Abstract
Sympathoneuronal outflow into dorsal root ganglia (DRG) is suggested to be involved in sympathetically maintained chronic pain, which is mediated by norepinephrine (NE) action on DRG cells. The present study combined in vitro and in vivo approaches to identify the cell types of DRG that received NE action and examined cell-type specific expression of adrenergic receptors (ARs) in DRG. Using DRG explants, we identified that NE acted on satellite glial cells (SGCs) to induce the phosphorylation of cAMP response element-binding (CREB). Using primarily cultured SGCs, we identified that beta (β)2AR but not alpha (α)AR nor other βAR isoforms mediated NE-induced CREB phosphorylation and CRE-promoted luciferase transcriptional activity. Using fluorescence in situ hybridization and affinity purification of mRNA from specific cell types, we identified that β2AR was expressed by SGCs but not DRG neurons. We further examined β2AR expression and CREB phosphorylation in vivo in a model of colitis in which sympathetic nerve sprouting in DRG was observed. We found that β2AR expression and CREB phosphorylation were increased in SGCs of thoracolumbar DRG on day 7 following colitis induction. Inhibition but not augmentation of β2AR reduced colitis- induced calcitonin gene-related peptide (CGRP) release into the spinal cord dorsal horn and colonic pain responses to colorectal distention. Prolonged activation of β2AR in naïve DRG increased CGRP expression in DRG neurons. These findings provide molecular basis of sympathetic modulation of sensory activity and chronic pain that involves β2AR-mediated signaling in SGCs of DRG.
Keywords: β2 adrenergic receptor, sympathoneuronal outflow, dorsal root ganglia, satellite glial cells, pain
INTRODUCTION
The sympathetic nervous system, traditionally identified in fight-flight response, is increasingly recognized for its role in modulation of pain [8; 21; 39]. In response to inflammation or nerve injury, the unmyelinated postganglionic sympathetic nerve fibers that use norepinephrine (NE) as their neurotransmitters pass the gray rami communicans to sprout into dorsal root ganglia (DRG) [35; 60]. A localized sympathectomy that cuts the sympathetic postganglionic axons in gray rami reduces mechanical hypersensitivity in neuropathic pain [53; 62]. Inhibition of sympathoneuronal outflow with either surgical sympathectomy or pharmacological intervention also blocks the development of chronic visceral hypersensitivity [21]. The sympathetic nerve fibers interact with sensory neurons by forming a basket-like structure [60; 61] and are coupled to satellite glial cells (SGCs) [60], a major non-neuronal cell type that forms a thin sheet around sensory neurons with synapse-like gaps (about 20 nm) [40; 46]. The activity of SGCs is regulated by paracrine factors within the sensory ganglia and bi-directionally interact with sensory neurons [44]. The close contact of SGCs to sympathetic fibers in chronic pain may endow noradrenergic regulation of SGCs in DRG. In basal physiological states, the glial cells including SGCs are normally quiescent however they can be activated during disease states [18; 47] such as in exaggerated pain [51], and could be regulated by noradrenergic signals.
All adrenergic receptors (ARs) are found to be expressed in DRG [4; 12; 33; 34]. The alpha (α)1ARs couple to Gq to facilitate rapid, robust Ca2+ release [14; 52]. The beta (β)ARs are primarily coupled to Gs protein to activate adenylyl cyclase to evoke cAMP-dependent signaling cascades such as activation of protein kinase A (PKA) [36] and small Ca2+ transients [14], together they may lead to activation of the cAMP-response element binding protein (CREB) [14; 19; 55]. β2AR-mediated activation of the cAMP-PKA-CREB cascade further promotes β2AR transcription, forming an autoregulation loop [13]. These studies on AR-mediated signaling are performed in HEK 293 cells and other cell types [14; 17; 19] and not much information is available on AR-mediated NE action in DRG and especially in SGCs.
Our previous study demonstrates sympathetic fiber sprouting into DRG during chronic visceral hypersensitivity post resolution of 2,4,6- trinitrobenzenesulfonic acid (TNBS) - induced colitis [60]. The present study is undertaken to identify the cell types and adrenergic receptors in DRG that respond to sympathoneuronal input. Using this animal model in combination with in vitro culture and genetic approaches, we reveal that β2AR is expressed by SGCs but not DRG neurons to respond to NE. β2AR has been suggested to participate in sympathetically engaged chronic pain, including neuropathic pain resulting from diabetes or nerve injury [3; 10; 11; 64], stress-induced itch hypersensitivity [42], and visceral pain [66]. Activation of endogenous β2AR by inhibiting catechol-O-methyltransferase also enhances inflammatory somatic pain sensitivity [12; 38]. Moreover, βAR pan inhibitor propranolol has prophylactic therapy for joint pain [26], fibromyalgia [24], and migraine headache [2]. The identification of β2AR in SGCs of DRG provides molecular basis for sympathetically regulated β2AR-mediated chronic pain process.
METHODS
Experimental animals
Adult male Sprague-Dawley rats (180–200 g) were purchased from Harlan Sprague Dawley, Inc. (Indianapolis, IN). Genetically modified C57BL/6 mice (22–25 g) were purchased from the Jackson Laboratory and bred in house. Mice were kept 2–5 per cage to ensure adequate social environment and numbered by ear clips. Standard husbandry conditions with 12:12-h light cycles and free access to regular food/water were provided. All experimental protocols involving animal use were approved by the university Institutional Animal Care and Use Committee (IACUC). Animal care was in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. All experiments were performed in rats except those specifically indicated when using transgenic mice.
Induction of colonic inflammation
Intracolonic installation of TNBS (Sigma-Aldrich, St. Louis, MO) was used to induce colonic inflammation and visceral hypersensitivity as described in our previous publication [49]. Under anesthesia (2 % isoflurane), rats were treated by a single dose of TNBS (90 mg/kg of 60 mg/mL in 50 % EtOH solution) through a transanal polyethylene (PE)-50 catheter. TNBS was infused to 6 cm inside from the anus. The animal tail was lifted for 1 min after TNBS installation to avoid drug leakage from the anus. The same amount of EtOH was used as vehicle treatment in control animals. TNBS induced a long-lasting visceral hypersensitivity during and after colonic inflammation was resolved [16; 23; 49; 67].
Tissue preparation
Freshly isolated DRGs were used in a variety of preparations. The thoracolumbar T13-L2 DRGs were chosen to align with the in vivo animal model of colitis-induced visceral hypersensitivity in which increased sympathetic fiber sprouting in T13-L2 DRGs was identified [60]. For immunohistochemistry and in situ hybridization, DRGs were post-fixed in 4 % paraformaldehyde (Sigma-Aldrich). For real-time PCR, DRGs were freshly homogenized in mRNA extraction buffer. For cell culture, DRGs were subjected to enzymatic digestion.
DRG explant culture
The segmentally matched DRG pairs were used for comparison between drug treatment and control. As described in our previous study [59], freshly dissected DRG pairs were immediately cultured in Dulbecco’s Modified Eagle Medium (DMEM) for 2–4 hours (fasting). One of the DRG explant of each pair was randomly chosen for treatment by a specific drug such as NE. The contralateral DRG served as control. After a designated time period of incubation, the DRGs were subjected to further biochemical analysis.
Immunohistochemistry
After fixation, DRGs and the spinal cord were incubated in 25 % sucrose overnight at 4 °C for cryoprotection. Rat DRGs were sectioned at 20 μm thickness and mouse DRGs at 10 μm thickness. The spinal cord was at 25 μm thickness. The tissue sections were processed for on-slide immunostaining. Sections were incubated in primary antibodies diluted in PBST (0.3 % Triton X-100 in 0.1 M PBS, pH 7.4) containing 5 % normal donkey serum overnight at room temperature followed by incubation with fluorescence-conjugated species-specific secondary antibody (1:500, Molecular Probes, Eugene, OR) for 2 h. Slides were coverslipped with Citifluor (Citifluor Ltd., London). Immunostaining in the absence of primary or secondary antibody was assessed for background evaluation. The specificity of the primary antibodies used were also validated in our previous studies using western blot or pre-absorption assay [43; 59]. The processed sections were visualized under a Zeiss fluorescent microscope.
Enzymatic isolation for SGC culture
Adult DRGs were disassociated by 4 mg/mL collagenase in DMEM at 32 °C for 1 hour followed by 0.25 % Gibco™ Trypsin-EDTA (Thermo Fisher Scientific, Waltham, MA) at room temperature for 5 min. Tissue/cells were pipetted up-and-down to completely disassociate the cells. After centrifugation, cells were re-suspended into DMEM containing 10% fetal bovine serum (FBS) for culture. After 4–6 hours glial cells but not neurons attached to the bottom of the dish. Neurons were removed after changing into fresh culture medium. The glial cells from DRG were mainly SGCs [30; 54] and were validated for their expression of glial fibrillary acidic protein (GFAP) but not neuronal marker protein gene product (PGP)9.5.
Cell-type specific mRNA extraction
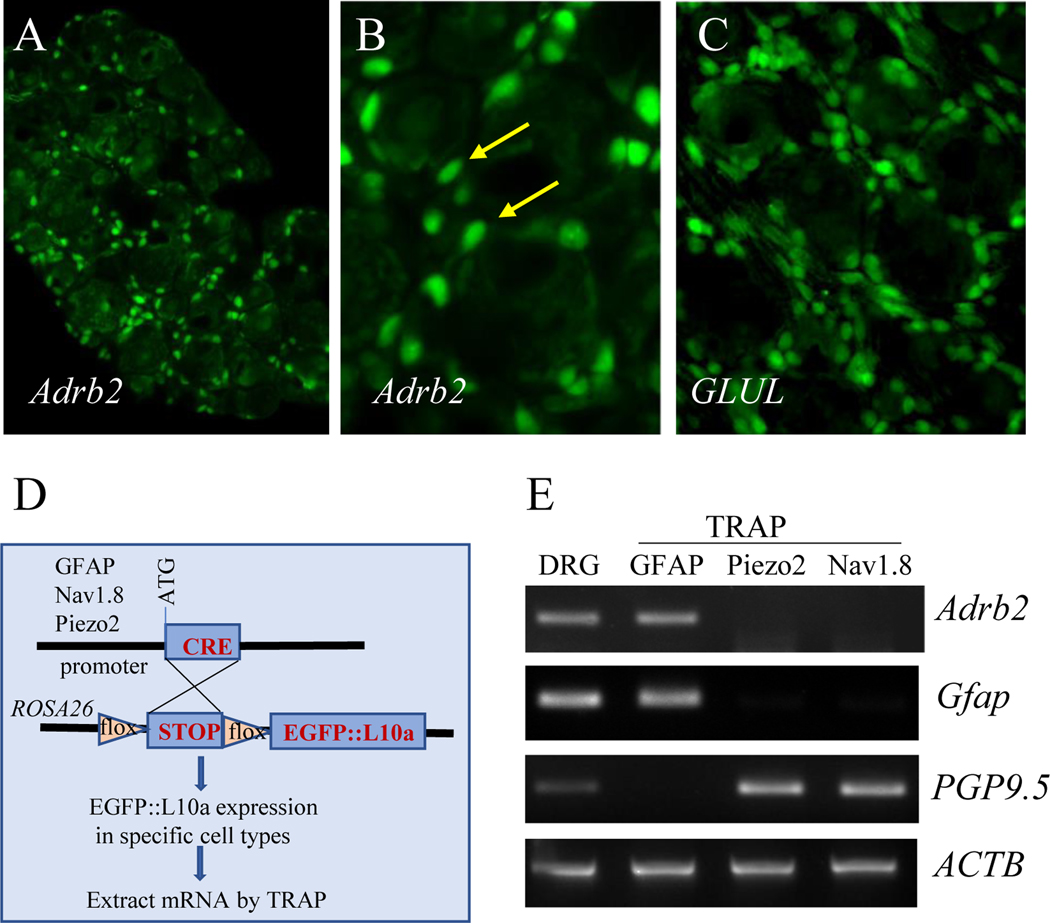
Translating ribosome affinity purification (TRAP) technique [22; 24] was used to extract mRNA from specific cell types. Specifically, GFAP;EGFP::L10a mice were generated by crossing GFAP-Cre mice (JAX Stock # 012886) with EGFP::L10a mice (JAX Stock #024750) for extraction of mRNA from GFAP-expressing SGCs of DRG. Piezo2;EGFP::L10a mice were generated by crossing Piezo2-EGFP-IRES-Cre (JAX Stock #027719) and EGFP::L10a mice for mRNA extraction from Piezo2-expressing DRG neurons. Nav1.8;EGFP::L10a mice were generated by crossing Nav1.8-Cre mice (a line that was created by Dr. John Wood, Wolfson Inst. UK) [9] with EGFP::L10a mice for mRNA extraction from Nav1.8-expressing DRG neurons. For cell type-specific mRNA extraction, freshly isolated DRGs from Cre-driven EGFP::L10a mice were homogenized according to published protocols [22; 24] and subject to immunoprecipitation of the GFP::L10a-mRNA complex using a specific GFP antibody (1:100, Thermo Fisher Scientific). The immunoprecipitates were re-suspended in RNA extraction buffer. The mRNA was extracted using RNAqueous™ Total RNA Isolation Kit. The mRNA extracts were validated by PCR for their expression of GFAP (SGCs) or PGP9.5 (neurons).
Total RNA extraction, conventional PCR and real-time PCR
Total RNA from DRG was extracted using a RNAqueous™ Total RNA Isolation Kit (Thermo Fisher Scientific). RNA concentration was determined spectrophotometrically. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). The products of conventional PCR were detected by agarose gel electrophoresis. Realtime PCR was performed using SYBR Green as indicator on StepOnePlus™ Systems (Applied Biosystems). Dissociation curve post-PCR reaction was monitored to verify the specificity of the real-time PCR reaction. The level of target mRNA was normalized against the expression of the internal control β-actin and was calculated with ΔCt method and expressed as fold changes (2-ΔΔCt fold). The primers used for conventional PCR in rats and mice were listed in Table 1. Real-time PCR was performed in rat tissues for tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), β2AR, and GFAP. Their primers are TNFα: AGCCCGTAGCCCACGTCGTA and ATGCCATTGGCCAGG AGGGC; IL-6: TGTTGACAGCCACTGCCTTCCC and ACTGGTCTGTTGTGGGTGGTATCC T; β2AR: GTTATCGTCCTGGCCATCGT and AAGTCCAGAACTCGCACCAG; GFAP: ATG GAGCTCAATGACCGCTT and ATCTTGGAGCTTCTGCCTCAG; and β-actin: CAGGGTGT GATGGTGGGTATGG and AGTTGGTGACAATGCCGTGTTC.
Table 1.
Primers used for PCR and real-time PCR.
| Primers used for PCR in rat | |
| α1A Forward | CACCCAGAGGAGGGGCGTCA |
| αlA Reverse | CGGGAAGAAAGACCCAATGGGCA |
| αlB Forward | ATCGCTCTCCCGCTTGGCTC |
| αlB Reverse | GGTCGGTCGTGGTGTCGCAG |
| αlD Forward | TGCCTCTGGGCTCTCTGTTCCC |
| αl D Reverse | TCTCTGTAGCGGCCCGAGCA |
| α2A Forward | AAAGCGCCCCAGAACCTCTT |
| α2A Reverse | ATGAGTGGCGGGAAGGAGAT |
| α2B Forward | AGTCAGTTCTGTGCGTCCTG |
| α2B Reverse | GTAGCCACTAGGATGTCGGC |
| α2C Forward | ATCGTTTTCACCGTGGTGGG |
| α2C Reverse | CATTCTCTGTGGTCGGGGAC |
| β1 Forward | GCTCTGGACTTCGGTAGACG |
| β1 Reverse | CCCAGCCAGTTGAAGAAGAC |
| β2 Forward | GGAACGGGACGAAGCGTGGG |
| β2 Reverse | GCTTGCTTGTGGGTGGCACG |
| β3 Forward | GGGAGCTGGGCCGTTTTCCG |
| β3 Reverse | GCCATCAAACCTGTTGAGCGGTG |
| GFAP Forward | TTGCGCGGCACGAACGAGTC |
| GFAP Reverse | ACTGACCGAGCCGTGGGCAT |
| PGP9.5 Forward | CCCTCACGGCCCAGCATGAAAA |
| PGP9.5 Reverse | GAGCCACTGCGGAGAAGCGG |
| β-actin Forward | AGCCATGTACGTAGCCATCC |
| β-actin Reverse | CAGTGAGGCCAGGATAGAGC |
| Primers used for PCR in mice | |
| β-actin Forward | CGCAGCCACTGTCGAGTC |
| β-actin Reverse | AAGGTCTCAAACATGATCTGGGT |
| GFAP Forward | CCCTGGCTCGTGTGGATTT |
| GFAP Reverse | GACCGATACCACTCCTCTGTC |
| PGP9.5 Forward | GATGCTGAACAAAGTGTTGGC |
| PGP9.5 Reverse | GGAGTTTCCGATGGTCTGCTT |
| β2AR Forward | GGGAACGACAGCGACTTCTT |
| β2AR Reverse | GCCAGGACGATAACCGACAT |
In situ hybridization
DRG sections (10 μm thickness) were mounted onto poly-L-lysine-coated slides and fixed in 4 % paraformaldehyde for 10 min. Slides were subsequently treated by acetic anhydride (0.25 % v/v) and proteinase K (10 μg/mL) followed by dehydration in a graded series of ethanol (70 % for 1 min, 80 % for 1 min, 95 % for 1 min x 2, 100 % for 1 min x 2) prior to hybridization in buffer containing 50 % formamide, 5xSSC, 500 ug/mL yeast tRNA, 0.1% Tween-20 (pH=6.0 adjusted by citric acid) and RNA probes. Adapted from published methods [7], the complementary RNA (cRNA, antisense) probes for fluorescence in situ hybridization (FISH) were generated by in vitro transcription using FISH Tag™ RNA Green Kit, with Alexa Fluor™ 488 dye (Thermo Fisher Scientific). The sense strain of RNA was used as negative control. The primers for PCR of cDNA of interests as templates for generating FISH RNA probes were that rat β2AR Forward GCCGAGCTCTGTCCACGTCATCCGGGCCA and Reverse CGGGGTACCGTCCTGTCAGG GAGGGGCCG; rat glutamine synthetase (GS) Forward GCCGAGCTCTGGACCCCAAGGACC CTATT and Reverse CGGGGTACCCAATCCGGGGAATGCGGATA. The cloning vector pSP73 (Promega Corporation) via restriction enzymatic sites SacI and KpnI was used for cDNA cloning and amplification, and in vitro transcription. After hybridization, the slides were visualized on a Zeiss fluorescent microscope.
Western blot
The supernatant of protein extracts was separated on a 10 % SDS-PAGE gel and transferred to a nitrocellulose membrane. The membrane was blocked with 5 % milk in Tris-buffered saline for 1 hour and then incubated with specific primary antibodies. After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary antibody. The bands were identified by ECL-exposed films that were then digitized and performed for densitometric quantification using ImageJ. The expression level of the protein of interest in control samples from each independent experiment was considered as 1, and the relative expression level of the protein of interest in experimental samples was adjusted as a ratio to control.
Luciferase assay to examine CREB transcriptional activity
The plasmid pCRE-MetLuc2-Reporter Vector (Clontech Laboratories, Inc.) was transfected into cultured SGCs or HEK 293 cells using Invitrogen™ Lipofectamine™ LTX Reagent (ThermoFisher Scientific). To transfect SGCs, the plasmid and transfection reagent were mixed with enzymatically isolated DRG cells prior to initial plating. Transfection of HEK 293 cells followed standard protocol in which confluent HEK 293 culture was trypsinized and mixed with plasmid and transfection reagent. Transfected cells were seeded evenly into experimental wells. Upon ligand stimulation, the level of luciferase secreted into the culture medium was determined by Ready-To-Glow™ Secreted Luciferase Reporter Assay (Clontech Laboratories, Inc.).
Drug treatment
Drugs used for in vitro studies included forskolin (1 μM), NE (25 μM), serotonin (5-HT: 10 μM) in presence of pargyline (1 mM), formoterol (1 μM), pan α inhibitor phentolamine (40 μM), pan β inhibitor propranolol (40 μM), β1 inhibitor CGP20712 (10 μM), β2 inhibitor ICI118551 (20 μM), and β3 inhibitor SR59230A (20 μM). The dosage of forskolin, NE and 5-HT were chosen according to our preliminary dose-responsive studies (e.g., for NE, we tested concentrations of 0.5 μM, 1 μM, 10 μM, 25 μM, 50 μM, and 100 μM, and chose the minimum concentration that produced highest p-CREB expression in DRG glial cells measured by immunostaining). The dosage of inhibitors to adrenergic receptors were chosen according to their IC50 and previous publications [19]. The inhibitors were applied 30 min prior to NE treatment. For in vivo studies, ICI118551 or formoterol was injected on the same day when colitis was induced via either intravenous (ICI118551:1 μmole (277 μg)/kg; formoterol: 10 μg/kg) or intrathecal (ICI118551: 30 μg/30 μL/animal; formoterol: 1 μg/30 μL/animal) routine. All injections were performed under 2.5 % isoflurane. The intrathecal injection was conducted by inserting the needle (27-gauge) of a 50 μL Hamilton syringe beween the L5 and L6 vertebrae. The placement of the needle to be delivered was checked by tail flick movement. The dosage of drugs was chosen according to and slightly modified from previous studies for their effectiveness in similar applications [5; 32; 63]. Due to the long-lasting act of formoterol, a single dose of formoterol was injected. A second dose of ICI118551 was injected on day 3 following colitis induction.
Measurement of colonic pain
Colonic pain responses was assessed by observing abdominal withdrawal reflex (AWR) during colorectal distension (CRD) as described previously by us and others [1; 49]. Briefly, a mini-distention balloon was placed into the descending colon under light anesthesia (1.5–2 % isoflurane). The balloon catheter was securely fixed onto the rat tail with tapes. The rat was then placed in an acrylic cage and allowed to wake up and adapt to the environment for 30 min. CRD balloon catheter was connected to a portable sphygmomanometer to gradually induce a constant pressure 40 mmHg or 60 mmHg. The CRD was performed three times at 3-min intervals for each rat with each measurement lasting 20 s under each designated pressure. The AWR scores were recorded as 0: no behavioral response to CRD; 1: immobile during the CRD; 2: a mild abdominal muscle contraction but no lifting of the abdomen; 3: a strong abdominal muscle contraction and lifting of the abdomen, but no lifting of the pelvic structure; and 4: body arching and lifting of the pelvic structure. The assessments were performed in a blind manner.
Hematoxylin and eosin (H&E) stain
The distal colon was sectioned transversely at a thickness of 7 μm and fixed with 4 % paraformaldehyde at room temperature for 30 min. Slides were stained with an H&E staining kit according to the protocol provided by the manufacture (Richard-Allan-Scientific, Kalamazoo, MI,). The sections were examined with a Nikon brightfield microscope. The histology score was graded to reflect the severity of the colonic inflammation (1, no inflammation; 2, very low inflammation; 3, low level of infiltration; 4, high level of infiltration and vascular density; 5, transmural infiltrations, loss of goblet cells, and high vascular density). The thickness of the muscular wall, the width of the submucosal space, the depth of the mucosal layer and the average width of the crypts were also measured independently with the built-in measurement software.
Data analysis
At least 3 independent experiments were performed for all levels of assessments. GraphPad Prism 5 was used for data analysis. The processed data were presented as mean ± SEM. When comparison was made between two groups, unpaired t test was used. For comparison among 3 or more groups, One-way ANOVA with Newman-Keuls Multiple Comparison Test was used. p ≤ 0.05 was considered significant.
RESULTS
NE increased CREB phosphorylation in satellite glial cells of DRG
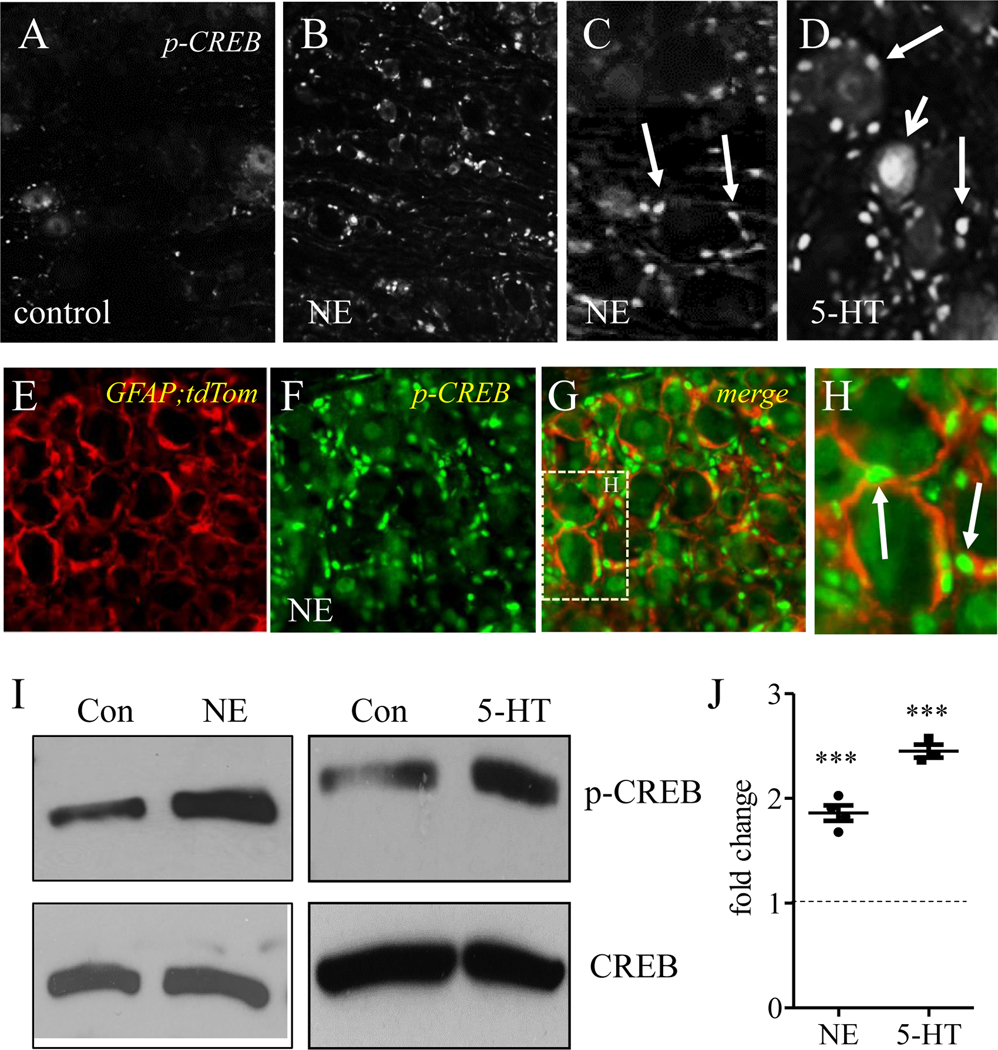
To identify what type of cells the sympathoneuronal outflow acted on within the DRG, we used DRG explant culture and treated them with NE. DRG explant culture ensured the integrity of DRG structures and was also easily manipulated by exogenous drugs. We examined whether and where NE regulated CREB phosphorylation in DRG. To our surprise, we found that NE (10 min treatment) increased the level of p-CREB mainly in perineuronal cells, morphologically resembling SGCs (compare Figure 1A to 1B; Figure 1C for higher magnification, perineuronal cells were indicated by arrows). As a comparison, 5-HT (10 min treatment) increased p-CREB in both perineuronal cells (Figure 1D, indicated by arrows) and DRG neurons (Figure 1D, indicated by vee arrow). We further treated mouse DRG explants with NE. Mouse DRGs were obtained from GFAP reporter mice that were generated by crossing GFAP-Cre mice with Ai27D mice (JAX Stock # 012567) therefore GFAP-expressing SGCs were labeled by tdTomato (Figure 1E, red cells). NE treatment of mouse DRG also increased perineuronal expression of p-CREB (Figure 1F, green cells), similar as in rat DRG (compare to Figure 1B, 1C). Merge (Figure 1G) of photographs of GFAP;tdTomato (Figure 1E) and p-CREB (Figure 1F) revealed that NE-induced p-CREB expression was localized in GFAP-expressing SGCs (Figure 1H, arrows indicated p-CREB in SGCs). Western blot confirmed that both NE and 5-HT increased p-CREB levels in DRG by 1.86- and 2.45-fold, respectively (Figure 1I-J).
Figure 1. NE increased CREB phosphorylation in satellite glial cells of DRG.
DRG explants were divided by pairs as control (A) or NE (B) treatment. The expression of p-CREB was examined by immunostaining and was shown to be increased in perineuronal cells by NE (C: indicted by arrows). 5-HT treatment increased p-CREB in both DRG neurons (D: indicated by short arrow) and perineuronal cells (D: indicated by long arrows). DRG explants from GFAP;tdTomato mice showed SGCs were labeled by tdTomato (E, red cells). NE-induced p-CREB expression in perineuronal cells of mouse DRG (F: green cells) was shown to be in GFAP-expressing cells (G-H: indicated by arrows). Western blot (I) confirmed p-CREB up-regulation in DRG by both NE and 5-HT (J). n=3–4. p<0.001.
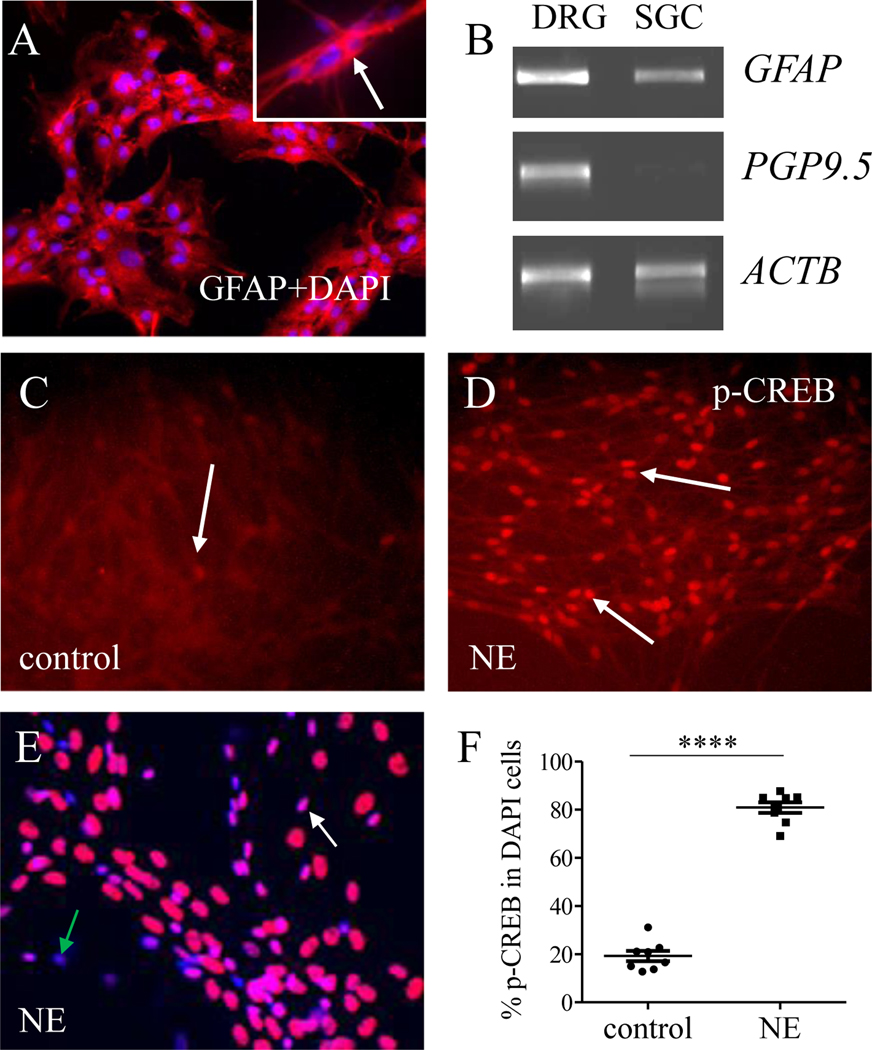
We next used cultured SGCs in which majority of the cells expressed GFAP immunoreactivity (Figure 2A: GFAP shows as red cytoplasmic stain indicated by arrow (inset); cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) to show nucleus). The cultured SGCs also expressed GFAP mRNA but not PGP9.5 mRNA (Figure 2B: a whole DRG was used as positive control and ACTB (gene for beta (β)-actin) was used as internal control), suggesting no contamination of DRG neurons in the culture. After treating these cells with NE for 5 min, we found robust increment of p-CREB in the nucleus of cultured SGCs (Figure 2C-D, indicated by arrows). For quantification, we stained SGC culture with DAPI (Figure 2E) and assessed that the percentage of DAPI-identified cells that expressed p-CREB was markedly up-regulated by NE (Figure 2F).
Figure 2. NE increased CREB phosphorylation in primarily cultured SGCs.
SGC single cell culture was characterized to express GFAP immunoreactivity (A, red stain and counterstained by DAPI), GFAP mRNA (B) but not PGP9.5 mRNA (B). Cell culture was evenly split and divided as control (C) and NE treatment (D). The expression of p-CREB (C-E, indicated by white arrows) was normalized as the percentage of DAPI-stained cells (E, blue stain, green arrow indicated a cell that did not have p-CREB) that expressed p-CREB (F). n=8. p<0.0001.
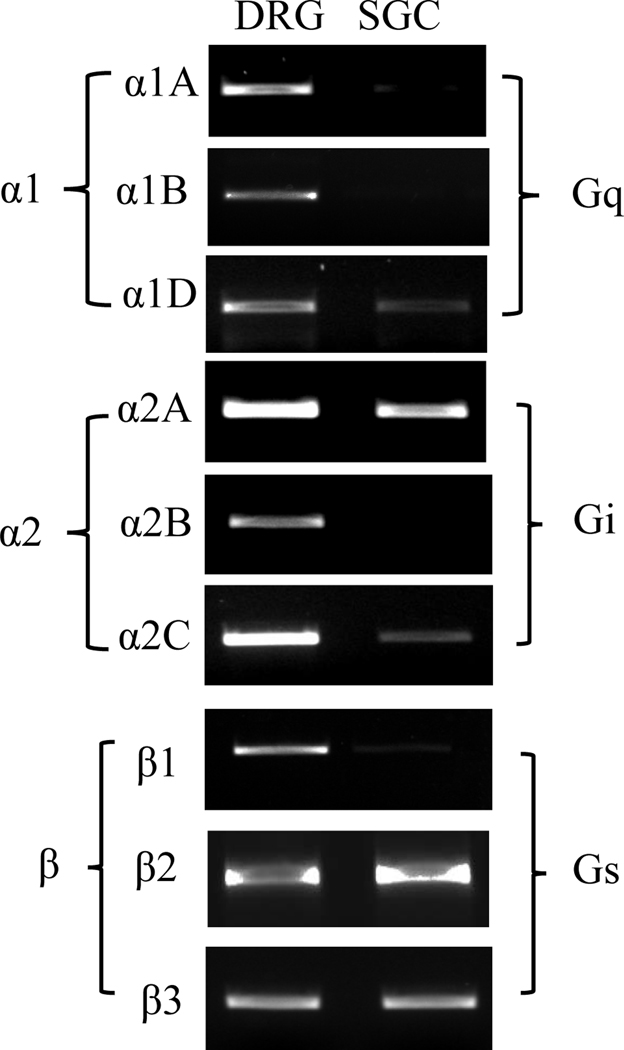
β2AR mediated NE-induced CREB phosphorylation in SGCs
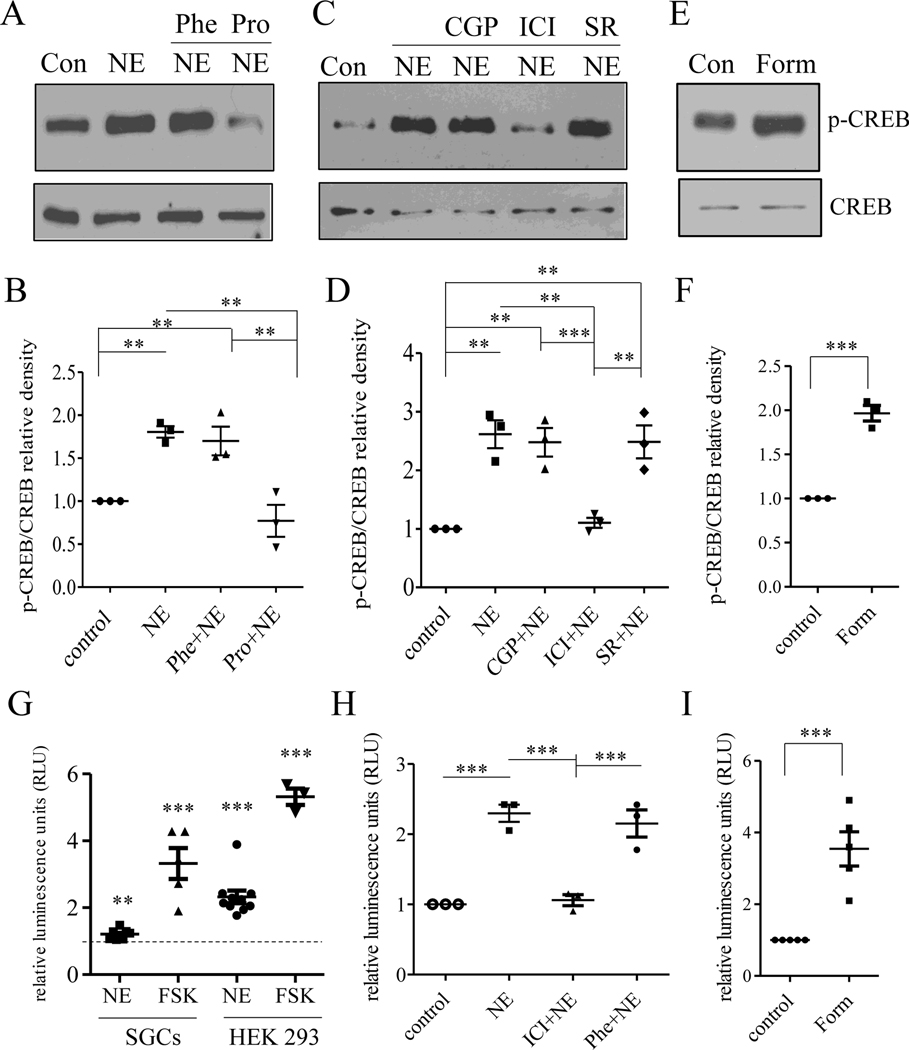
Using conventional PCR, we found that all adrenergic receptors were present in whole DRG but only some of them in cultured SGCs (Figure 3). To characterize which adrenergic receptors mediated NE-induced CREB phosphorylation in SGCs, we treated the cultured SGCs with NE in presence or absence of specific inhibitors of adrenergic receptors. We found that NE-induced p-CREB up-regulation was not affected by pan-αAR inhibitor (Phe: phentolamine) but was blocked by pan-βAR inhibitor (Pro: propranolol) (Figure 4A-B). We next identified the subtypes of βAR that mediated NE-induced p-CREB up-regulation in SGCs by applying specific antagonists for β1, β2, or β3 prior to NE treatment. We found that NE-induced p-CREB up-regulation was not affected by β1AR inhibitor (CGP: CGP20712) nor by β3AR inhibitor (SR: SR59230A) while it was blocked by β2AR inhibitor (ICI: ICI118551) (Figure 4C-D). These results suggest that β2AR mediated NE-induced p-CREB up-regulation in SGCs. We therefore treated the cultured SGCs with β2AR agonist formoterol (Form) and found that direct activation of β2AR also increased p-CREB expression in SGCs (Figure 4E-F).
Figure 3. Expression of adrenergic receptors in SGCs.
Cultured SGCs were processed for total RNA extraction followed by PCR. A whole DRG was processed simultaneously. The expression of adrenergic receptor subtypes was examined by PCR. The cultured SGCs were validated to be neuron-free (see Figure 2B).
Figure 4. NE-induced p-CREB in SGCs was mediated by β2AR.
Primarily cultured SGCs were treated with NE in presence or absence of pan α inhibitor (Phe) or pan β inhibitor (Pro) and were subject to p-CREB western blot (A). NE-induced p-CREB up-regulation was attenuated by β inhibitor but not α inhibitor (B). Cultured SGCs were also treated with NE in presence or absence of β1 (CGP), β2 (ICI), or β3 (SR) inhibitor and were subject to p-CREB western blot (C). NE-induced p-CREB up-regulation was attenuated by β2 inhibitor but not β1 or β3 inhibitor (D). Formoterol (Form) treatment of cultured SGCs also increased p-CREB expression examined by western blot (E, F). Luciferase assay showed that NE and FSK increased Cre-dependent transcriptional activity (CREB activity) in both SGCs and HEK 293 cells (G), and NE-induced CREB activity examined in HEK 293 cells was blocked by β2 inhibitor (ICI) bot not α inhibitor (H). Formoterol (Form) treatment of HEK 293 cells also increased CREB transcriptional activity (I). n>3. p<0.01 (**) or 0.001 (***).
In measuring CREB transcriptional activity in SGCs via luciferase assay, we found that NE-induced a very mild (1.37-fold) increase in CREB activity (Figure 4G). We then turned to HEK 293 cells that contained endogenous β2AR [12] and were routinely used for plasmid transfection when the transfection efficiency was low in primarily cultured cells (i.e., SGCs). We found that NE increased CREB activity in HEK 293 cells by 2.32-fold (Figure 4G). The adenylyl cyclase activator forskolin (FSK) that bypassed the receptors to act on the cAMP/PKA pathway also increased CREB transcriptional activity in SGCs and HEK 293 cells. Consistent to NE treatment, a bigger increase of luciferase activity in HEK 293 cells than in SGCs was stimulated by FSK (Figure 4G). Using HEK 293 cells, we also found that NE-induced CREB transcriptional activity was inhibited by β2AR antagonist (Figure 4H: ICI+NE). HEK 293 cells also contained low amount of αAR [11]. However, NE-induced CREB activity in HEK 293 cells was not inhibited by pan-αAR inhibitor (Figure 4H: Phe+NE). Consistently, β2AR agonist formoterol also induced robust CREB activity in HEK 293 cells (Figure 4I).
β2AR was expressed by SGCs but not neurons of DRG
To confirm the expression of β2AR in DRG SGCs, we performed FISH to detect β2AR transcripts (Adrb2) in DRG. We found that Adrb2 was exclusively expressed by cells that surrounded DRG neurons (Figure 5A-B, green cells indicated by arrows), morphologically resembling SGCs that were identified by their molecular marker GS (encoded by GLUL) in FISH (Figure 5C). Using TRAP techniques in transgenic mice [22; 24], we isolated mRNA from specific cell types of DRG that expressed either GFAP, Nav1.8, or Piezo2 (Figure 5D). We found that Adrb2 mRNA was expressed specifically in GFAP-expressing SGCs but not in Nav1.8- or Piezo2-expressing DRG neurons (Figure 5E, ACTB was used as internal control).
Figure 5. Expression of β2AR in SGCs.
FISH analysis showed that β2AR transcripts (Adrb2) was present in SGCs (A, B showed higher magnification, SGCs were indicated by arrows). The GS transcripts (GLUL) in SGCs were characterized (C) as positive control. The GFAP;TRAP, Nav1.8;TRAP, and Piezo2;TRAP mice were generated for cell-type specific mRNA extraction (D) and Adrb2 was detected in GFAP-expressing cells but not Nav1.8- or Piezo2- expressing cells (E). The mRNA extracts were validated by GFAP or PGP9.5 expression (E).
β2AR and p-CREB were up-regulated in SGCs in vivo in colitis
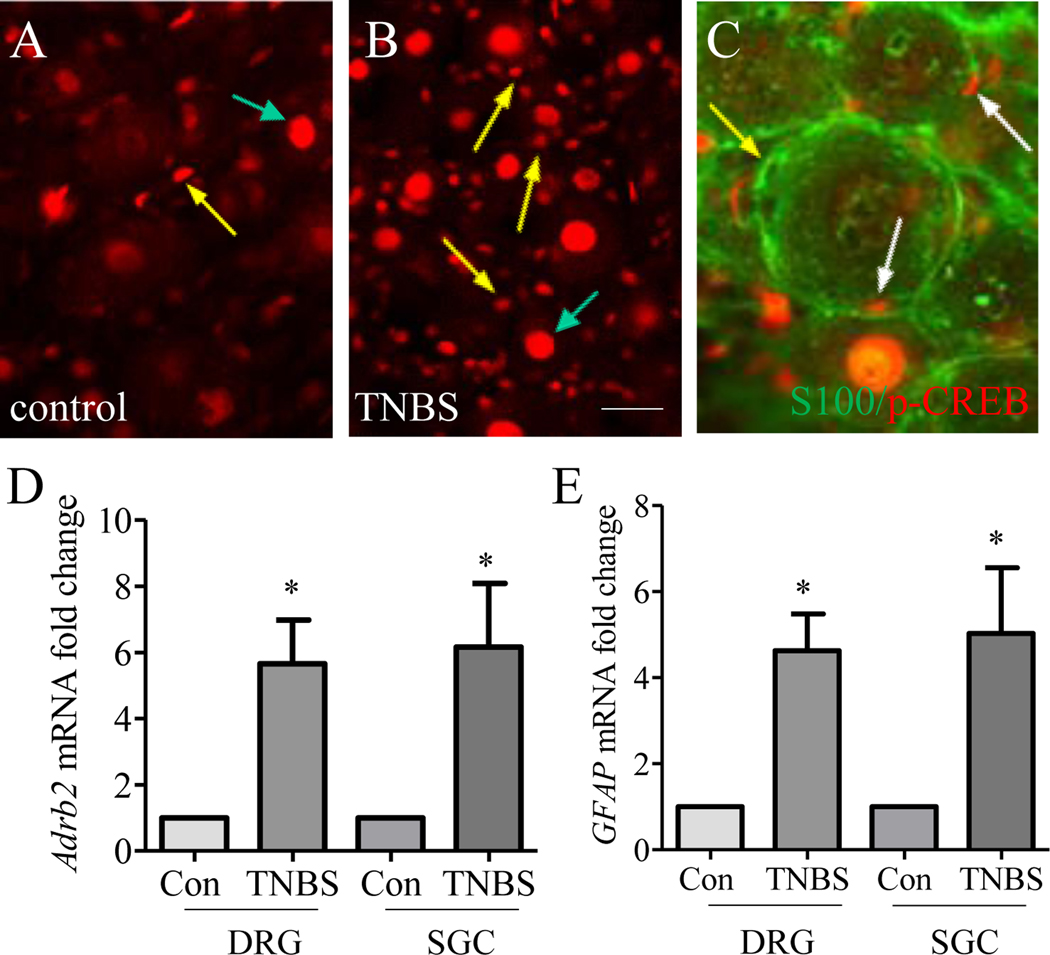
We previously showed that the level of tyrosine hydroxylase (TH), a rate limiting enzyme in NE synthesis [27], was persistently increased in thoracolumbar DRGs on day 7 and day 21 post colitis induction, which was demonstrated as sympathetic nerve sprouting around SGCs [60]. We therefore investigated whether the β2AR/p-CREB axis changed in vivo in DRG SGCs post colitis induction. On day 7 post TNBS treatment, the expression of p-CREB in thoracolumbar DRG was not only increased in DRG neurons (Figure 6A-B, indicated by green arrows) as we reported previously [23; 59], but also was there an apparent increase in p-CREB in SGCs (compare Figure 6B to A, indicated by yellow arrows). The p-CREB immunoreactivity (Figure 6C, red stain indicted by white arrows) was present in the nucleus of S-100 positive cells (Figure 6C, green stain, yellow arrow indicated an SGC without p-CREB) that surrounded DRG neurons. Concomitantly, we found that the level of Adrb2 was also increased in thoracolumbar whole DRG as well as in SGCs on day 7 post colitis induction (Figure 6D). In these studies, the mRNA from SGCs was extracted via TRAP-based affinity purification of mRNA from GFAP-expressing DRG cells. The levels of Adrb2 mRNA in DRG neurons (TRAP-based mRNA extracts from Nav1.8- or Piezo2-expressing DRG neurons) with or without colitis were not detectable (The ct values for Adrb2 in real-time PCR measurement were >40). The total RNA and mRNA extracts from SGCs were subject to examination of GFAP levels which showed an upregulation in whole DRG and in SGCs on day 7 following TNBS treatment when compared to control (Figure 6F).
Figure 6. Up-regulation of β2AR and p-CREB in SGCs in colitis.
Immunostaining of thoracolumbar DRG showed that p-CREB expression was not only increased in DRG neurons (A-B, indicated by green arrows) but also in SGCs (A-B, indicated by yellow arrows) following TNBS treatment. Co-stain of p-CREB with S-100 showed presence of p-CREB in the nucleus of SGCs (C, indicated by white arrows) and not all SGCs had p-CREB (C, indicated by yellow arrow). TNBS colitis also increased the expression of β2AR (Adrb2) (D) and GFAP (E) in whole DRG and SGCs. n>3. p<0.05.
β2AR regulated colonic inflammation and visceral hypersensitivity
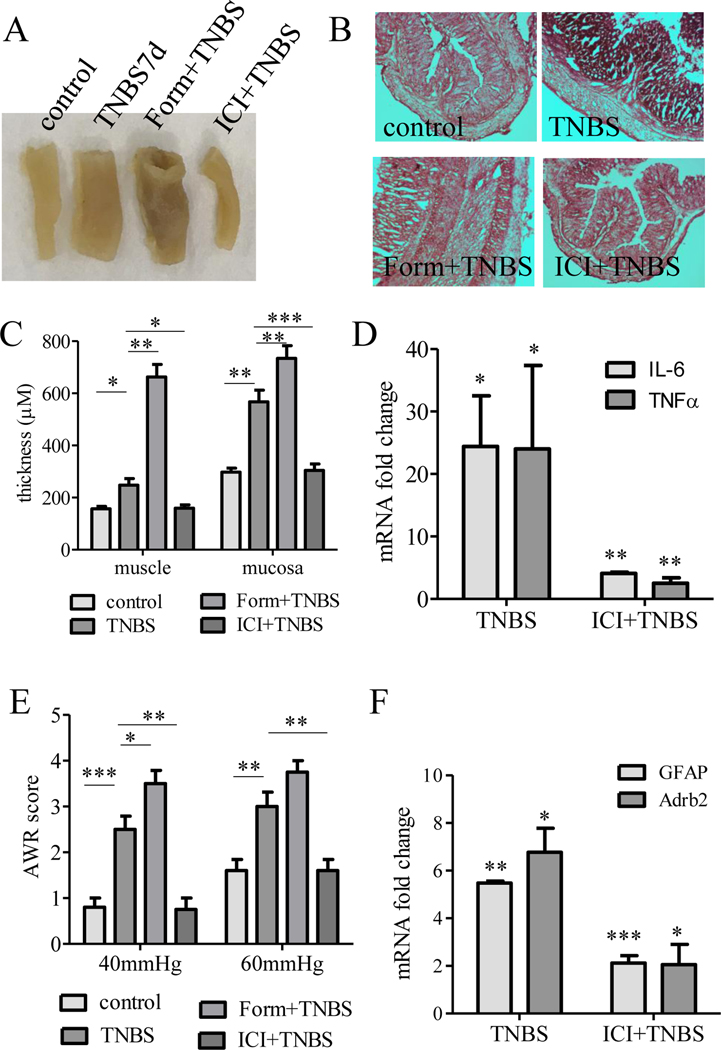
Since β2AR was up-regulated in SGCs in colitis, we sought to examine the functional role of β2AR in colitis and colitis-induced visceral hypersensitivity by augmenting or inhibiting β2AR in vivo. We first tested the effects of systemic β2AR modulation by intravenous injection of β2AR agonist formoterol or β2AR antagonist ICI118551. We found that formoterol (Form) exacerbated while ICI118551 (ICI) reduced colonic inflammation examined on day 7 post TNBS treatment, which was determined by macroscopic examination (Figure 7A) and histology (Figure 7B). The thickness of the muscular layer and the mucosal layer were measured to confirm the effects of β2AR inhibition but not augmentation on blocking the development of colonic inflammation (Figure 7C). β2AR inhibition also reduced the levels of pro-inflammatory cytokines in the colon that was evoked by colitis (Figure 7D). In the measurement of visceral hypersensitivity, we found that systemic β2AR inhibition but not augmentation also reduced colonic pain in response to noxious CRD (40 mmHg and 60 mmHg: Figure 7E) stimulation. In DRG, β2AR inhibition reduced colitis-evoked up-regulation of GFAP and Adrb2 mRNA (Figure 7F), suggesting that inhibition of β2AR reduced the activity of SGCs during colitis.
Figure 7. β2AR regulated colonic inflammation, visceral hypersensitivity, and activity of SGCs of DRG.
TNBS-induced colonic inflammation was characterized by macroscopic examination (A), H & E stain (B), analysis of colonic structures (C), and expression of pro-inflammatory factors in the colon (D). TNBS-induced visceral hypersensitivity was characterized by painful behavior in response to noxious CRD at 40 mmHg or 60 mmHg (E). TNBS-induced and β2AR-mediated SGC activity was characterized by the expression of GFAP (F). β2AR inhibitor also suppressed β2AR levels in DRG (F). The activity of β2AR was modulated by its agonist (Form) or antagonist (ICI). n>3. p<0.05 (*), 0.01 (**), or 0.001 (***).
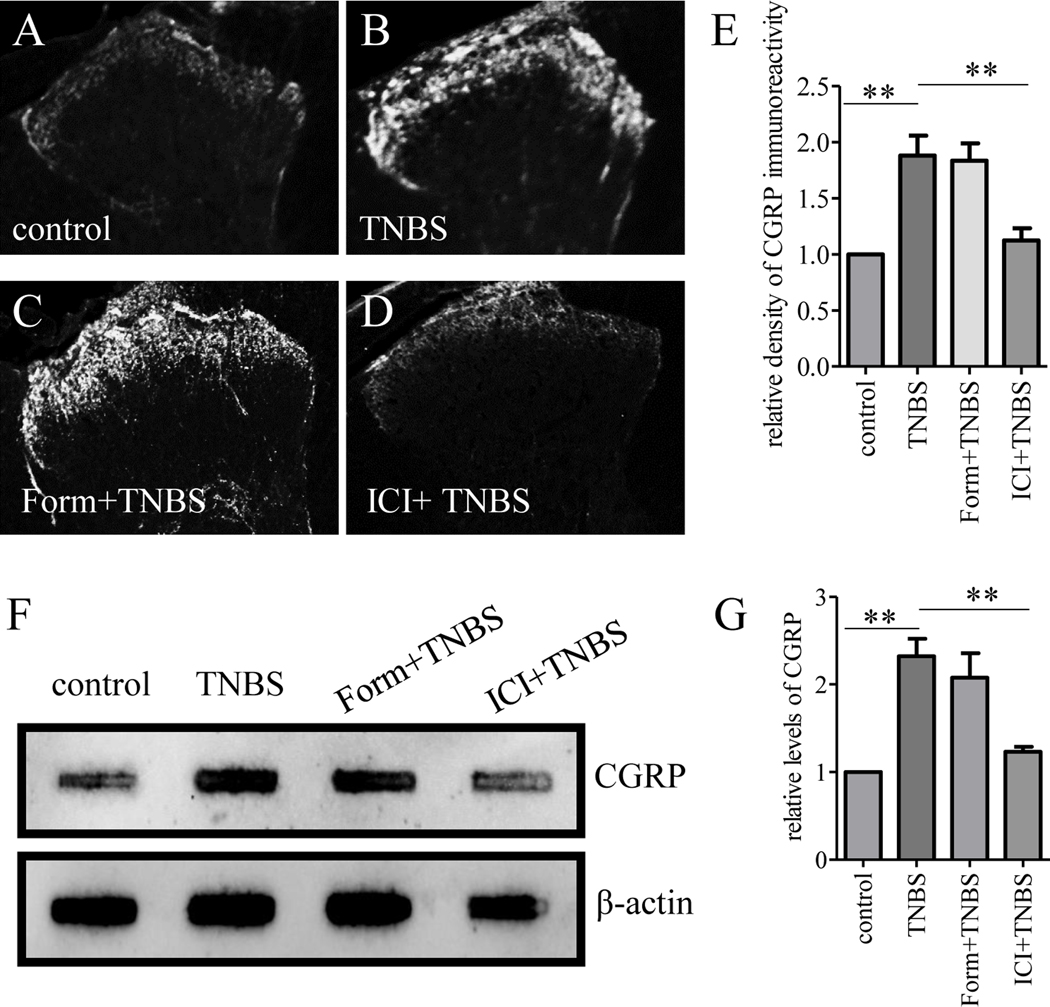
We next performed intrathecal injection of formoterol or ICI118551 to colitic animals in order to more specifically target cells in the primary afferent pathways including SGCs. We found that colitis on day 7 induced calcitonin gene-related peptide (CGRP) upregulation in the region of the spinal cord dorsal horn (compare Figure 8A to 8B), which was not affected by formoterol (compare Figure 8C to 8B) but was attenuated by ICI118551 (compare Figure 8D to 8B, summarized in Figure 8E). Using slot blot, we confirmed that β2AR antagonist but not agonist reduced colitis-evoked CGRP release into the spinal cord (Figure 8F-G).
Figure 8. β2AR regulated CGRP release to the spinal cord.
TNBS-induced CGRP release to the spinal cord was characterized by immunostaining (A-D) showing that β2AR inhibition but not activation blocked CGRP release (E). CGRP levels in the spinal cord were also characterized by slot blot (F) showing that TNBS evoked CGRP increase which was blocked by β2AR inhibition but not activation (G). n=3. p<0.01.
CGRP is generated by nociceptive neurons and its release to the spinal cord causes spinal central sensitization [28], a hallmark of pain [45]. In colitis, β2AR could be expressed by accumulated macrophages in DRG or cells in the spinal cord. To examine whether β2AR in SGCs had a role in inducing CGRP expression in DRG neurons, we used DRG explants from naïve animals in which the level of macrophages was scarce [44] and β2AR was identified in SGCs (Figure 5). We treated the DRG explants with formoterol overnight (16 hours) and examined CGRP expression (Figure 9). We found that CGRP expression was up-regulated by formoterol in both rat (Figure 9A-C) and mouse (Figure 9D-F) DRGs. Since we used the mouse DRGs from GFAP reporter mice and β2AR was expressed in GFAP-expressing SGCs, we characterized the CGRP expression in DRG neurons that were adjacent to GFAP-expressing SGCs and found an upregulation of CGRP in these neurons (Figure 9G-I).
Figure 9. β2AR regulated CGRP expression in DRG neurons.
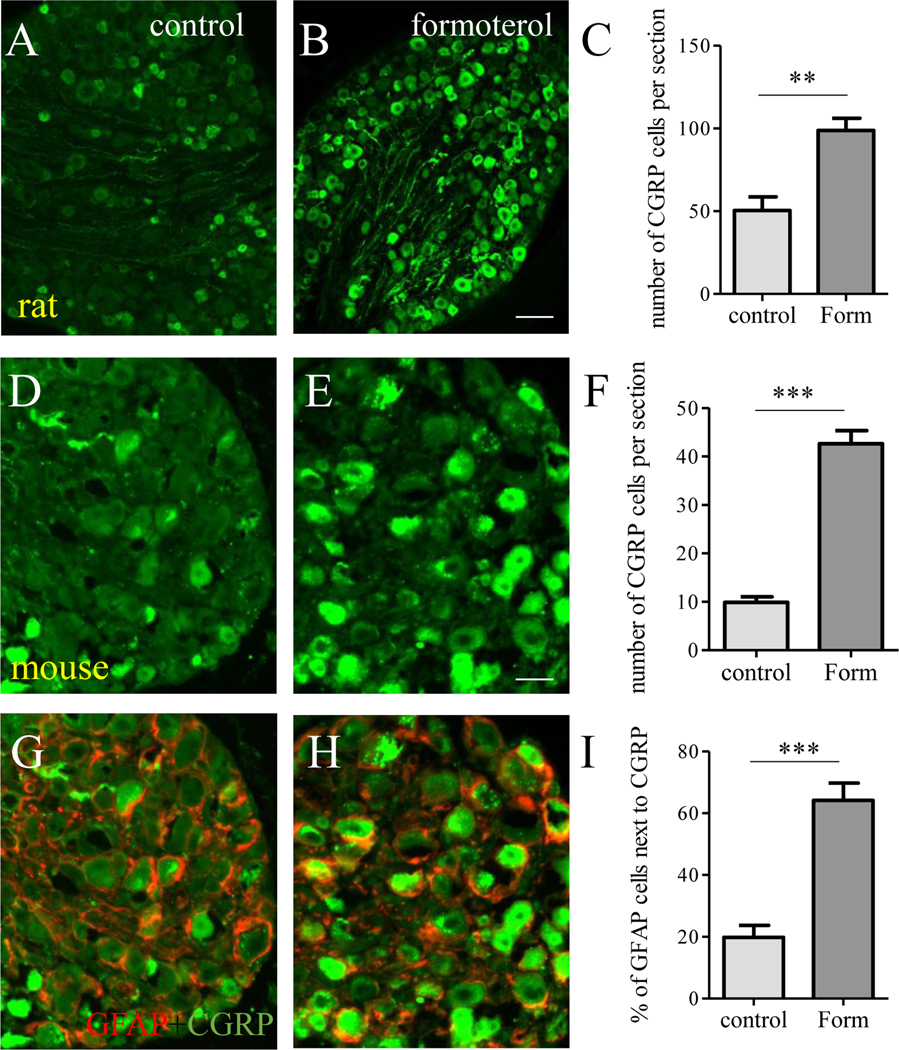
Naïve rat DRGs (A, B) were treated by formoterol and showed upregulation of CGRP in DRG neurons (C). Naïve mouse DRG (D, E) were treated by formoterol and showed upregulation of CGRP in DRG neurons (F). Upregulation of CGRP was adjacent to GFAP-expressing SGCs (G-I, red cells were SGCs and green cells were CGRP expressing cells). Calibration bar = 80 μm in A and B, and 40 μm in D, E, G and H. n>3. p< 0.01 (**) or 0.001 (***).
DISCUSSION
In searching for the cell types and receptors that mediate sympathoneuronal outflow in DRG, we demonstrate that β2AR is exclusively expressed in SGCs of DRG and mediates NE-induced CREB activity. Using DRG explants, we unexpectedly found that NE increased CREB phosphorylation mainly in SGCs surrounding DRG neurons. Using primarily cultured SGCs and HEK 293 cells, we revealed that β2AR was the only adrenergic receptor that mediated NE-induced CREB phosphorylation and transcriptional activity. In combination of FISH and affinity purification of mRNA from specific cell types, we identified β2AR to be exclusively expressed by SGCs in DRG but not DRG neurons that participate in pain processes. We took advantage of an in vivo colitic model in which sympathetic nerve fiber sprouting was prolongedly increased in thoracolumbar DRG and was surrounding SGCs [60] and found that β2AR and p-CREB were also up-regulated in SGCs of thoracolumbar DRG at the time point (day 7) when TH was up-regulated by colitis. Concomitantly the expression of GFAP was increased in thoracolumbar DRG on day 7 post colitis induction, which was suppressed by inhibition of β2AR. Inhibition of β2AR in vivo also reduced colitis-induced colonic pain responses to noxious CRD stimulation and attenuated colitis-facilitated CGRP release to the spinal cord on day 7 of colitis. CGRP expression in DRG neurons is evoked by treatment of naïve DRG explants with β2AR specific agonist formoterol. These data suggest that β2AR in SGCs of DRG receives noradrenergic signals and participates in sympathetically maintained chronic visceral hypersensitivity.
It is well characterized that chronic pain is accompanied with sympathetic nerve sprouting into DRG [60]. Several recent studies using localized microsympathectomy to block sympathetic fibers entering the spinal nerves and sprouting into DRG reveal its effectiveness in reducing pain [53; 62]. The molecular process within DRG in response to sympathoneuronal outflow has not been investigated. Our lab is routinely using DRG explant culture to examine paracrine actions among different types of cells within DRG [49; 59; 65]. When we treat DRG explants with NE, the neurotransmitter used by postganglionic sympathetic fibers, it reveals an interesting phenomenon that NE increases p-CREB mainly in SGCs that are identified by GFAP expression. To rule out this is not an artifact, we treat DRG explants with a different monoamine, 5-HT, which increases p-CREB in both DRG neurons and SGCs. These results suggest that SGCs are the primary cell types in DRG to receive noradrenergic signals. In our previous study in examining sympathetic sprouting into DRG in colitis, we find that sympathetic nerve fibers wraps around SGCs [60]. An interesting observation in colitis is that p-CREB is also highly expressed by SGCs at the time point when TH is up-regulated. This suggests that SGCs are an integral component in sympathetic regulation of sensory activity in which NE promotes p-CREB upregulation and subsequent Cre-promoted transcriptional activity. Indeed, in cultured SGCs in the present study and in cultured cortical astrocytes by others, the phosphorylation and activity of CREB are evoked by NE treatment [6; 41].
We next move to characterize the adrenergic receptor(s) that participate in NE-CREB axis in SGCs of DRG. Using primary SGC culture and HEK 293 cell line in combination with specific inhibitors, we reveal β2AR in mediation of NE-induced CREB phosphorylation and transcriptional activity. The role of β2AR in p-CREB upregulation in SGCs is also confirmed by using β2AR agonist formoterol. β2AR couples to Gs protein and activates adenylyl cyclase to stimulate cAMP-dependent pathway. Direct activation of adenylyl cyclase by FSK also increases CREB activity in SGCs and HEK 293 cells; this is consistent to those in cultured astrocytes in which FSK increases CREB activity [6; 41] suggesting a cAMP-dependent CREB activation [25]. It is noted that SGCs also contain other adrenergic receptors such as α1AR and β3AR although they do not mediate NE-induced CREB activity. αAR is coupled to Gq protein and mediates Ca2+ mobilization. Our unpublished data (reported by us in an abstract format, not included in the present study) shows that NE treatment of SGCs evokes intracellular Ca2+ transients. While in cultured astrocytes, NE-induced CREB transcriptional activity is not Ca2+ dependent [6]. Interestingly, NE-induced CREB activity in astrocytes is independent of βAR or PKA regardless that FSK promotes CREB activity in astrocytes [6]. The reasons behind the discrepancy in the receptors and signaling pathways that mediate NE-induced CREB activity between SGCs and astrocytes are unknown, but it may reflect cell type specificity.
The most surprising result in the present study is that β2AR is not expressed by DRG neurons. In FISH experiment, β2AR transcripts are only present in SGCs. The cell type-specific mRNA extraction approach confirms that β2AR transcripts are identified in GFAP-expressing DRG cells but not Piezo2- or Nav1.8- expressing DRG neurons that are key players in pain processes. In a previous study, β2AR exogenous agonist terbutaline also activates SGCs in DRG/SGCs co-culture [4]. It is noted that β2AR exogenous agonist such as terbutaline and isoproterenol may function differently from β2AR endogenous ligand NE. For example, terbutaline at a high concentration (100 μM) increases Ca2+ levels in SGCs [4]. Isoproterenol also induces robust Ca2+ release from stores in an cAMP/PKA-independent fashion [19] in HEK 293 cells where endogenous β2AR is found [15]. However, β2AR-mediated NE action involves the cAMP/PKA pathway but not Ca2+ [14]. One interesting feature about β2AR is its bidirectional function resulting from Gs-to-Gi switch in response to exogenous agonists to lead to desensitization [15; 17; 57]. The paradoxical effects of β2AR complicate its functional role in vivo which could be pro-inflammatory and anti-inflammatory, and is disease condition- and immune cell type-dependent [20; 37; 48; 50]. In the early stage of rheumatoid arthritis (RA) activation of β2AR has beneficial effects in reducing inflammation, while in chronic stage of RA β2AR stimulation promotes production of pro-inflammatory cytokines to maintain the inflammatory process [58].
Sympathetic fiber sprouting to DRG is often seen during the chronic but not acute state [31; 35]. Pharmacological studies have suggested β2AR in modulation of pain process, while information obtained so far are not consistent. In general, it is shown that chronic treatment with β2AR agonists is beneficial in neuropathic pain [3; 11], however, β2AR antagonism is effective in reducing other types of sensory hypersensitivity including visceral pain and itch [42; 56; 66]. It is not clear whether chronic β2AR agonists treatment would cause β2AR desensitization in vivo. Prolonged β2AR agonist treatment of airway smooth muscles down regulate β2AR expression through cAMP/PKA/CREB mediated miRNA let-7f expression to suppress β2AR expression [29]. It is noted that in addition to SGCs and astrocytes that express β2AR, immune cells are also major carrier of β2AR in response to stress and inflammation [58]. Although macrophages in naïve DRGs are very low, TNBS treatment can evoke macrophages accumulating around DRG neurons [44]. At this moment we have no data on whether macrophages in DRG during colitis also express β2AR, however, using naïve DRG explants in which macrophages are very scarce we find that activation of β2AR which is expressed by SGCs facilitates CGRP expression in DRG neurons. These findings suggest that inhibition of β2AR in colitis that reduces GFAP expression in DRG and attenuates CGRP release into the spinal cord may involve, at least in part, β2AR in SGCs, although this does not preclude a role of β2AR in DRG macrophages and cells in the spinal cord in visceral hypersensitivity. Interestingly, β2AR inhibitor also reduced β2AR levels in DRG during colitis, suggesting that β2AR-induced autoregulation loop [13] also occurred in vivo.
In summary, β2AR has been suggested to participate in chronic pain and its expression in DRG is now identified in SGCs to mediate NE-induced CREB activation. Since β2AR is not expressed in DRG neurons, sympathetically maintained chronic pain may involve SGCs to integrate the sympathetic nervous system and sensory neurons. Target of SGCs for treating chronic pain could be a potential therapeutic approach.
Acknowledgments
Grant Support:
NIH R01 DK118137; Virginia’s Commonwealth Health Research Board (CHRB) 236-06-18; CTSA 5KL2TR002648; NIH P30 DA033934 (Sub)
Footnotes
Disclosures:
The authors declare no conflict of interest.
REFERENCE
- [1].Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology 2000;119(5):1276–1285. [DOI] [PubMed] [Google Scholar]
- [2].Bakhshandeh Bali M, Rahbarimanesh AA, Sadeghi M, Sedighi M, Karimzadeh P, Ghofrani M. Comparison of propranolol and pregabalin for prophylaxis of childhood migraine: a randomised controlled trial. Acta medica Iranica 2015;53(5):276–280. [PubMed] [Google Scholar]
- [3].Baraka AM, Darwish IE, Ghoneim MT, Korayem HK. beta2-Adrenoceptor agonists as potential therapeutic drugs in diabetic peripheral neuropathy. European journal of pharmacology 2015;746:89–95. [DOI] [PubMed] [Google Scholar]
- [4].Bohren Y, Tessier LH, Megat S, Petitjean H, Hugel S, Daniel D, Kremer M, Fournel S, Hein L, Schlichter R, Freund-Mercier MJ, Yalcin I, Barrot M. Antidepressants suppress neuropathic pain by a peripheral beta2-adrenoceptor mediated anti-TNFalpha mechanism. Neurobiology of disease 2013;60:39–50. [DOI] [PubMed] [Google Scholar]
- [5].Bowden JJ, Anderson GP, Lefevre PM, Sulakvelidze I, McDonald DM. Characterization of tolerance to the anti-leakage effect of formoterol in rat airways. European journal of pharmacology 1997;338(1):83–87. [DOI] [PubMed] [Google Scholar]
- [6].Carriba P, Pardo L, Parra-Damas A, Lichtenstein MP, Saura CA, Pujol A, Masgrau R, Galea E. ATP and noradrenaline activate CREB in astrocytes via noncanonical Ca(2+) and cyclic AMP independent pathways. Glia 2012;60(9):1330–1344. [DOI] [PubMed] [Google Scholar]
- [7].Carter BS, Fletcher JS, Thompson RC. Analysis of messenger RNA expression by in situ hybridization using RNA probes synthesized via in vitro transcription. Methods 2010;52(4):322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Charrua A, Pinto R, Birder LA, Cruz F. Sympathetic nervous system and chronic bladder pain: a new tune for an old song. Translational andrology and urology 2015;4(5):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen L, Huang J, Zhao P, Persson AK, Dib-Hajj FB, Cheng X, Tan A, Waxman SG, Dib-Hajj SD. Conditional knockout of NaV1.6 in adult mice ameliorates neuropathic pain. Scientific reports 2018;8(1):3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Choucair-Jaafar N, Salvat E, Freund-Mercier MJ, Barrot M. The antiallodynic action of nortriptyline and terbutaline is mediated by beta(2) adrenoceptors and delta opioid receptors in the ob/ob model of diabetic polyneuropathy. Brain research 2014;1546:18–26. [DOI] [PubMed] [Google Scholar]
- [11].Choucair-Jaafar N, Yalcin I, Rodeau JL, Waltisperger E, Freund-Mercier MJ, Barrot M. Beta2-adrenoceptor agonists alleviate neuropathic allodynia in mice after chronic treatment. British journal of pharmacology 2009;158(7):1683–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ciszek BP, O’Buckley SC, Nackley AG. Persistent Catechol-O-methyltransferase-dependent Pain Is Initiated by Peripheral beta-Adrenergic Receptors. Anesthesiology 2016;124(5):1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Collins S, Altschmied J, Herbsman O, Caron MG, Mellon PL, Lefkowitz RJ. A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. The Journal of biological chemistry 1990;265(31):19330–19335. [PubMed] [Google Scholar]
- [14].Copik AJ, Ma C, Kosaka A, Sahdeo S, Trane A, Ho H, Dietrich PS, Yu H, Ford AP, Button D, Milla ME. Facilitatory interplay in alpha 1a and beta 2 adrenoceptor function reveals a non-Gq signaling mode: implications for diversification of intracellular signal transduction. Molecular pharmacology 2009;75(3):713–728. [DOI] [PubMed] [Google Scholar]
- [15].Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the beta2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997;390(6655):88–91. [DOI] [PubMed] [Google Scholar]
- [16].Deiteren A, van der Linden L, de Wit A, Ceuleers H, Buckinx R, Timmermans JP, Moreels TG, Pelckmans PA, De Man JG, De Winter BY. P2X3 receptors mediate visceral hypersensitivity during acute chemically-induced colitis and in the post-inflammatory phase via different mechanisms of sensitization. PloS one 2015;10(4):e0123810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Molecular pharmacology 2001;60(3):577–583. [PubMed] [Google Scholar]
- [18].Fiacco TA, McCarthy KD. Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. J Neurosci 2018;38(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Galaz-Montoya M, Wright SJ, Rodriguez GJ, Lichtarge O, Wensel TG. beta2-Adrenergic receptor activation mobilizes intracellular calcium via a non-canonical cAMP-independent signaling pathway. The Journal of biological chemistry 2017;292(24):9967–9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Galvez I, Martin-Cordero L, Hinchado MD, Alvarez-Barrientos A, Ortega E. Anti-inflammatory effect of beta2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain, behavior, and immunity 2019;80:564–572. [DOI] [PubMed] [Google Scholar]
- [21].Gil DW, Wang J, Gu C, Donello JE, Cabrera S, Al-Chaer ED. Role of sympathetic nervous system in rat model of chronic visceral pain. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2016;28(3):423–431. [DOI] [PubMed] [Google Scholar]
- [22].Gracida X, Calarco JA. Cell type-specific transcriptome profiling in C. elegans using the Translating Ribosome Affinity Purification technique. Methods 2017;126:130–137. [DOI] [PubMed] [Google Scholar]
- [23].Hashmi F, Liu M, Shen S, Qiao LY. EXPRESS: Phospholipase C gamma mediates endogenous brain-derived neurotrophic factor - regulated calcitonin gene-related peptide expression in colitis - induced visceral pain. Molecular pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heiman M, Kulicke R, Fenster RJ, Greengard P, Heintz N. Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP). Nature protocols 2014;9(6):1282–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cellular and molecular neurobiology 2003;23(3):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaplan R, Robinson CA, Scavulli JF, Vaughan JH. Propranolol and the treatment of rheumatoid arthritis. Arthritis and rheumatism 1980;23(2):253–255. [DOI] [PubMed] [Google Scholar]
- [27].Kaufman S. Tyrosine hydroxylase. Advances in enzymology and related areas of molecular biology 1995;70:103–220. [DOI] [PubMed] [Google Scholar]
- [28].Kay JC, Xia CM, Liu M, Shen S, Yu SJ, Chung C, Qiao LY. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Experimental neurology 2013;250:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim D, Cho S, Woo JA, Liggett SB. A CREB-mediated increase in miRNA let-7f during prolonged beta-agonist exposure: a novel mechanism of beta2-adrenergic receptor down-regulation in airway smooth muscle. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2018;32(7):3680–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kim YS, Anderson M, Park K, Zheng Q, Agarwal A, Gong C, Saijilafu Young L, He S, LaVinka PC, Zhou F, Bergles D, Hanani M, Guan Y, Spray DC, Dong X. Coupled activation of primary sensory neurons contributes to chronic pain. Neuron 2016;91(5):1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Experimental brain research 1998;120(4):432–438. [DOI] [PubMed] [Google Scholar]
- [32].Malinowska B, Zakrzeska A, Kurz CM, Gothert M, Kwolek G, Wielgat P, Braszko JJ, Schlicker E. Involvement of central beta2-adrenergic, NMDA and thromboxane A2 receptors in the pressor effect of anandamide in rats. Naunyn-Schmiedeberg’s archives of pharmacology 2010;381(4):349–360. [DOI] [PubMed] [Google Scholar]
- [33].Maruo K, Yamamoto H, Yamamoto S, Nagata T, Fujikawa H, Kanno T, Yaguchi T, Maruo S, Yoshiya S, Nishizaki T. Modulation of P2X receptors via adrenergic pathways in rat dorsal root ganglion neurons after sciatic nerve injury. Pain 2006;120(1–2):106–112. [DOI] [PubMed] [Google Scholar]
- [34].Matsushita Y, Manabe M, Kitamura N, Shibuya I. Adrenergic receptors inhibit TRPV1 activity in the dorsal root ganglion neurons of rats. PloS one 2018;13(1):e0191032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McLachlan EM, Janig W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 1993;363(6429):543–546. [DOI] [PubMed] [Google Scholar]
- [36].Milligan G, Svoboda P, Brown CM. Why are there so many adrenoceptor subtypes? Biochemical pharmacology 1994;48(6):1059–1071. [DOI] [PubMed] [Google Scholar]
- [37].Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, Yudanin NA, Monticelli LA, Putzel GG, Rodewald HR, Artis D. beta2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018;359(6379):1056–1061. [DOI] [PubMed] [Google Scholar]
- [38].Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 2007;128(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nazarewicz J, Verdejo-Garcia A, Giummarra MJ. Sympathetic pain? A role of poor parasympathetic nervous system engagement in vicarious pain states. Psychophysiology 2015;52(11):1529–1537. [DOI] [PubMed] [Google Scholar]
- [40].Pannese E. The satellite cells of the sensory ganglia. Adv Anat Embryol Cell Biol 1981;65:1–111. [DOI] [PubMed] [Google Scholar]
- [41].Pardo L, Valor LM, Eraso-Pichot A, Barco A, Golbano A, Hardingham GE, Masgrau R, Galea E. CREB regulates distinct adaptive transcriptional programs in astrocytes and neurons. Scientific reports 2017;7(1):6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Peng XY, Huang Y, Wang XL, Cao LF, Chen LH, Luo WF, Liu T. Adrenergic beta2-receptor mediates itch hypersensitivity following heterotypic chronic stress in rats. Neuroreport 2015;26(17):1003–1010. [DOI] [PubMed] [Google Scholar]
- [43].Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Experimental neurology 2007;204(2):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Qiao LY, Tiwari N. Spinal neuron-glia-immune interaction in cross-organ sensitization. American journal of physiology Gastrointestinal and liver physiology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Raja SN, Sivanesan E, Guan Y. Central sensitization, N-methyl-D-aspartate Receptors, and human experimental pain models: bridging the gap between target discovery and drug development. Anesthesiology 2019;131(2):233–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rozanski GM, Nath AR, Adams ME, Stanley EF. Low voltage-activated calcium channels gate transmitter release at the dorsal root ganglion sandwich synapse. J Physiol-London 2013;591(22):5575–5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Savtchouk I, Volterra A. Gliotransmission: Beyond Black-and-White. J Neurosci 2018;38(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sharma M, Arbabzada N, Flood PM. Mechanism underlying beta2-AR agonist-mediated phenotypic conversion of LPS-activated microglial cells. Journal of neuroimmunology 2019;332:37–48. [DOI] [PubMed] [Google Scholar]
- [49].Shen S, Al-Thumairy HW, Hashmi F, Qiao LY. Regulation of transient receptor potential cation channel subfamily V1 protein synthesis by the phosphoinositide 3-kinase/Akt pathway in colonic hypersensitivity. Experimental neurology 2017;295:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sitkauskiene B, Sakalauskas R. The role of beta(2)-adrenergic receptors in inflammation and allergy. Current drug targets Inflammation and allergy 2005;4(2):157–162. [DOI] [PubMed] [Google Scholar]
- [51].Stern P. Glial cells contribute to pain. Science 2016;354(6316):1114–1115. [DOI] [PubMed] [Google Scholar]
- [52].Thonberg H, Fredriksson JM, Nedergaard J, Cannon B. A novel pathway for adrenergic stimulation of cAMP-response-element-binding protein (CREB) phosphorylation: mediation via alpha1-adrenoceptors and protein kinase C activation. The Biochemical journal 2002;364(Pt 1):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tonello R, Xie W, Lee SH, Wang M, Liu X, Strong JA, Zhang JM, Berta T. Local sympathectomy promotes anti-inflammatory responses and relief of paclitaxel-induced mechanical and cold allodynia in mice. Anesthesiology 2020;132(6):1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Tongtako W, Lehmbecker A, Wang Y, Hahn K, Baumgartner W, Gerhauser I. Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and oligodendrocytic properties. Scientific reports 2017;7(1):13915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proceedings of the National Academy of Sciences of the United States of America 2001;98(20):11024–11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 2010;138(1):294–304 e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Woo AY, Jozwiak K, Toll L, Tanga MJ, Kozocas JA, Jimenez L, Huang Y, Song Y, Plazinska A, Pajak K, Paul RK, Bernier M, Wainer IW, Xiao RP. Tyrosine 308 is necessary for ligand-directed Gs protein-biased signaling of beta2-adrenoceptor. The Journal of biological chemistry 2014;289(28):19351–19363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Wu L, Tai Y, Hu S, Zhang M, Wang R, Zhou W, Tao J, Han Y, Wang Q, Wei W. Bidirectional role of beta2-adrenergic receptor in autoimmune diseases. Frontiers in pharmacology 2018;9:1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Xia C, Shen S, Hashmi F, Qiao LY. Colitis-induced bladder afferent neuronal activation is regulated by BDNF through PLCgamma pathway. Experimental neurology 2016;285(Pt B):126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Xia CM, Colomb DG Jr., Akbarali HI, Qiao LY. Prolonged sympathetic innervation of sensory neurons in rat thoracolumbar dorsal root ganglia during chronic colitis. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society 2011;23(8):801–e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xie W, Strong JA, Mao J, Zhang JM. Highly localized interactions between sensory neurons and sprouting sympathetic fibers observed in a transgenic tyrosine hydroxylase reporter mouse. Molecular pain 2011;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xie W, Strong JA, Zhang JM. Localized sympathectomy reduces peripheral nerve regeneration and pain behaviors in 2 rat neuropathic pain models. Pain 2020;161(8):1925–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Yalcin I, Tessier LH, Petit-Demouliere N, Doridot S, Hein L, Freund-Mercier MJ, Barrot M. Beta2-adrenoceptors are essential for desipramine, venlafaxine or reboxetine action in neuropathic pain. Neurobiology of disease 2009;33(3):386–394. [DOI] [PubMed] [Google Scholar]
- [64].Yalcin I, Tessier LH, Petit-Demouliere N, Waltisperger E, Hein L, Freund-Mercier MJ, Barrot M. Chronic treatment with agonists of beta(2)-adrenergic receptors in neuropathic pain. Experimental neurology 2010;221(1):115–121. [DOI] [PubMed] [Google Scholar]
- [65].Yu SJ, Grider JR, Gulick MA, Xia CM, Shen S, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Experimental neurology 2012;238(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhang C, Rui YY, Zhou YY, Ju Z, Zhang HH, Hu CY, Xiao Y, Xu GY. Adrenergic beta2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PloS one 2014;9(4):e94726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in TNBS-induced colitis in rats. Digestive diseases and sciences 2008;53(2):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]