Abstract
Heightened aggressive behavior is considered as one of the central symptoms of many neuropsychiatric disorders including autism, schizophrenia, and dementia. The consequences of aggression pose a heavy burden on patient’s families, clinicians, and the patients themselves. At the same time, we have limited treatment options for aggression and lack mechanistic insight into the causes of aggression needed to inform new efforts in drug discovery and development. Levels of proinflammatory cytokines in the periphery or cerebrospinal fluid were previously reported to correlate with aggressive traits in humans. However, it is still unknown whether cytokines affect brain circuits to modulate aggression. Here, we examined the functional role of interleukin 1β (IL-1β) in mediating individual differences in aggression using a resident-intruder mouse model. We found that non-aggressive mice exhibit higher levels of IL-1β in the dorsal raphe nucleus (DRN), the major source of forebrain serotonin (5-HT), compared to aggressive mice. We then examined the effect of pharmacological antagonism and viral-mediated gene knockdown of the receptors for IL-1 within the DRN and found that both treatments consistently increased aggressive behavior of male mice. Aggressive mice also exhibited higher c-Fos expression in 5-HT neurons in the DRN compared to non-aggressive mice. In line with these findings, deletion of IL-1 receptor in the DRN caused enhanced c-Fos expression in 5-HT neurons during aggressive encounters, suggesting that modulation of 5-HT neuronal activity by IL-1β signaling in the DRN controls expression of aggressive behavior.
Introduction
Aggressive behavior is expressed in the state of competition between individuals to acquire and protect resources including territories, food, mates, and offspring. Therefore, aggressive behavior is generally considered to be adaptive and is observed in many animal species from insects to mammals and humans. At the same time, there are large individual differences in aggression within a species; some animals show strong aggressive behavior toward conspecific rivals, while others rarely show aggressive behavior [1]. Although aggressive individuals have some evolutionary advantages over less aggressive individuals, they also assume higher risk of injury or death when they encounter similarly aggressive individuals. By contrast, low aggression individuals can maintain an advantage in non-competitive situations in which resources are shared [2]. It is therefore likely that evolutionary forces influence individual differences in aggression within a species.
The immune system is the body’s primary defense against infectious organisms, but it has also been shown to respond to psychological stress [3–7]. Increasing evidence from animal models supports the concept that peripheral and/or central immune responses are linked to individual differences in social stress susceptibility [8–10]. Although less defined, recent evidence suggests there is also a link between the immune system and aggression, since human studies suggest a relationship between the level of peripheral cytokines and aggression/anger/hostility (for review, see [11]). Interleukin 1 beta (IL-1β), a proinflammatory cytokine, induces sickness behaviors and reduces social interaction in rodents when elevated [12–14]. In contrast, studies in humans demonstrate positive correlations between the history of aggressive acts and the expression of receptors for IL-1β in the cerebrospinal fluid [15]. Furthermore, an expectation of upcoming aggressive encounters (i.e., a rugby game) increases circulating IL-1β levels in athletes [16]. These results support involvement of IL-1β in aggressive behavior, however, it is unclear from correlative human studies whether peripheral or central IL-1 β signaling is causally linked to aggressive behavior.
In this study, we examined whether variations of IL-1β levels in the periphery and central nervous system produces individual differences in aggressive behavior in male mice. Our results indicate that there were similar phasic increases of peripheral cytokines, including but not limited to IL-1β, during aggressive encounters in both aggressive and non-aggressive mice. In contrast, non-aggressive mice showed higher elevation of central IL-1β in the dorsal raphe nucleus (DRN) compared to aggressive mice. The DRN is located in the midbrain, contains the largest accumulation of 5-HT neurons [17], and has been shown to regulate a wide array of social behaviors, including aggression [18–23]. Pharmacological antagonism and viral-mediated gene knockdown of the receptors for IL-1 in the DRN caused an increase in aggressive behavior, suggesting an inhibitory role of IL-1β signaling in the DRN on aggression. Our result also revealed that aggressive mice exhibit higher c-Fos expression in 5-HT neurons in the DRN compared to non-aggressive mice, and that this can be modulated by IL-1β signaling.
Materials and Methods
Animals
CD-1 male mice were sexually experienced breeders ~4 months of age (Charles River Laboratory, Wilmington, MA). Resident CD-1 males were acclimated to the animal facility 1 week before the resident-intruder test. All mice were housed individually throughout the experiment in standard mouse cages with corn cobb bedding material. For intruders, intact BALB/cByJ (The Jackson Laboratory, Bar Harbor, Maine) or ICR/Jcl male mice (Charles River Laboratory; Atsugi, Japan) with olfactory bulbectomy (OBX) to eliminate aggressive behavior, at least 10 weeks old, were used. Males of BALB/cByJ and OBX-treated ICR/Jcl mouse strain are less aggressive than CD-1 males. None of the intruders showed aggressive behaviors toward resident CD-1 males. Intruders were housed in a group (n=4-5) in standard mouse cages throughout the experiment.
All mice were maintained on a 12 h light-dark cycle with ad libitum access to food and water. Mouse procedures were performed in accordance with the National Institute of Health Guide for Care and Use of Laboratory animals, the Icahn School of Medicine at Mount Sinai (ISMMS) Animal Care and Use Committee (approval number LA10-00266), and the Animal Care and Use Committee at the University of Tsukuba (approval number 14-391).
Resident-intruder (RI) test
A male intruder mouse was introduced into the home cage of a CD-1 resident males and their behavior was videotaped from the side of cage. The latency to first attack was recorded during the experiment. In each test, a novel intruder male mouse was used.
For the pretest session, a 3-min RI test was conducted once a day for 3 days according to our previously published protocols [24–26]. We defined animals that exhibited aggressive behavior toward intruders in all three aggressive encounters as aggressors (AGG), and animals that did not show any attack bites throughout the three encounters as non-aggressors (NON). Animals that showed aggressive behavior once or twice among three aggressive encounters were defined as variable aggressors. For the manipulation test session (IL-1RA microinjection, IL-R1 knockdown, and c-Fos expression experiment), a 5-min RI test was conducted (detailed in the following sections).
Frequency and duration of behaviors during the RI test were scored from the video by a well-trained observer using free software established by A. Tanave (TanaMove_v0.01). Behaviors analyzed include aggressive behaviors (attack latency, attack bites, sideways threats, tail rattles, and pursuits) and non-aggressive behaviors (locomotion, rearing, self-grooming, and social contacts) [27, 28]. Total aggressive behavior was defined as the duration of aggressive acts including attack bite, sideways threat, and pursuit (tail rattle was not included).
Blood sampling
Blood samples were collected by submandibular bleed 3-5 days prior to the RI test and either 20 minutes or 24 hours after the end of the last RI test. To isolate serum, whole blood samples were collected into protein lo-bind tubes (Eppendorf), incubated at room temperature for 1 hour, and centrifuged at 956 × g for 15 min at 4 °C. Serum was collected in new protein lo-bind tubes and stored at −80 °C until ELISA assay. For lipopolysaccharide (LPS) stimulation assay, whole blood was collected into heparin-lined tubes (Heparin-lithium coat, Milian), and stored overnight at room temperature in the dark.
Brain sampling for cytokine measurement
One hour after the last RI test, brains were rapidly removed after cervical dislocation and sliced to 1mm thick coronal sections using the mouse brain slicer matrix (Alto Stainless Steel Coronal 1.00mm Brain Matrix, CellPoint Scientific) on ice. The medial prefrontal cortex, ventral striatum, hypothalamus, and DRN were removed using 2 mm punch cannula (Harris Uni-Core-2.00, Electron Microscopy Sciences) in ice-cold PBS. Samples were collected into 1.5 mL tubes, immediately frozen on dry ice, and stored at −80 °C. To measure brain IL-1β level, 100 μL extraction solution (20 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 % Triton-X100, and 1 μg/mL protease inhibitor cocktail [Complete tablet; Roche Diagnostics] in distilled water) was added to each frozen brain sample, and samples homogenized on ice. Then, samples were agitated for 90 min at 4 °C, centrifuged at 956 × g for 20 min at 4 °C, and the supernatants were collected into new tube. They were stored at −80 °C until ELISA assays were performed. For multiplex ELISA analysis, the DRN sample was collected the same as described above for IL-1β measurement, except the tissue was homogenized with ProcartaPlex cell lysis buffer (ThermoFisher) and left on ice for 30 min before centrifugation and collection of supernatant.
LPS stimulation of cultured leukocytes
Blood samples were obtained from naïve CD-1 mice 3-5 days prior to their first RI test. Leukocytes were isolated from whole blood by Ficoll density gradient [8]. Briefly, whole blood (200 μL) was mixed with 2 mL of culture medium (RPMI 1640 (Sigma), 20% horse serum, 10 % FBS, 2 mM l-glutamate, 100 units/mL of penicillin, and 100 ug/mL streptomycin), layered on top of 2 mL Ficoll-Paque Plus (GE Healthcare), and centrifuged at 790 × g for 15 min at 25 °C. The buffy coat interphase was collected, washed in BEP (0.5 % BSA and 2 mM EDTA in PBS), and centrifuged at 529 × g for 8 min at 25 °C. The mononuclear cells were resuspended in 200 uL of BEP. Cells were counted using a hemocytometer. 1 x 106 viable cells were plated in each well, and 1 mL media with or without LPS (34 μg/mL) was added. Cells were incubated for 24 hours at 37 °C with 5% CO2. On the following day, media was collected, centrifuged (2348 × g, 5 min), and supernatant was stored at −80 °C until ELISA assays were performed.
IL-1β ELISA
Serum samples collected before any aggressive encounter, 20 min and 24 hours after the RI test were used to measure IL-1β level. Frozen samples were thawed and brought to room temperature. IL-1β level was measured using Mouse IL-1β/IL-1F2 Quantikine ELISA (R&D Systems). Serum samples were measured in duplicate, but brain samples were measured as single samples due to the small volume. Plates were read on microplate reader (Spectramax 340PC, Molecular Devices) according to the manufacturer’s specifications. Mean minimum detectable dose was 2.31 pg/mL. In brain samples, total protein concentration was measured using a DC Protein Assay (Bio-Rad) and IL-1β concentration is expressed as a % of total protein.
Multiplex ELISA
Serum samples were used to measure cytokines and chemokines. A total of 32 cytokines and chemokines were measured using MILLIPLEX MAP Mouse Cytokine/Chemokine magnetic bead panel (MCYTOMAG-70K, Milipore). All samples were thawed and brought to room temperature, vortexed briefly, centrifuged at 956 × g, 5 min, and 25 μl of supernatant (1/2 dilution with assay buffer) was measured in duplicate according to the manufacturer’s instructions. For DRN samples, a total of 6 cytokines were measured using MILLIPLEX MAP Mouse Cytokine/Chemokine magnetic bead panel (MCYTOMAG-70K, Milipore). 20 μg protein were loaded per well and assay results read on a Luminex 200 milliplex analyzer.
Quantification of mRNA expression in the DRN
One day after the last RI test, brains were rapidly removed after cervical dislocation and then sliced to 1mm thick coronal sections using a mouse brain slicer matrix (Alto Stainless Steel Coronal 1.00mm Brain Matrix, CellPoint Scientific) on ice. The DRN was removed using a 2-mm punch biopsy tool (Harris Uni-Core-2.00, Electron Microscopy Sciences) in ice-cold PBS. Samples were collected into 1.5 mL tubes, immediately frozen on dry ice, and stored at −80 °C until cDNA synthesis. For RNA extraction and cDNA synthesis, DRN samples were homogenized in TRIzol Reagent (Invitrogen) and then chloroform was added to isolate RNA. The RNA layer was processed with RNeasy Micro Kit (Qiagen) to extract total RNA and the total RNA concentration was measured using Nanodrop (Thermo Fisher Scientific). cDNA was synthesized from 500 ng of total RNA by reverse transcription using qSCRIPT cDNA SuperMix (Quanta Biosciences). Quantitative PCR reaction was conducted with Perfecta SYBR Green (Quanta Biosciences). Analysis was done using the ΔΔ Ct method and samples were normalized to Gapdh.
Isolation of microglia and endothelial cells from the DRN
Test mice were characterized as AGG or NON suing the 3-day RI test described above. Under deep anesthesia with isoflurane, animals were transcardially perfused with ice-cold PBS, and the brains were rapidly removed. Brains were sliced to 1mm thick coronal sections using the mouse brain slicer matrix (Alto Stainless Steel Coronal 1.00mm Brain Matrix, CellPoint Scientific) on ice, and the DRN were removed using 2 mm punch cannula (Harris Uni-Core-2.00, Electron Microscopy Sciences) in ice-cold DPBS(+) with 0.5% BSA. The DRN samples from 3 animals were combined to one tube. Cell dissociation was performed using the Adult Brain Dissociation Kit (P) (Miltenyi Biotec) with the gentleMACS Dissociator Octo with Heaters (Miltenyi Biotec) according to the manufacturer’s protocol. The dissociated cells were passed through the 70 μm strainer (Falcon). The cells were incubated with CD11b Microbeads (130-093-634, Miltenyi Biotec) for 15 min at 4°C and the microglial (CD11b+) cells were isolated by using LS column on the magnet (MACS MultiStand, Miltenyi Biotec). Both CD11b+ cells and CD11b− cells were collected in the separated tubes, and CD11b− cells were then incubated with CD31 Microbeads (130-097-418, Miltenyi Biotec) for 15 min at 4°C. Then endothelial cells (CD31+) and the other (CD31−) cells were isolated by LS column on the magnet into the separated tubes. Isolated microglial cells (CD11b+), endothelial cells (CD31+, CD11b−) and other cells (CD11b−, CD31−) were pelleted down by centrifuge, 400 μL TRIzol (Invitrogen) was added to each tube and they were kept at −80 °C until RNA extraction. Methods for the RNA extraction, cDNA synthesis and qPCR analysis were the same as described in the previous section.
Stereotaxic surgery and intracranial microinjection of IL-1RA and IL-1β
Following day 3 of aggression screening, stereotaxic surgery was conducted for DRN cannulation. Animals with variable aggression or aggressors with low to intermediate level of aggressive behavior were used in this experiment to increase or decrease aggressive behavior by pharmacological manipulation. In a separate experiment, we used non-aggressor animals to see whether intra-DRN IL-1RA injection can promote aggressive behaviors in these animals.
Animals were anesthetized by intraperitoneal injection of a mixture of 100 mg/kg Ketamine HCl and 10 mg/kg xylazine, and the analgesic drug bupivacaine was locally applied before the surgery. A 26-gauge cannula (Plastics One) was inserted aimed at either the lateral ventricle (AP, −0.6 mm; ML, +1.2 mm; DV, −1.8 mm) or the DRN (AP, −4.6 mm, ML, +1.5 mm, DV −1.9 mm, Angle 26°). A 33-gauge dummy cannula (Plastics One) was inserted after the surgery. After 5 days of recovery from the surgery, we started to handle the animals and moved the obturator every day to prevent blockage of the cannula and to habituate animals to handling.
Drug microinjection experiment started ten days after the surgery. Animals were injected with IL-1 receptor antagonist (IL-1RA; Recombinant Mouse IL-1ra/IL-1F3, R&D System, USA) into either the lateral ventricle or the DRN. For microinjection, a 33-gauge microinjector (Plastics One) was attached to a polyethylene tube, and connected to 10 μL Hamilton mycrosyringe placed on a microinfusion pump (Harvard PHD2000 syringe pump, Harvard Apparatus). For intracerebroventricular (i.c.v.) injection, IL-1RA (250 ng) was injected in a volume of 1 μL over 4 min, and the injector was removed 1 min after the end of the infusion. Fifteen minutes after the injection, an intruder was introduced into the home cage and aggressive behavior was videotaped for 5 min. This experiment was conducted three times over three-days; the drug group received IL-1RA every time, while the control group received a vehicle (sterile 0.1 M PBS) for 3 days. On day 4, we tested their aggressive behavior without any injection. In a separate group of mice, we examined 5-HT neuron activation after i.c.v. IL-1RA injection and aggressive interaction. Animals received a single IL-1RA injection and brain samples were collected 90 min after the aggressive encounter (see details in the later section). For intra-DRN injection, IL-1RA (50 ng) was injected in a volume of 0.2 μL over 2 min, and the injector was removed 1 min after the end of the infusion. An intruder was introduced into the homecage of test animals 10 min after the microinjection, and aggressive behavior was observed for 5 min over 2 consecutive days.
For IL-1β intra-DRN injection experiment, 200 pg of IL-1β (Recombinant Mouse IL-1β/IL-1F2 Protein, R&D System, USA) was microinjected into the DRN in a volume of 0.2 μL over 2 min, and the injector was removed 1 min after the end of the infusion. Ten minutes after the injection an intruder was introduced into the home cage and aggressive behavior was videotaped for 5 min. For IL-1β injection, animals received drug or vehicle (sterile 0.1 M PBS) injection only on Day 1, and aggressive behaviors were tested on Day 2 and Day 3 without injection.
IL-1R1 shRNA construction
DNA oligos encoding small hairpin RNAs (shRNA) targeting mouse IL-1R1 were annealed and cloned immediately downstream of the H1 promoter into an adeno associated virus (rAAV) vector plasmid. Two target shRNA sequences were cloned for IL-1R1 transcript separately (IL1R1-shRNA1: GTGCCTCTGCTGTCGCTGGA, IL1R1-shRNA2: GTCTTCAAGGTTGACATAGTG). As a control, a rAAV2 vector expressing shRNA targeting luciferase (Luc) was used (AAV.H1.Luc; [29]). All of these vectors expressed yellow fluorescent protein (YFP) under the CBA promotor in order to visualize the transfected cells. The viral stocks were generated by packaging the vector plasmids encoding the shRNA into AAV serotype 2 particles using a helper-free system. Titers of AAVs were determined by quantitative PCR (qPCR) using WPRE (Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element)-specific primers and adjusted to 1012 genomic particles per ml. 1:1 mixture of IL1R1-shRNA1 AAV and IL1R1-shRNA2 AAV were used in all experiments (Supplementary Table 1).
IL-1R1 shRNA knockdown (KD) in the DRN
Mice were tested for aggressive behavior over three consecutive days before stereotaxic surgery. Animals with variable aggression or aggressors with low to intermediate level of aggressive behavior were used in this experiment to prevent floor and ceiling effects that might interfere with our ability to uncover an increase or decrease in aggressive behavior following treatment. Animals were anesthetized by intraperitoneal injection of a mixture of 100 mg/kg Ketamine HCl and 10 mg/kg xylazine, and analgesic drug bupivacaine was locally applied before the surgery. An 33-gauge needle attached to a glass Hamilton syringe was stereotaxically inserted into the DRN (AP, −4.6 mm, ML, +1.5 mm, DV −4.0 mm, Angle 26°). Either IL-1R1 shRNA AAV or Luc shRNA AAV vector was infused in a volume of 1.0 ul over 3 min, and the needle was left in place for 10 min after the injection. Two-weeks after the AAV injection, aggressive behavior of these mice was examined using the RI test over 3 consecutive days. In a separate group of mice, we examined 5-HT neuron activation after aggressive interaction in the IL-1R1 KD animals by c-Fos immunohistochemistry.
Immunohistochemistry, in situ hybridization and histology
To examine c-Fos expression in 5-HT neurons, brain samples were collected 90 min after the RI test on the third day. Mice were anesthetized by i.p. injection of a euthanizing dose of 15% Chloral hydrate and perfused intracardially with PBS and then with 4 % paraformaldehyde (PFA) in PBS. Brains were postfixed overnight in 4% PFA in PBS and placed in 30% sucrose in PBS for cytoprotection for 2 nights at 4 °C. Brains were then frozen in isopentane on dry ice and kept at −80 °C until slicing on a cryostat (30 μm coronal sections). A free-floating staining method was used for c-Fos and 5-HT staining. After 2 hours of incubation in blocking solution (3% normal donkey serum + 0.3% Triton-X100) at 4 °C, sections were incubated with c-Fos antibody (1:250 goat anti-c-Fos antibody (sc-52-G), Santa Cruz Biotechnology, Santa Cruz, CA) for 3 night at 4 °C. To examine the co-localization of c-Fos with 5-HT neurons, sections were then incubated with a mixture of c-Fos antibody (1:250) and 5-HT antibody (1:1000 5-HT rabbit antibody (20080), Immunostar, Hudson, WI) for 1 night at 4 °C. After washing with PBS, sections were incubated with a mixture of secondary antibodies (1: 400 anti-rabbit Cy2; 1:400 anti-goat Cy5, Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature, washed again with PBS, then incubated with DAPI for 15 min and finally coverslipped with DPX mounting media after ethanol dehydration.
For c-Fos analysis in the IL-1R1 shRNA KD animals, immunohistochemistry was performed as described above with a different secondary antibody mixture (1:1000 anti-rabbit Alexa Fluor 594 and 1:1000 anti-goat Alexa Fluor 680, Jackson ImmunoResearch). To confirm the infection site of IL-1R1 KD AAV, sections containing the DRN were stained with a mixture of 5-HT (1:1000) and GFP (1:3000 chicken anti-GFP (GFP-1020), Aves Labs, inc.) primary antibodies overnight at 4°C. Sections were then incubated in a mixture of 1:400 anti-chicken Cy2 and 1:400 anti-rabbit Cy5 secondary antibodies.
For histological verification of drug microinjection site, animals were deeply anesthetized with chloral hydrate and perfused with saline then 4 % PFA at the end of intracranial microinjection experiment. After overnight postfixation with 4 % PFA, brains were sliced into 60 um sections with a vibratome and then stained with cresyl violet to verify cannula placement.
For in situ hybridization, RNAscope Multiplex Fluorescent in situ kit (Advanced Cell Diagnostics) was used according to the manufacturer’s instructions. Briefly, brain samples were collected 30 min after the last RI test. Fresh-frozen sections were fixed in the ice-cold 4% PFA for 15 min, dehydrated with EtOH, and pretreated with a protease for 30 min. RNAscope probes for IL-1R1 (cat. 413211), serotonin transporter (SERT, cat. 315851-C3), GAD67 (cat. 400951-C2), c-Fos (cat. 316921, vesicular glutamate transporter 3 (vGlut3, cat. 431261-C3) were hybridized at 40oC for 2h, serially amplified, counterstained with DAPI, and coverslipped with EcoMount mounting media (Biocare Medical).
Microscopic images of the DRN were acquired on a LSM-780 confocal microscope (Carl Zeiss) using a 40X oil immersion objective. 5x5 tile scanning images were taken to cover the entire DRN using ZEN software. In IL-1R1 KD experiment, 3X3 tile scanning images were acquired using an All-In-One Fluorescence Microscope (BZ-X710, Keyence) with 10X objective.
Data analysis
GraphPad Prism 5 software was used for statistical analysis. One-way ANOVA followed by Tukey’s multiple comparison tests were conducted to compare group differences in attack latency between AGG, NON and variable AGG animals. Two-way ANOVA was performed to examine the temporal change blood levels of IL-1β. Two-way repeated measures ANOVA was conducted to assess IL-1β between AGG, NON and variable AGG animals following ex vivo leukocyte LPS stimulation. When F value were significant, t tests with Bonferroni correction were performed. Pearson correlation coefficients (r) were calculated to examine the correlation between aggressive behaviors and either the number of leukocytes or the level of IL-1β in the DRN. Unpaired t tests were used to compare brain IL-1β level between AGG and NON. In IL-1RA and IL-1β intracranial injection and IL-1R1 shRNA KD experiments, group difference of aggressive behaviors in the pretest were examined by using unpaired t test. Two-way repeated measures ANOVA followed by t test with Bonferroni correction was performed to examine the effect of IL-1RA or IL-1β injection or IL-1R1 shRNA KD. For detailed behavioral analysis, average data over 2-day tests (i.c.v. and intra-DRN IL-1RA microinjection) or 3-day tests (IL-1R1 KD) were analyzed with unpaired t test to compare the control group with either the IL-1RA or IL-1R1 shRNA group. For intra-DRN IL-1β injection, we separately analyzed the results of Day1 (with IL-1β injection) and Day 2-3 (without IL-1β injection). For immunohistochemistry, qPCR and in situ hybridization data, unpaired t tests were used to compare two groups. For MACS analysis, two-way repeated measures ANOVA followed by t test with Bonferroni correction was performed to examine the effect of cell type and group on IL-1β mRNA expression.
Results
Individual difference of aggression and the level of IL-1β in the periphery and brain
As reported previously [24–26], we observed individual differences in the initiation of aggressive behavior among CD-1 male mice (n=424) when evaluated in the resident-intruder (RI) test. Approximately half of all animals attacked BALB/cByJ intruder males consistently during each encounter from day 1 to day 3 (AGG; Fig. 1a). Animals that showed aggressive attack in 1 or 2 days among three aggressive encounters were defined as variable aggressors (VAR; 36.6%). A smaller percentage of mice (17.2 %) did not exhibit any attack behavior over three resident-intruder encounters (NON). AGG mice showed significantly lower attack latency than NON and VAR mice (F(2,423) = 631.5, p < 0.0001; Fig. 1b). Detailed behavior analysis over 3-day encounters (n=123) revealed that AGG mice also exhibit more attack bites (F(2,246) = 62.58, p < .0001), sideways threats (F(2,246) = 68.15, p < .0001), tail rattles (F(2,246) = 40.73, p < .0001), pursuits (F(2,246) = 9.197, p = .0002), locomotion (F(2,246) = 6.752, p = .0017) and grooming (F(2,246) = 5.659, p = .0036), whereas the AGG mice exhibited significantly less non aggressive-social contact (F(2,246) = 19.13, p < .0001) compared to NON in the RI test (Supplementary Fig. 1, Supplementary Table 2).
Figure 1. Individual difference of aggressive behavior and IL-1β response in the periphery and central nervous system.
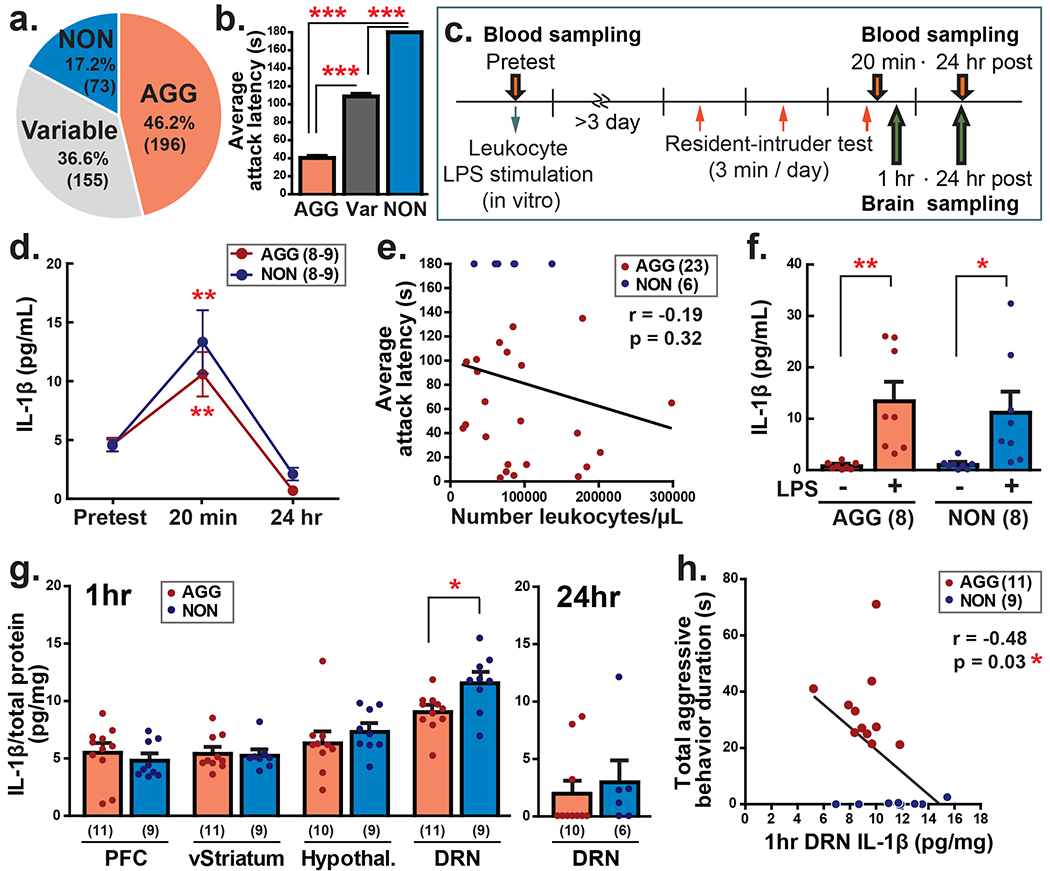
a Ratio of aggressors (AGG), non-aggressors (NON), and variable aggression animals (Variable) in 424 resident CD-1 male mice. b Average attack latency over 3 days of resident-intruder encounters in AGG, Variable (Var) and NON animals (right). c Schematic timeline of experiments. d Blood IL-1β level before, 20 min, and 24 hrs after the aggressive encounter in AGG and NON animals. Dunnet’s t-test was conducted in each group to compare 20 min and 24 hrs samples with the base level (pre). e IL-1β production from cultured leukocytes by LPS stimulation in AGG and NON. f There was no correlation between number of leukocytes and average attack latency over 3 days of resident-intruder test. g IL-1β level in the prefrontal cortex (PFC), ventral striatum (vStriatum), hypothalamus (hypothal.), and dorsal raphe nucleus (DRN) 1 hour after the aggressive encounter (Left). IL-1β level in the DRN decreased 24 hours after aggressive encounter (right). h Negative correlation was observed between duration of total aggressive behaviors and IL-1β level in the DRN 1 hour after the aggressive encounter. Numbers in the parenthesis indicate the number of animals in each group. *p<0.05, **p<0.01, ***p<0.001.
To examine differences in serum levels of the proinflammatory cytokine IL-1β following resident-intruder encounter, we collected blood samples from both AGG and NON 3-5 days prior to the RI test and then again 20 min or 24 hours after the RI test (Fig. 1c). Two-way ANOVA indicated a significant main effect of time on blood IL-1β level (F(2,44) = 26.17, p < 0.0001). IL-1β level was significantly increased 20 min after the resident-intruder encounter from its basal level in both AGG and NON and it returned to basal level 24 hours after the aggressive encounter (Fig. 1d). There was no group difference between AGG and NON in peripheral IL-1β level at any time points. We also examined the level of other cytokines and chemokines by using multiplex ELISA assays (Supplementary Table 3) and found IL-4, IL-6, IL-7, IL-18, KC, and MCP-1 were increased to higher circulating levels 20 minutes after the encounter and fell to lower level 24 hours after the encounter. IL-1α and RANTES increased to a higher level 24 hours after the aggressive encounter compared to the 20-minute time point in both AGG and NON mice. Again, there was no significant group difference between AGG and NON. Because NON mice do not exhibit any physical attack behaviors during the intruder encounter, it is likely that changes in peripheral cytokines are induced by the psychological stress of territorial intrusion by another male, rather than by the aggressive act itself.
Previous studies have shown that the number of circulating leukocytes as well as leukocyte-derived cytokine release via ex vivo stimulation with LPS can predict individual differences in stress susceptibility in the social defeat stress model [8]. Thus, we tested whether there might be any differences in these leukocyte properties between AGG and NON that predict their response in the RI test. First, we found no difference in the number of leukocytes between AGG and NON (10.9 ± 1.9 ×104 cells and 9.4 ± 2.2 ×104 cells, AGG and NON, respectively), nor did we observe a correlation between the number of leukocytes and attack latency (Fig. 1e). We then measured cytokine release from cultured leukocytes in response to LPS. Similar to results from in vivo cytokine analysis, LPS stimulation significantly increased production of IL-1β from cultured leukocytes (F(1,14) = 18.95, p = 0.0007; Fig. 1f); however, there were no differences between AGG and NON.
Given the fact that AGG and NON mice did not differ in peripheral IL-1β production, we next tested whether IL-1β or its receptor expression differed in any brain regions known to regulate aggression. We first collected tissue from the medial prefrontal cortex, ventral striatum, hypothalamus, and DRN 1 hour after the RI test and measured IL-1β protein. We found that NON mice had higher levels of IL-1β in the DRN compared to AGG (t(18) = 2.679, p = 0.0153; Fig. 1g). Levels of IL-1β normalized 24 hours after the aggressive encounter (Fig. 1g, right). There was no difference in IL-1β protein between AGG and NON in other brain regions (Fig. 1g, left). We also found a significant negative correlation between the total duration of aggressive behaviors and IL-1β in the DRN one hour after the RI test (Fig. 1h). No differences between AGG and NON were observed in levels of other cytokines in the DRN (Supplementary Table 4). We also examined gene expression between AGG and NON in the DRN. Similar to protein levels, we found that NON had higher IL-1β mRNA in the DRN compared to AGG when sampled 24 hours after the last fight (Fig. 2a). We did not see any significant differences in mRNA for IL-1 receptors, IL-1 receptor adaptive proteins, or the endogenous IL-1 receptor antagonist (IL-1RA) (Fig. 2a, Supplementary Table 5). These results suggest that increased IL-1β in the DRN of NON might suppress aggressive behavior.
Figure 2. Expression of IL-1β and IL-1 receptor type 1 (IL-1R1) mRNA in the DRN of AGG and NON animals.
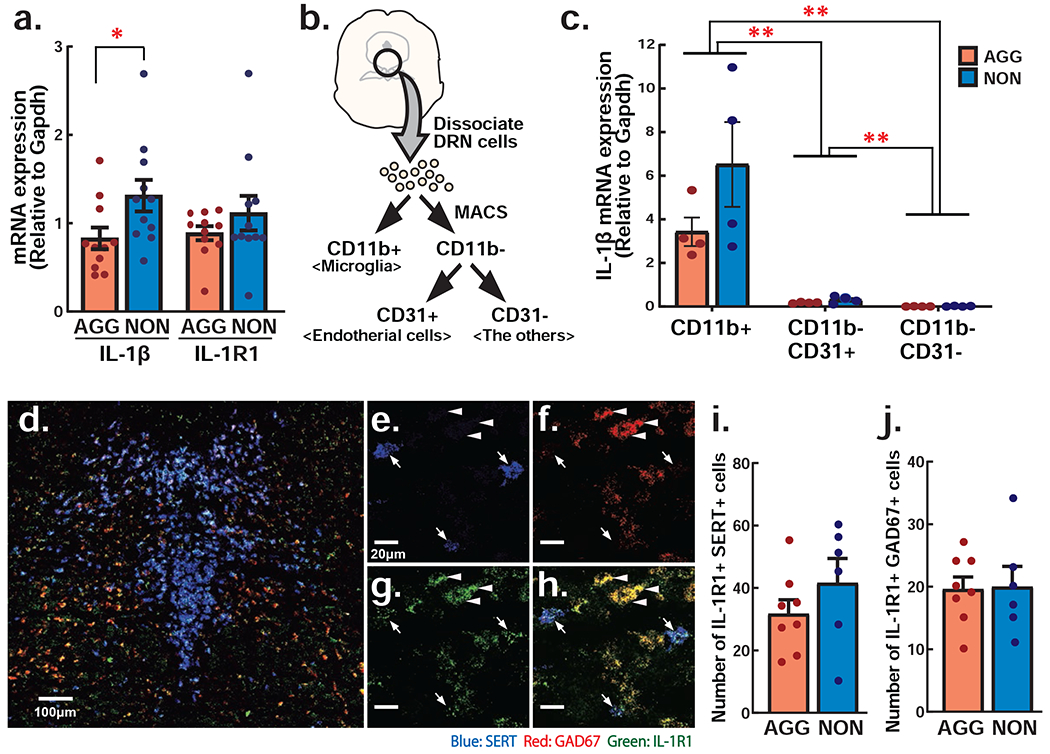
a IL-1β and IL-1R1 mRNA expression in the DRN. b Schematics of MACS analysis to isolate microglial cells (CD11b+), endotherial cells (CD31+, CD11b−) and the other cells including neurons (CD11b−, CD31−). c IL-1β expression relative to Gapdh in the CD11b+ cells (microglia), CD31+ cells (endotherial cells) and CD11b− & CD31− cells (the others). CD11b+ cells express the highest amount of IL-1β mRNA compared to other cell types. IL-1β mRNA expression in CD11b−,CD31+ cells were very low compared to CD11b+ cells, but it was higher than CD11b−,CD31− cells. One data point represents a combined sample from 3 animal’s DRN. Although it did not reach statistical significance because of small number of data points, NON showed higher IL-1β mRNA expression in any cell types compared to AGG. d Representative picture of the expression of IL-1R1 (green), SERT (blue) and GAD67 (red) in the DRN. e-h Enlarged pictures of the DRN showing SERT (e), GAD67 (f), IL-1R1 (g), and their co-localization (h). Arrows indicate SERT-positive 5-HT neurons and arrow heads indicate GAD67-positive GABA neurons. i-j Total number of IL-1R1 positive 5-HT neurons (i) and IL-1R1 positive GABA neurons (j) in the DRN of AGG an NON animals. * p<.05, ** p<.01.
Cell-type specific expression of IL-1β and IL-1 receptor in the DRN
Because IL-1β mRNA expression in the DRN was increased in the NON compared to AGG, it is likely that the IL-1β is produced locally rather than derived from peripheral blood. To examine which cell types are producing individual difference of IL-1β in the DRN, we isolated the microglial cells and endothelial cells from the DRN of AGG and NON (Fig. 2b). The IL-1β mRNA expression was condensed in the CD11b+ microglial cells; 20.5 times higher than the CD11b−,CD31+ endothelial cells and 485.2 times higher than CD11b−,CD31− cells including neurons (Fig. 2c). Indeed, two-way ANOVA showed significant main effect of cell-type, and CD11b+ cells showed significantly higher IL-1β expression than other cell types. However, we failed to detect significant main effect or interaction of group (AGG vs NON). In average, NON showed higher IL-1β mRNA expression in the microglia, though it failed to reach statistical significance compared to AGG because of small number of samples. Due to high level of IL-1β mRNA expression in the microglia of the DRN, higher level of DRN IL-1β proteins observed in NON mice may be originated from microglia.
We next examined the expression of IL-1 receptors on neurons in the DRN by in situ hybridization. We confirmed expression of IL-1R1 in both GAD67-positive GABA neurons and SERT-positive 5-HT neurons (Fig. 2d–h). We also examined the number of 5-HT neurons and GABA neurons that express IL-1R1 between AGG and NON, but there were no group differences in either subtype (Fig. 2i, 2j). These results indicate that higher IL-1β production from microglial cells in the DRN of NON mice has the potential to affect activity of both 5-HT neurons and GABA neurons leading to suppression of aggressive behavior.
Blocking of IL-1β receptor in the brain caused increased aggressive behavior
To confirm a causal role for IL-1β in the brain in aggressive behavior regulation, we microinjected an IL-1 receptor antagonist (IL-1RA) either into the lateral ventricle or directly into the DRN (Fig. 3a, f). Prior to cannulation surgery, each animal’s aggressive behavior was characterized for 3 consecutive days and they were assigned to either the control group or IL-1RA group (Fig. 3b, c, Pretest). Aggression levels were fully balanced across the 2 groups. Following i.c.v. injection we found that aggression levels were increased in the group receiving IL-1RA. Two-way repeated measures ANOVA revealed a significant main effect of drug on the latency to first attack (F(1,15) = 8.508, p = 0.0106; Fig. 3b), and post hoc tests confirmed that IL-1RA injection caused a significant reduction of attack latency compared to vehicle-injected controls. There was also a significant main effect of drug on the duration of aggressive behaviors (F(1,15) = 5.209, p = 0.0375; Fig. 3c), where animals receiving IL-1RA showed a longer duration of aggressive behaviors compared to vehicle-injected controls. A more detailed behavioral analysis showed that i.c.v. IL-1RA injection caused a significant increase in the frequency of attack bites (t(15) = 2.772, p = 0.0142) compared to vehicle-treated controls (Fig. 3d). We did not observe any significant change in non-aggressive behaviors (Fig. 3e). On day 4, after the drug was no longer present, we again tested these animals and found no difference in any behaviors between IL-1RA group and vehicle control (Supplementary Fig. 2).
Figure 3. Inhibition of IL-1β receptors in the brain increased aggressive behavior of male mice.
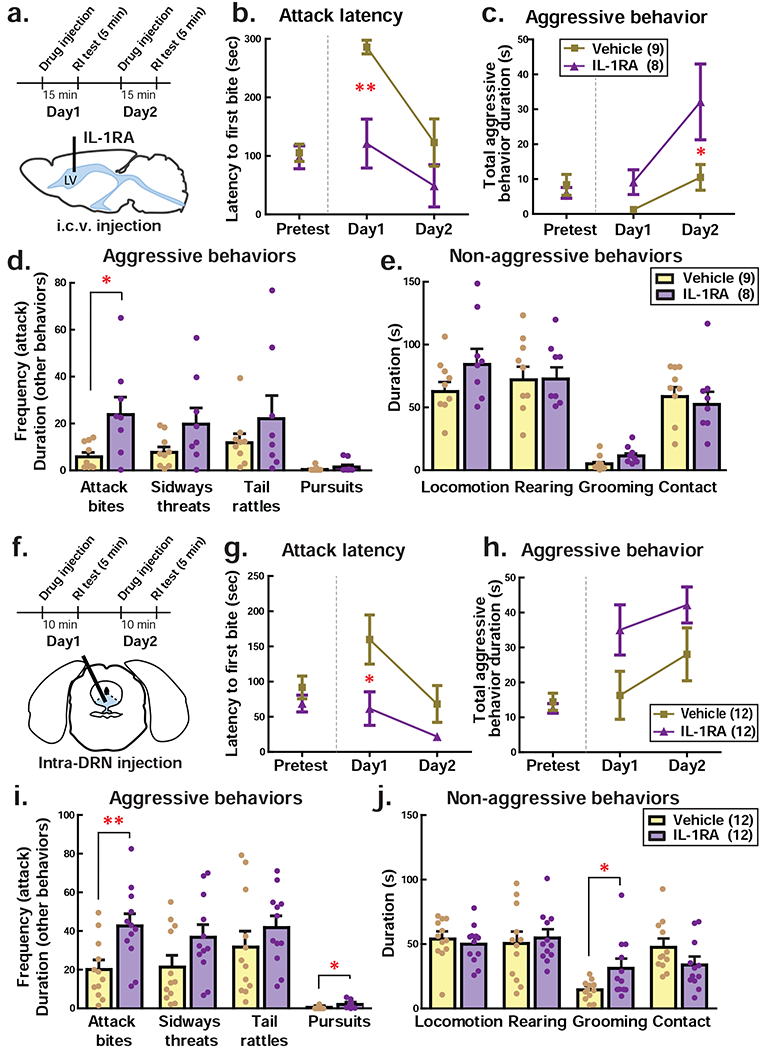
a Schematic timeline of i.c.v. IL-1RA injection experiment. RI test: resident-intruder test. b-c Effect of i.v.c. IL-1RA injection on the attack latency (b) and duration of aggressive behaviors (c). d-e Detailed behavioral analysis for aggressive behaviors (d) and non-aggressive behaviors (e). Average data of first 2 days of resident intruder test was presented. f Schematic timeline of intra-DRN IL-1RA injection experiment. g-h Effect of intra-DRN IL-1RA injection on the attack latency (g) and duration of aggressive behaviors (h). i-j Detailed behavioral analysis for aggressive behaviors (i) and non-aggressive behaviors (j). Average data of 2-day resident intruder test was presented. Numbers in the parenthesis indicate the number of animals used in each group. +p<.10, *p<0.05, **p<0.01.
We next wanted to confirm a specific role for IL-1β signaling directly within the DRN, so we cannulated mice and directly injected IL-1RA into the DRN (Fig. 3f). Two-way repeated measured ANOVA showed a significant main effect of drug on the attack latency (F(1,22) = 7.560, p = 0.0117) and post hoc tests confirmed that intra-DRN IL-1RA injection shortened attack latency compared to the vehicle-treated control mice (Fig. 3g). There was a trend towards increased duration of aggressive behaviors following intra-DRN IL-1RA injection when compared to control (F(1,22) = 4.119, p = 0.0547; Fig. 3h). Detailed behavioral analysis showed significant increases in attack bites (t(22) = 3.128, p = 0.0049), and pursuits (t(22) = 2.543, p = 0.0185) following intra-DRN IL-1RA injection compared to vehicle control (Fig. 3i, Supplementary Fig. 3). We also observed a significant increase in grooming behavior (t(22) = 2.404, p = 0.0251) with intra-DRN IL-1RA injection compared to control (Fig. 3j). It is important to note that intra-DRN injection of IL-1RA did not initiate aggressive behaviors in NON mice (Supplementary Fig. 4). These results indicated that endogenous IL-1β in the DRN has a suppressive effect on the level of aggressive behavior in AGG animals.
Knockdown of IL-1β receptor in the DRN caused increased aggressive behavior
To further confirm the inhibitory role of IL-1β signaling in the DRN on aggressive behavior, we suppressed the expression of IL-1R1 by injecting IL-1R1 shRNA-expressing AAVs into the DRN (Fig. 4a). Two weeks after AAV injection, we observe strong viral expression in both serotonergic and non-serotonergic neurons within the DRN (Fig. 4b, c), with ~4 times more non-serotonergic neurons colocalized with YFP than serotonergic neurons (Fig. 4d). To confirm efficiency of IL-1R1 knockdown (KD) by IL-1R1 shRNA AAV injection, we dissected the DRN of IL-1R1 shRNA AAV injected animals (n=9) and control Luc shRNA injected animals (n=10) and extracted RNA for qPCR. IL-1R1 shRNA AAV injected animals showed ~41% reduction of IL-1R1 mRNA in the DRN compared to Luc shRNA AAV injected controls (t(17) = 2.741, p = 0.0139; Fig. 4e). IL-1R1 KD also led to a reduction of IL-1 receptor adaptor protein (IL-1RaP) mRNA expression; however, there were no significant differences in IL-1β, IL-1RA and TNFα mRNAs between IL-1R1 KD and control (Supplementary Table 6). We then examined aggressive behavior in the three-day RI test as described above. Two-way repeated measures ANOVA showed a significant interaction between drug and time (F(2,68) = 5.721, p = 0.0051) and a significant main effect of virus (F(1,68) = 6.780, p = 0.0021) on the attack latency (Fig. 4f). Post hoc tests confirmed that intra-DRN IL-1R1 KD group exhibited shorter attack latency on Day 1. We also observed a significant main effect of virus on the duration of aggressive behaviors (F(1,68) = 7.843, p = 0.0084; Fig. 4g). Detailed behavioral analysis showed significant increases in attack bites (t(34) = 2.735, p = 0.0098) and pursuits (t(34) = 2.358, p = 0.0243) in intra-DRN IL-1R1 KD-injected animals when compared to Luc control (Fig. 4h, Supplementary Fig. 5). We also observed a significant reduction in rearing (t(34) = 2.468, p = 0.0188) and a significant increase in grooming (t(34) = 2.528, p = 0.0163) by intra-DRN IL-1R1 KD compared to Luc control (Fig. 4i). In conclusion, consistent with pharmacological antagonism experiments, these data showed that IL-1β signaling through IL-1R1 in the DRN has a suppressive effect on aggressive behavior.
Figure 4. Knockdown of IL-1 receptor type I (IL-1R1) expression in the DRN facilitated aggressive behavior of male mice.
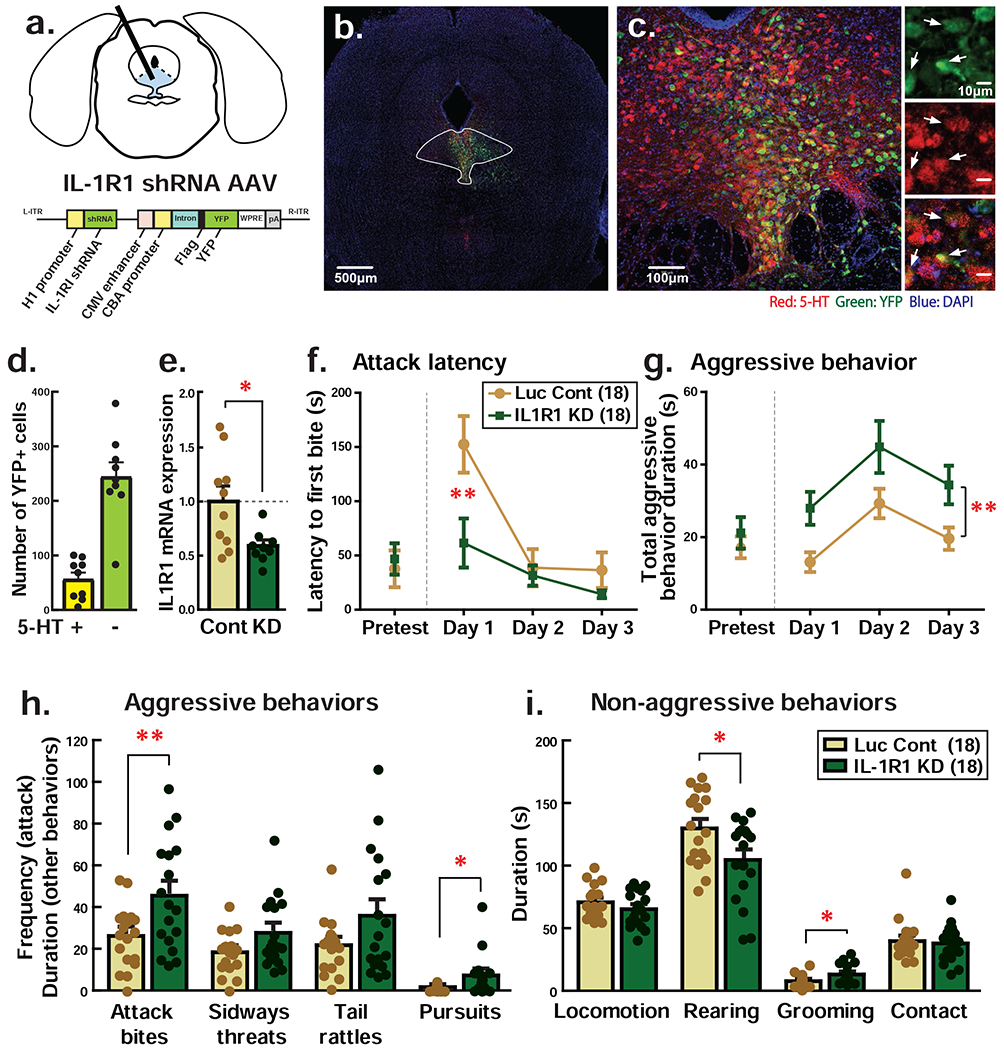
a Schematics of IL-1R1 knockdown (KD) in the DRN and IL-1R1 shRNA expressing AAV construct. IL-1R1 shRNA AAV also expresses YFP as a marker under the different promoter. b-c Representative pictures of IL-1R1 shRNA AAV infection in the DRN. Green: YFP, Red: serotonin (5-HT), Blue: DAPI. Inserted pictures in the right side of c showed enlarged pictures of YFP-positive cells (top), 5-HT-positive cells (middle) and their co-localization in the merged picture (bottom). White arrows indicate YFP-positive 5-HT neurons. d Number of YFP-positive cells on 5-HT-immunopositive neurons (5-HT+) and non-serotonergic neurons (5-HT−). e Quantitative PCR expression analysis of IL-1R1 mRNA in the DRN. Cont: Luciferase (Luc) shRNA expressing AAV injection control, KD: IL-1R1 shRNA expressing AAV injection into the DRN. f-g Effect of IL-1R1 KD in the DRN on the attack latency (f) and duration of aggressive behaviors (g). h-i Detailed behavioral analysis for aggressive behaviors (h) and non-aggressive behaviors (i). Average data of 3-days resident intruder test was presented. Numbers in the parenthesis indicate the number of animals used in each group. *p<0.05, **p<0.01.
To examine if an acute increase of IL-1β during aggressive encounter, which was observed in NON animals, suppresses aggressive behavior, we examined the priming effect of direct microinjection of IL-1β into the DRN on subsequent aggressive behaviors 2-3 day later. We found that intra-DRN injection of a low dose of IL-1β (200 pg) did not affect aggressive behaviors acutely (Day1, Supplementary Fig. 6), but it suppressed aggressive behaviors during subsequent encounters without IL-1β injection (Day2 and 3, Supplementary Fig. 6). Therefore, previous elevations of IL-1β in the DRN during aggressive encounter has long-term effects that suppress aggressive behaviors.
5-HT c-Fos expression is suppressed following RI in NON compared to AGG, and increased by genetic and pharmacological IL-1β receptor antagonism
5-TH has been considered a key neurotransmitter that controls aggression [30–35]. It has been reported that acute application of IL-1β suppresses the activity of DRN 5-HT neurons [36,37]. To examine possible differences in 5-HT neuron activation between AGG and NON during aggressive encounters, we compared expression of the immediate early gene, c-Fos, 90 minutes after the RI test in NON vs AGG mice (Fig. 5a, b). There was no difference in the number of 5-HT neurons between AGG and NON (759.4±41.3 and 739.4±26.4 cells, respectively) and c-Fos expression was observed in both 5-HT cells and non-5HT cells (Fig. 5c—e). While there was no difference in c-Fos expression in non 5-HT expressing DRN neurons between AGG and NON (Fig. 5g), we found lower c-Fos expression in 5-HT neurons in NON compared to AGG mice (t(19) = 2.132, p = 0.0463; Fig. 5h), suggesting that 5-HT neuron activation was reduced by social encounters in NON mice compared to AGG mice. This group difference was specific to the medial subdivision of the DRN (t(19) = 3.709, p = 0.0015) and was not observed in the anterior and lateral subdivisions (Fig. 5i). Correlation analysis revealed a significant positive correlation between total duration of aggressive behaviors and number of c-Fos expressing 5-HT neurons in the medial subdivision of the DRN (r=.6738, p=0.0008; Fig. 5j). We confirmed that there were no differences in the number of c-Fos expressing GAD67-positive GABA neurons (Fig. 5k) and vGlut3-positive glutamatergic neurons (Fig. 5l) by RNAscope analysis.
Figure 5. Individual difference and the effect of IL-1R1 KD in the aggressive encounter-induced activation of serotonin (5-HT) neurons in the DRN.
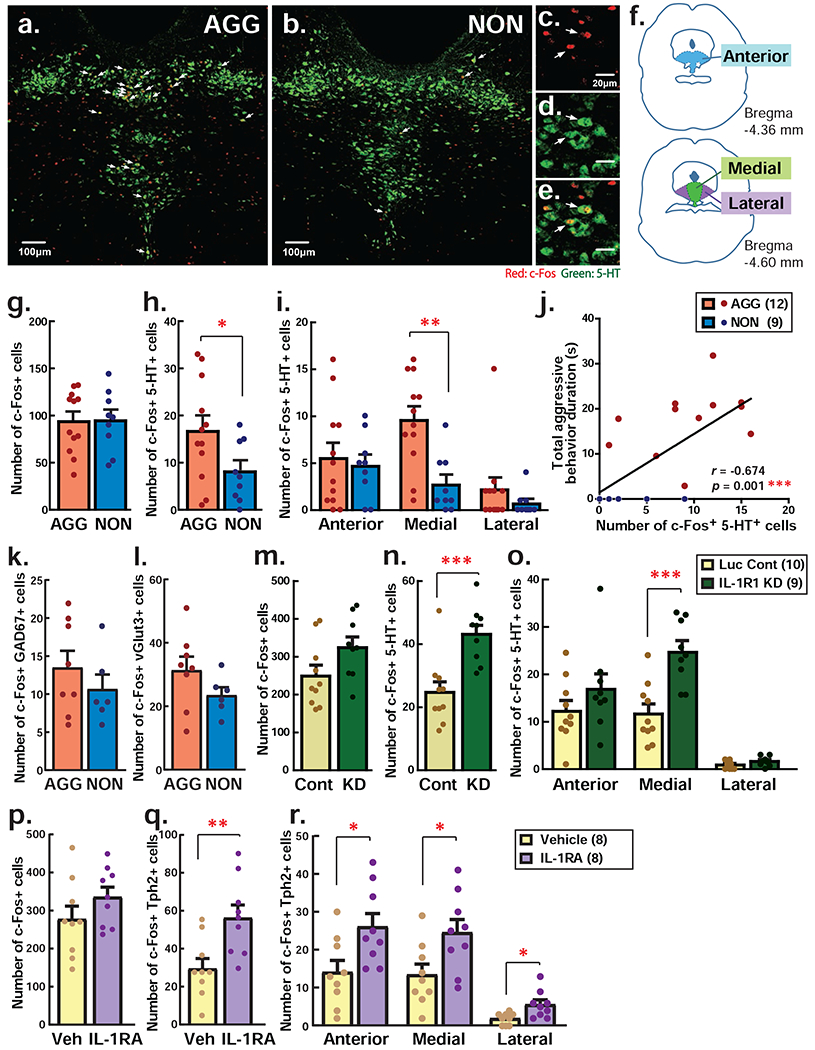
a-j c-Fos expression analysis in AGG and NON male mice by immunohistochemistry. Representative picture of the expression of 5-HT (green) and c-Fos (red) in the DRN in AGG (a) and NON (b) individuals. c-e Enlarged picture of a representative picture of the DRN showing c-Fos (c), 5-HT (d) and their co-localization (e). White arrows indicate activated 5-HT neurons that co-express both 5-HT and c-Fos protein. f Schematics of brain atlas for anterior, medial, and lateral subregions of the DRN where c-Fos and 5-HT expression was examined in this study. g-h Total number of c-Fos positive cells (g) and c-Fos expressing 5-HT neurons (h) in the DRN of AGG an NON animals. i Number of c-Fos expressing 5-HT neurons in each subregion of the DRN. j Positive correlation was observed between the number of c-Fos positive 5-HT neurons in the medial subregion of the DRN and total duration of aggressive behaviors. k-l Total number of c-Fos expressing GAD67-positive GABA neurons (k) and c-Fos expressing vGlut3-positive glutamatergic neurons (l) in the DRN of AGG an NON animals by RNAscope analysis. m-o c-Fos expression analysis in IL-1R1 KD and Luc control animals. Total number of c-Fos positive cells (m) and c-Fos expressing 5-HT neurons (n) in the DRN and each subregion of the DRN (o). p-r c-Fos expression analysis in i.c.v. IL-1RA injection and vehicle (Veh) injection control animals. Total number of c-Fos positive cells (p) and c-Fos expressing Tph2-positive 5-HT neurons (q) in the DRN and each subregion of the DRN (r). *p<0.05, **p<0.01. ***p<0.001.
To examine whether this differential c-Fos expression in 5-HT neurons between AGG and NON is mediated by IL-1β signaling, we examined c-Fos expression in the DRN of IL-1R1 KD animals following RI test. Again, there was no difference in the number of c-Fos-expressing non-5HT neurons in the DRN between IL-1R1 KD and control groups (Fig. 6m). However, IL-1R1 KD animals showed more c-Fos positive 5-HT neurons in the DRN compared to the Luc control group (t(17)=4.010, p=0.0009; Fig. 6n). This effect was prominent in the medial DRN (t(17)=4.413, p=0.0004) but was not significant in other DRN subdivisions (Fig. 6o). We also examined c-Fos induction by i,c.v. IL-1RA injection and RI test. Consistently, IL1RA injection significantly increased c-Fos expression in 5-HT cells in the DRN compared to vehicle treatment (t(16)=2.827, p=0.0121) without affecting c-Fos+ expression in non-5-HT cells(Fig. 6p, q). In this case, significant c-Fos expression was observed in all subdivisions of the DRN (anterior: t(16)=2.626, p=0.0184, medial: t(16)=2.547, p=0.0215, lateral: t(16)=2.827, p=0.0121; Fig. 6r). Therefore, both genetic and pharmacological blockade of IL-1β receptor increased c-Fos expression in 5-HT neurons in the DRN. These results suggest that the IL-1β receptor can mediate suppression of 5-HT neuronal activation during the RI test to dampen aggressive behavior.
Discussion
In this study, we identified a novel role for IL-1β signaling in the DRN in mediating individual differences in aggressive behaviors. Our results indicated that DRN IL-1β has a suppressive effect on aggressive behavior. We observed a phasic increase of IL-1β in blood after an aggressive encounter, but this response was not dependent on the execution of aggressive acts per se since animals who did not show any aggressive behaviors also exhibited a similar increase of IL-1β blood levels. Analysis of additional cytokines confirmed a general inflammatory response to social encounters during the RI test with no difference between AGG and NON mice. Thus, peripheral cytokines are elevated as a result of social stress due to the introduction of a novel conspecific male, but none of them were directly correlated with aggressive behaviors. Blood leukocytes express receptors for several types of stress hormones such as adrenocorticotropic, glucocorticoids, noradrenaline and adrenaline [38], which have collectively been shown to regulate peripheral inflammation [39, 40]. It is possible that the acute cytokine responses observed in our study reflect some type of fight/flight response of the sympathetic nervous system or stress-induced activation of the HPA axis. Various kinds of acute psychological stress have been shown to increase peripheral IL-1β and IL-6 in humans [41]. Interestingly, the expectation of an aggressive encounter (rugby game) also increases IL-1β level in the blood of rugby athletes [16]. This expectation-induced increase of peripheral IL-1β level was positively correlated with anger level (rage state score), with individuals who experienced higher anger level before the game expressing the highest elevation of blood IL-1β [16]. It will be interesting to examine in future studies if an expectation of aggressive encounter changes peripheral IL-1β in the mice, and whether that is correlated with the resulting behavioral output.
In the brain we found that there was a region-specific phasic increase of IL-1β after an aggressive encounter in NON, but not AGG, mice. Previous reports have suggested that central IL-1β is generally expressed at very low levels under basal conditions and can be upregulated in response to pathological conditions such as stroke, head injury or a peripheral immune challenge [42, 43]. However, psychological stress also increases IL-1β levels in several brain areas including the hypothalamus, hippocampus, and locus coeruleus [44–47]. Central IL-1β can trigger fever and an array of sickness behaviors including loss of appetite, anhedonia, increases in non-REM sleep and anxiety, as well as a reduction of social behaviors [12, 13]. Peripheral injection of IL-1β causes prolonged reductions of social interaction and aggressive behavior, and i.c.v. injection of IL-1RA can block the effect of IL-1β on social behaviors, indicating that the suppressive effect of IL-1β on social behavior is mediated in the brain [14, 48–50]. Indeed, we observed intra-DRN injection of IL-1β caused sustained reduction of aggressive behaviors in subsequent aggressive encounters. But, more importantly, our pharmacological and genetic antagonism experiments indicate that physiological levels of IL-1β inside the brain can directly modulate aggressive behavior—independent of sickness behavior—and this might be one of the underlying mechanisms of individual differences in aggression.
Within the brain, IL-1β can be produced by many cell types including microglia, endothelial cells and even neurons. Given that we find higher levels of IL-1β mRNA expression in DRN of NON versus AGG mice, we hypothesized that it is likely being produced locally by one of these cell types. This hypothesis was bolstered by the fact that we found no differences in peripheral levels of IL-1β between NON and AGG mice. Cell type specific transcriptional profiling in DRN showed high levels of IL-1β in microglia compared to all other cell types (20.5 times higher than endothelial cells, and 485.2 times higher than the other cells). In addition, IL-1β mRNA was only elevated by social encounters in microglia of NON versus AGG mice, further supporting the hypothesis that central microglia are likely the primary source of IL-1β in the DRN that suppresses aggressive behavior.
While IL-1 receptors are expressed throughout the body, previous studies have shown that in the brain, they are densely expressed in the pituitary, dentate gyrus, and DRN [51, 52]. Given the fact that the DRN has been previously associated with aggressive behavior [18–23] and our data showed higher IL-1R mRNA within the DRN of NON mice, we hypothesized that IL-1β may be acting on DRN neurons to control aggressive behavior. Indeed, previous electrophysiological studies reported that acute bath application of IL-1 to DRN slices suppresses neural firing of 5-HT neurons by increasing GABAergic inhibitory post-synaptic potentials [36, 37]. Here we found that physiological levels of IL-1β suppress c-Fos expression induced by aggressive encounters in the DRN of 5-HT neurons, an effect that was dependent on neuronally-expressed IL-1R. Both pharmacological inhibition and viral mediated knockdown of IL-1R was sufficient to increase aggressive behavior in AGG mice during the RI test. Interestingly, we did not observe a clear effect of IL-1RA injection on aggressive behavior of NON animals. The fact that we were unable to convert NON mice into AGG mice with a DRN-specific manipulation is consistent with our previous work [24, 26] and suggests that the circuitry defining individual differences in aggressive behavior is complex and likely requires dynamic activity across the brain.
Indeed, studies have shown that IL-1β signaling can act in different ways to control aggressive behavior depending upon where in the brain it is acting. For example, microinjection of IL-1β into the medial hypothalamus caused an increase of defensive rage responses induced by periaqueductal gray stimulation in cats [53, 54]. Conversely, here we observed that IL-1β in the DRN suppressed territorial intermale aggressive behavior in mice. We also show that c-Fos expression in 5-HT neurons in the DRN is suppressed by physiological levels of IL-1β. In contrast, previous studies found peripheral and i.c.v. administration of IL-1β stimulated 5-HT release in the hippocampus [55, 56]. Injection (i.c.v.) of IL-1β also enhanced 5-HT release in the medial preoptic areas [57], suggesting opposing effects of IL-1β on 5-HTergic systems when acting directly within the DRN versus a projection site [58]. Thus, IL-1β has a differential role on 5-HT neuronal activity and aggressive behaviors depending on where receptors in the cell body or on terminals are exposed.
While the downstream mechanisms of IL-1β are not well worked out in the DRN, in the hippocampus, it has been shown to activate both nuclear factor κB (NF-κB) pathway and p38 MAPK pathway (Allan et al 2005 Nature Reviews Immunology). NF-κB is a transcription factor that induces expression of inflammatory cytokines (Srinivasan et al 2004 J Neurosci). In addition, IL-1β treatment has been shown to induce c-Fos expression through a p38 MAPK-dependent pathway involving CREB (Srinivasan et al 2004) or through the IL-1 receptor accessory protein isoform b (IL-1RAcPb)-Src pathway (Huang et al 2011 J Neurosci). To test whether any of these pathways were differentially affected by IL-1R1 KD, we performed qPCR on components of the p38-MAPK pathway or IL-1RAcPb-Src pathway in NON and AGG mice following social interaction. However, our qPCR analysis did not find differences in levels of IL-1RAcPb (Supplemental Figure 2) or MAPK p36 mRNA (data not shown). Future studies will be needed to examine post-translational modifications as well as total level of these downstream proteins to better understand the molecular mechanisms of IL-1β mediated inhibition of aggressive behavior.
Overall our data identifies a novel role for IL-1β signaling within the DRN which may represent an important biological target to suppress aggressive behavior in male mice. Since our behavioral assay examined territorial aggression of male mice, which may in fact be considered as adaptive behavior, future studies will be required to understand if IL-1β signaling in the DRN is involved in pathological forms of aggression observed in human neuropsychiatric syndromes.
Supplementary Material
Acknowledgements
This research was supported by US National Institutes of Health grants R01 MH090264-06 and R01 MH104559-02 to SJR. The study was also supported by JSPS KAKENHI Grant Number JP17H04766, JP15K12773, and JP19H05202 to AT. We would like to thank Dr. Tsuyoshi Koide for sharing experimental equipment (realtime PCR), and Dr. Shiho Kitaoka for giving advice on microglia isolation.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. [DOI] [PubMed] [Google Scholar]
- 2.Maynard Smith J, Price GR. The logic of animal conflict. Nature. 1973;246:15–18. [Google Scholar]
- 3.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. [DOI] [PubMed] [Google Scholar]
- 4.Zalcman SS, Siegel A. The neurobiology of aggression and rage: Role of cytokines. Brain Behav Immun. 2006;20:507–514. [DOI] [PubMed] [Google Scholar]
- 5.Koolhaas JM. Coping style and immunity in animals: Making sense of individual variation. Brain Behav Immun. 2008;22:662–667. [DOI] [PubMed] [Google Scholar]
- 6.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. [DOI] [PubMed] [Google Scholar]
- 7.Réus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. [DOI] [PubMed] [Google Scholar]
- 8.Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci. 2014;111:16136–16141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, et al. Social stress induces neurovascular pathology promoting depression. Nat Neurosci. 2017;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. Establishment of a repeated social defeat stress model in female mice. Sci Rep. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi A, Flanigan ME, McEwen BS, Russo SJ. Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci. 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson SJ, Dunn AJ. Behavioral Effects of Cytokines. Brain Behav Immun. 2001;15:371–387. [DOI] [PubMed] [Google Scholar]
- 14.Cirulli F, De Acetis L, Alleva E. Behavioral effects of peripheral interleukin-1 administration in adult CD-1 mice: specific inhibition of the offensive components of intermale agonistic behavior. Brain Res. 1998;791:308–312. [DOI] [PubMed] [Google Scholar]
- 15.Coccaro EF, Lee R, Coussons-Read M. Cerebrospinal fluid inflammatory cytokines and aggression in personality disordered subjects. Int J Neuropsychopharmacol. 2015;18:pyv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pesce M, Speranza L, Franceschelli S, Ialenti V, Iezzi I, Patruno A, et al. Positive Correlation Between Serum Interleukin-1β and State Anger in Rugby Athletes. Aggress Behav. 2013;39:141–148. [DOI] [PubMed] [Google Scholar]
- 17.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology. 2011;213:243–264. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs BL, Cohen A. Differential behavioral effects of lesions of the median or dorsal raphe nuclei in rats: open field and pain-elicited aggression. J Comp Physiol Psychol. 1976;90:102–108. [DOI] [PubMed] [Google Scholar]
- 19.van der Vegt BJ, Lieuwes N, van de Wall EHEM, Kato K, Moya-Albiol L, Martínez-Sanchis S, et al. Activation of serotonergic neurotransmission during the performance of aggressive behavior in rats. Behav Neurosci. 2003; 117:667–674. [DOI] [PubMed] [Google Scholar]
- 20.Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology. 2007;193:295–304. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi A, Shimamoto A, Boyson CO, DeBold JF, Miczek KA. GABAB receptor modulation of serotonin neurons in the dorsal raphé nucleus and escalation of aggression in mice. J Neurosci. 2010;30:11771–11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faccidomo S, Quadros IMH, Takahashi A, Fish EW, Miczek KA (2012) Infralimbic and dorsal raphé microinjection of the 5-HT(1B) receptor agonist CP-93,129: attenuation of aggressive behavior in CFW male mice. Psychopharmacology. 2012;222:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi A, Lee RX, Iwasato T, Itohara S, Arima H, Bettler B, et al. Glutamate input in the dorsal raphe nucleus as a determinant of escalated aggression in male mice. J Neurosci. 2015;35:6452–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden SA, Heshmati M, Flanigan M, Christoffel DJ, Guise K, Pfau ML, et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature. 2016;534:688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golden SA, Aleyasin H, Heins R, Flanigan M, Heshmati M, Takahashi A, et al. Persistent conditioned place preference to aggression experience in adult male sexually-experienced CD-1 mice. Genes, Brain Behav. 2017;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanigan ME, Aleyasin H, Li L, Burnett CJ, Chan KL, LeClair KB, et al. Orexin signaling in GABAergic lateral habenula neurons modulates aggressive behavior in male mice. Nat Neurosci. 2020;23:638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21:246–259. [Google Scholar]
- 28.Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology (Berl). 1978;57:47–55. [DOI] [PubMed] [Google Scholar]
- 29.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hen R. Mean genes. Neuron. 1996;16:17–21. [DOI] [PubMed] [Google Scholar]
- 31.Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154:1430–1435. [DOI] [PubMed] [Google Scholar]
- 32.Olivier B. Serotonin and aggression. Ann N Y Acad Sci. 2004;1036:382–392. [DOI] [PubMed] [Google Scholar]
- 33.de Boer SF, Koolhaas JM. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur J Pharmacol. 2005;526:125–139. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi A, Quadros IM, de Almeida RMM, Miczek KA. Brain serotonin receptors and transporters: initiation vs. termination of escalated aggression. Psychopharmacology 2011;213:183–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suri D, Teixeira CM, Cagliostro MK, Mahadevia D, Ansorge MS. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology. 2015;40:88–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manfridi A, Brambilla D, Bianchi S, Mariotti M, Opp MR, Imeri L. Interleukin-1beta enhances non-rapid eye movement sleep when microinjected into the dorsal raphe nucleus and inhibits serotonergic neurons in vitro. Eur J Neurosci. 2003;18:1041–1049. [DOI] [PubMed] [Google Scholar]
- 37.Brambilla D, Franciosi S, Opp MR, Imeri L. Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials. Eur J Neurosci. 2007;26:1862–1869. [DOI] [PubMed] [Google Scholar]
- 38.Glaser R, Kiecolt-Glaser JK. Science and society: Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. [DOI] [PubMed] [Google Scholar]
- 39.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. [DOI] [PubMed] [Google Scholar]
- 41.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. [DOI] [PubMed] [Google Scholar]
- 42.Loddick SA, Liu C, Takao T, Hashimoto K, De Souza EB. Interleukin-1 receptors: Cloning studies and role in central nervous system disorders. Brain Res. Rev, vol. 26, 1998. p. 306–319. [DOI] [PubMed] [Google Scholar]
- 43.Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: Biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. [DOI] [PubMed] [Google Scholar]
- 44.Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, et al. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki E. Immobilization stress increases mRNA levels of interleukin-1 receptor antagonist in various rat brain regions. Cell Mol Neurobiol. 1997;17:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1β protein in the rat. J Neurosci. 1998;18:2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood SK, Wood CS, Lombard CM, Lee CS, Zhang XY, Finnell JE, et al. Inflammatory factors mediate vulnerability to a social stress-induced depressive-like phenotype in passive coping rats. Biol Psychiatry. 2015;78:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bluthé RM, Dantzer R, Kelley KW. Central mediation of the effects of interleukin-1 on social exploration and body weight in mice. Psychoneuroendocrinology. 1997;22:1–11. [DOI] [PubMed] [Google Scholar]
- 49.Kent S, Bluthe RM, Dantzer R, Hardwick AJ, Kelley KW, Rothwell NJ, et al. Different receptor mechanisms mediate the pyrogenic and behavioral effects of interleukin 1. Proc Natl Acad Sci U S A. 1992;89:9117–9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crestani F, Seguy F, Dantzer R. Behavioural effects of peripherally injected interleukin-1: role of prostaglandins. Brain Res. 1991;542:330–335. [DOI] [PubMed] [Google Scholar]
- 51.Cunningham ET, Wada E, Carter DB, Tracey DE, Battey JF, De Souza EB. In situ histochemical localization of type I interleukin-1 receptor messenger RNA in the central nervous system, pituitary, and adrenal gland of the mouse. J Neurosci. 1992;12:1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schöbitz B, de Kloet ER, Holsboer F. Gene expression and function of interleukin I, interleukin 6 and tumor necrosis factor in the brain. Prog Neurobiol. 1994;44:397–432. [DOI] [PubMed] [Google Scholar]
- 53.Hassanain M, Zalcman S, Bhatt S, Siegel A. Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: role of serotonin-2 receptors. Neuroscience. 2003;120:227–233. [DOI] [PubMed] [Google Scholar]
- 54.Hassanain M, Bhatt S, Zalcman S, Siegel A. Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res. 2005;1048:1–11. [DOI] [PubMed] [Google Scholar]
- 55.Linthorst AC, Flachskamm C, Müller-Preuss P, Holsboer F, Reul JM. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J Neurosci. 1995;15:2920–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linthorst AC, Flachskamm C, Holsboer F, Reul JM. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic-pituitary-adrenocortical axis activity, and body temperature. Endocrinology. 1994;135:520–532. [DOI] [PubMed] [Google Scholar]
- 57.Gemma C, Imeri L, de Simoni MG, Mancia M. Interleukin-1 induces changes in sleep, brain temperature, and serotonergic metabolism. Am J Physiol. 1997;272:R601--6. [DOI] [PubMed] [Google Scholar]
- 58.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


