Abstract
Alstonia scholaris, Cardiospermum halicacabum, Hydrocotyle sibthorpioides, and Hypericum japonicum are important folk medicinal plants used by tribal communities of Bodoland region of Assam to treat helminth infections. Because of their ethnomedicinal values, the present study was designed to investigate the antioxidant, antiproliferative, and anthelmintic activities of the plants. The antioxidant activity was measured by total antioxidant capacity, total phenolics (TPC), total flavonoid (TFC), FRAP, DPPH, ABTS, and TBARS assay. Antiproliferative and apoptosis-inducing activities of plants were conducted in Dalton’s lymphoma (DL) cells. Cells were treated for 24 h with different doses (25–200 mg/mL) of plant extracts. Anthelmintic study was conducted by treating the Paramphistomum sp. at different doses of plant extracts. Phytochemical and antioxidant studies showed rich TPC, TFC, and free radical scavenging activity in H. japonicum and H. sibthorpioides. Both the antiproliferative and anthelmintic bioassays showed a dose-dependent efficacy in all plants. H. japonicum showed the strongest anthelmintic activity (LC50 0.21 mg/mL) followed by H. sibthorpioides (5.36 mg/mL), C. halicacabum (13.40 mg/mL), and A. scholaris (18.40 mg/mL). Evidently, H. sibthorpioides showed the strongest antiproliferative and apoptosis-inducing activities among all the plants. The study observed a positive correlation between the antioxidant properties and antiproliferative and anthelmintic activities of the plants. We, therefore, conclude that the phytocompounds present in the crude extracts along with antioxidant molecules may have combined effects contributing to the antiproliferative and anthelmintic activities of the plants.
Keywords: Medicinal plant, Antioxidant, Antiproliferative, Apoptosis, Anthelmintic, Paramphistomum sp.
Introduction
Helminths are a group of eukaryotic organisms that affect millions of people worldwide and cause severe economic loss (Qamar et al. 2011). There are about 3.5 lakh species of endo-parasites of vertebrates many of which can complete their life cycle either in humans or in other animals (Carlson et al. 2020). Among the major helminthiases, soil-transmitted helminthiases and schistosomiasis account for more than 1 billion infections worldwide, mainly in economically poor countries (James et al. 2018). Helminth infections put children at high risk of stunted growth, impaired cognitive development, and life-threatening complications (Dixon et al. 2019). They can also lead to epilepsy, liver and bladder cancer leading to chronic health and economic burden (Mosthafa et al. 1999). Helminth infections of ruminants are a major constraint on efficient livestock production. It has been estimated that the mass deworming programs of helminth infection cost about US$300 million annually, while the cost of treatment by screening programs would likely be US$2 billion annually (UCNTD 2014). Since several decades ago, the use of commercial anthelmintics such as benzimidazoles, macrocyclic lactones, praziquantel, etc. was the most common practice of controlling helminthiasis. However, there is a growing report of drug resistance in helminth parasites leading to ineffective control of helminthiasis (Mphahlele et al. 2019). Plants are known for their rich phytochemicals and medicinal values and are therefore explored for their anthelmintic properties as an alternative to the existing commercial drugs. Several medicinal plants are screened for their anthelmintic property by many researchers (Akhtar et al. 2000; Roy and Swargiary 2009; Tandon et al. 2011).
Plants are a rich source of phytochemicals and possess several medicinal properties such as antioxidants, anti-diabetes, anti-inflammatory, anti-cancer, anti-bacterial activities, etc. (Basu et al. 2020; Fahad et al. 2021). Plants possess strong antioxidant properties because of their innate ability to synthesize non-enzymatic antioxidant molecules such as ascorbic acid, glutathione, and phenolic compounds. At high concentrations, free radicals can damage cells, including DNA, proteins, and other biomolecules, and play a major role in the development of cancer and other health conditions (Valko et al. 2007). Phenolics and flavonoids are important chemical entities with several medicinal values and antioxidant properties (Horwitt 1991). They act as strong antioxidant agents by donating hydrogen to the reactive oxygen and nitrogen molecules (Pereira et al. 2009). Plants and plant-derived products can supplement the external need for antioxidant molecules to control the continuous generation of free radicals in the body. Many studies have reported the presence of strong antioxidant properties in several plants. Polyphenols and flavonoids have been highlighted not only as antioxidant molecules but also as potential anticancer substances and inhibit cell proliferation (Singh et al. 2014; Grigalius and Petrikaite 2017). Plants having strong antioxidant properties also exhibit strong cytotoxicity and anticancer activities (Sammar et al. 2019). Several plants and phytocompounds have been reported to exhibit antiproliferative and anticancer activity (Kamaruddin et al. 2019; Erdogan et al. 2020).
Assam is one of the northeastern states of India with rich flora and fauna. People living in this part of India, especially the tribal communities, follow several traditional practices to control common ailments, including helminth infection. Several plants traditionally used by tribal people are documented by many researchers from this part of India (Swargiary et al. 2017; Panda et al. 2018; Daimari et al. 2019). In our earlier studies, we have reported several medicinal plants traditionally used as antidiabetic and anthelmintic agents by the tribal communities of Kokrajhar, Chirang, Baksa, and Udalguri districts of Assam (Swargiary et al. 2016, 2020; Swargiary et al. 2019a, b). Alstonia scholaris, Cardiospermum halicacabum, Hydrocotyle sibthorpioides, and Hypericum japonicum are some of the important ethnomedicinal plants used as deworming agents. A. scholaris has ethnomedicinal values such as general tonic, aphrodisiac, antidysentery, antipyretic, emmenagogue, and vulnerary agents (Khyade et al. 2014). Similarly, C. halicacabum is used as a folk medicine for treating cough, hyperthermia, rheumatism, lumbago, nervous illnesses, ear ache, and fever (Krishna Murti et al. 2010). H. sibthorpioides is an important wild vegetable of Assam. The plant has several ethnomedicinal values and is used for treating cough, jaundice, dysentery, fever, throat pain, and edema (Barukial and Sarmah 2011). Recent pharmacological studies have shown antibacterial, anticancer, hepatoprotective, antiviral, and immunoregulatory properties of H. japonicum (Liu et al. 2014). Though these plants are popular in folk medicine, little work has been conducted to investigate their bioactivity. The present study, therefore, attempts to investigate the antioxidant, antiproliferative, and anthelmintic activities of A. scholaris, C. halicacabum, H. sibthorpioides, and H. japonicum.
Materials and methods
Chemicals and drugs
Chemicals were procured from SRL, HIMEDIA, and SIGMA. Different solvents were procured from MERCK. All chemicals and solvents were analytical grades (> 99% purity). Reference anthelmintic drug, albendazole (Manufacturer: Zydus Healthcare Ltd.) was purchased from a local pharmacy at Kokrajhar town.
Collection and identification plants
Four medicinal plants used by the traditional healers of Kokrajhar—Alstonia scholaris (L.) R. Br. (BUBH2018040) (family Apocynaceae), Cardiospermum halicacabum L. (BUBH2018001) (family Sapindaceae), Hydrocotyle sibthorpioides Lam. (BUBH2018019) (family Araliaceae), and Hypericum japonicum Thunb. (BUBH0000129) (family Hypericaceae) were collected from Kokrajhar town (Latitude 26° 24′ 5.1696'' N and Longitude 90° 16′ 0.1236'' E). Herbarium sheets were prepared and submitted to the Department of Botany, Bodoland University for taxonomic identification.
Preparation of crude plant extracts
Bark of A. scholaris, and leaves of C. halicacabum, H. sibthorpioides, and H. japonicum were washed properly to remove dirt particles and processed for methanolic crude extraction following the method described by Seidel (2005). Briefly, plant parts were dried completely in a hot-air oven at 45 ± 2 °C. Dried plants were powdered using a mixer grinder. Plant powder was macerated with 80% methanol (1:5, w/v) and kept for 72 h. Next, the solutions were filtered with Whatman filter paper No. 1, and the filtrate obtained was dried in a rotary evaporator. The solid material was collected as crude extract and kept at -20 °C until further use.
Antioxidant studies
Total phenolic content (TPC)
The total phenolic content was estimated using Folin-Ciocalteu reagent (Iloki-Assanga et al. 2013). Briefly, 1 mL of plant extracts (5 mg/mL) was mixed with 3 mL of 10% Folin-Ciocalteu reagent and 0.5 mL of sodium carbonate (10% w/v). The mixture was vortexed for 15 s and incubated at 40ºC for 30 min. The absorbance was measured at 765 nm using UV/VIS double beam spectrophotometer (Systronics 2206). TPC was calculated from a calibration curve of gallic acid and results were expressed as µg gallic acid equivalent (µgGAE/mg) of plant extract.
Total flavonoid content (TFC)
The flavonoid content was determined following spectrophotometric method (Ordonez et al. 2006). Briefly, 1 mL of plant extract (5 mg/mL, prepared in 80% ethanol) was mixed with 0.5 mL of 2% AlCl3 (prepared in 80% ethanol). The assay mixture was made 3 mL by adding distilled water. The mixture was incubated at room temperature for 30 min and the formation of yellow colour was measured at 430 nm. TFC was calculated from the standard curve of quercetin and the values were expressed as µg quercetin equivalent (µgQE/mg) of plant extract.
Total antioxidant capacity (TAC) assay
TAC of the plant extract was done by phosphomolybdate method using ammonium molybdate reagent (Huda-Faujan et al. 2009). Briefly, 1 mL of plant extracts (5 mg/mL) was mixed with 1 mL distilled water and 1 mL of reagent solution (600 mM H2SO4, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The reaction mixture was incubated at 95ºC for 30 min and absorbance was measured at 765 nm against a blank solution. TAC was expressed as µg ascorbic acid equivalent (µgAAE/mg) of plant extract.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was performed using FRAP reagent (Iloki-Assanga et al. 2015). Briefly, 1 mL of standard ascorbic acid (5–100 µg/mL) and plant extracts (5–100 µg/mL) was mixed with 2 mL of FRAP reagent (prepared by mixing 10 mL of acetate buffer pH 3.6, 1 mL of 10 mM TPTZ solution in 40 mM HCl and 1 mL of 20 mM ferric chloride). After 30 min of incubation at 50ºC, the absorbance of the mixture was measured using a spectrophotometer at 593 nm. The FRAP activity is compared with the standard ascorbic acid and values were expressed as μg Fe2+ equivalent (µgFE/mg) of plant extract.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
The DPPH scavenging activity of methanolic plant extracts was estimated using DPPH reagent (Mamta et al. 2015). Briefly, 2 mL DPPH (0.135 mM, prepared in methanol) was added to 1 mL of ascorbic acid and plant extracts (25–500 µg/mL). After 30 min of incubation at room temperature decrease in absorbance was observed at 517 nm. The scavenging activity of plant extract was calculated using the formula:
Abs control = absorbance of DPPH and methanol.
Abs sample = absorbance of DPPH and plant extract or ascorbic acid.
Lipid peroxidation inhibition study (Thiobarbituric acid reactive species) assay
Lipid peroxidation inhibitory activity was studied by following the modified thiobarbituric acid reactive species (TBARS) assay to measure lipid peroxide formation using egg yolk homogenates as lipid-rich media (Ohkawa et al. 1979). Lipid peroxidation was induced in 0.1 mL of egg homogenate (10% v/v) by adding 1 mL plant extract/standard (concentration range 0.05–1.0 mg/mL) and 0.05 mL of 75 mM FeSO4. The mixture was incubated for 30 min at 37ºC. Then, 1 mL 10% TCA and 0.8% TBA (prepared in 1.1% SDS) was added and the mixture was vortexed and heated for 1 h at 95 °C. After cooling, the mixture was centrifuged at 3,000 rpm for 10 min. The colour developed was measured at 532 nm using a spectrophotometer. Ascorbic acid was used as standard. TBARS activity was calculated by the following equation:
Abs control = absorbance of assay mixture without plant extract and reference chemical.
Abs sample = absorbance of assay mixture with plant extract and reference chemical.
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) Assay
The ABTS activity was measured using ABTS reagent (Re et al. 1999). Equal volumes of stock solutions of ABTS (7 mM) and potassium persulphate (2.5 mM) were mixed and allowed to react for 12–16 h in the dark at room temperature to generate the free radical. The ABTS solution was then diluted with 60% methanol and the absorbance of the working solution was adjusted to 0.70 ± 0.02 at 734 nm using a spectrophotometer. A series of five concentrations of plant extract (50–1000 µg/mL) was prepared in 1 mL of distilled water. Next, 2 mL of working ABTS solution was added and the absorbance was read at 734 nm. Gallic acid was used as standard. The ABTS radical scavenging activity was calculated by the following equation:
Abs control = absorbance of assay mixture without plant extract and reference chemical.
Abs sample = absorbance of assay mixture with plant extract and reference chemical.
In vitro anthelmintic bioassay
The in vitro anthelmintic study was performed following the method of Eguale and Giday (2009) and Belemlilga et al. (2016) with little modification in the concentrations of plant extracts. Plant extracts and albendazole were dissolved in 100 µL dimethyl sulfoxide and PBS. Live, adult trematode parasites, Paramphistomum sp. were collected from the rumen of the cow slaughtered at different places of Kokrajhar town. Soon after the slaughter, rumens were separated from the main body, cut open, cleaned the dirt materials, and parasites were collected in phosphate-buffered saline (1xPBS, pH 7.4). The parasites were brought to the laboratory and acclimatised for ~ 30 min at 37 ± 1ºC. Next, 10–15 parasites were incubated in a series of extract concentrations (A. scholaris and H. sibthorpioides, 1–20 mg/mL; C. halicacabum, 2–40 mg/mL, and H. japonicum, 0.05–2 mg/mL). After 24 h of treatment, mortality of parasites was observed and the lethal concentration at 50% mortality (LC50) was calculated. Albendazole, a broad-spectrum anthelmintic drug was used as a reference chemical. Control parasites were incubated in PBS alone. For each set of experiments, three replicates were performed.
Cell proliferation and apoptosis study
The antiproliferative and apoptosis-inducing activities of plant extracts was studied using Dalton’s lymphoma (DL) cell line as described in our earlier publication (Swargiary et al. 2021). Cell proliferation was measured by 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (Mosmann 1983; Verma and Prasad 2013). In brief, the cells were treated with different doses (10–200 mg/mL) of plant extracts for 24 h in a 96-well plate. Next, 10 µL of MTT reagent was added and incubated for 4 h at 5% CO2 and 95% air at 37ºC followed by the addition of 100 µL DMSO. The colour developed was measured at 570 nm using a microplate reader. Furthermore, for the apoptosis study, both the control and plant extract treated cells were stained with acridine orange/ethidium bromide (AO/Eb) for 5 min in the dark cold room (Squier and Cohen 2001). The cells were then thoroughly examined for three replicates under a fluorescence microscope and photographed. About 1000 cells were counted, and the percentage of apoptotic nuclei was determined based on differential staining pattern (red/green) of the nucleus.
Statistical analysis
All statistical calculations were conducted in Microsoft Excel. LC50 and IC50 were calculated using OriginPro-8.5 software. The test of significance and analysis of variance study was performed using OriginPro-8.5 at P = 0.05 probability level. Pearson correlation study was performed in SPSS-13.0. All experiments were conducted in triplicate (n = 3) and the results were represented as mean ± standard deviation (SD).
Results
Plant extracts and phytochemical content
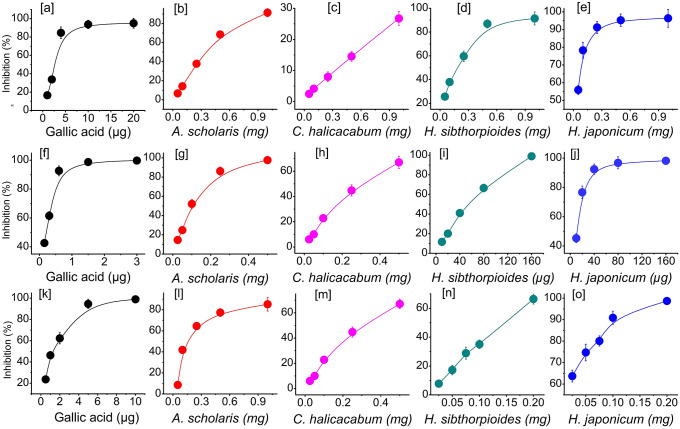
Plants are rich in phytochemicals and contain various primary and secondary metabolites. The medicinal properties of a plant can be attributed to its secondary metabolites—phenolics, flavonoid, etc. The TPC, TFC, FRAP, and total antioxidant properties of the studied plants are shown in Fig. 1. A. scholaris showed the highest TPC (86.37 ± 7.29 µgGAE/mg) followed by H. japonicum, C. halicacabum, and H. sibthorpioides. The values of TFC ranged from 5.84 ± 0.95 to 83.76 ± 4.27 µgQE/mg. In TFC, FRAP, and TAC study, H. japonicum showed the strongest activity followed by H. sibthorpioides. The values were found to be 83.76 ± 4.27 µgQE/mg, 287.58 ± 8.33 µgFE/mg, and 115.65 ± 7.34 µgAAE/mg, respectively, for TFC, FRAP, and TAC in H. japonicum. Values were found to be significantly different compared to the other three plants at P ≤ 0.05 level. A. scholaris and C. halicacabum showed almost similar activities in TFC, TAC, and FRAP activities. The free radical scavenging activities of all four plants are shown in Fig. 2. Antioxidant studies showed a concentration-dependent inhibition of the plants. Similar to TPC and TFC, the strongest free radical scavenging activity was observed in H. japonicum. H. sibthorpioides also showed strong free radical scavenging activity followed by A. scholaris. The IC50 values ranged from 28.45 ± 1.70 µg (H. japonicum) to 4.27 ± 0.39 mg (C. halicacabum) for DPPH scavenging property. The IC50 values of H. sibthorpioides and A. scholaris were found to be 231.34 ± 17.30 and 349.64 ± 14.10 µg. For ABTS assay, H. japonicum showed the strongest activity with IC50 value 9.97 ± 1.77 µg followed by H. sibthorpioides (61.32 ± 2.22 µg), A. scholaris (118.28 ± 6.16 µg), and C. halicacabum (287.79 ± 9.77 µg). All four plants showed strong ABTS radical scavenging activity. Similar to DPPH and ABTS assays, H. japonicum showed the strongest lipid peroxidation inhibition property (IC50, 57.10 ± 3.06 µg) followed by H. sibthorpioides (148.13 ± 1.0 µg), A. scholaris (169.22 ± 6.88 µg), and the weakest in C. halicacabum (IC50 372.79 ± 19.62 µg). The correlation study among the different parameters of antioxidant activities is shown in Table 2. Both TPC and TFC showed significant correlation to FRAP activity (at P = 0.05 level), while others showed positive but insignificant correlation. DPPH, ABTS, and TBARS showed strong correlation to each other. The reference chemical showed the strongest DPPH, ABTS, and TBARS activity with IC50 values of 2.27 ± 0.11 µg, 1.15 ± 0.01 µg, and 24.33 ± 1.13 µg, respectively.
Fig. 1.
Total phenolic (TPC), flavonoid (TFC), total antioxidant (TAC), and ferric reducing (FRAP) capacity of the plants. Values were expressed as mean ± SD, n = 3
Fig. 2.
Antioxidant activity of plants a–e DPPH, f–j ABTS and k–o TBARS activity. Values were expressed as mean ± SD, n = 3
Table 2.
Two-tailed Pearson correlation study of phytochemical content, antioxidant, antiproliferative and anthelmintic activities of the plants
| MTT | APO | ANT | TPC | TFC | TAC | FRAP | DPPH | ABTS | TBARS | |
|---|---|---|---|---|---|---|---|---|---|---|
| MTT | 1 | .992** | .425 | .261 | .349 | .518 | .225 | .755 | .694 | .669 |
| APO | .992** | 1 | .452 | .331 | .377 | .476 | .271 | .784 | .706 | .732 |
| ANT | .425 | .452 | 1 | .923 | .997** | .846 | .969* | .906 | .945 | .853 |
| TPC | .261 | .331 | .923 | 1 | .932 | .576 | .972* | .817 | .806 | .880 |
| TFC | .349 | .377 | .997** | .932 | 1 | .828 | .983* | .869 | .915 | .823 |
| TAC | .518 | .476 | .846 | .576 | .828 | 1 | .718 | .781 | .883 | .581 |
| FRAP | .225 | .271 | .969* | .972* | .983* | .718 | 1 | .809 | .842 | .810 |
| DPPH | .755 | .784 | .906 | .817 | .869 | .781 | .809 | 1 | .982* | .956* |
| ABTS | .694 | .706 | .945 | .806 | .915 | .883 | .842 | .982* | 1 | .894 |
| TBARS | .669 | .732 | .853 | .880 | .823 | .581 | .810 | .956* | .894 | 1 |
**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
APO—apoptosis, ANT—anthelmintic
Antiproliferative and apoptosis study
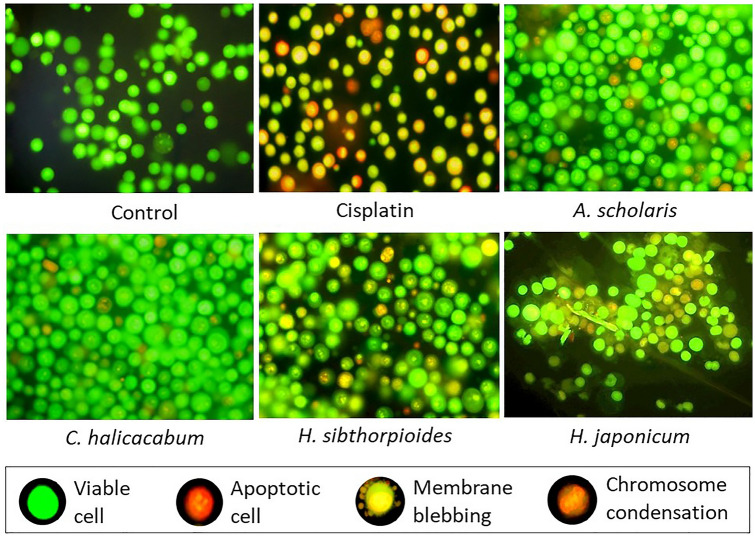
The antiproliferative and apoptosis-inducing properties of the four plants are presented in Table 1. The study revealed a dose-dependent cytotoxicity effect of the plants on DL cells. Of the four plants, H. sibthorpioides showed strongest cytotoxic activity. MTT assay showed that the percentage death of DL cells ranging from 23 ± 20% to 58.67 ± 4.73%, 17.33 ± 1.53 to 43 ± 4.36%, 13 ± 2.64 to 27.67 ± 3.05%, and 3 ± 1 to 15 ± 3.61% for H. sibthorpioides, H. japonicum, A. scholaris, and C. halicacabum, respectively at the concentration range of 25–200 mg/mL after 24 h treatment. Similar results were seen in the apoptosis study indicating the potency of plant extracts to induce apoptosis and cell death. At 200 mg/mL concentration of plant extract, the cell death was found to be 42.33 ± 3.21%, 32.37 ± 2.52%, 22 ± 4%, and 9.0 ± 1.0% for H. sibthorpioides, H. japonicum, A. scholaris, and C. halicacabum, respectively. The percentage cell death of MTT assay and cytotoxicity assays were compared using ANOVA analysis. Pearson’s correlation study showed a positive and significant correlation (P = 0.01 level) between antiproliferative and apoptotic properties of the plants (Table 2). Although insignificant, antiproliferative study showed positive correlation to the antioxidant properties of the plants. Changes in the morphological features with apoptotic characteristics with red/orange nuclei, membrane blebbing, chromatin condensation, and formation of apoptotic bodies were observed in DL cells treated with plant extracts and reference drug, cisplatin (Fig. 3). Control cells showed green nuclei with intact membrane. AO/Eb staining showed higher density of apoptotic cells in H. sibthorpioides treatment followed by H. japonicum, and A. scholaris. C. halicacabum extract showed the lowest apoptosis-inducing property.
Table 1.
Percentage mortality of cells by MTT and Apoptosis assay on exposure to different concentrations of plant extracts
| Conc. (mg) | A. scholaris | C. halicacabum | H. sibthorpioides | H. japonicum | Cisplatin |
|---|---|---|---|---|---|
| MTT | |||||
| 10 | 13 ± 2.65 | 3 ± 1.0 | 23 ± 2.0 | 17.33 ± 1.53 | 16.21 ± 1.77 |
| 25 | 15.67 ± 1.15 | 7 ± 1.0 | 34.67 ± 2.08 | 25.33 ± 1.15 | 24.73 ± 2.2 |
| 50 | 23.33 ± 2.52 | 12 ± 2.64 | 44 ± 3.0 | 29 ± 2.64 | 53.8 ± 4.32 |
| 100 | 25.67 ± 2.08 | 12.67 ± 3.05 | 52.33 ± 4.51 | 40 ± 5.73 | 62.29 ± 6.33 |
| 200 | 27.67 ± 3.05 | 15 ± 3.6 | 58.67 ± 4.72 | 43 ± 4.36 | 81.82 ± 6.04 |
| Apoptosis | |||||
| 10 | 6.67 ± 1.15 | 2.67 ± 1.15 | 14.67 ± 1.53 | 6.67 ± 1.15 | 11.82 ± 1.02 |
| 25 | 13 ± 3.0 | 2.67 ± 0.58 | 25.33 ± 3.51 | 18.67 ± 3.05 | 16.92 ± 2.08 |
| 50 | 18.67 ± 3.05 | 3.67 ± 1.08 | 34.33 ± 2.31 | 21.67 ± 2.08 | 39.44 ± 4.32 |
| 100 | 21 ± 3.0 | 5.67 ± 1.54 | 40.67 ± 3.78 | 29.33 ± 4.16 | 53.72 ± 4.83 |
| 200 | 22 ± 4.0 | 9 ± 1.0 | 42.33 ± 3.21 | 32.67 ± 2.51 | 63.54 ± 4.23 |
Values are represented as mean ± SD, n = 3
Fig. 3.
Apoptotic features of plant extract-treated cells observed under fluorescence microscope after AO/Eb staining. Cisplatin is used as the reference drug. Apoptotic cells are shown in red/orange nucleus, control cells showed green nucleus (Color figure online)
Anthelmintic activity
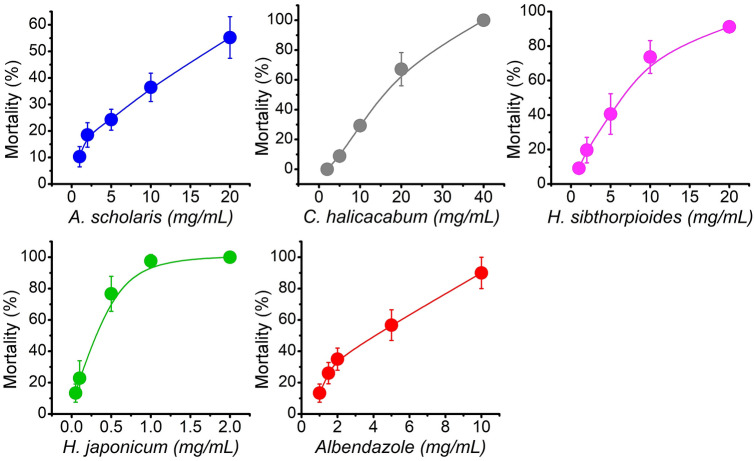
The trematode parasite, Paramphistomum sp. when exposed to different concentrations of plant extract showed dose-dependent mortality after 24 h of treatment. Figure 4 showed the percent mortality of the parasite treated with different concentrations of extracts. H. japonicum showed the strongest parasite mortality followed by H. sibthorpioides, C. halicacabum, and A. scholaris. The plant also showed better activity compared to reference drug, albendazole. At the highest dose of 2 mg/mL, H. japonicum showed almost 100% mortality in helminth parasites. Similarly, at 20 mg/mL plant extract of H. sibthorpioides and A. scholaris, helminth parasites showed almost 100% and 60% mortality. While C. halicacabum attained almost 100% mortality at 40 mg/mL. The LC50 values were found to be 0.21 mg/mL, 5.36 mg/mL, 13.40 mg/mL, and 18.40 mg/mL for H. japonicum, H. sibthorpioides, C. halicacabum, and A. scholaris, respectively. Similarly, the LC50 value of reference drug, albendazole was found to be 3.69 mg/mL. The control, untreated parasite lived up to ~ 73 h. Anthelmintic property of plants showed significant correlation to flavonoid and FRAP activity (Table 2). Similarly, phenolic content and other antioxidant activities also showed good correlation with anthelmintic property of plants.
Fig. 4.
Mortality of helminth parasites at different test concentrations of plant extracts. Values were represented as mean ± SD, n = 10
Discussion
Free radicals are by-products of biological reactions, which produce several health effects. Biomolecules, called antioxidants, can neutralise and scavenge free radicals. Plants have strong antioxidant properties due to their rich secondary metabolites. Polyphenolics and flavonoids are among the most important phytochemicals with rich antioxidant properties. Phytocompounds can provide health benefits in many ways, such as substrates for biochemical reactions, co-factors of enzymatic reactions, enzyme inhibitors or stimulators, scavengers of reactive or toxic chemicals, and many more (Dillard and German 2000). Plants, in addition to primary metabolites, also produce secondary metabolites that help in normal growth, development, and defense system of plants. Among the different types of secondary metabolites, phenolics are the most important because of their promising antioxidant properties (Horwitt 1991). The antioxidant properties of plants may be attributed to their innate ability to synthesize non-enzymatic antioxidants such as ascorbic acid, glutathione as well as secondary metabolites such as phenolic compounds. The present study revealed a considerable amount of phenolics and flavonoid content in all four plants. Many findings suggest that high TPC and TFC can improve biochemical indices of oxidative damage (Serafini et al. 1996; Stein et al. 1999). The continuous generation of free radicals may cause severe complications, if in excess. To minimize such complications, antioxidant intake is always beneficial. In this study, H. japonicum, and H. sibthorpioides showed higher phenolic and flavonoid contents among the four plants. A similar kind of study showed comparable data of phenolics and flavonoid content (Irshad et al. 2012). The methanolic peel extract of Citrus grandis also showed comparable data with our findings (Divya et al. 2016). Like most of the other studies, we also found potent antioxidant properties in all plants. Statistical analysis revealed that the in vitro antioxidant study by TAC, FRAP, DPPH, ABTS, and TBARS assays showed a positive correlation with the TPC and TFC, which reinstates the function of phenolics as potent antioxidant molecules.
Growth and development of an organism depend on the proper regulation of cell division and death. Any deviation from the normal physiology of cell growth, division, and death leads to the development of disease and complications. Unregulated cell division and growth are important characteristics of cancer (Wong 2011). Apoptosis is a cellular mechanism that causes normal cell death, also known as programmed cell death. Cancer cells are known to avoid apoptosis and normal cell death cascade leading to uncontrolled cell division. Because of their cellular importance, apoptosis-inducing drugs are the centre of new anticancer therapy (Elmore 2007). Several studies have been carried out to explore the antiproliferative and apoptosis-inducing properties of several medicinal plants (Rais et al. 2019; Khurshid et al. 2020). Phytochemicals and bioactive compounds isolated from plants were also investigated for anticancer activity in numerous cell lines (Kamaruddin et al. 2019; Erdogan et al. 2020). In vitro study reported the antiproliferative activity of aqueous and ethanolic extracts of H. sibthorpioides in human hepatoma Hep3B cells (Huang et al. 2008). Similarly, alcoholic crude extract of the plant showed potent inhibitory effects on the growth of tumors and immunomodulatory effects in mice (Yu et al. 2007). Recent studies have revealed four phytocompounds viz. propanoic acid, 3-nitro-, methyl ester (C1), 5-hepten-3-one, 5-ethyl-4-methyl-(C2), 1-cyclohexyl-2-methyl-2-propanol (C3), and 2-methyl-5-(1-adamantyl)pentan-2-ol (C4) from the crude extracts of H. sibthorpioides (Swargiary and Daimari 2021). Similarly, UPLC–MS/MS analysis identified catechin, epicatechin, quercetin, and chlorogenic acid as the major antioxidant molecules of H. sibthorpioides (Kumari et al. 2016). In a similar study, Surya Surendren et al. (2011) reported the anticancer activity of leaf extract of A. scholaris. Wang et al. (2017) isolated eight triterpinoids and five sterols from A. scholaris of which ursolic acid, betulinic acid, betulin, and 2β,3β,28-lup-20(29)-ene-triol showed antiproliferative activity against NSCLC, with IC50 of 39.8, 40.1, 240.5, and 172.6 μM, respectively. Five novel terpionoids, hyperjapones A–E reported from H. japonicum and compound A, B, and D were found to possess moderate antitumor activities in vitro (Yang et al. 2016). Similarly, 39 compounds, including nine undescribed compounds have been reported from H. japonicum and 18 compounds showed good inhibitory activities against the HEL cell line, with IC50 values of 3.53–18.7 μM (Zhu et al. 2019). Similarly, strong cytotoxicity property of n-hexane extract of C. halicacabum was reported against MCF-7 breast cancer cells (Mohaddesi et al. 2015). Mathan Kumar et al. (2019) reported the anticancer activity of isolated flavonoids (apigenin, luteolin, and chryseriol) from C. halicacabum. In the present study, H. sibthorpioides and H. japonicum showed better antiproliferative and apoptosis-inducing properties compared to other plants. Both the plants showed high cell mortality (~ 50%) at the highest dose of plant extract. The various phytocompounds present in the plant extract and their synergistic effects may have contributed to the antiproliferative and apoptotic activity. It is reported that the crude extracts of plants show lesser biological activity compared to the isolated compounds (Lowe et al. 2013; Alasmary et al. 2018). Cisplatin, the reference chemical showed a significant difference (at P ≤ 0.05 level) in both antiproliferative and apoptotic-inducing capacity compared to medicinal plants.
Helminth infection is one of the 17 neglected tropical diseases (NTD) of the world (WHO 2010). According to recent studies, there is a high prevalence of many NTDs including helminthiasis and other vector-borne diseases in India (Hotez et al. 2018). Most of the time development of drug resistance by helminth parasites is recognised as the major hurdle in effective controlling of helminthiasis. As an alternative or supplement to the existing system of synthetic medicines, plants are regarded as an effective tool for dealing with helminthiasis. In the present study, we have investigated the anthelmintic property of four medicinal plants consumed by the tribal groups of Bodoland region of Assam. In a similar study, Kumar et al. (2013) established strong anthelmintic property of the bark extract of A. scholaris. Leaves of H. japonicum showed the strongest anthelmintic activity among all plants while A. scholaris showed the weakest activity. H. japonicum is also known to have several pharmacological properties such as anticancer, hepatoprotective, antiviral, and immune-boosting activities (Liu et al. 2014). Several other studies have reported the anthelmintic activity of many plants (Roy and Swargiary 2009; Irshad et al. 2010; Swargiary et al. 2017). Wahyuni et al. (2019) also reported in vivo anthelmintic activity of four plants belonging to Cassia sp. from Indonesia showing the highest activity in Cassia surattensis. Similarly, several species of citrus, C. sinensis, C. medica, and C. reticulata have been reported to possess good anthelmintic property against several helminth parasites (Gainza et al. 2015; Aryal et al. 2017). Studies have revealed a positive relationship between the phenolics and pharmacological properties of the plants (Akkari et al. 2016). The phenolic compounds of plants can interfere with the oxidative phosphorylation pathway of parasites leading to the inhibition of ATP synthesis and induce mortality (Athnasiadou et al. 2001). Studies have also reported that the phenolic compounds can bind to glycoproteins on the cuticle of helminth parasites and causes death (Salhan et al. 2011). The secondary metabolites responsible of high antioxidant property may also have contributed to strong antiproliferative and anthelmintic properties of the plants in the present study. The cytotoxicity property of the plants is directly correlated to the mortality of cells; the higher the cytotoxicity, the higher the cell death. The present study also revealed that cytotoxicity of the plant is correlated to the anthelmintic activity.
Conclusions
The current study supports the traditional usage of Alstonia scholaris, Cardiospermum halicacabum, Hydrocotyle sibthorpioides, and Hypericum japonicum for the treatment of helminthiasis and other diseases. All four plants showed promising antiproliferative and anthelmintic activities. H. sibthorpioides and H. japonicum showed better activity among the four plants. The present study observed a strong correlation between the phenolic, flavonoid, and antioxidant properties with the antiproliferative and anthelmintic activity of the plants. However, whether the phytocompounds responsible for antioxidant activity also contributed to the bioactivity of the plants is not known. Therefore, further phytochemical characterisation and in vivo bioassay need to be carried out to explore the bioactive compounds responsible for the antiproliferative and anthelmintic activities of the plants.
Acknowledgements
AS would like to thank SERB, Govt. of India for providing financial assistance in the form of research project to carry out the present work. The authors would also like to thank the traditional healer and elderly people for providing ethnomedicinal information. The authors also acknowledge Dr. Sanjib Baruah, Department of Botany for helping in the scientific validation of the sample plants. The authors also acknowledge the infrastructural facilities provided by the Department of Zoology, Bodoland University.
Author’s contributions
AS involved in designing the study, statistical calculations, and writing of the manuscript, MKR carried out the antioxidant and anthelmintic study, and AKV conducted the antiproliferative and apoptosis study. All authors read and reviewed the final manuscript.
Funding
The present study was funded by Science and Engineering Research Board, Government of India (File no. EEQ/2017/000071).
Availability of data and material
The authors confirm that the data supporting the findings of the study are available within the manuscript.
Declarations
Conflict of interest
Authors do not have any conflict of interest.
Consent for publication
All the authors gave their consent for publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Akhtar MS, Iqbal Z, Khan MN, et al. Anthelmintic activity of medicinal plants with particular reference to their use in animals in the Indo-Pakistan subcontinent. Small Ruminant Res. 2000;38(2):99–107. doi: 10.1016/S0921-4488(00)00163-2. [DOI] [Google Scholar]
- Akkari H, B’chir F, Hajaji H, et al. Potential anthelmintic effect of Capparis spinosa (Capparidaceae) as related to its polyphenolic content and antioxidant activity. Vet Med. 2016;61:308–316. doi: 10.17221/169/2015-VETMED. [DOI] [Google Scholar]
- Alasmary FAS, Awaad AS, Kamal M, et al. Antitumor activity of extract and isolated compounds from Drechslera rostrata and Eurotium tonophilum. Saudi Pharm J. 2018;26(2):279–285. doi: 10.1016/j.jsps.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal A, Upreti S, Das K. Evaluation of anthelmintic activity of Citrus reticulata: In vitro and its phytochemical investigation. Asian J Pharm Clin Res. 2017;10(5):278–280. doi: 10.22159/ajpcr.2017.v10i5.17406. [DOI] [Google Scholar]
- Athnasiadou S, Kyriazakis I, Jackson F, et al. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo studies. Vet Parasitol. 2001;99:205–219. doi: 10.1016/s0304-4017(01)00467-8. [DOI] [PubMed] [Google Scholar]
- Barukial J, Sarmah JN. Ethnomedicinal plants used by the people of Golaghat district, Assam, India. Int J Med Aromat Plants. 2011;1(3):203–211. [Google Scholar]
- Basu P, Meza E, Bergel M, et al. Estrogenic, antiestrogenic and antiproliferative activities of Euphorbia bicolor (Euphorbiaceae) latex extracts and its phytochemicals. Nutrients. 2020;12(1):59. doi: 10.3390/nu12010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belemlilga MB, Traore A, Ouedraogo S, et al. Anthelmintic activity of Saba senegalensis (A.DC.) Pichon (Apocynaceae) extract against adult worms and eggs of Haemonchus contortus. Asian Pac J Trop Biomed. 2016;6(11):945–949. doi: 10.1016/j.apjtb.2016.07.015. [DOI] [Google Scholar]
- Carlson CJ, Phillips AJ, Dallas TA, et al. What would it take to describe the global diversity of parasites? Proc R Soc B. 2020;287:20201841. doi: 10.1098/rspb.2020.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daimari M, Roy MK, Swargiary A, et al. An ethnobotanical survey of antidiabetic medicinal plants used by the Bodo tribe of Kokrajhar district. Assam Indian J Tradit Know. 2019;18(3):421–429. [Google Scholar]
- Dillard CJ, German JB. Phytochemicals: Nutraceuticals and human health. J Sci Food Agric. 2000;80:1744–1756. doi: 10.1002/1097-0010(20000915)80:12<1744::AID-JSFA725>3.0.CO;2-W. [DOI] [Google Scholar]
- Divya PJ, Jamuna P, Jyothi LA. Antioxidant properties of fresh and processed Citrus aurantium fruit. Cogent Food Agric. 2016;2:1184119. doi: 10.1080/23311932.2016.1184119. [DOI] [Google Scholar]
- Dixon MA, Braae UC, Winskill P, et al. Strategies for tackling Taenia solium taeniosis/cysticercosis: a systematic review and comparison of transmission models, including an assessment of the wider Taeniidae family transmission models. PLOS Negl Trop Dis. 2019;13(4):1–24. doi: 10.1371/journal.pntd.0007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguale T, Giday M. In vitro anthelmintic activity of three medicinal plants against Haemonchus contortus. Int J Green Pharm. 2009;3(1):29–34. doi: 10.4103/0973-8258.49371. [DOI] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan MK, Geçibesler IH, Behcet L. Chemical constituents, antioxidant, anti-proliferative and apoptotic effects of a new endemic Boraginaceae species: Paracaryum bingoelianum. Results Chem. 2020 doi: 10.1016/j.rechem.2020.100032. [DOI] [Google Scholar]
- Fahad FI, Barua N, Islam MS, et al. Investigation of the pharmacological properties of Lepidagathis hyaline Nees through experimental approaches. Life. 2021;11(3):180. doi: 10.3390/life11030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainza YA, Domingues LF, Perez OP, et al. Anthelmintic activity in vitro of Citrus sinensis and Melaleuca quinquenervia essential oil from Cuba on Haemonchus contortus. Ind Crop Prod. 2015;76:647–652. doi: 10.1016/j.rechem.2020.100032. [DOI] [Google Scholar]
- Grigalius I, Petrikaite V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules. 2017;22(12):2169. doi: 10.3390/molecules22122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitt MK. Data supporting supplementation of humans with vitamin E. J Nutr. 1991;121:424–429. doi: 10.1093/jn/121.3.424. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Pecoul B, Rijal S, et al. Eliminating the neglected tropical diseases: translational science and new technologies. PLoS Negl Trop Dis. 2018;10(3):e0003895. doi: 10.1371/journal.pntd.0003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Huang G, Ho Y, et al. Antioxidant and antiproliferative activities of the four Hydrocotyle species from Taiwan. Bot Stud. 2008;49:311–322. [Google Scholar]
- Huda-Faujan N, Noriham A, Norrakiah AS, et al. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr J Biotechnol. 2009;8(3):484–489. [Google Scholar]
- Iloki-Assanga SB, Lewis L, Rivera EG, et al. Effect of maturity and harvest season on antioxidant activity, phenolic compounds and ascorbic acid of Morinda citrifolia L (Noni) grown in Mexico. Afr J Biotechnol. 2013;12(29):4630–4639. [Google Scholar]
- Iloki-Assanga SB, Lewis-Lujan LM, Lara-Espinoza CL, et al. Solvent effects on phytochemical constituent profiles and antioxidant activities, using four different extraction formulations for analysis of Bucida buceras L. and Phoradendron californicum. BMC Res Notes. 2015;8:396. doi: 10.1186/s13104-015-1388-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M, Singh M, Rizvi M. Assessment of anthelmintic activity of Cassia fistula L. Middle East J Sci Res. 2010;5(5):346–349. doi: 10.5455/javar.2019.f338. [DOI] [Google Scholar]
- Irshad M, Zafaryab M, Singh M, et al. Comparative analysis of the antioxidant activity of Cassia fistula extracts. Int J Med Chem. 2012 doi: 10.1155/2012/157125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamaruddin MF, Hossain MZ, Mohamed Alabsi A, et al. The antiproliferative and apoptotic effects of capsaicin on an oral squamous cancer cell line of Asian Origin, ORL-48. Medicina. 2019;55(7):322. doi: 10.3390/medicina55070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid Y, Syed B, Simjee SU, et al. Antiproliferative and apoptotic effects of proteins from black seeds (Nigella sativa) on human breast MCF-7 cancer cell line. BMC Complem Med Ther. 2020;20(5):1–11. doi: 10.1186/s12906-019-2804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khyade MS, Kasote DM, Vaikos NP. Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: a comparative review on traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2014;153(1):1–18. doi: 10.1016/j.jep.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Krishna Murti K, Panchal MA, Lambole V, et al. Pharmacological properties of Cardiospermum halicacabum—a review. Pharmacologyonline. 2010;2:1005–1009. [Google Scholar]
- Kumar HN, Kulkarni BD, Hoskeri JH, et al. Bactericidal, fungicidal and anthelmintic activities of Alstonia scholaris bark extracts. Int J Phytomed. 2013;5:18–25. [Google Scholar]
- Kumari S, Elancheran R, Kotoky J, et al. Rapid screening and identification of phenolic antioxidants in Hydrocotyle sibthorpioides Lam. by UPLC–ESI-MS/MS. Food Chem. 2016;203:521–529. doi: 10.1016/j.foodchem.2016.02.101. [DOI] [PubMed] [Google Scholar]
- Liu L, Liu M, He J. Hypericum japonicum Thunb. ex Murray: phytochemistry, pharmacology, quality control and pharmacokinetics of an important herbal medicine. Molecules. 2014;19(8):10733–10754. doi: 10.3390/molecules190810733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe HI, Toyang NJ, Watson C, et al. In vitro anticancer activity of the crude extract and two dicinnamate isolates from the Jamaican Ball Moss (Tillandsia recurvata L) Am Int J Contemp Res. 2013;3(1):93–96. [PMC free article] [PubMed] [Google Scholar]
- Mamta MS, Mehrotra S, Amitabh, et al. Phytochemical and antimicrobial activities of Himalayan Cordyceps sinensis (Berk.) Sacc. Indian J Exp Biol. 2015;53(1):36–43. [PubMed] [Google Scholar]
- Mathan Kumar S, Juju V, Chamundeeswari D. Investigation of phytoconstituents of Cardiospermum halicacabum and its efficacy as a potential anti-cancer drug candidate. J Drug Deliv Ther. 2019;9(4-s):252–257. doi: 10.22270/jddt.v9i4-s.2592. [DOI] [Google Scholar]
- Mohaddesi B, Dudhrejiya A, Sheth NR. Anticancer screening of various seed extract of Cardiospermum halicacabum on human colorectal, skin and breast cancer cell lines. Arch Breast Cancer. 2015;2(3):91–95. doi: 10.19187/abc.20152391-95. [DOI] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mosthafa MH, Sheweita S, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111. doi: 10.1128/CMR.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mphahlele M, Molefe N, Tsotetsi-Khambule A, et al. Anthelmintic resistance in livestock, helminthiasis, omolade olayinka Okwa. IntechOpen. 2019 doi: 10.5772/intechopen.87124. [DOI] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay of lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Ordonez AAL, Gomez JD, Vattuone MA, et al. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97(3):452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- Panda SK, Das R, Leyssen P, et al. Assessing medicinal plants traditionally used in the Chirang Reserve Forest, Northeast India for antimicrobial activity. J Ethnopharmacol. 2018;225:220–233. doi: 10.1016/j.jep.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Pereira DM, Valentão P, Pereira JA, et al. Phenolics: from chemistry to biology. Molecules. 2009;14(6):2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- Qamar MF, Maqbool A, Ahmad N. Economic losses due to haemonchosis in sheep and goats. Sci Intern. 2011;23(4):321–324. [Google Scholar]
- Rais J, Jafri A, Bano S, et al. Antiproliferative and apoptotic effects of Rheum emodi on human breast adeno carcinoma, MCF-7 Cells, and antimicrobial effectiveness against selected bacterial strains. Phcog Mag. 2019;15(64):237–242. doi: 10.4103/pm.pm_674_18. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26(9–10):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Roy B, Swargiary A. Anthelmintic efficacy of ethanolic shoot extract of Alpinia nigra on tegumental enzymes of Fasciolopsis buski, a giant intestinal parasite. J Parasit Dis. 2009;33(1–2):48–53. doi: 10.1007/s12639-009-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhan M, Kumar B, Tiwari P, et al. Comparative anthelmintic activity of aqueous and ethanolic leaf extracts of Clitoria ternatea. Int J Drug Dev Res. 2011;3(1):62–69. [Google Scholar]
- Sammar M, Abu-Farich B, Rayan I, et al. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: in vitro evaluation of 57 medicinal and edible plant extracts. Oncol Lett. 2019;18:6563–6571. doi: 10.3892/ol.2019.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel V. Initial and bulk extract. In: Satyajit D, Sarker SD, Latif Z, Gray AI, editors. Natural product research. 2. Totowa, New Jersey: Humana Press; 2005. pp. 29–33. [Google Scholar]
- Serafini M, Ghiselli A, Ferro-Luzzi A. In-vivo antioxidant effect of green and black tea in man. Eur J Clin Nutr. 1996;50:28–32. [PubMed] [Google Scholar]
- Singh M, Kaur M, Silakari O. Flavones: An important scaffold for medicinal chemistry. Eur J Med Chem. 2014;84:206–239. doi: 10.1016/j.ejmech.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Squier MKT, Cohen JJ. Standard quantitative assays for apoptosis. Mol Biotechnol. 2001;19:305. doi: 10.1385/MB:19:3:305. [DOI] [PubMed] [Google Scholar]
- Stein JH, Keevil JG, Wiebe DA, et al. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999;100(10):1050–1055. doi: 10.1161/01.cir.100.10.1050. [DOI] [PubMed] [Google Scholar]
- Surya Surendren P, Jayanthi G, Smitha KR. In vitro evaluation of the anticancer effect of methanolic extract of Alstonia scholaris leaves on mammary carcinoma. J Appl Pharm Sci. 2011;2(5):142–149. doi: 10.7324/JAPS.2012.2526. [DOI] [Google Scholar]
- Swargiary A, Daimari A, Daimari M, et al. Phytochemicals, antioxidant and anthelmintic activity of selected traditional wild edible plants of Lower Assam. Indian J Pharmacol. 2016;48(4):418–423. doi: 10.4103/0253-7613.186212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swargiary A, Daimari M. GC–MS analysis of phytocompounds and antihyperglycemic property of Hydrocotyle sibthorpioides Lam. SN Appl Sci. 2021 doi: 10.1007/s42452-020-04101-2. [DOI] [Google Scholar]
- Swargiary A, Daimari M, Roy MK. Survey and documentation of anthelmintic plants used in traditional medicine system of tribal communities of Udalguri district of Assam. India. J Appl Pharm Sci. 2020;10(1):46–54. doi: 10.7324/JAPS.2020.101006. [DOI] [Google Scholar]
- Swargiary A, Nath P, Basumatary B, et al. Phytochemical, antioxidant, and trace element analysis of anthelmintic plants of north-east India. Int J Pharm Pharm Sci. 2017;9(9):228–232. doi: 10.22159/ijpps.2017v9i9.20668. [DOI] [Google Scholar]
- Swargiary A, Roy MK, Daimari M. Survey and documentation of ethnobotanicals used in the traditional medicines system of tribal communities of Chirang district of Assam against helminthiasis. Biomed Pharmacol J. 2019;12(4):1923–1935. doi: 10.13005/bpj/1824. [DOI] [Google Scholar]
- Swargiary A, Roy MK, Daimari M. Survey and documentation of putative anthelmintic plants used in ethnomedicinal systems of tribal communities of Baksa district of Assam. Med Plants. 2019;11(4):368–379. doi: 10.5958/0975-6892.2019.00048.0. [DOI] [Google Scholar]
- Swargiary A, Verma AK, Singh S, et al. Antioxidant and antiproliferative activity of selected medicinal plants of lower Assam, India: an in vitro and in-silico study. Anticancer Agent Med Chem. 2021;21(2):267–277. doi: 10.2174/1871520620666200719000449. [DOI] [PubMed] [Google Scholar]
- Tandon V, Yadav AK, Roy B, et al. Phytochemicals as cure of worm infections in traditional medicine systems. In: Kumar S, et al., editors. Srivastava UC. Narendra Publishing House: Emerging trends in zoology; 2011. pp. 351–378. [Google Scholar]
- Uniting to Combat Neglected Tropical Diseases (2014) Delivering on promises and driving progress. https://unitingtocombatntds.org/wp-content/uploads/2017/11/2nd_report_summary_english.pdf
- Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Verma AK, Prasad SB. Changes in glutathione, oxidative stress and mitochondrial membrane potential in apoptosis involving the anticancer activity of cantharidin isolated from redheaded blister beetles, Epicauta hirticornis. Anticancer Agent Med Chem. 2013;13:1096. doi: 10.2174/18715206113139990131. [DOI] [PubMed] [Google Scholar]
- Wahyuni S, Sunarso S, Prasetiyono BWHE, et al. Exploration of anthelmintic activity of Cassia spp. extracts on gastrointestinal nematodes of sheep. J Adv Vet Anim Res. 2019;6:236–240. doi: 10.5455/javar.2019.f338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM, Tsai SJ, Jhan YL, et al. Anti-proliferative activity of triterpenoids and sterols isolated from Alstonia scholaris against non-small-cell lung carcinoma cells. Molecules. 2017;22(12):2119. doi: 10.3390/molecules22122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2010) Working to overcome the global impact of neglected tropical diseases: First WHO report on Neglected Tropical Diseases. WHO Press, World Health Organization, Switzerland. https://apps.who.int/iris/handle/10665/70503
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(87):1–14. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li Y, Su J, et al. Hyperjapones A-E, terpenoid polymethylated acylphloroglucinols from Hypericum japonicum. Org Lett. 2016;18(8):1876–1879. doi: 10.1021/acs.orglett.6b00650. [DOI] [PubMed] [Google Scholar]
- Yu F, Yu F, McGuire PM, et al. Effects of Hydrocotyle sibthorpioides extract on transplanted tumors and immune function in mice. Phytomedicine. 2007;14(2–3):166–171. doi: 10.1016/j.phymed.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Zhu W, Qiu J, Zeng Y, et al. Cytotoxic phenolic constituents from Hypericum japonicum. Phytochemistry. 2019;164:33–40. doi: 10.1016/j.phytochem.2019.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of the study are available within the manuscript.