Abstract
Staphylococcus schleiferi is a coagulase-negative staphylococcus infrequently reported as a human pathogen. We report a case of prosthetic valve endocarditis attributed to this organism, contrast it to another Staphylococcus species that gives similar clumping factor results (S. lugdunensis), and propose a simple, effective identification scheme for identification of clumping factor-positive staphylococci.
Staphylococcus schleiferi is a recently described (6) coagulase-negative staphylococcus (CoNS) that has rarely been reported in human infections. We report what we believe is the first described case of S. schleiferi endocarditis.
A 78-year-old man presented with a 2-day history of intermittent rigors, night sweats, urinary and fecal incontinence on one occasion, and urinary retention at presentation. There was no history of dysuria, frequent urination, or abdominal pain. He reported an influenza-like illness with rhinorrhea, cough, myalgia, and vertigo 3 weeks prior to presentation.
His medical history included a Starr-Edwards mitral valve replacement for myxomatous valve degeneration and coronary artery bypass grafting to three vessels 4 years previously, chronic atrial fibrillation, hypertension, and one transient ischemic attack. His regular oral medication included digoxin (250 μg per day [q.d.]), amiodarone (100 mg q.d.), warfarin (2 mg q.d.), and captopril (50 mg/12 h). He was an ex-smoker.
On examination, he had a temperature of 38.5°C, a heart rate of 90 to 100 in atrial fibrillation, and a blood pressure of 115/74 mm Hg. The prosthetic valve sounds were normal; no murmurs or added sounds were heard. The rest of the clinical examination was unremarkable; in particular, there were no peripheral signs of endocarditis.
Investigation demonstrated a subtherapeutic international normalized ratio of 1.2 (recommended therapeutic range, 3.0 to 4.5), and urinalysis was positive for blood. Full blood count, creatinine, electrolyte, and liver function tests were all within reference ranges. The chest X-ray was reported as normal. The C-reactive protein level was 150 mg/liter (normal, <10 mg/liter). Despite the lack of clinical signs to support a diagnosis of endocarditis, the occurrence of fevers in a patient with a mitral valve prosthesis in situ necessitated antimicrobial therapy. He was given gentamicin (180 mg, stat) and amoxicillin (1 g/6 h) intravenously (i.v.). A transthoracic echocardiogram did not demonstrate any vegetations. Blood cultures yielded staphylococci after 48 h, and flucloxacillin (1 g/4 h given i.v.) was substituted for the amoxicillin. A transesophageal echocardiogram (TOE) showed a small (5 by 3 by 4 mm) vegetation on the prosthetic mitral valve, with independent mobility and different echodensity. Valve function was normal, and there was no evidence of paravalvular regurgitation or abscess, so conservative therapy with antimicrobials was continued in lieu of urgent valve replacement.
All four sets of blood cultures (BacT/Alert FAN; Organon Teknika Corporation, Durham, N.C.) yielded gram-positive cocci in clusters that were catalase positive, consistent with staphylococci. Growth on solid media (chocolate agar [Oxoid GC agar base with growth supplement; Unipath Ltd., Basingstoke, United Kingdom] and horse blood agar) produced colony variation consisting of large and small morphotypes; pure subcultures of both colonial morphologies also produced colony variation with identical biochemical reactions. The clumping factor (coagulase rabbit plasma with EDTA; BBL Becton Dickinson, Cockeysville, Md.), STAPH-A-LEX latex agglutination (Trinity Laboratories Inc., Raleigh, N.C.), and tube coagulase tests were all negative. The clumping factor test using human plasma was positive. The isolate produced a heat-stable nuclease when commercial media were used (10). The RBH-STAPH system that utilizes Rosco diagnostic tablets, the Murex PYR (1-pyrrolidonyl-β-naphthylamide) reagent (Murex Biotech Ltd., Dartford, United Kingdom), and antibiotic susceptibility testing for identification of staphylococci (15) showed the isolate to be furazolidone susceptible, to be desferrioxamine resistant, to be novobiocin susceptible, to be PYR positive, to be beta-hemolytic on horse blood agar after 18 h of incubation at 37°C, to be polymyxin susceptible, to be resistant to 0.04 U of bacitracin but susceptible to 10 U of bacitracin, to exhibit a zone of inhibition greater than 30 mm in diameter (susceptible) around a fosfomycin tablet, to be ornithine decarboxylase (ODC) negative, to be alkaline phosphatase (ALP) positive, and to be urease negative. These results were consistent with those for S. schleiferi. The ID32 STAPH identification system (bioMérieux Vitek Inc., Hazelwood, Mo.) gave an identification profile of 26112640, consistent with 99.99% certainty of identification as S. schleiferi. The MicroScan WalkAway Rapid Pos Breakpoint 1 Panel (Dade-Behring, West Sacramento, Calif.) gave an identification profile of 040075762000-110, consistent with 99.9% certainty of identification as S. schleiferi. The Staph-Zym identification method (Rosco Diagnostica, Taastrup, Denmark) gave an identification profile of 2171-3, consistent with unequivocal identification as S. schleiferi, after additional tests recommended by the manufacturer (acetoin production and lactose and sucrose fermentation) were performed. The isolate was not identified by Vitek GPI cards, as S. schleiferi is not in that gram-positive database.
A nested PCR using primers specific for the S. aureus thermonuclease gene (nuc) and primers for the gene encoding penicillin-binding protein 2a and conferring methicillin resistance (mecA) (2) produced no amplicons. Susceptibility testing using the Kirby-Bauer disc diffusion method (14), the Vitek GPS-IX card (bioMérieux Vitek Inc.), and the MicroScan WalkAway Rapid Pos Breakpoint 1 Panel (Dade-Behring) showed the isolate to be susceptible to benzylpenicillin, oxacillin, ciprofloxacin, rifampin, tetracycline, erythromycin, and vancomycin. The β-lactamase tests in the Vitek GPS-IX card (bioMérieux Vitek Inc.) and the MicroScan panel (Dade-Behring) were negative and were confirmed negative by using growth at the margin of the zone of inhibition around a 0.5-U penicillin disk to inoculate a nitrocefin disk (Cefinase; BBL Becton Dickinson).
After confirmation of the isolate’s identity, the patient was treated with benzylpenicillin (1.8 g/4 h) i.v. and rifampin (300 mg/8 h) orally with cessation of flucloxacillin. Gentamicin (80 mg/8 h) was given i.v. for the first 2 weeks of treatment. He received benzylpenicillin and rifampin for a total of 6 weeks. A follow-up TOE showed resolution of the vegetation. The patient made a complete recovery with a C-reactive protein level of <4 mg/liter at follow-up 6 weeks after presentation.
We believe that this case represents the first report of S. schleiferi endocarditis. Blood samples collected by four separate percutaneous venipunctures (eight bottles) all grew the organism, and TOE evidence was consistent with a vegetation on the prosthetic mitral valve. These findings fulfilled the Duke clinical criteria for definite endocarditis (4). A recent paper (9) suggested that endocarditis due to S. schleiferi has been previously reported. The references given were two that reported blood culture isolates of S. schleiferi. Fleurette et al. (5) briefly mentioned one patient with a single blood culture positive for S. schleiferi and possible vertebral osteomyelitis; the possibility of endocarditis was not raised. Jean-Pierre et al. (7) described a patient with eight blood cultures positive for S. schleiferi; echocardiography excluded endocarditis, and the probable source of the organism was extensive venous thrombophlebitis. At least three other papers have reported the isolation of S. schleiferi from blood cultures, but none reported associated endocarditis. Latorre et al. (12) described a patient with three blood cultures positive for S. schleiferi; again, the possibility of endocarditis was not mentioned. Célard et al. (1) described four pacemaker infections with S. schleiferi, including one in a patient with six positive blood cultures, without mentioning endocarditis. Da Costa et al. (3) examined the role of preaxillary flora in pacemaker infections and described two patients with S. schleiferi bacteremia resulting from pacemaker infection. Endocarditis was not listed as a complication in these patients.
A recent paper described biochemical tests that helped to differentiate S. schleiferi subsp. schleiferi and S. schleiferi subsp. coagulans (18). Only seven different S. schleiferi strains were tested, making it difficult to attribute defining characteristics to individual subspecies, and the isolates were not correlated with human infections. We did not identify our isolate to the subspecies level, but it was considered most likely to belong to the subspecies schleiferi since it was tube coagulase and urease negative (S. schleiferi subsp. coagulans is tube coagulase and urease positive) and there is only a single report of S. schleiferi subsp. coagulans being isolated from humans (18).
Nine S. schleiferi isolates from six distinct geographical regions of Australia were tested for common phenotypic characteristics. This data is presented in Table 1. In summary, all of the isolates were tube coagulase negative, clumping factor positive (using human plasma), heat-stable nuclease positive, ALP positive, and urease and maltose fermentation negative. Eight of the nine isolates were PYR positive. All of the isolates could be definitively identified with the ID32 STAPH (bioMérieux Vitek Inc.) and RBH-STAPH systems. The Staph-Zym (Rosco Diagnostica) system gave unequivocal identification of all isolates after additional tests (acetoin production and lactose and sucrose fermentation) recommended by the manufacturer were performed.
TABLE 1.
Geographical origins, sites of isolation, and phenotypic characteristics of nine Australian S. schleiferi isolatesa
| Geographic origin | Source | Tube coagulase test result | Human clumping factor | Rabbit clumping factor | Thermonuclease | PYR | ALP | Urease | Mannose | Maltose | ID32 profile | Staph-Zym profile |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Southeastern Queensland | Blood culture | Neg | Pos | Neg | Pos | Pos | Pos | Neg | Neg | Neg | 26103640 | 2071-3 |
| Southeastern Queensland | Peritoneal dialysate | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26113640 | 2171-3 |
| Melbourne, Victoria | Eye swab | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26113640 | 2171-3 |
| Far northern Queensland | Blood culture | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26112640 | 2171-3 |
| Far northern Queensland | Catheter tip | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26102600 | 2071-3 |
| Sydney, New South Wales | Blood culture | Neg | Pos | Pos | Pos | Negb | Pos | Neg | Neg | Neg | 26113240 | 2171-3b |
| Northern Queensland | Catheter tip | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26113640 | 2171-3 |
| Perth, Western Australia | Pacemaker | Neg | Pos | Pos | Pos | Pos | Pos | Neg | Neg | Neg | 26112640 | 2171-3 |
| Perth, Western Australia | Blood culture | Neg | Pos | Neg | Pos | Pos | Pos | Neg | Neg | Neg | 26112640 | 2171-3 |
Neg, negative; Pos, positive.
PYR positive by the Staph-Zym kit.
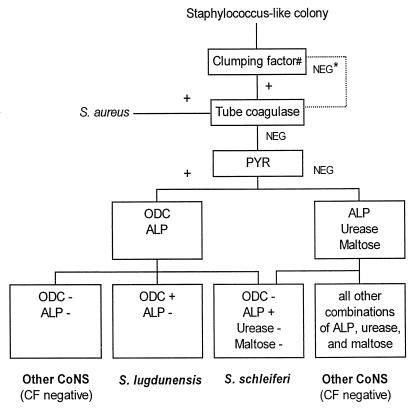
S. schleiferi and S. lugdunensis are the only two CoNS species that frequently give positive clumping factor reactions. We tested 146 staphylococcal strains with a variety of phenotypic and biochemical tests (using Rosco diagnostic tablets and the Murex PYR reagent). Results are presented in Table 2. From these results, a 4-h screening scheme (Fig. 1) was derived for the identification of staphylococci that yield positive clumping factor results (using human plasma). None of 25 strains of S. haemolyticus gave a weak positive ODC reaction although this phenomenon has been reported previously (16). Three of 37 isolates of S. epidermidis did yield a weak positive ODC reaction, but all S. epidermidis isolates were clumping factor and PYR negative. We believe that this screening strategy will accurately identify S. schleiferi and S. lugdunensis and differentiate them from other tube coagulase-negative staphylococci.
TABLE 2.
Key reactions for differentiation of S. schleiferi and S. lugdunensis from other staphylococci
| Species | No. of isolates tested | % Positivity
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tube coagulase | Human clumping factor | Rabbit clumping factor | Thermonuclease | PYR | ALP | ODC | Urease | Maltose | ||
| S. aureus | 20 | 100 | 100 | 100 | 100 | 0 | 95 | 0 | 95 | 90 |
| S. schleiferi | 9 | 0 | 100 | 78 | 100 | 89 | 100 | 0 | 0 | 0 |
| S. lugdunensis | 15 | 0 | 87 | 73 | 0 | 100 | 100 | 100 | 81 | 100 |
| S. haemolyticus | 25 | 0 | 0 | 0 | NTa | 100 | 0 | 0 | 3 | 96 |
| S. epidermidis | 37 | 0 | 0 | 0 | NT | 0 | 92 | 11b | 86 | 100 |
| Other CoNS spp. | 40 | 0 | 0 | 0 | NT | 40 | 23 | 0 | 70 | 63 |
NT, not tested.
Weak reaction.
FIG. 1.
Screening scheme for the identification of clumping factor (CF)-positive staphylococci. Symbols: NEG *, some strains of S. lugdunensis are clumping factor negative; #, tested by using human plasma; NEG, negative reaction; +, positive reaction.
S. lugdunensis has increasingly been reported in endocarditis, characteristically an aggressive form with poor clinical outcome similar to that of S. aureus rather than the better outcome generally associated with other CoNS species (13, 17). The more aggressive endocarditis associated with S. lugdunensis has been attributed to the expression by the organism of virulence factors similar to those of S. aureus (11). It is of interest that the same group reported similar virulence factors in strains of S. schleiferi, yet severe infections caused by S. schleiferi seem to be underrepresented compared to infections caused by S. lugdunensis. Moreover, S. schleiferi has not previously been reported to cause endocarditis. S. schleiferi subsp. schleiferi is indigenous to carnivores but may be transferred from carnivore pets to their owners or handlers (8). An earlier article that reviewed a large number of S. schleiferi isolates reported that almost all were considered to be part of the skin flora of some humans (5). One report suggested that the preaxillary skin is a preferred site, although prospective cultures yielded only five strains from 104 patients (1). In a more recent study, S. schleiferi was isolated from preaxillary skin in a similar number of patients undergoing pacemaker insertion (3 of 104) (3).
Colony variation was noted in the strain of S. schleiferi in this report and also in another isolate from a patient at one of our institutions with an infected pacemaker. The feature of colony variation has not been previously documented in S. schleiferi isolates. We reported a similar observation in S. lugdunensis strains and question whether colony variation is also underreported in S. schleiferi, although all three of the other S. schleiferi strains in our previous report did not show colony variation (13).
S. schleiferi appears to have a propensity to cause infection associated with implanted foreign material and should be considered when a CoNS is isolated from implants. We believe that this is the first report of S. schleiferi endocarditis. Because S. schleiferi has virulence factors similar to those of S. lugdunensis, a CoNS isolated from blood cultures from a patient with suspected endocarditis needs to be accurately identified. It is possible that S. schleiferi was previously incorrectly identified due to overlap of phenotypic characteristics with those of S. aureus and other CoNS species. Application of a simple identification method as presented in this report should enhance the identification of S. schleiferi. We expect more reports of human infections caused by S. schleiferi in the future.
REFERENCES
- 1.Célard M, Vandenesch F, Darbas H, Grando J, Jean-Pierre H, Kirkorian G, Etienne J. Pacemaker infection caused by Staphylococcus schleiferi, a member of the human preaxillary flora: four case reports. Clin Infect Dis. 1997;24:1014–1015. doi: 10.1093/clinids/24.5.1014. [DOI] [PubMed] [Google Scholar]
- 2.Coombs G W, Kay I D, Pearman J W, Christiansen K J. Programme and abstracts of the 20th International Congress of Chemotherapy. Sydney, Australia: International Congress of Chemotherapy; 1997. The role of multiplex mecA/nuc PCR for routine detection of methicillin resistance in staphylococci, abstr. 2292; p. 38. [Google Scholar]
- 3.Da Costa A, Lelièvre H, Kirkorian G, Célard M, Chevalier P, Vandenesch F, Etienne J, Touboul P. Role of the preaxillary flora in pacemaker infections. Circulation. 1998;97:1791–1795. doi: 10.1161/01.cir.97.18.1791. [DOI] [PubMed] [Google Scholar]
- 4.Durack D T, Lukes A S, Bright D K The Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med. 1994;96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 5.Fleurette J, Bès M, Brun Y, Freney J, Forey F, Coulet M, Reverdy M E, Etienne J. Clinical isolates of Staphylococcus lugdunensis and S. schleiferi: bacteriological characteristics and susceptibility to antimicrobial agents. Res Microbiol. 1989;140:107–118. doi: 10.1016/0923-2508(89)90044-2. [DOI] [PubMed] [Google Scholar]
- 6.Freney J, Brun Y, Bes M, Meugnier H, Grimont F, Grimont P A, Nervi C, Fleurette J. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38:168–172. [Google Scholar]
- 7.Jean-Pierre H, Darbas H, Jean-Roussenq A, Boyer G. Pathogenicity in two cases of Staphylococcus schleiferi, a recently described species. J Clin Microbiol. 1989;27:2110–2111. doi: 10.1128/jcm.27.9.2110-2111.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloos W. Taxonomy and systematics of staphylococci indigenous to humans. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 113–137. [Google Scholar]
- 9.Kluytmans J, Berg H, Steegh P, Vandenesch F, Etienne J, Van Belkum A. Outbreak of Staphylococcus schleiferi wound infections: strain characterization by randomly amplified polymorphic DNA analysis, PCR ribotyping, conventional ribotyping, and pulsed-field gel electrophoresis. J Clin Microbiol. 1998;36:2214–2219. doi: 10.1128/jcm.36.8.2214-2219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachica R V, Hoeprich P D, Genigeorgis C. Metachromatic agar-diffusion microslide technique for detecting staphylococcal nuclease in foods. Appl Microbiol. 1972;23:168–169. doi: 10.1128/am.23.1.168-169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambe D W, Ferguson K P, Keplinger J L, Gemmell C G, Kalbfleisch J H. Pathogenicity of Staphylococcus lugdunensis, Staphylococcus schleiferi and three other coagulase-negative staphylococci in a mouse model and possible virulence factors. Can J Microbiol. 1991;36:455–463. doi: 10.1139/m90-080. [DOI] [PubMed] [Google Scholar]
- 12.Latorre M, Rojo P M, Unzaga M J, Cisterna R. Staphylococcus schleiferi: a new opportunistic pathogen. Clin Infect Dis. 1993;16:589–590. doi: 10.1093/clind/16.4.589. [DOI] [PubMed] [Google Scholar]
- 13.Leung M J, Nuttall N, Pryce T M, Coombs G W, Pearman J W. Colony variation in Staphylococcus lugdunensis. J Clin Microbiol. 1998;36:3096–3098. doi: 10.1128/jcm.36.10.3096-3098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Sixth informational supplement. NCCLS document M100-S8. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 15.Nuttall N. Programme and abstracts of the 8th International Symposium on Staphylococci and Staphylococcal Infections. 1996. RBH-STAPH: a simple, effective method to identify coagulase negative staphylococci of clinical significance, abstr. O-18; p. 67. [Google Scholar]
- 16.Schnitzler N, Meilicke R, Conrads G, Frank D, Haase G. Staphylococcus lugdunensis: report of a case of peritonitis and an easy-to-perform screening strategy. J Clin Microbiol. 1998;36:812–813. doi: 10.1128/jcm.36.3.812-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandenesch F, Etienne J, Reverdy M E, Eykyn S J. Endocarditis due to Staphylococcus lugdunensis: report of 11 cases and review. Clin Infect Dis. 1993;17:871–876. doi: 10.1093/clinids/17.5.871. [DOI] [PubMed] [Google Scholar]
- 18.Vandenesch F, Lebeau C, Bes M, Lina G, Lina B, Greenland T, Benito Y, Brun Y, Fleurette J, Etienne J. Clotting activity in Staphylococcus schleiferi subspecies from human patients. J Clin Microbiol. 1994;32:388–392. doi: 10.1128/jcm.32.2.388-392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]