, Bakkerwoudenberg I, Kluft C, Storm G, SPECIFIC TARGETING WITH POLY(ETHYLENE GLYCOL)-MODIFIED LIPOSOMES - COUPLING OF HOMING DEVICES TO THE ENDS OF THE POLYMERIC CHAINS COMBINES EFFECTIVE TARGET BINDING WITH LONG CIRCULATION TIMES, Biochimica Et Biophysica Acta, 1149 (1993) 180–184. [DOI] [PubMed] [Google Scholar]
- [126].Maurer N, Fenske DB, Cullis PR, Developments in liposomal drug delivery systems, Expert Opin Biol Ther, 1 (2001) 923–947. [DOI] [PubMed] [Google Scholar]
- [127].Sawant RR, Torchilin VP, Liposomes as ‘smarť pharmaceutical nanocarriers, Soft Matter, 6 (2010) 4026–4044. [Google Scholar]
- [128].Sawant RR, Torchilin VP, Challenges in Development of Targeted Liposomal Therapeutics, Aaps Journal, 14 (2012) 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Torchilin V, Multifunctional and stimuli-sensitive pharmaceutical nanocarriers, European Journal of Pharmaceutics and Biopharmaceutics, 71 (2009) 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Eloy JO, Petrilli R, Trevizan LNF, Chorilli M, Immunoliposomes: A review on functionalization strategies and targets for drug delivery, Colloids and Surfaces B-Biointerfaces, 159 (2017) 454–467. [DOI] [PubMed] [Google Scholar]
- [131].Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B, Ligand-targeted liposome design: challenges and fundamental considerations, Trends Biotechnol, 32 (2014) 32–45. [DOI] [PubMed] [Google Scholar]
- [132].Hermanson GT, Chapter 18 - PEGylation and Synthetic Polymer Modification, in: Hermanson GT (Ed.) Bioconjugate Techniques (Third Edition), Academic Press, Boston, 2013, pp. 787–838. [Google Scholar]
- [133].Hermanson GT, Chapter 3 - The Reactions of Bioconjugation, in: Hermanson GT (Ed.) Bioconjugate Techniques (Third Edition), Academic Press, Boston, 2013, pp. 229–258. [Google Scholar]
- [134].Blenke EO, Klaasse G, Merten H, Pluckthun A, Mastrobattista E, Liposome functionalization with copper-free “click chemistry”, Journal of Controlled Release, 202 (2015) 14–20. [DOI] [PubMed] [Google Scholar]
- [135].Hood ED, Greineder CF, Shuvaeva T, Walsh L, Villa CH, Muzykantov VR, Vascular targeting of radiolabeled liposomes with bio-orthogonally conjugated ligands: single chain fragments provide higher specificity than antibodies, Bioconjug Chem, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Howard MD, Greineder CF, Hood ED, Muzykantov VR, Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation, Journal of Controlled Release, 177 (2014) 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K, Liposome: classification, preparation, and applications, Nanoscale Research Letters, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bak M, Jolck RI, Eliasen R, Andresen TL, Affinity Induced Surface Functionalization of Liposomes Using Cu-Free Click Chemistry, Bioconjugate Chemistry, 27 (2016) 1673–1680. [DOI] [PubMed] [Google Scholar]
- [139].Guo XQ, Wu ZM, Guo ZW, New Method for Site-Specific Modification of Liposomes with Proteins Using Sortase A-Mediated Transpeptidation, Bioconjugate Chemistry, 23 (2012) 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Hassane FS, Frisch B, Schuber F, Targeted liposomes: Convenient coupling of ligands to preformed vesicles using “click chemistry”, Bioconjugate Chemistry, 17 (2006) 849–854. [DOI] [PubMed] [Google Scholar]
- [141].Hood ED, Greineder CF, Dodia C, Han J, Mesaros C, Shuvaev VV, Blair IA, Fisher AB, Muzykantov VR, Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo, J Control Release, 163 (2012) 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Cheng WW, Allen TM, The use of single chain Fv as targeting agents for immunoliposomes: an update on immunoliposomal drugs for cancer treatment, Expert Opinion on Drug Delivery, 7 (2010) 461–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Koren E, Apte A, Jani A, Torchilin VP, Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity, Journal of Controlled Release, 160 (2012) 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Li XM, Ding LY, Xu YL, Wang YL, Ping QN, Targeted delivery of doxorubicin using stealth liposomes modified with transferrin, International Journal of Pharmaceutics, 373 (2009) 116–123. [DOI] [PubMed] [Google Scholar]
- [145].Suzuki R, Takizawa T, Kuwata Y, Mutoh M, Ishiguro N, Utoguchi N, Shinohara A, Eriguchi M, Yanagie H, Maruyama K, Effective anti-tumor activity of oxaliplatin encapsulated in transferrin-PEG-liposome, International Journal of Pharmaceutics, 346 (2008) 143–150. [DOI] [PubMed] [Google Scholar]
- [146].Xu L, Huang CC, Huang WQ, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM, Pirollo KF, Chang EH, Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes, Molecular Cancer Therapeutics, 1 (2002) 337–346. [PubMed] [Google Scholar]
- [147].Arpicco S, Lerda C, Pozza ED, Costanzo C, Tsapis N, Stella B, Donadelli M, Dando I, Fattal E, Cattel L, Palmieri M, Hyaluronic acid-coated liposomes for active targeting of gemcitabine, European Journal of Pharmaceutics and Biopharmaceutics, 85 (2013) 373–380. [DOI] [PubMed] [Google Scholar]
- [148].Gabizon A, Horowitz AT, Goren D, Tzemach D, Mandelbaum-Shavit F, Qazen MM, Zalipsky S, Targeting folate receptor with folate linked to extremities of poly(ethylene glycol-grafted liposomes: In vitro studies, Bioconjugate Chemistry, 10 (1999) 289–298. [DOI] [PubMed] [Google Scholar]
- [149].Kim EM, Jeong HJ, Liposomes: Biomedical Applications, Chonnam Med J, 57 (2021) 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Li Z, Tan S, Li S, Shen Q, Wang K, Cancer drug delivery in the nano era: An overview and perspectives (Review), Oncol Rep, 38 (2017) 611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Ventola CL, Progress in Nanomedicine: Approved and Investigational Nanodrugs, P T, 42 (2017) 742–755. [PMC free article] [PubMed] [Google Scholar]
- [152].Beltrán-Gracia E, López-Camacho A, Higuera-Ciapara I, Velázquez-Fernández JB, Vallejo-Cardona AA, Nanomedicine review: clinical developments in liposomal applications, Cancer Nanotechnology, 10 (2019) 1–40. [Google Scholar]
- [153].Anselmo AC, Mitragotri S, Nanoparticles in the clinic: An update, Bioeng Transl Med, 4 (2019) e10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Filipczak N, Pan J, Yalamarty SSK, Torchilin VP, Recent advancements in liposome technology, Adv Drug Deliv Rev, 156 (2020) 4–22. [DOI] [PubMed] [Google Scholar]
- [155].Alvarez FJ, Herraez A, Murciano JC, Jordan JA, Diez JC, Tejedor MC, In vivo survival and organ uptake of loaded carrier rat erythrocytes, J Biochem, 120 (1996) 286–291. [DOI] [PubMed] [Google Scholar]
- [156].Perez MT, Alvarez FJ, Garcia-Perez AI, Lucas L, Tejedor MC, Sancho P, Heterogeneity of hypotonically loaded rat erythrocyte populations as detected by counter-current distribution in aqueous polymer two-phase systems, J Chromatogr B Biomed Appl, 677 (1996) 45–51. [DOI] [PubMed] [Google Scholar]
- [157].Ktavtzoff R, Desbois I, Doinel C, Colombat P, Lamagnere JP, Chassaigne M, Ropars C, Immunological response to L-asparaginase loaded into red blood cells, Adv Exp Med Biol, 326 (1992) 175–182. [DOI] [PubMed] [Google Scholar]
- [158].Rossi L, Serafini S, Cappellacci L, Balestra E, Brandi G, Schiavano GF, Franchetti P, Grifantini M, Perno CF, Magnani M, Erythrocyte-mediated delivery of a new homodinucleotide active against human immunodeficiency virus and herpes simplex virus, J Antimicrob Chemother, 47 (2001) 819–827. [DOI] [PubMed] [Google Scholar]
- [159].Tonetti M, Astroff B, Satterfield W, De Flora A, Benatti U, DeLoach JR, Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system, Biotechnol Appl Biochem, 12 (1990) 621–629. [PubMed] [Google Scholar]
- [160].Kim SH, Kim EJ, Hou JH, Kim JM, Choi HG, Shim CK, Oh YK, Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides, Biomaterials, 30 (2009) 959–967. [DOI] [PubMed] [Google Scholar]
- [161].Teisseire B, Ropars C, Villereal MC, Nicolau C, Long-term physiological effects of enhanced O2 release by inositol hexaphosphate-loaded erythrocytes, Proc Natl Acad Sci U S A, 84 (1987) 6894–6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Bax BE, Bain MD, Fairbanks LD, Webster AD, Chalmers RA, In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase, Br J Haematol, 109 (2000) 549–554. [DOI] [PubMed] [Google Scholar]
- [163].Hambye AS, Verbeke KA, Vandermeiren RP, Joosens EJ, Verbruggen AM, De Roo MJ, Comparison of modified technetium-99m albumin and technetium-99m red blood cells for equilibrium ventriculography, J Nucl Med, 38 (1997) 1521–1528. [PubMed] [Google Scholar]
- [164].Kravtzoff R, Colombat PH, Desbois I, Linassier C, Muh JP, Philip T, Blay JY, Gardenbas M, Poumier-Gaschard P, Lamagnere JP, Chassaigne M, Ropars C, Tolerance evaluation of L-asparaginase loaded in red blood cells, Eur J Clin Pharmacol, 51 (1996) 221–225. [DOI] [PubMed] [Google Scholar]
- [165].Kravtzoff R, Desbois I, Lamagnere JP, Muh JP, Valat C, Chassaigne M, Colombat P, Ropars C, Improved pharmacodynamics of L-asparaginase-loaded in human red blood cells, Eur J Clin Pharmacol, 49 (1996) 465–470. [DOI] [PubMed] [Google Scholar]
- [166].Hinderling PH, Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics, Pharmacol Rev, 49 (1997) 279–295. [PubMed] [Google Scholar]
- [167].Muzykantov VR, Zaltsman AB, Smirnov MD, Samokhin GP, Morgan BP, Target-sensitive immunoerythrocytes: interaction of biotinylated red blood cells with immobilized avidin induces their lysis by complement, Biochim Biophys Acta, 1279 (1996) 137–143. [DOI] [PubMed] [Google Scholar]
- [168].Beppu M, Mizukami A, Nagoya M, Kikugawa K, Binding of anti-band 3 autoantibody to oxidatively damaged erythrocytes. Formation of senescent antigen on erythrocyte surface by an oxidative mechanism, J Biol Chem, 265 (1990) 3226–3233. [PubMed] [Google Scholar]
- [169].Chiarantini L, Droleskey R, Magnani M, DeLoach JR, In vitro targeting of erythrocytes to cytotoxic T-cells by coupling of Thy-1.2 monoclonal antibody, Biotechnol Appl Biochem, 15 (1992) 171–184. [PubMed] [Google Scholar]
- [170].Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM, Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide, Am J Pathol, 128 (1987) 276–285. [PMC free article] [PubMed] [Google Scholar]
- [171].Muzykantov VR, Smirnov MD, Zaltzman AB, Samokhin GP, Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement, Anal Biochem, 208 (1993) 338–342. [DOI] [PubMed] [Google Scholar]
- [172].Godfrey W, Doe B, Wallace EF, Bredt B, Wofsy L, Affinity targeting of membrane vesicles to cell surfaces, Exp Cell Res, 135 (1981) 137–145. [DOI] [PubMed] [Google Scholar]
- [173].Orr GA, The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components, J Biol Chem, 256 (1981) 761–766. [PubMed] [Google Scholar]
- [174].Roffman E, Meromsky L, Ben-Hur H, Bayer EA, Wilchek M, Selective labeling of functional groups on membrane proteins or glycoproteins using reactive biotin derivatives and 125I-streptavidin, Biochem Biophys Res Commun, 136 (1986) 80–85. [DOI] [PubMed] [Google Scholar]
- [175].Bayer EA, Safars M, Wilchek M, Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes, Anal Biochem, 161 (1987) 262–271. [DOI] [PubMed] [Google Scholar]
- [176].Wilchek M, Ben-Hur H, Bayer EA, p-Diazobenzoyl biocytin--a new biotinylating reagent for the labeling of tyrosines and histidines in proteins, Biochem Biophys Res Commun, 138 (1986) 872–879. [DOI] [PubMed] [Google Scholar]
- [177].Muzykantov VR, Smirnov MD, Klibanov AL, Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes, J Immunol Methods, 158 (1993) 183–190. [DOI] [PubMed] [Google Scholar]
- [178].Samokhin GP, Smirnov MD, Muzykantov VR, Domogatsky SP, Smirnov VN, Red blood cell targeting to collagen-coated surfaces, FEBS Lett, 154 (1983) 257–261. [DOI] [PubMed] [Google Scholar]
- [179].Cowley H, Wojda U, Cipolone KM, Procter JL, Stroncek DF, Miller JL, Biotinylation modifies red cell antigens, Transfusion, 39 (1999) 163–168. [DOI] [PubMed] [Google Scholar]
- [180].Magnani M, Chiarantini L, Mancini U, Preparation and characterization of biotinylated red blood cells, Biotechnol Appl Biochem, 20 (1994) 335–345. [DOI] [PubMed] [Google Scholar]
- [181].Magnani M, Rossi L, D’Ascenzo M, Panzani I, Bigi L, Zanella A, Erythrocyte engineering for drug delivery and targeting, Biotechnol Appl Biochem, 28 (1998) 1–6. [PubMed] [Google Scholar]
- [182].Muzykantov VR, Sakharov DV, Smirnov MD, Domogatsky SP, Samokhin GP, Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface, FEBS Lett, 182 (1985) 62–66. [DOI] [PubMed] [Google Scholar]
- [183].Smirnov MD, Samokhin GP, Muzykantov VR, Idelson GL, Domogatsky SP, Smirnov VN, Type I and III collagens as a possible target for drug delivery to the injured sites of vascular bed, Biochem Biophys Res Commun, 116 (1983) 99–105. [DOI] [PubMed] [Google Scholar]
- [184].Muzykantov VR, Smirnov MD, Samokhin GP, Streptavidin-induced lysis of homologous biotinylated erythrocytes. Evidence against the key role of the avidin charge in complement activation via the alternative pathway, FEBS Lett, 280 (1991) 112–114. [DOI] [PubMed] [Google Scholar]
- [185].Medof ME, Kinoshita T, Nussenzweig V, Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes, J Exp Med, 160 (1984) 1558–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hoffman JF, On red blood cells, hemolysis and resealed ghosts, Adv Exp Med Biol, 326 (1992) 1–15. [DOI] [PubMed] [Google Scholar]
- [187].Muzykantov VR, Smirnov MD, Samokhin GP, Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement, Blood, 78 (1991) 2611–2618. [PubMed] [Google Scholar]
- [188].Muzykantov VR, Seregina N, Smirnov MD, Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes, Int J Artif Organs, 15 (1992) 622–627. [PubMed] [Google Scholar]
- [189].Muzykantov VR, Smirnov MD, Klibanov AL, Avidin attachment to biotinylated amino groups of the erythrocyte membrane eliminates homologous restriction of both classical and alternative pathways of the complement, FEBS Lett, 318 (1993) 108–112. [DOI] [PubMed] [Google Scholar]
- [190].Muzykantov VR, Murciano JC, Attachment of antibody to biotinylated red blood cells: immuno-red blood cells display high affinity to immobilized antigen and normal biodistribution in rats, Biotechnol Appl Biochem, 24 (1996) 41–45. [PubMed] [Google Scholar]
- [191].Muzykantov VR, Taylor RP, Attachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complement, Anal Biochem, 223 (1994) 142–148. [DOI] [PubMed] [Google Scholar]
- [192].Muzykantov VR, Smirnov MD, Samokhin GP, Avidin acylation prevents the complement-dependent lysis of avidin-carrying erythrocytes, Biochem J, 273(Pt 2) (1991) 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Zaltzman AB, Van den Berg CW, Muzykantov VR, Morgan BP, Enhanced complement susceptibility of avidin-biotin-treated human erythrocytes is a consequence of neutralization of the complement regulators CD59 and decay accelerating factor, Biochem J, 307 (Pt 3) (1995) 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Muzykantov VR, Smirnov MD, Samokhin GP, Avidin-induced lysis of biotinylated erythrocytes by homologous complement via the alternative pathway depends on avidin’s ability of multipoint binding with biotinylated membrane, Biochim Biophys Acta, 1107 (1992) 119–125. [DOI] [PubMed] [Google Scholar]
- [195].Muzykantov VR, Murciano JC, Taylor RP, Atochina EN, Herraez A, Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin, Anal Biochem, 241 (1996) 109–119. [DOI] [PubMed] [Google Scholar]
- [196].Suzuki K, Okumura Y, GPI-linked proteins do not transfer spontaneously from erythrocytes to liposomes. New aspects of reorganization of the cell membrane, Biochemistry, 39 (2000) 9477–9485. [DOI] [PubMed] [Google Scholar]
- [197].Civenni G, Test ST, Brodbeck U, Butikofer P, In vitro incorporation of GPI-anchored proteins into human erythrocytes and their fate in the membrane, Blood, 91 (1998) 1784–1792. [PubMed] [Google Scholar]
- [198].Medof ME, Nagarajan S, Tykocinski ML, Cell-surface engineering with GPI-anchored proteins, FASEB J, 10 (1996) 574–586. [DOI] [PubMed] [Google Scholar]
- [199].Hill A, Ridley SH, Esser D, Oldroyd RG, Cullen MJ, Kareclas P, Gallagher S, Smith GP, Richards SJ, White J, Smith RA, Hillmen P, Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy, Blood, 107 (2006) 2131–2137. [DOI] [PubMed] [Google Scholar]
- [200].Chen R, Walter EI, Parker G, Lapurga JP, Millan JL, Ikehara Y, Udenfriend S, Medof ME, Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation, Proc Natl Acad Sci U S A, 95 (1998) 9512–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Kooyman DL, Byrne GW, McClellan S, Nielsen D, Tone M, Waldmann H, Coffman TM, McCurry KR, Platt JL, Logan JS, In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium, Science, 269 (1995) 89–92. [DOI] [PubMed] [Google Scholar]
- [202].Mukthavaram R, Shi G, Kesari S, Simberg D, Targeting and depletion of circulating leukocytes and cancer cells by lipophilic antibody-modified erythrocytes, J Control Release, 183 (2014) 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Zhang X, Luo M, Dastagir SR, Nixon M, Khamhoung A, Schmidt A, Lee A, Subbiah N, McLaughlin DC, Moore CL, Gribble M, Bayhi N, Amin V, Pepi R, Pawar S, Lyford TJ, Soman V, Mellen J, Carpenter CL, Turka LA, Wickham TJ, Chen TF, Engineered red blood cells as an off-the-shelf allogeneic anti-tumor therapeutic, Nat Commun, 12 (2021) 2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Russo V, Barker-Gear R, Gates R, Franco R, Studies with biotinylated RBC: (1) use of flow cytometry to determine posttransfusion survival and (2) isolation using streptavidin conjugated magnetic beads, Adv Exp Med Biol, 326 (1992) 101–107. [DOI] [PubMed] [Google Scholar]
- [205].Mock DM, Matthews NI, Zhu S, Strauss RG, Schmidt RL, Nalbant D, Cress GA, Widness JA, Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities, Transfusion, 51 (2011) 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Mock DM, Widness JA, Veng-Pedersen P, Strauss RG, Cancelas JA, Cohen RM, Lindsell CJ, Franco RS, Measurement of posttransfusion red cell survival with the biotin label, Transfus Med Rev, 28 (2014) 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Landsteiner K, Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymphe, Zentralbl. Bakteriol, 27 (1900) 357–362. [Google Scholar]
- [208].Landsteiner K, Levine P, On Individual Differences in Human Blood, J Exp Med, 47 (1928) 757–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Villa CH, Pan DC, Johnston IH, Greineder CF, Walsh LR, Hood ED, Cines DB, Poncz M, Siegel DL, Muzykantov VR, Biocompatible coupling of therapeutic fusion proteins to human erythrocytes, Blood Adv, 2 (2018) 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Chiarantini L, Argnani R, Zucchini S, Stevanato L, Zabardi P, Grossi MP, Magnani M, Manservigi R, Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice, Vaccine, 15 (1997) 276–280. [DOI] [PubMed] [Google Scholar]
- [211].Muzykantov VR, Sakharov DV, Smirnov MD, Samokhin GP, Smirnov VN, Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot, Biochim Biophys Acta, 884 (1986) 355–362. [DOI] [PubMed] [Google Scholar]
- [212].Smirnov VN, Domogatsky SP, Dolgov VV, Hvatov VB, Klibanov AL, Koteliansky VE, Muzykantov VR, Repin VS, Samokhin GP, Shekhonin BV, et al. , Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall, Proc Natl Acad Sci U S A, 83 (1986) 6603–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Gyimesi E, Bankovich AJ, Schuman TA, Goldberg JB, Lindorfer MA, Taylor RP, Staphylococcus aureus bound to complement receptor 1 on human erythrocytes by bispecific monoclonal antibodies is phagocytosed by acceptor macrophages, Immunol Lett, 95 (2004) 185–192. [DOI] [PubMed] [Google Scholar]
- [214].Kuhn SE, Nardin A, Klebba PE, Taylor RP, Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model, J Immunol, 160 (1998) 5088–5097. [PubMed] [Google Scholar]
- [215].Taylor RP, Sutherland WM, Reist CJ, Webb DJ, Wright EL, Labuguen RH, Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment, Proc Natl Acad Sci U S A, 88 (1991) 3305–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Spitzer D, Unsinger J, Bessler M, Atkinson JP, ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement, Mol Immunol, 40 (2004) 911–919. [DOI] [PubMed] [Google Scholar]
- [217].Muller M, Buchi L, Woodtli K, Haeberli A, Beer JH, Preparation and characterization of ‘heparinocytes’: erythrocytes with covalently bound low molecular weight heparin, FEBS Lett, 468 (2000) 115–119. [DOI] [PubMed] [Google Scholar]
- [218].Holvoet P, Laroche Y, Stassen JM, Lijnen HR, Van Hoef B, De Cock F, Van Houtven A, Gansemans Y, Matthyssens G, Collen D, Pharmacokinetic and thrombolytic properties of chimeric plasminogen activators consisting of a single-chain Fv fragment of a fibrin-specific antibody fused to single-chain urokinase, Blood, 81 (1993) 696–703. [PubMed] [Google Scholar]
- [219].Topol EJ, Morris DC, Smalling RW, Schumacher RR, Taylor CR, Nishikawa A, Liberman HA, Collen D, Tufte ME, Grossbard EB, et al. , A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction, J Am Coll Cardiol, 9 (1987) 1205–1213. [DOI] [PubMed] [Google Scholar]
- [220].Narita M, Bu G, Herz J, Schwartz AL, Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo, J Clin Invest, 96 (1995) 1164–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Collen D, Van Hoef B, Schlott B, Hartmann M, Guhrs KH, Lijnen HR, Mechanisms of activation of mammalian plasma fibrinolytic systems with streptokinase and with recombinant staphylokinase, Eur J Biochem, 216 (1993) 307–314. [DOI] [PubMed] [Google Scholar]
- [222].Holvoet P, Dewerchin M, Stassen JM, Lijnen HR, Tollenaere T, Gaffney PJ, Collen D, Thrombolytic profiles of clot-targeted plasminogen activators. Parameters determining potency and initial and maximal rates, Circulation, 87 (1993) 1007–1016. [DOI] [PubMed] [Google Scholar]
- [223].Muzykantov VR, Barnathan ES, Atochina EN, Kuo A, Danilov SM, Fisher AB, Targeting of antibody-conjugated plasminogen activators to the pulmonary vasculature, J Pharmacol Exp Ther, 279 (1996) 1026–1034. [PubMed] [Google Scholar]
- [224].Runge MS, Harker LA, Bode C, Ruef J, Kelly AB, Marzec UM, Allen E, Caban R, Shaw SY, Haber E, Hanson SR, Enhanced thrombolytic and antithrombotic potency of a fibrin-targeted plasminogen activator in baboons, Circulation, 94 (1996) 1412–1422. [DOI] [PubMed] [Google Scholar]
- [225].Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA, Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice, Nat Med, 4 (1998) 228–231. [DOI] [PubMed] [Google Scholar]
- [226].Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM, Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions, J Clin Invest, 89 (1992) 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Gersh KC, Zaitsev S, Muzykantov V, Cines DB, Weisel JW, The spatial dynamics of fibrin clot dissolution catalyzed by erythrocyte-bound vs. free fibrinolytics, J Thromb Haemost, 8 (2010) 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Gersh KC, Zaitsev S, Cines DB, Muzykantov V, Weisel JW, Flow-dependent channel formation in clots by an erythrocyte-bound fibrinolytic agent, Blood, 117 (2011) 4964–4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Ganguly K, Krasik T, Medinilla S, Bdeir K, Cines DB, Muzykantov VR, Murciano JC, Blood clearance and activity of erythrocyte-coupled fibrinolytics, J Pharmacol Exp Ther, 312 (2005) 1106–1113. [DOI] [PubMed] [Google Scholar]
- [230].Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR, Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes, Nat Biotechnol, 21 (2003) 891–896. [DOI] [PubMed] [Google Scholar]
- [231].Armstead WM, Christine AJ, Higazi AA, Cines DB, Urokinase plasminogen activator impairs SNP and PGE2 cerebrovasodilation after brain injury through activation of LRP and ERK MAPK, J Neurotrauma, 25 (2008) 1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Armstead WM, Cines DB, Higazi AA, Plasminogen activators contribute to impairment of hypercapnic and hypotensive cerebrovasodilation after cerebral hypoxia/ischemia in the newborn pig, Stroke, 36 (2005) 2265–2269. [DOI] [PubMed] [Google Scholar]
- [233].Armstead WM, Ganguly K, Kiessling JW, Chen XH, Smith DH, Higazi AA, Cines DB, Bdeir K, Zaitsev S, Muzykantov VR, Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation, J Cereb Blood Flow Metab, 29 (2009) 1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Armstead WM, Ganguly K, Riley J, Kiessling JW, Cines DB, Higazi AA, Zaitsev S, Muzykantov VR, Red blood cell-coupled tissue plasminogen activator prevents impairment of cerebral vasodilatory responses through inhibition of c-Jun-N-terminal kinase and potentiation of p38 mitogen-activated protein kinase after cerebral photothrombosis in the newborn pig, Pediatr Crit Care Med, 12 (2011) e369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Ganguly K, Murciano JC, Westrick R, Leferovich J, Cines DB, Muzykantov VR, The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition, J Pharmacol Exp Ther, 321 (2007) 158–164. [DOI] [PubMed] [Google Scholar]
- [236].Murciano JC, Higazi AA, Cines DB, Muzykantov VR, Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile, J Control Release, 139 (2009) 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Danielyan K, Ganguly K, Ding BS, Atochin D, Zaitsev S, Murciano JC, Huang PL, Kasner SE, Cines DB, Muzykantov VR, Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator, Circulation, 118 (2008) 1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Pisapia JM, Xu X, Kelly J, Yeung J, Carrion G, Tong H, Meghan S, El-Falaky OM, Grady MS, Smith DH, Zaitsev S, Muzykantov VR, Stiefel MF, Stein SC, Microthrombosis after experimental subarachnoid hemorrhage: time course and effect of red blood cell-bound thrombin-activated pro-urokinase and clazosentan, Exp Neurol, 233 (2012) 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Stein SC, Ganguly K, Belfield CM, Xu X, Swanson EW, Chen XH, Browne KD, Johnson VE, Smith DH, LeBold DG, Cines DB, Muzykantov VR, Erythrocyte-bound tissue plasminogen activator is neuroprotective in experimental traumatic brain injury, J Neurotrauma, 26 (2009) 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Biagiotti S, Rossi L, Bianchi M, Giacomini E, Pierige F, Serafini G, Conaldi PG, Magnani M, Immunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugs, J Control Release, 154 (2011) 306–313. [DOI] [PubMed] [Google Scholar]
- [241].Sabatino R, Antonelli A, Battistelli S, Schwendener R, Magnani M, Rossi L, Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells, PLoS One, 9 (2014) e101260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Boberg A, Dominici S, Brave A, Hallermalm K, Hinkula J, Magnani M, Wahren B, Immunization with HIV protease peptides linked to syngeneic erythrocytes, Infect Agent Cancer, 2 (2007) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Magnani M, Chiarantini L, Vittoria E, Mancini U, Rossi L, Fazi A, Red blood cells as an antigen-delivery system, Biotechnol Appl Biochem, 16 (1992) 188–194. [PubMed] [Google Scholar]
- [244].Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA, Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens, Sci Rep, 5 (2015) 15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA, Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase, Sci Adv, 1 (2015) e1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Dominici S, Laguardia ME, Serafini G, Chiarantini L, Fortini C, Tripiciano A, Brocca-Cofano E, Scoglio A, Caputo A, Fiorelli V, Gavioli R, Cafaro A, Ensoli B, Magnani M, Red blood cell-mediated delivery of recombinant HIV-1 Tat protein in mice induces anti-Tat neutralizing antibodies and CTL, Vaccine, 21 (2003) 2073–2081. [DOI] [PubMed] [Google Scholar]
- [247].Kontos S, Kourtis IC, Dane KY, Hubbell JA, Engineering antigens for in situ erythrocyte binding induces T-cell deletion, Proc Natl Acad Sci U S A, 110 (2013) E60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Ji W, Smith PN, Koepsel RR, Andersen JD, Baker SL, Zhang L, Carmali S, Myerson JW, Muzykantov V, Russell AJ, Erythrocytes as carriers of immunoglobulin-based therapeutics, Acta Biomater, 101 (2020) 422–435. [DOI] [PubMed] [Google Scholar]
- [249].Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li ZY, Deshycka R, Bousbaine D, Li H, Patterson HC, Dougan SK, Maruyama T, Lodish HF, Ploegh HL, Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease (vol 114, pg 3157, 2017), Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) E3583–E3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Glassman PM, Villa CH, Ukidve A, Zhao Z, Smith P, Mitragotri S, Russell AJ, Brenner JS, Muzykantov VR, Vascular Drug Delivery Using Carrier Red Blood Cells: Focus on RBC Surface Loading and Pharmacokinetics, Pharmaceutics, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Rossi L, Pierige F, Aliano MP, Magnani M, Ongoing Developments and Clinical Progress in Drug-Loaded Red Blood Cell Technologies, BioDrugs, 34 (2020) 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Leuzzi V, Rossi L, Gabucci C, Nardecchia F, Magnani M, Erythrocyte-mediated delivery of recombinant enzymes, J Inherit Metab Dis, 39 (2016) 519–530. [DOI] [PubMed] [Google Scholar]
- [253].Updike SJ, Wakamiya RT, Lightfoot EN Jr., Asparaginase entrapped in red blood cells: action and survival, Science, 193 (1976) 681–683. [DOI] [PubMed] [Google Scholar]
- [254].Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, Auvrignon A, Corm S, Dombret H, Chevallier P, Galambrun C, Huguet F, Legrand F, Mechinaud F, Vey N, Philip I, Liens D, Godfrin Y, Rigal D, Bertrand Y, l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial, Br J Haematol, 153 (2011) 58–65. [DOI] [PubMed] [Google Scholar]
- [255].Bax BE, Levene M, Bain MD, Fairbanks LD, Filosto M, Kalkan Ucar S, Klopstock T, Kornblum C, Mandel H, Rahman S, Roubertie A, Scarpelli M, Sedgwick PM, Baru M, Sellos-Moura M, Price J, Horn P, Nirmalananthan N, Erythrocyte Encapsulated Thymidine Phosphorylase for the Treatment of Patients with Mitochondrial Neurogastrointestinal Encephalomyopathy: Study Protocol for a Multi-Centre, Multiple Dose, Open Label Trial, J Clin Med, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Levene M, Bain MD, Moran NF, Nirmalananthan N, Poulton J, Scarpelli M, Filosto M, Mandel H, MacKinnon AD, Fairbanks L, Pacitti D, Bax BE, Safety and Efficacy of Erythrocyte Encapsulated Thymidine Phosphorylase in Mitochondrial Neurogastrointestinal Encephalomyopathy, J Clin Med, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Pascucci T, Rossi L, Colamartino M, Gabucci C, Carducci C, Valzania A, Sasso V, Bigini N, Pierige F, Viscomi MT, Ventura R, Cabib S, Magnani M, Puglisi-Allegra S, Leuzzi V, A new therapy prevents intellectual disability in mouse with phenylketonuria, Mol Genet Metab, 124 (2018) 39–49. [DOI] [PubMed] [Google Scholar]
- [258].Annese V, Latiano A, Rossi L, Lombardi G, Dallapiccola B, Serafini S, Damonte G, Andriulli A, Magnani M, Erythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients-a pilot uncontrolled study, Am J Gastroenterol, 100 (2005) 1370–1375. [DOI] [PubMed] [Google Scholar]
- [259].Shi J, Kundrat L, Pishesha N, Bilate A, Theile C, Maruyama T, Dougan SK, Ploegh HL, Lodish HF, Engineered red blood cells as carriers for systemic delivery of a wide array of functional probes, Proc Natl Acad Sci U S A, 111 (2014) 10131–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Pishesha N, Bilate AM, Wibowo MC, Huang NJ, Li Z, Deshycka R, Bousbaine D, Li H, Patterson HC, Dougan SK, Maruyama T, Lodish HF, Ploegh HL, Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease, Proc Natl Acad Sci U S A, 114 (2017) 3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Huang NJ, Pishesha N, Mukherjee J, Zhang S, Deshycka R, Sudaryo V, Dong M, Shoemaker CB, Lodish HF, Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin, Nat Commun, 8 (2017) 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Hawksworth J, Satchwell TJ, Meinders M, Daniels DE, Regan F, Thornton NM, Wilson MC, Dobbe JG, Streekstra GJ, Trakarnsanga K, Heesom KJ, Anstee DJ, Frayne J, Toye AM, Enhancement of red blood cell transfusion compatibility using CRISPR-mediated erythroblast gene editing, EMBO Mol Med, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Koleva L, Bovt E, Ataullakhanov F, Sinauridze E, Erythrocytes as Carriers: From Drug Delivery to Biosensors, Pharmaceutics, 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Antonelli A, Sfara C, Mosca L, Manuali E, Magnani M, New biomimetic constructs for improved in vivo circulation of superparamagnetic nanoparticles, J Nanosci Nanotechnol, 8 (2008) 2270–2278. [DOI] [PubMed] [Google Scholar]
- [265].Antonelli A, Pacifico S, Sfara C, Tamma M, Magnani M, Ferucarbotran-loaded red blood cells as long circulating MRI contrast agents: first in vivo results in mice, Nanomedicine (Lond), 13 (2018) 675–687. [DOI] [PubMed] [Google Scholar]
- [266].Antonelli A, Sfara C, Manuali E, Bruce IJ, Magnani M, Encapsulation of superparamagnetic nanoparticles into red blood cells as new carriers of MRI contrast agents, Nanomedicine (Lond), 6 (2011) 211–223. [DOI] [PubMed] [Google Scholar]
- [267].Antonelli A, Sfara C, Weber O, Pison U, Manuali E, Salamida S, Magnani M, Characterization of ferucarbotran-loaded RBCs as long circulating magnetic contrast agents, Nanomedicine (Lond), 11 (2016) 2781–2795. [DOI] [PubMed] [Google Scholar]
- [268].Milanick MA, Ritter S, Meissner K, Engineering erythrocytes to be erythrosensors: first steps, Blood Cells Mol Dis, 47 (2011) 100–106. [DOI] [PubMed] [Google Scholar]
- [269].Ritter SC, Milanick MA, Meissner KE, Encapsulation of FITC to monitor extracellular pH: a step towards the development of red blood cells as circulating blood analyte biosensors, Biomed Opt Express, 2 (2011) 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Kim I, Lee D, Lee SW, Lee JH, Lee G, Yoon DS, Coagulation-Inspired Direct Fibrinogen Assay Using Plasmonic Nanoparticles Functionalized with Red Blood Cell Membranes, ACS Nano, 15 (2021) 6386–6394. [DOI] [PubMed] [Google Scholar]
- [271].Kim I, Kim C, Lee D, Lee SW, Lee G, Yoon DS, A bio-inspired highly selective enzymatic glucose sensor using a red blood cell membrane, Analyst, 145 (2020) 2125–2132. [DOI] [PubMed] [Google Scholar]
- [272].Sarin H, Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability, J Angiogenes Res, 2 (2010) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, Group CBCS, Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer, Ann Oncol, 15 (2004) 440–449. [DOI] [PubMed] [Google Scholar]
- [274].Gabizon A, Shmeeda H, Barenholz Y, Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies, Clin Pharmacokinet, 42 (2003) 419–436. [DOI] [PubMed] [Google Scholar]
- [275].Dams ET, Laverman P, Oyen WJ, Storm G, Scherphof GL, van Der Meer JW, Corstens FH, Boerman OC, Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes, J Pharmacol Exp Ther, 292 (2000) 1071–1079. [PubMed] [Google Scholar]
- [276].Laverman P, Carstens MG, Boerman OC, Dams ET, Oyen WJ, van Rooijen N, Corstens FH, Storm G, Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection, J Pharmacol Exp Ther, 298 (2001) 607–612. [PubMed] [Google Scholar]
- [277].Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H, Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes, J Control Release, 112 (2006) 15–25. [DOI] [PubMed] [Google Scholar]
- [278].Wang X, Ishida T, Kiwada H, Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes, J Control Release, 119 (2007) 236–244. [DOI] [PubMed] [Google Scholar]
- [279].Juliano RL, Stamp D, Lectin-mediated attachment of glycoprotein-bearing liposomes to cells, Nature, 261 (1976) 235–238. [DOI] [PubMed] [Google Scholar]
- [280].Heath TD, Fraley RT, Papahdjopoulos D, Antibody targeting of liposomes: cell specificity obtained by conjugation of F(ab’)2 to vesicle surface, Science, 210 (1980) 539–541. [DOI] [PubMed] [Google Scholar]
- [281].Martin FJ, Hubbell WL, Papahadjopoulos D, Immunospecific targeting of liposomes to cells: a novel and efficient method for covalent attachment of Fab’ fragments via disulfide bonds, Biochemistry, 20 (1981) 4229–4238. [DOI] [PubMed] [Google Scholar]
- [282].Singhal A, Bali A, Gupta CM, Antibody-mediated targeting of liposomes to erythrocytes in whole blood, Biochim Biophys Acta, 880 (1986) 72–77. [DOI] [PubMed] [Google Scholar]
- [283].Singhal A, Gupta CM, Antibody-mediated targeting of liposomes to red cells in vivo, FEBS Lett, 201 (1986) 321–326. [DOI] [PubMed] [Google Scholar]
- [284].Peeters PA, Claessens CA, Eling WM, Crommelin DJ, Immunospecific targeting of liposomes to erythrocytes, Biochem Pharmacol, 37 (1988) 2215–2222. [DOI] [PubMed] [Google Scholar]
- [285].Peeters PA, Oussoren C, Eling WM, Crommelin DJ, Immunospecific targeting of immunoliposomes, F(ab’)2 and IgG to red blood cells in vivo, Biochim Biophys Acta, 943 (1988) 137–147. [DOI] [PubMed] [Google Scholar]
- [286].Hall SS, Mitragotri S, Daugherty PS, Identification of peptide ligands facilitating nanoparticle attachment to erythrocytes, Biotechnol Prog, 23 (2007) 749–754. [DOI] [PubMed] [Google Scholar]
- [287].Sahoo K, Koralege RS, Flynn N, Koteeswaran S, Clark P, Hartson S, Liu J, Ramsey JD, Pope C, Ranjan A, Nanoparticle Attachment to Erythrocyte Via the Glycophorin A Targeted ERY1 Ligand Enhances Binding without Impacting Cellular Function, Pharm Res, 33 (2016) 1191–1203. [DOI] [PubMed] [Google Scholar]
- [288].Heikham KD, Gupta A, Kumar A, Singh C, Saxena J, Srivastava K, Puri SK, Dwivedi AK, Habib S, Misra A, Preferential targeting of human erythrocytes infected with the malaria parasite Plasmodium falciparumvia hexose transporter surface proteins, Int J Pharm, 483 (2015) 57–62. [DOI] [PubMed] [Google Scholar]
- [289].Agrawal AK, Singhal A, Gupta CM, Functional drug targeting to erythrocytes in vivo using antibody bearing liposomes as drug vehicles, Biochem Biophys Res Commun, 148 (1987) 357–361. [DOI] [PubMed] [Google Scholar]
- [290].Singh AM, Owais M, Varshney GC, Use of specific polyclonal antibodies for site specific drug targeting to malaria infected erythrocytes in vivo, Indian J Biochem Biophys, 30 (1993) 411–413. [PubMed] [Google Scholar]
- [291].Owais M, Varshney GC, Choudhury A, Chandra S, Gupta CM, Chloroquine encapsulated in malaria-infected erythrocyte-specific antibody-bearing liposomes effectively controls chloroquine-resistant Plasmodium berghei infections in mice, Antimicrob Agents Chemother, 39 (1995) 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Agrawal AK, Gupta CM, Antibody-bearing liposomes as chloroquine vehicles in treatment of murine malaria, Methods Mol Med, 25 (2000) 227–239. [DOI] [PubMed] [Google Scholar]
- [293].Moles E, Urban P, Jimenez-Diaz MB, Viera-Morilla S, Angulo-Barturen I, Busquets MA, Fernandez-Busquets X, Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy, J Control Release, 210 (2015) 217–229. [DOI] [PubMed] [Google Scholar]
- [294].Moles E, Moll K, Ch’ng JH, Parini P, Wahlgren M, Fernandez-Busquets X, Development of drug-loaded immunoliposomes for the selective targeting and elimination of rosetting Plasmodium falciparum-infected red blood cells, J Control Release, 241 (2016) 57–67. [DOI] [PubMed] [Google Scholar]
- [295].Moles E, Galiano S, Gomes A, Quiliano M, Teixeira C, Aldana I, Gomes P, Fernandez-Busquets X, ImmunoPEGliposomes for the targeted delivery of novel lipophilic drugs to red blood cells in a falciparum malaria murine model, Biomaterials, 145 (2017) 178–191. [DOI] [PubMed] [Google Scholar]
- [296].Biosca A, Dirscherl L, Moles E, Imperial S, Fernandez-Busquets X, An ImmunoPEGliposome for Targeted Antimalarial Combination Therapy at the Nanoscale, Pharmaceutics, 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Singh MP, Flynn NH, Sethuraman SN, Manouchehri S, Ritchey J, Liu J, Ramsey JD, Pope C, Ranjan A, Reprogramming the rapid clearance of thrombolytics by nanoparticle encapsulation and anchoring to circulating red blood cells, J Control Release, 329 (2021) 148–161. [DOI] [PubMed] [Google Scholar]
- [298].Rossi L, Pierige F, Antonelli A, Bigini N, Gabucci C, Peiretti E, Magnani M, Engineering erythrocytes for the modulation of drugs’ and contrasting agents’ pharmacokinetics and biodistribution, Adv Drug Deliv Rev, 106 (2016) 73–87. [DOI] [PubMed] [Google Scholar]
- [299].Philips GR, Gleich B, Paredes-Juarez GA, Antonelli A, Magnani M, Bulte JWM, Magnetic Manipulation of Blood Conductivity with Superparamagnetic Iron Oxide-Loaded Erythrocytes, ACS Appl Mater Interfaces, 11 (2019) 11194–11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Antonelli A, Szwargulski P, Scarpa ES, Thieben F, Cordula G, Ambrosi G, Guidi L, Ludewig P, Knopp T, Magnani M, Development of long circulating magnetic particle imaging tracers: use of novel magnetic nanoparticles and entrapment into human erythrocytes, Nanomedicine (Lond), 15 (2020) 739–753. [DOI] [PubMed] [Google Scholar]
- [301].Villa CH, Anselmo AC, Mitragotri S, Muzykantov V, Red blood cells: Supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems, Adv Drug Deliv Rev, 106 (2016) 88–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R, Biodegradable long-circulating polymeric nanospheres, Science, 263 (1994) 1600–1603. [DOI] [PubMed] [Google Scholar]
- [303].Mozar FS, Chowdhury EH, Impact of PEGylated Nanoparticles on Tumor Targeted Drug Delivery, Curr Pharm Des, 24 (2018) 3283–3296. [DOI] [PubMed] [Google Scholar]
- [304].Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP, The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation, Polymers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Chambers E, Mitragotri S, Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes, J Control Release, 100 (2004) 111–119. [DOI] [PubMed] [Google Scholar]
- [306].Chambers E, Mitragotri S, Long circulating nanoparticles via adhesion on red blood cells: mechanism and extended circulation, Exp Biol Med (Maywood), 232 (2007) 958–966. [PubMed] [Google Scholar]
- [307].Anselmo AC, Gupta V, Zern BJ, Pan D, Zakrewsky M, Muzykantov V, Mitragotri S, Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells, ACS Nano, 7 (2013) 11129–11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Marcos-Contreras OA, Brenner JS, Kiseleva RY, Zuluaga-Ramirez V, Greineder CF, Villa CH, Hood ED, Myerson JW, Muro S, Persidsky Y, Muzykantov VR, Combining vascular targeting and the local first pass provides 100-fold higher uptake of ICAM-1-targeted vs untargeted nanocarriers in the inflamed brain, J Control Release, 301 (2019) 54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Muzykantov VR, Brenner JS, Vascular Immunotargeting: Take the Highway to the First Exit, Hepatology, 68 (2018) 1672–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Danielyan K, Ding BS, Gottstein C, Cines DB, Muzykantov VR, Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema, J Pharmacol Exp Ther, 321 (2007) 947–952. [DOI] [PubMed] [Google Scholar]
- [311].Scherpereel A, Rome JJ, Wiewrodt R, Watkins SC, Harshaw DW, Alder S, Christofidou-Solomidou M, Haut E, Murciano JC, Nakada M, Albelda SM, Muzykantov VR, Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature, J Pharmacol Exp Ther, 300 (2002) 777–786. [DOI] [PubMed] [Google Scholar]
- [312].Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao JQ, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, Muzykantov V, Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude, Nat Commun, 9 (2018) 2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Kuebler WM, Goetz AE, The marginated pool, Eur Surg Res, 34 (2002) 92–100. [DOI] [PubMed] [Google Scholar]
- [314].Zelepukin IV, Yaremenko AV, Shipunova VO, Babenyshev AV, Balalaeva IV, Nikitin PI, Deyev SM, Nikitin MP, Nanoparticle-based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth, Nanoscale, 11 (2019) 1636–1646. [DOI] [PubMed] [Google Scholar]
- [315].Zhao Z, Ukidve A, Gao Y, Kim J, Mitragotri S, Erythrocyte leveraged chemotherapy (ELeCt): Nanoparticle assembly on erythrocyte surface to combat lung metastasis, Sci Adv, 5 (2019) eaax9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Greineder CF, Johnston IH, Villa CH, Gollomp K, Esmon CT, Cines DB, Poncz M, Muzykantov VR, ICAM-1-targeted thrombomodulin mitigates tissue factor-driven inflammatory thrombosis in a human endothelialized microfluidic model, Blood Adv, 1 (2017) 1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Calderon AJ, Bhowmick T, Leferovich J, Burman B, Pichette B, Muzykantov V, Eckmann DM, Muro S, Optimizing endothelial targeting by modulating the antibody density and particle concentration of anti-ICAM coated carriers, J Control Release, 150 (2011) 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [318].Greineder CF, Chacko AM, Zaytsev S, Zern BJ, Carnemolla R, Hood ED, Han J, Ding BS, Esmon CT, Muzykantov VR, Vascular immunotargeting to endothelial determinant ICAM-1 enables optimal partnering of recombinant scFv-thrombomodulin fusion with endogenous cofactor, PLoS One, 8 (2013) e80110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Brenner JS, Bhamidipati K, Glassman PM, Ramakrishnan N, Jiang D, Paris AJ, Myerson JW, Pan DC, Shuvaev VV, Villa CH, Hood ED, Kiseleva R, Greineder CF, Radhakrishnan R, Muzykantov VR, Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions, Nanomedicine, 13 (2017) 1495–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, Mitragotri S, Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium, Proc Natl Acad Sci U S A, 110 (2013) 10753–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Champion JA, Katare YK, Mitragotri S, Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers, J Control Release, 121 (2007) 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Anselmo AC, Kumar S, Gupta V, Pearce AM, Ragusa A, Muzykantov V, Mitragotri S, Exploiting shape, cellular-hitchhiking and antibodies to target nanoparticles to lung endothelium: Synergy between physical, chemical and biological approaches, Biomaterials, 68 (2015) 1–8. [DOI] [PubMed] [Google Scholar]
- [323].Brenner JS, Pan DC, Myerson JW, Marcos-Contreras OA, Villa CH, Patel P, Hekierski H, Chatterjee S, Tao JQ, Parhiz H, Bhamidipati K, Uhler TG, Hood ED, Kiseleva RY, Shuvaev VS, Shuvaeva T, Khoshnejad M, Johnston I, Gregory JV, Lahann J, Wang T, Cantu E, Armstead WM, Mitragotri S, Muzykantov V, Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude, Nat Commun, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Zhao ZM, Ukidve A, Krishnan V, Fehnel A, Pan DC, Gao YS, Kim J, Evans MA, Mandal A, Guo JL, Muzykantov VR, Mitragotri S, Systemic tumour suppression via the preferential accumulation of erythrocyte-anchored chemokine-encapsulating nanoparticles in lung metastases, Nat Biomed Eng, (2020). [DOI] [PubMed] [Google Scholar]
- [325].Ukidve A, Zhao ZM, Fehnel A, Krishnan V, Pan DIC, Gao YS, Mandal A, Muzykantov V, Mitragotri S, Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function, Proceedings of the National Academy of Sciences of the United States of America, 117 (2020) 17727–17736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, van Bruggen R, Of macrophages and red blood cells; a complex love story, Front Physiol, 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Hess C, Schifferli JA, Immune adherence revisited: Novel players in an old game, News Physiol Sci, 18 (2003) 104–108. [DOI] [PubMed] [Google Scholar]
- [328].Nelson RA, The Immune-Adherence Phenomenon - an Immunologically Specific Reaction between Microorganisms and Erythrocytes Leading to Enhanced Phagocytosis, Science, 118 (1953) 733–737. [DOI] [PubMed] [Google Scholar]
- [329].Pan D, Vargas-Morales O, Zern B, Anselmo AC, Gupta V, Zakrewsky M, Mitragotri S, Muzykantov V, The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells, Plos One, 11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Pan DC, Myerson JW, Brenner JS, Patel PN, Anselmo AC, Mitragotri S, Muzykantov V, Nanoparticle Properties Modulate Their Attachment and Effect on Carrier Red Blood Cells, Sci Rep-Uk, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Grimm AJ, Kontos S, Diaceri G, Quaglia-Thermes X, Hubbell JA, Memory of tolerance and induction of regulatory T cells by erythrocyte-targeted antigens, Sci Rep-Uk, 5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA, Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase, Science Advances, 1 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Kontos S, Kourtis IC, Dane KY, Hubbell JA, Engineering antigens for in situ erythrocyte binding induces T-cell deletion, P Natl Acad Sci USA, 110 (2013) E60–E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Zhang X, Luo M, Dastagir SR, Nixon M, Khamhoung A, Schmidt A, Lee A, Subbiah N, McLaughlin DC, Moore CL, Gribble M, Bayhi N, Amin V, Pepi R, Pawar S, Lyford TJ, Soman V, Mellen J, Carpenter CL, Turka LA, Wickham TJ, Chen TF, Engineered red blood cells as an off-the-shelf allogeneic anti-tumor therapeutic, Nat Commun, 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Hamid O, Luke J, Spira A, Kuesters G, Sienczylo I, Gordon G, Johnson M, A phase 1 trial of RTX-240, an allogeneic engineered red blood cell with cell-surface expression of 4-1BBL and trans-presented IL-15, in patients with advanced solid tumors, AACR, 2021. [Google Scholar]
- [336].Dugast A-S, McArdel SL, Hong E, Bollampalli A, Hoover ME, Pawar S, Amin V, Qiao K, Ta C, Turka LA, Wickham TJ, Elloul S, RTX-240, an allogeneic engineered red blood cell expressing 4-1BBL and IL-15TP, promotes NK cell functionality in vitro and in vivo, SITC, 2020. [Google Scholar]
- [337].Dugast A-S, Hong E, Hoover ME, Bollampalli A, McLaughlin DC, Bhate O, Lyford TJ, Nissen TS, Carpenter CL, Wickham TJ, Melancon L, Elloul S, RTX-224, an allogeneic red cell therapeutic expressing 4-1BBL and IL-12, exhibits potent in vitro and in vivo activity and a favorable preclinical safety profile, AACRAtlanta, 2019. [Google Scholar]
- [338].Brenner JS, Mitragotri S, Muzykantov VR, Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers, Annu Rev Biomed Eng, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339].Muzykantov VR, Drug delivery carriers on the fringes: natural red blood cells versus synthetic multilayered capsules, Expert Opin Drug Deliv, 10 (2013) 1–4. [DOI] [PubMed] [Google Scholar]
- [340].Villa CH, Seghatchian J, Muzykantov V, Drug delivery by erythrocytes: “Primum non nocere”, Transfus Apher Sci, 55 (2016) 275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Corsi D, Paiardini M, Crinelli R, Bucchini A, Magnani M, Alteration of alpha-spectrin ubiquitination due to age-dependent changes in the erythrocyte membrane, Eur J Biochem, 261 (1999) 775–783. [DOI] [PubMed] [Google Scholar]
- [342].Chiarantini L, Antonelli A, Rossi L, Fraternale A, Magnani M, Red blood cell phagocytosis following hexokinase inactivation, Cell Biochem Funct, 12 (1994) 217–220. [DOI] [PubMed] [Google Scholar]
- [343].Magnani M, Papa S, Rossi L, Vitale M, Fornaini G, Manzoli FA, Membrane-bound immunoglobulins increase during red blood cell aging, Acta Haematol, 79 (1988) 127–132. [DOI] [PubMed] [Google Scholar]
- [344].Magnani M, Stocchi V, Cucchiarini L, Chiarantini L, Fornaini G, Red blood cell phagocytosis and lysis following oxidative damage by phenylhydrazine, Cell Biochem Funct, 4 (1986) 263–269. [DOI] [PubMed] [Google Scholar]
- [345].Magnani M, Piatti E, Serafini N, Palma F, Dacha M, Fornaini G, The age-dependent metabolic decline of the red blood cell, Mech Ageing Dev, 22 (1983) 295–308. [DOI] [PubMed] [Google Scholar]
- [346].Chiarantini L, Rossi L, Fraternale A, Magnani M, Modulated red blood cell survival by membrane protein clustering, Mol Cell Biochem, 144 (1995) 53–59. [DOI] [PubMed] [Google Scholar]
- [347].Suzuki T, Dale GL, Senescent erythrocytes: isolation of in vivo aged cells and their biochemical characteristics, Proc Natl Acad Sci U S A, 85 (1988) 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [348].Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL, Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age, Blood, 79 (1992) 1351–1358. [PubMed] [Google Scholar]
- [349].Pan D, Vargas-Morales O, Zern B, Anselmo AC, Gupta V, Zakrewsky M, Mitragotri S, Muzykantov V, The Effect of Polymeric Nanoparticles on Biocompatibility of Carrier Red Blood Cells, PLoS One, 11 (2016) e0152074. [DOI] [PMC free article] [PubMed] [Google Scholar]


