Abstract
Is the brain bipotential or is sex-typical behavior determined during development? Thirty years of research in whiptail lizards transformed the field of behavioral neuroscience to show that the brain is indeed bipotential, producing behaviors along a spectrum of male-typical and female-typical behavior via a parliamentary system of neural networks and not a predetermined program of constrained behavioral output. The unusual clade of whiptails gave these insights as there are several parthenogenetic all female species that display both male-typical and female-typical sexual behavior. These descendant species exist alongside their ancestors, allowing a unique perspective into how brain-behavior relationships evolve. In this review, we celebrate the over fourty year career of David Crews, beginning with the story of how he established whiptails as a model system through serendipitous behavioral observations and ending with advice to young scientists formulating their own questions. In between these personal notes, we discuss the discoveries that integrated hormones, neural activity, and gene expression to provide transformative insights into how brains function and reshaped our understanding of sexuality.
Keywords: evolution, parthenogenesis, dopamine, neuronal nitric oxide synthase, social behavior network, hormones
Graphical Abstract
Research in reptiles is like a magic well - the more you draw from them in your research, the more they have to tell you about the way brains and organisms evolve and function. It should be made clear that neither of us can be considered a herpetologist. Rather, we use herps in our research because they often give special insight into the evolution of brain-behavior mechanisms that are not easily accessible in other animals. Granted we are biased, but we see these animals as a source of unique perspectives in our efforts to understand the biological bases of behavior within evolutionary and ecologically relevant contexts. Reptiles, along with birds and mammals, are amniotes and offer simpler behaviors and neural systems that allow for easier analysis of the structure and function of the vertebrate brain. For example, the role of the limbic system in the control of species-typical behaviors were made clear in reptilian research. Diamond (1983) captured this perspective in an essay, concluding that “natural experiments permit one to examine conditions that cannot…be created experimentally…and reveal the end results of ecological and evolutionary processes. In this review we provide both the context and interpretation of three decades of research with whiptail lizards. This research has overturned many concepts that were based on the studies of the reproductive biology of the lab rat, mouse, or gerbil. Overall, this major contribution to science has changed our foundational understanding of sexuality and how the neural circuits that control these behaviors evolve.
We begin with the personal story of how this model system was established and how D. Crews chose parthenogenetic lizards as the best clade of animals to address questions about how brain-behavior relationships evolve. We then review the major contributions of whiptail research to the behavioral neuroendocrinology field, specifically with using parthenogenetic lizards to understand sexual behavior and sex specificity in neuronal circuits. We end with how this research is continuing to draw from the magic well of whiptails and how modern technologies, including advanced molecular and computational methods, can be applied to this system to continue to ask how circuits underlying sexual behavior and other social behaviors evolve and diversify.
The personal story of making a model system: David Crews and a “natural experiment” in behavioral evolution using whiptail lizards
In the early 1970’s, I entered the Institute of Animal Behavior (IAB) at Rutgers as the student of Daniel (Danny) S. Lehrman and Jay S. Rosenblatt and spent my first year learning an entirely new and exciting scientific area that combined neuroanatomy, endocrinology, and ethology and spent many hours at the American Museum of Natural History (AMNH) in the stacks. During this period was a great debate on the ontogeny of the mechanisms regulating behavior. A hotly debated topic was whether there might be brain structural differences that are functionally related to differences in sexual behavior versus the previous experience and immediate context determining the individual’s response. The two opposing schools of thought were led by Frank A. Beach and William C. Young. Beach considered the brain to be bisexual, even after the organizing effects of hormones had finished their work, where behavioral output is determined by context. Young believed that structural differences may be present in brain mechanisms controlling sexual behavior, just as they are in the gonadal differentiation that follows sex determination. Rather than study neurotransmitters (the “hot topic” at the time), I wanted to do ‘frontier behavioral endocrinology’ to determine if the principles developed in conventional animal models can be applied broadly to other animals. However, I was not particularly thrilled by the choice of animal models at the IAB, which focused on ring doves, rats, hamsters, guinea pigs, and primates. While learning from my mentors about challenges and dogma of the field, I began to search for a model system best suited for asking my questions.
Early in my graduate school period, I came upon an original reprint of Bernard Greenberg and G. Kingsley Noble’s review of their research on the mating and other social behaviors of male and female green anoles at the AMNH (Greenberg and Noble, 1944). I was enthralled – I had watched these animals for hours as an avid fisherman and hunter during my youth in South Carolina and Florida. I prepared a research proposal for Danny, arguing why a psychobiological perspective with modern methods and knowledge was likely to yield fresh results. He agreed and financed the construction of a laboratory greenhouse on the roof of our building, thus allowing me to focus my thesis research on the green anole (Anolis carolinensis). I did not find out until much later that Danny had been a high school volunteer assisting Noble at the greenhouse atop AMNH! As my exposure to herpetology was limited to the literature, I traveled to Berkeley (to visit Paul Licht), the swamps of Louisiana, and in the summer of 1975 I participated in the Summer Training Institute in Behavioral Genetics at the Institute for Behavioral Genetics, University of Colorado, Boulder. In my spare time I would hang out with Richard (Dick) E. Jones and his lab in the Department of Biology. Dick was using the green anole as a model for ovulation, as this animal is the only vertebrate other than the human that ovulates a single egg alternating between ovaries. We collaborated to merge my work on psychobiology with the anole follicular cycle (Jones et al., 1983).
I then moved to Harvard University and set up my lizard lab on top of the cyclotron; this space had the distinction of being an explosion proof top that had already been tested once (one fatality). After setting up my anole colony and getting my research going, my thoughts kept turning to a different lizard species that could provide an inroad to addressing the fundamental debate between Beach and Young on how sexual behavior is governed by the brain. According to the literature, the clade of whiptail lizards (genus Cnemidophorus) contained some species that produced sexually (male and female) and some female-only species (no males) that produced by parthenogenesis (Figure 1A-B). These parthenogenetic species are now known to be derived from hybridization of the sexual species and are obligate parthenogens (Maslin, 1971). For example, the all-female Desert Grasslands whiptail lizard (C. uniparens) is derived from a hybrid union followed by a backcross that resulted in only females being produced, with both steps involving C. inornatus (Figure 1D). Indeed, of the 45 nominate species of Cnemidophorus, at least 15 are obligate parthenogens and consist of only female individuals.
Figure 1. Whiptail lizards as a model system to study the evolution of sexual behavior.
(A) The Little Striped Whiptail (Cnemidophorus inornatus) and the (B) Desert Grassland Whiptail (Cnemidophorus uniparens) have been used in behavioral neuroscience research by (C) David Crews (far right) and his colleagues. (D) The parthenogenetic C. uniparens species arose through hybridization and a backcross of two whiptail species, including C. inornatus, and all three are still present today, enabling research on both the ancestral and descendant species. (E) Hybridization events have been observed both in the lab and field.
I confess that I did not believe that Cnemidophorus uniparens reproduced by parthenogenesis. I had to see it for myself. I asked a friend (Liz Smith) to collect some animals and send them back east, which arrived very healthy and several gravid. One day while I was observing the anoles, I glanced to the side where the whiptails were housed. I promptly fell out of my chair and rushed to get film from the freezer before the animals stopped. This is where the quote of Louis Pasteur is so apt. “Dans les champs de l'observation le hasard ne favorise que les esprits préparés.” (Translation: “In the fields of observation, chance favors only the prepared mind"). I was observing the bisexuality of the brain! Here were animals that developed only as a female, did not need males to reproduce, yet were showing the classic mating posture of sexual whiptail species. In other words, here was a bisexual brain capable of displaying both male-like and female-like pseudosexual behavior, which alternated according to their follicular state. It was my opinion that research on this animal would address the original and unresolved Beach and Young debate on the fundamental organization of the brain in different sexes. When I phoned Dick Jones about this observation, he was not surprised and told me his student, Kevin Fitzgerald, had observed this same behavior in additional unisexual Cnemidophorus species. Fitzgerald’s thesis topic was a comparison between the mate selection in the male green anole, where sex differences in size are profound and as females grow they continue to lay one egg at a time. He contrasted this anole ovulation pattern with three Cnemidophorus species, where the number of eggs laid in a clutch is a reflection of the size of the female. Our combined observations, experiments, and speculations were published (Crews and Fitzgerald, 1980). We labeled the behavior ‘pseudosexual’ or ‘pseudocopulatory’ behavior since no males existed and, as females, there was no intromission or sperm transferred. The final posture assumed with copulation we labeled the doughnut for obvious reasons (Figure 1E).
From those serendipitous moments doing animal behavior observations in the lab, I formulated a strategy to discover how pseudosexual behavior is governed by the brain (binary output from unitary network) and the extent to which sex differences were latent, awaiting release. I focused on the Desert Grassland whiptail (C. uniparens) because of its abundance. This species is believed to be descended from a hybridization event between two sexually reproducing species, the Texas Spotted whiptail (C. gularis) and the Little Striped whiptail (C. inornatus) (Figure 1D). This represents a snapshot of evolution in which we could compare the unisexual descendant with its direct sexual ancestors. This ability to compare ancestral and descendant species is incredibly rare and represents a major strength in studying the evolution of behavior in these animals.
I have had the privilege to follow my thoughts and energies over 30 years of research. In all of my research programs, I have worked with extremely talented individuals, some of whom have continued in science and others who have pursued their passions in other professions (for example, Kevin is now a practicing vet in Denver as well as a successful stand-up comedian). Our fundamental contributions to the field of behavioral neuroendocrinology have established the brain as bipotential, where both male- and female-typical behavioral circuits exist in each individual and underlying physiological and neural factors facilitate the display of one behavioral action over another.
Hormonal insights into the neural control of sexual behavior
Like all other vertebrates, sexual behavior in whiptails is regulated by sex steroid hormones (reviewed in (Crews and Silver, 1985; Kabelik and Crews, 2017)). Early work showed that pseudocopulatory behavior in parthenogenetic whiptails occurred during specific phases of the ovarian cycle (Figure 2A). Parthenogens display female-typical receptivity before ovulation, similar to the ancestral species, whereas male-like copulatory behavior occurs after ovulation. Conversely, in the sex-typical ancestral species, the postovulatory period is marked by rejection of mounting by males, but the females do not display male-like mounting behavior. Although no sperm is transferred in parthenogens, pseudosexual behavior increases fecundity by reducing the time to ovulation in the mounted animal (Gustafson and Crews, 1981; Crews et al., 1986), which partly explains why the behavioral trait has persisted in these species. Working with unisexual lizards inevitably lead to questions such as the purpose of pseudosexual behavior in a species that lack males. We find the functional outcome is similar to that of male courtship behavior in ancestral sexual species, namely that pseudocopulatory behavior is stimulatory to follicular growth regardless of the mode of reproduction (Crews et al., 1985, 1986).
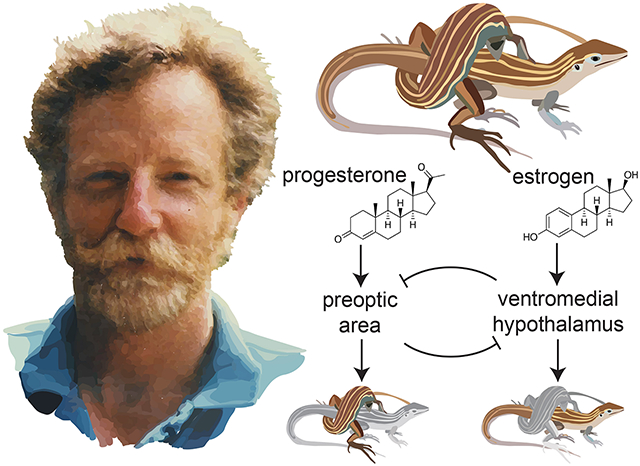
Figure 2. Female hormonal cycling of behavior in the ancestral sexual species and the descendent parthenogen species.
(A) In the females of the ancestral species (Cnemidophorus inornatus), females cycle between being receptive to mounting by males and rejecting males. The all female parthenogen species (C. uniparens) cycles between being receptive to mounting and mounting others. (B) Estrogen (solid lines) and progesterone (dashed lines) are typical of vertebrates, where estrogen is higher prior to ovulation whereas progesterone peaks post ovulation. Androgens are undetectable in both species. (C) Brain region activity in the preoptic area (POA, solid line) and the ventromedial hypothalamus (VMH, dashed line) also cycles with hormones and behavior. (D) In the parthenogen species, estrogen receptor (ER, solid line) gene expression in the POA increases during pre-ovulation and progesterone receptor (PR, dashed line) gene expression increases during post-ovulation when mounting behavior is observed. This estrogen-driven increase in PR expression within the POA is not observed in C. inornatus females.
Another fascinating link is with the work of Joe Thornton. I happened to be presenting a seminar in the Department of Biology at Columbia on the topic of the evolution of steroid hormone-hormone receptors. My bottom line was that sex steroid hormones led the evolutionary interaction of hormones with their receptors. Later in the day I served as outside examiner of Joe’s dissertation defense (he was a student of Darcy Kelley). Joe had recently written a paper that came to the opposite conclusion (Thornton, 2001), namely that a primitive estrogen hormone receptor arose first that then exploited the follicular surges in estrogen as a means to signal this critical event of ovulation. To find out who was correct, we were able to show that the estrogen receptor is the most ancient transcription factor and accounts for its various endocrine roles in a wide variety of taxa, from sea stars to vertebrates (Thornton et al., 2003). We demonstrated that the next hormone-hormone receptor unit to evolve was progesterone and progesterone receptor (PR), a functional association that signals ovulation. Coincident with progesterone was the evolution of glucocorticoid and its receptor. The evolution of the androgen receptor (AR) arose approximately 300 mya after the origin of the estrogen-estrogen receptor (ER). This is mostly likely coincident with the advent of colorful male individuals delivering sperm to the females.
The next 20 years of research in the CrewsLab focused on how sex steroid hormones regulate behavior and ushered in a golden age of understanding how the neuroendocrine systems control sexual behavior in vertebrates. The ancestral C. inornatus has sex-typical hormonal control of copulatory behavior (Kabelik and Crews, 2017). In males, castration ablates male mounting behavior and subsequent androgen treatment reinstates this behavior. In C. inornatus females, receptive behavior is regulated by estrogen, which is released by the developing follicle in the preovulatory stage of the ovarian cycle (Figure 2B). With ovulation, progesterone is released by the corpus luteum and further receptive behavior is inhibited. Estrogen modulation of female-like receptive behavior is maintained in the parthenogen C. uniparens. In contrast, instead of progesterone inhibiting receptive behavior in the postovulatory phase of the decedent parthenogen species, mounting behavior is induced (Grassman and Crews, 1986). Although androgen implants also induce mounting behavior in the parthenogens, androgens are not detectable at any stage of the ovarian cycle. This body of hormone work shows that androgen sensitivity remains in the descendent parthenogen species, but that in a naturally cycling animal, progesterone has taken on an androgen-like role. How could progesterone evolve an entirely different function in behavior? This question was answered through the work of Jonathan Lindzey, who showed that roughly 40% of castrated C. inornatus males will display mounting behavior when implanted with non-metabolizable progesterone and a progesterone receptor antagonist blocks this effect (Lindzey and Crews, 1986). Thus, progesterone sensitivity in the C. uniparens parthenogen has an evolutionary antecedent in the C. inornatus ancestral species with natural variation in progesterone-sensitivity across individuals.
Hormones secreted by the gonads have wide ranging functions on physiology and behavior. Once the relationship between sex steroid hormones and psuedocopulatory behavior became clear, the next step was to determine which brains regions were responsive to sex steroid hormones and regulated male-typical or female-typical sexual behavior. Quantifying 2-deoxyglucose uptake was, at the time, a state-of-the-art approach, especially in an unusual research organism like a reptile. This molecule is taken up by active neurons via glucose transporters, but cannot be metabolized, which traps this compound inside the neurons with high energy needs. Using this approach, the preoptic area (POA) was identified as more active during male-like copulatory behavior whereas the ventromedial hypothalamus (VMH) was identified as more active during female-like copulatory behavior (Figure 2C) (Rand and Crews, 1994). The volume of these brain regions are sexually dimorphic in C. inornatus, but are similar sizes in C. uniparens (Crews et al., 1990; Wade and Crews, 1991). Lesion studies in both the ancestral sexual and descendent parthenogen species established the necessity of these brain regions in governing sexual behavior, where POA lesions abolished male-typical mounting behavior (Kingston and Crews, 1994) and VMH lesions abolished female receptivity (Kendrick et al., 1995). These brain regions were then established to be hormone sensitive, where intracranial implants of androgens into the POA is sufficient to stimulate mounting behavior in castrated males (Crews, 2013). Implants of progesterone into the POA of castrated and progesterone-sensitive C. inornatus males was also sufficient to stimulate mounting behavior (Crews et al., 1996), establishing that testosterone and progesterone act within the POA to govern male sexual behavior. Complementary to this, intracranial implants of estradiol directly into the VMH elicits receptivity in both C. uniparens and female C. inornatus (Wade, 1991; Godwin and Crews, 1997).
After connecting the action of hormones to specific brain regions, CrewsLab members Larry Young and John Godwin set out on a series studies linking these observations to variation in expression of sex steroid hormone receptor genes, which was transformative to the field at that time and coincident with the landmark studies in mammals. All three sex steroid hormone receptors were found to be present in both the POA and VMH (Young et al., 1994; Young and Crews, 1995), providing a molecular link between gonadal hormones and brain regions necessary for sexual behavior. Moreover, the expression of these sex steroid hormone receptors were under hormonal control in interesting species- and sex-specific ways (Figure 2D). As in other vertebrates, preovulatory estrogen levels increase expression of the progesterone receptor in the VMH to elicit receptive behavior in both C. uniparens and C. inornatus females, but not in C. inornatus males (Godwin and Crews, 1999). In relation to species differences in male-typical sexual behavior, estrogen increases the abundance of the progesterone receptor in the POA of C. uniparens, but not the ancestral female C. inornatus (Young et al., 1994; Godwin and Crews, 1995). This suggests that in a naturally cycling parthenogen, estradiol primes the progesterone system in the POA to facilitate male-typical copulatory behavior. In summary, up until this point, the most important difference between the ancestral and descendent species is how gene expression in the POA is regulated by estrogen.
One knowledgeable about sex steroid hormone metabolism may ask at this point about the potential role of aromatase in whiptail sexual behavior. Aromatase converts estradiol into testosterone and plays an important role in both gonadal development and adult sexual behavior (Balthazart and Foidart, 1993). Unfortunately, a study on the role of aromatase in regulating whiptail sexual behavior has never been attempted in adult animals. However, aromatase is present in brain regions important for social behavior, including the VMH and POA. Expression of aromatase increases in the POA during the postovulatory period of C. uniparens when male pseudocopulatory behavior is typically observed (Dias et al., 2009), although the functional significance of this pattern is unknown. Interestingly, when aromatase inhibitors is applied to C. uniparens eggs during development, males with fully functional testes hatch (Figure 3) (Wennstrom and Crews, 1995), demonstrating that the ability to make males is still encoded within the C. uniparens genome. This also established that during normal development, estrogen is the primary organizing hormone. The fact that an aromatase inhibitor is needed to alter gene expression further emphasizes that the female (and estrogen) is the fundamental and organizational gonad.
Figure 3. Making males in an all-female species.
The all-female parthenogen species C. uniparens maintains the capacity to make males, where treatment of eggs with aromatase inhibitors make males (Virago) that display mounting behavior (left) and have fully functional testes (right). These animals never show receptive behavior.
As sex steroid hormones classically exert their influence through sex steroid hormone receptors that modulate gene expression within specific brain regions, the next logical step was to examine which genes are under hormonal control to influence neuronal function. Although David Crews worked to avoid the “hot topic” of neurotransmitters in behavior when establishing whiptail lizards as a model system in behavioral neuroendocrinology, the science inevitably led there 20 years later.
Hormonal tuning of neurotransmitter function
In the mid 2000s, Dominguez and Hull (2005) put forth a circuit model for male-typical sexual behavior in rodents that included olfactory information being processed by the amygdala, and then routed to the POA, where the switch to turn on or off motor patterns associated with copulation occurred. The gating mechanism in the POA was proposed to be a testosterone-dependent increase in dopamine that altered the probability of a copulatory motor response. The key that opened this gate was proposed to be nitric oxide, a signaling molecule that reshaped the field's definition of neuronal signaling (Esplugues, 2002). Nitric oxide cannot be stored like traditional neurotransmitters and thus its production is directly coupled to the presence of neuronal nitric oxide synthase (nNOS), whose activity is regulated by the calcium influx from the NMDA receptor. However, other neurotransmitters can influence this simple framework as well, most notably serotonin (Hull et al., 2004). Work in whiptail lizards in the late 2000’s focused on testing the generalizability of this POA gating mechanism as a potential inroad to understanding the evolutionary mechanisms of male-typical sexual behavior within the clade (Figure 4). Unfortunately, whiptail work in the past 25 years has primarily focused only on male-typical sexual behavior using this gating model framework, and although work on female-typical sexual behavior will be mentioned when information is available, it is an area that needs more investigation.
Figure 4. Model of how hormones influence neurotransmitter function to promote male-typical sexual behavior in whiptail lizards.
Dopamine and nitric oxide facilitate male-typical copulatory behavior in both C. uniparens parthenogens and C. inornatus males while serotonin inhibits male-typical copulatory behavior. Research suggests that progesterone, via the progesterone receptor, takes on an androgen-like role in the parthenogens to stimulate male-typical copulatory behavior during the postovulatory progesterone surge. This includes stimulation of neuronal nitric oxide synthase (nNOS) that facilitates dopamine release in the POA.
Dopamine signaling plays a well established role in promoting sexual behavior in vertebrates, including in Japanese quail (Kleitz-Nelson et al., 2010) and rodents (reviewed in (Melis and Argiolas, 1995). Sarah Woolley’s dissertation work focused on the role of dopamine in whiptail sexual behavior, finding many parallels with rodents and some hints at how the ancestral and descendent whiptail species differed in dopamine regulation. In the ancestral male C. inornatus, sexual vigor is associated with increased number of dopaminergic cells, where decreased latency to display male-typical copulatory behavior is correlated with increased number of hypothalamic cells positive for tyrosine hydroxylase, a rate-limiting enzyme in dopamine synthesis (Woolley et al., 2004). In the descendent C. uniparens, the number of tyrosine hydroxylase cells also increases from preovulatory to postovulatory animals as progesterone levels increase (Woolley and Crews, 2004). This pattern is not observed in ancestral C. inornatus females that do not display male-typical sexual behavior at any time in their ovarian cycle. Administration of a dopamine D1 receptor agonist increases mounting behavior in both C. uniparens and progesterone-sensitive male C. inornatus (Woolley et al., 2001), suggesting that the progesterone system interacts with dopamine to facilitate male-typical sexual behavior. At the receptor level, androgen treatment changes the expression levels of dopamine D1 and D2 receptors in the nucleus accumbens, but not the POA (O’Connell et al., 2012), and so it is likely the regulation of dopamine release, rather than changes in dopamine receptor abundance, influences male-typical behavior in whiptails. Overall, this work suggests that dopamine signaling in both the nucleus accumbens and POA are important for male-typical sexual behavior in whiptails, as has been described in rodents (Pfaus and Phillips, 1991).
A question that has not been addressed in whiptails within this model is the role of glutamate, where in male rodents, sexual behavior is mediated through a glutamate-induced dopamine release. In this context, glutamatergic inputs from the medial amygdala activate NMDA receptors in the POA, which then stimulates nNOS to induce release of dopamine (Dominguez et al., 2007). Expression of the obligate NMDA subunit NR1 decrease with androgen treatment in C. inornatus males within the nucleus accumbens and medial amygdala, but not in the POA or VMH (O’Connell et al., 2012), suggesting the NMDA receptor itself is not modulated by androgens in brain regions regulating sexual behavior. The role of glutamate and the function of NMDA receptors in both male-typical and female-typical sexual behavior is an area that is ripe for study in this comparative whiptail framework and would give additional insight into how glutamatergic regulation of sexual behavior evolves and whether sex differences in this circuitry is important for sex-typical behavior.
Serotonin has a well established inhibitory role in male-typical sexual behavior, although the precise function of serotonin depends on the receptor it binds (Hull et al., 2004). Brian Dias’ dissertation work focused on the inhibitory function of serotonin in whiptails and found many parallels between male sexual behavior in rodents and the male-like pseudocopulatory behavior of parthenogens. C. uniparens have increased serotonin in the POA during the preovulatory phase compared to the postovulatory phase, which is in line with the suppression of male-typical behavior during that time (Dias and Crews, 2008). Serotonin levels in the VMH do not change with natural cycling. However, serotonin in both the POA and VMH is under hormonal regulation, where ovariectomized animals implanted with testosterone or estradiol have decreased serotonin levels in both brain regions compared to ovariectomized animals without hormone implants. When serotonin is directly infused into the POA, male-typical copulatory behavior is inhibited in androgen-treated parthenogens, whereas infusing serotonin into the VMH inhibits estrogen-induced female-typical receptivity (Dias and Crews, 2006). The role of serotonin in sex-typical behaviors in the ancestral species was not investigated, which leaves evolutionary aspects of serotonergic modulation unknown.
The role of nitric oxide as the gate key to male sexual behavior was explored by Nicholas Sanderson in his dissertation work focusing on male-typical sexual behavior in the ancestral C. inornatus. Castration and testosterone implants increase nNOS expression within the POA within 18 days, which is around the same timeframe when males begin to show copulatory behavior post-castration (Sanderson et al., 2008). Testosterone implants in castrated males also increase nNOS expression in the nucleus accumbens and VMH (O’Connell et al., 2012), although its role in these other brain regions is unclear. These general patterns of testosterone-regulation of nNOS were replicated in ovariectomized female C. inornatus, where females with testosterone implants had more nNOS positive cells in the POA compared to control animals with blank implants (Sanderson et al., 2006). This study demonstrated two important things. First, testosterone implanted females were presented with either a receptive female or dummy control and nNOS expression increased in both groups, demonstrating that testosterone, and not social experience, was driving nNOS abundance. Second, it showed that androgens can induce nNOS expression in females as well as males, where females also have the capacity to increase nNOS expression depending on the hormonal environment. To functionally test the role of nNOS activity, androgen-implanted C. uniparens were treated with an nNOS inhibitor, which decreased male-like mounting behavior (Sanderson et al., 2005). Overall these studies provided support for nitric oxide functioning as an important regulator of androgen-dependent male sexual behavior in reptiles.
How could this framework be tinkered with to evolve the male-like sexual behavior observed in all-female parthenogenetic whiptails? Androgens are not detectable in parthenogens at any phase of their ovarian cycle and so we quantified the number of nNOS-positive cells in naturally cycling C. uniparens parthenogens and ancestral C. inornatus females. Postovulatory parthenogens have increased nNOS-positive cells in the POA compared to preovulatory parthenogens and this increase is not observed between pre- and post-ovulation in the ancestral C. inornatus females (O’Connell et al., 2011). These results suggest that nNOS is regulated in a different manner between these species, where androgens are sufficient, but not necessary, to drive nNOS expression. As progesterone has previously been linked to male-typical sexual behavior in male C. inornatus and postovulatory C. uniparens (Kabelik and Crews, 2017), we then asked how nNOS is regulated in progesterone-sensitive animals. First, we established that progesterone-sensitive C. inornatus males had a similar number of nNOS-positive cells compared to androgen treated males. Second, we found that both these groups had more nNOS-positive cells than progesterone-insensitive and blank-implanted males, demonstrating that individual variation in progesterone sensitivity in the ancestral species predicted the number of nNOS-positive cells in the POA. The molecular mechanisms underlying the individual variation in progesterone-regulation of nNOS abundance is unknown. Both androgen receptor and progesterone receptor response elements exist with the nNOS promoter next to CpG islands in whiptails, suggesting that transcriptional regulation may lie within individual variation in the promoter region, although this idea still remains to be tested.
In summary, these investigations have increased our understanding of how hormones alter neurotransmitter function to promote or inhibit male-typical sexual behavior. This work suggests that a progesterone-sensitive C. inornatus ancestor was involved in the hybridization events that led to the C. uniparens descendant parthenogen species displaying male-typical sexual behavior during the postovulatory progesterone surge. Complementary neurotransmitter research suggests the parthenogen display of progesterone-driven male-like sexual behavior is linked to expression of nNOS in the POA. We propose that progesterone, via the progesterone receptor, has taken on a new role in these parthenogens to facilitate male-like mounting behaviour, and that this behavioural display requires nitric oxide. This nitric oxide production then increases dopamine levels in the POA, leading to an increased probability of mounting behavior. Thus, this evolutionary behavioral change resulting from ancestral individual variation in hormonal sensitivity became fixed in the descendent species and shifted neural circuit function. Parallel studies focusing on how these systems regulate female-typical sexual behavior are needed to fully understand how these neural circuits influence the probability of displaying female-like versus male-like sexual behavior.
The bipotenial brain and the evolution of sex-specific behaviors
Research in whiptails shaped how the field of behavioral neuroscience regards sexual behavior and the organization of neural circuits that govern sex-typical behaviors. Long before neurogenetic tools were used to rediscover this concept in mice, research in whiptails already established that brains are bipotential. This dual circuit model, or brain bipotentiality, suggests that the neural circuits governing sex-typical behavior exist in both sexes and that internal hormone levels or other molecular factors can shift the display probability of male- or female-typical behavior. This work overturned an old dogma in the field that stated sex differences were established in infancy or puberty and cemented the display of male-typical behavior in males and female-typical behavior in females. It is now clear that sex-typical behavior is not a developmentally firm decision, but rather a parliamentary system in adulthood that involves networks of genes and neurons that produce behavior along a spectrum (Crews, 2012). This is not a species-specific feature of whiptail brains, but has also been demonstrated with sexual behavior in rodents (Kimchi et al., 2007). Moreover, this foundational concept is not restricted to sexual behavior, as parental behavior in rodents (Wu et al., 2014) and frogs (Fischer and O’Connell, 2020) also rely on neuronal circuits that exist in both sexes despite the probability of displaying the behavior being very different between sexes. Thus, this work that started with whiptails has been further enriched by molecular tools in other animals and highlights a generalizable feature of the vertebrate brain. Brains integrate external and internal factors to shift behavior along a spectrum of male-typical and female-typical behaviors, rather than dictating behavior along predetermined paths. The defining principles of how neural circuits present in both sexes are tuned by individual experiences, physiological state, and broader evolutionary processes to shift probability of occurrence in males and females remains an open question that would benefit from continued comparative research.
Whiptails are a magic well that never empties and we still have much to learn from them about the evolution of behavior and the architecture of neuronal circuits governing sex-typical behaviors. One question regarding the repeatability of evolution can be answered using a phylogenetically informed approach, as parthenogenetic species have evolved several times through hybridization in the Cnemidophorus clade and whether all parthenogens have male-typical sexual behavior and parallel mechanisms as we have described in C. uniparens remains to be investigated. The molecular mechanisms of individual variation in progesterone sensitivity in the ancestral and descendent species is still unknown and molecular sequencing tools currently available would be able to answer this question. Additionally, most of the neurotransmitter work in the last twenty years focused on male-typical behavior, leaving a major gap in our knowledge of how neurotransmitters function to influence female-typical behavior in whiptails, which is critical to painting a more complete picture of behavioral evolution. As the field is moving past the model system bottleneck that favored mice to an appreciation for other animals that can reshape our scientific viewpoint, the time is ripe for work in whiptails and other reptiles, whose simple brains can continue to transform our understanding of brain-behavior relationships.
Reflections on a pioneering career
There are several facets of D. Crews’s path into and through science that made his career and contributions remarkable. We list a few reflections here, from both D. Crews as a mentor and L. O’Connell as a mentee. We direct these notes to a young scientist, with the hopes that the next generation will be bold enough to pick up whatever model system is best suited to address their scientific question and “if they have the fire within, that is all that is needed”:
Academic performance does not define scientific success. I (D. Crews) was not a prodigy in school. I set records for detention and going to summer school. It was the first refrain of Paul Simon’s Kodachrome. Years later when I was in college and graduate school, my grandparents liked to show me off, saying that I was a late bloomer. Indeed, the only reason I advanced through the grades is that my father was in the United States Air Force and we moved every one or two years. It was only when my context changed that I decided to apply myself. No university in the United States would accept me, but it was possible to enroll in the University of Maryland, Munich campus. The faculty were mainly old expats or young newly graduated and still trying to figure out their own life. This is all to say that grades and academic performance are not a reflection of your ability as a scientist.
Be question-driven, not model system-driven. David and the many trainees that have contributed to whiptail work were not herpetologists and they were not bent on using the fanciest new tools to address questions that had already been answered (“old wine in new bottles”). His career was marked by having a question on the bipotentiality of the vertebrate brain and asking that question in a model system that was best suited to give answers. Established animal model systems in basic and biomedical research do not hold answers to all the important questions. Rather, taking a broader approach to learn basic principles about how brains function is a fruitful endeavor. Pioneering a new field or model system is difficult, but the rewards are plenty.
Be resilient – you will fail sometimes and that is normal. Self-efficiency is crucial for success in science. We see many trainees that struggle with self-efficiency and are afraid to begin experiments for fear that they will fail. First, you will fail – everyone does and the important thing is to move past that with resiliency. At some point in graduate school, L. O’Connell’s experiments on measuring neurotransmitter gene expression in whiptails failed and wasted an entire field season. In near tears, she delivered the news to D. Crews, who simply stated that mistakes and failures happen – what is important is that we move on – which she did, repeating the experiment and publishing the results the following year. That was incredibly liberating to an early graduate student, to be given the space to make mistakes and learn from them – instilling fearlessness in science.
Be bold and creative in your scientific approach. Starting a new model system or conducting experiments that address a major dogma in the field sometimes requires developing and applying new techniques or approaches that may be unfamiliar to you or your lab. We see many trainees hesitant to try new things and opting for established laboratory methods in their laboratories. The fundamental contributions of whiptail research to science were made possible by students asking the best questions and applying cutting-edge technologies to a non-traditional model system. This requires one to be bold in applying new technologies to a research organism, sometimes with only the methods section of a manuscript as a guide. We encourage students to be bold – being creative and naive can be a gift that breaks down scientific barriers and allows one to successfully approach problems from a different angle.
Acknowledgements
In a career that spans over 40 years of research I have had the privilege of working with many undergraduates from both Harvard and the University of Texas Austin; the total is well over 120 students. Invariably they are smart and bored. With direction and responsibility, they are soon doing graduate level work. Many of undergraduates became physician-scientists, some taking time off from studies to extend their research in the CrewsLab, like Brian Camazine. The many graduate students and postdocs that worked with the ‘Cnemis’ have all gone on to stellar careers, some working with unconventional animal models and others not. I would also mention the people, often unknown, who have supported the work and made sure that it could be conducted. Fred Stollnitz (NSF) and Izja Lerderhendler (NIMH) deserve accolades for their efforts to make sure that comparative behavioral neuroscience and neuroendocrinology had a mandate for support. Lastly, It is not often that you have a colleague that is always at the cutting edge of research and theory. I met Randy as he was looking for something to do as his mentor had absconded. He found my lab in the basement and I never let him leave. Randy is a wry and wonderful person; his adage of ‘educate through humor’ has great merit, particularly in these difficult days.
Lauren thanks David for introducing her to the fabulous world of studying brain-behavior relationships in weird critters, to which she has dedicated her life’s scientific work. Lauren O’Connell is a New York Stem Cell Foundation – Robertson Investigator and acknowledges that Stanford University resides on the ancestral and unceded land of the Muwekma Ohlone Tribe.
Funding
The Cnemidophorus work performed by the CrewsLab was supported by the National Science Foundation [BNS 82-0253] and the National Institutes of Health [2 R37 MH41770 (01-28 years) and by 2 K05 MH00135 Research Scientist Award (01-20 years)]. Lauren O’Connell is supported by the National Science Foundation [IOS-1845651, IOS-1827333, IOS-1822025], the National Institutes of Health [DP2HD102042], the Rita Allen Foundation, the McKnight Foundation, Pew Charitable Trusts, and the New York Stem Cell Foundation.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Data availability
This manuscript contains no new data.
References
- Balthazart J, Foidart A. 1993. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol 44:521–540. [DOI] [PubMed] [Google Scholar]
- Crews D 2012. The (bi)sexual brain: Science & Society Series on Sex and Science. EMBO Rep 13:779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D 2013. Binary Outputs from Unitary Networks. Integr Comp Biol 53:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Fitzgerald KT. 1980. “Sexual” behavior in parthenogenetic lizards (Cnemidophorus). Proc Natl Acad Sci 77:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Godwin J, Hartman V, Grammer M, Prediger EA, Sheppherd R. 1996. Intrahypothalamic Implantation of Progesterone in Castrated Male Whiptail Lizards ( Cnemidophorus inornatus ) Elicits Courtship and Copulatory Behavior and Affects Androgen Receptor- and Progesterone Receptor-mRNA Expression in the Brain. J Neurosci 16:7347–7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Grassman M, Lindzey J. 1986. Behavioral facilitation of reproduction in sexual and unisexual whiptail lizards. Proc Natl Acad Sci 83:9547–9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Silver R. 1985. Reproductive Physiology and Behavior Interactions in Nonmammalian Vertebrates. In: Adler N, Pfaff D, Goy RW, editors. Reproduction. Boston, MA: Springer US. p 101–182. Available from: 10.1007/978-1-4684-4832-0_5 [DOI] [Google Scholar]
- Crews D, Teramoto L, Carson H. 1985. Behavioral facilitation of reproduction in sexual and parthenogenetic Drosophila. Science 227:77–78. [DOI] [PubMed] [Google Scholar]
- Crews D, Wade J, Wilczynski W. 1990. Sexually Dimorphic Areas in the Brain of Whiptail Lizards. Brain Behav Evol 36:262–270. [DOI] [PubMed] [Google Scholar]
- Diamond JM. 1983. Ecology: Laboratory, field and natural experiments. Nature 304:586–587. [Google Scholar]
- Dias BG, Chin SG, Crews D. 2009. Steroidogenic enzyme gene expression in the brain of the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Brain Res 1253:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Crews D. 2006. Serotonergic modulation of male-like pseudocopulatory behavior in the parthenogenetic whiptail lizard, Cnemidophorus uniparens. Horm Behav 50:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias BG, Crews D. 2008. Regulation of Pseudosexual Behavior in the Parthenogenetic Whiptail Lizard, Cnemidophorus uniparens. Endocrinology 149:4622–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez J, Hull E. 2005. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav 86:356–368. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Balfour ME, Lee HS, Brown JL, Davis BA, Coolen LM. 2007. Mating activates NMDA receptors in the medial preoptic area of male rats. Behav Neurosci 121:1023–1031. [DOI] [PubMed] [Google Scholar]
- Esplugues JV. 2002. NO as a signalling molecule in the nervous system: NO in the nervous system. Br J Pharmacol 135:1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer EK, O’Connell LA. 2020. Hormonal and neural correlates of care in active versus observing poison frog parents. Horm Behav 120:104696. [DOI] [PubMed] [Google Scholar]
- Godwin J, Crews D. 1995. Sex Differences in Estrogen and Progesterone Receptor Messenger Ribonucleic Acid Regulation in the Brain of Little Striped Whiptail Lizards. Neuroendocrinology 62:293–300. [DOI] [PubMed] [Google Scholar]
- Godwin J, Crews D. 1997. Sex differences in the nervous system of reptiles. Cell Mol Neurobiol 17:649–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin J, Crews D. 1999. Hormonal regulation of progesterone receptor mRNA expression in the hypothalamus of whiptail lizards: regional and species differences. J Neurobiol 39:287–293. [PubMed] [Google Scholar]
- Grassman M, Crews D. 1986. Progesterone induction of pseudocopulatory behavior and stimulus-response complementarity in an all-female lizard species. Horm Behav 20:327–335. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Noble GK. 1944. Social Behavior of the American Chameleon (Anolis carolinensis Voigt). Physiol Zool 17:392–439. [Google Scholar]
- Gustafson JE, Crews D. 1981. Effect of group size and physiological state of a cagemate on reproduction in the parthenogenetic lizard, Cnemidophorous uniparens (Teiidae). Behav Ecol Sociobiol 8:267–272. [Google Scholar]
- Hull EM, Muschamp JW, Sato S. 2004. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav 83:291–307. [DOI] [PubMed] [Google Scholar]
- Jones RE, Guillette LJ, Summers CH, Tokarz RR, Crews D. 1983. The relationship among ovarian condition, steroid hormones, and estrous behavior inAnolis carolinensis. J Exp Zool 227:145–154. [DOI] [PubMed] [Google Scholar]
- Kabelik D, Crews D. 2017. Hormones, Brain, and Behavior in Reptiles. In: Hormones, Brain and Behavior. Elsevier. p 171–213. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128035924000274 [Google Scholar]
- Kendrick AM, Rand MS, Crews D. 1995. Electrolytic lesions to the ventromedial hypothalamus abolish receptivity in female whiptail lizards, Cnemidophorus uniparens. Brain Res 680:226–228. [DOI] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. 2007. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448:1009–1014. [DOI] [PubMed] [Google Scholar]
- Kingston PA, Crews D. 1994. Effects of hypothalamic lesions on courtship and copulatory behavior in sexual and unisexual whiptail lizards. Brain Res 643:349–351. [DOI] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Ball GF. 2010. Dopamine release in the medial preoptic area is related to hormonal action and sexual motivation. Behav Neurosci 124:773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindzey J, Crews D. 1986. Hormonal control of courtship and copulatory behavior in male Cnemidophorus inornatus, a direct sexual ancestor of a unisexual, parthenogenetic lizard. Gen Comp Endocrinol 64:411–418. [DOI] [PubMed] [Google Scholar]
- Maslin TP. 1971. Parthenogenesis in Reptiles. Am Zool 11:361–380. [Google Scholar]
- Melis MR, Argiolas A. 1995. Dopamine and sexual behavior. Neurosci Biobehav Rev 19:19–38. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Matthews BJ, Crews D. 2011. Neuronal Nitric Oxide Synthase as a Substrate for the Evolution of Pseudosexual Behaviour in a Parthenogenetic Whiptail Lizard: Role of nNOS in pseudosexual behaviour. J Neuroendocrinol 23:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Mitchell MM, Hofmann HA, Crews D. 2012. Androgens coordinate neurotransmitter-related gene expression in male whiptail lizards: Neurotransmitters and sexual behavior. Genes Brain Behav 11:813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Phillips AG. 1991. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav Neurosci 105:727–743. [DOI] [PubMed] [Google Scholar]
- Rand MS, Crews D. 1994. The bisexual brain: sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res 663:163–167. [DOI] [PubMed] [Google Scholar]
- Sanderson NSR, Le B, Zhou Z, Crews D. 2008. Preoptic neuronal nitric oxide synthase induction by testosterone is consistent with a role in gating male copulatory behavior: Preoptic nNOS up-regulation by testosterone. Eur J Neurosci 27:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson NSR, Le BD, Crews D. 2006. Testosterone induction of male-typical sexual behavior is associated with increased preoptic NADPH diaphorase and citrulline production in female whiptail lizards. J Neurobiol 66:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW. 2001. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci 98:5671–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. 2003. Resurrecting the Ancestral Steroid Receptor: Ancient Origin of Estrogen Signaling. Science 301:1714–1717. [DOI] [PubMed] [Google Scholar]
- Wade J 1991. The effects of intracranial implantation of estrogen on receptivity in sexually and asexually reproducing female whiptail lizards, Cnemidophorus inornatus and Cnemidophorus uniparens. Horm Behav 25:342–353. [DOI] [PubMed] [Google Scholar]
- Wade J, Crews D. 1991. The relationship between reproductive state and ?sexually? dimorphic brain areas in sexually reproducing and parthenogenetic whiptail lizards. J Comp Neurol 309:507–514. [DOI] [PubMed] [Google Scholar]
- Wennstrom KA, Crews D. 1995. Making Males from Females: The Effects of Aromatase Inhibitors on a Parthenogenetic Species of Whiptail Lizard. Gen Comp Endocrinol 99:316–322. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Crews D. 2004. Species differences in the regulation of tyrosine hydroxylase inCnemidophorus whiptail lizards. J Neurobiol 60:360–368. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Crews D. 2004. Tyrosine hydroxylase expression is affected by sexual vigor and social environment in maleCnemidophorus inornatus. J Comp Neurol 476:429–439. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Sakata JT, Gupta A, Crews D. 2001. Evolutionary Changes in Dopaminergic Modulation of Courtship Behavior in Cnemidophorus Whiptail Lizards. Horm Behav 40:483–489. [DOI] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. 2014. Galanin neurons in the medial preoptic area govern parental behaviour. Nature 509:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Crews D. 1995. Comparative neuroendocrinology of steroid receptor gene expression and regulation: Relationship to physiology and behavior. Trends Endocrinol Metab 6:317–323. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lopreato GF, Horan K, Crews D. 1994. Cloning and in situ hybridization analysis of estrogen receptor, progesterone and androgen receptor expression in the brain of whiptail lizards (Cnemidophorus uniparens andC. inornatus). J Comp Neurol 347:288–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript contains no new data.






