Abstract
The purpose of this study was to determine the course of infection in pigeons with Eimeria labbeana. Thirty-five squabs (4–6 weeks old) were brought from the local poultry market and examined for coccidial infection for 7 days to ensure they were coccidia free. A negative control group of five squabs was used, and thirty squabs were infected orally with 2.5 × 104 sporulated E. labbeana oocysts. From day 1–8 post-infection (PI), three squabs were scarified daily to track the endogenous stages in the intestinal tissue. Furthermore, six squabs were preserved to track the patent period and calculate daily oocyst shedding. The parasite stages were differentiated using paraffin-embedded intestinal tissues that were sectioned and stained. On day 5 PI, the infected squabs had greenish watery diarrhea, weakness, rough feathers, and decreased food intake. The pre-patent and patent durations were six and fourteen days PI, respectively. The shedding of oocysts began on day 6 PI and peaked on day 8 PI. In the duodenum and jejunum of the small intestine, histopathological investigation indicated the presence of three schizont stages, macro- and micro-gametes, and oocysts. To the best of our knowledge, this is the first study in Egypt to explore the course of E. labbeana infection in domestic pigeons.
Keywords: Eimeria labbeana, Piegons, Histopathology, Infection, Biology
Introduction
Domestic pigeons (Columba livia domestica) are classified in the order Columbiformes and the family Columbidae. It is raised for meat production, gambling, and, more recently, scientific study as laboratory animals (Radfar et al. 2011; Sood et al. 2017). Domestic pigeons are primarily raised for meat production and racing by small farms and some households in Egypt, as a source of nourishment for the local people (Elseify et al. 2018).
Pigeons are susceptible to a variety of parasitic diseases, including coccidiosis, which is caused by protozoan parasites of the genus Eimeria (Coccidia: Eimeriidae). Coccidiosis in pigeon may occasionally be observed in young squabs under intensive ring conditions, between the ages of 4 and 16 weeks. While the older pigeons act as asymptomatic carriers and remain apparently healthy (Soulsby 1982; Tenter et al. 2002; Ali et al. 2015). Clinical signs of coccidiosis in pigeon include rough feathers, anorexia, greenish watery diarrhea with mucus, dehydration, loss of body weight, and mortality (McDougald 2003; Abdul Latif et al. 2016; Dong et al. 2018). E. columbae, E. columbarum, E. labbeana, and E. labbeana-like are the four species of Eimeria known to infect domestic pigeons (Yang et al. 2016). These four species were previously discovered in Egypt by Gadelhaq and Abdelaty (2019) in the Minia governorate. In many parts of the globe, E. labbeana is the most common species.
Coccidiosis in pigeons is normally asymptomatic, although outbreaks may occur, resulting in significant mortality among nestlings and young birds (Yabsley 2008). As published data on E. labbeana infections in Egyptian pigeons are sparse. Thus, the purpose of this study was to monitor and examine the progression of E. labbeana infection in experimentally infected pigeons.
Materials and methods
Ethical approval
All experiments were done in accordance with ethical standards and procedures authorized by the Animal Experimentation Committee of Beni-Suef University's Faculty of Veterinary Medicine (BSUV- 33/2019).
Source of E. labbeana oocysts
Eimeria oocysts were collected from naturally infected pigeons in Minia province, Egypt, that Gadelhaq and Abdelaty (2019) had previously identified (a mix of E. labbeana, 95% and E. columbarum + E. columbae, 5%). To establish a continuous source of E. labbeana oocysts, sporulated oocysts were propagated in coccidia-free 30 day old squabs.
On the eighth day PI, fecal samples were collected, and then the pigeons were sacrificed and their intestinal contents were collected to recover the purified oocysts. To prepare the inoculums, Eimeria oocysts were collected, concentrated in saturated sodium chloride solution, washed, sporulated in 2% potassium dichromate, and then counted using the McMaster Technique as described previously (Aboelhadid et al. 2019). Purified oocysts obtained in the second time after propagation in five pigeon squabs were primarily sporulated E. labbeana oocysts (more than 95%). Purified oocysts were stored at 4 °C until used in the current study.
Experimental infection
Thirty-five apparently healthy squabs (4–6 weeks old) were obtained from a local poultry market and were examined for 7 days for coccidial infection to confirm they were coccidia-free. Prior to infection, birds were housed in metal cages for one week with ad libitum access to food and water. Anticoccidial-free diets were fed to birds (a 20% protein diet). Thirty squabs were infected experimentally with 2.5 × 104 sporulated eimerian oocysts, with five pigeon squabs serving as a negative control. Thirty squabs infected experimentally were divided into six replicates (5 birds per replicate). Daily scarification of three squabs was performed from day one to day eight PI. The remaining six birds were retained to monitor the oocyst shedding. From day 6 to day 14 PI, clinical signs and oocyst count in bird droppings were recorded daily.
We determined the daily oocyst count per gram feces (OPG). Briefly, 6 g of fresh feces were collected and homogenized in 60 ml of saturated sodium chloride solution. The number of oocysts per gram of feces was determined using a McMaster slide (mean count of six fields (dilution) 100/6). (Soulsby, 1968). Tissue samples were taken from all parts of the intestine (duodenum, jejunum, ileum, colon, cecum, and rectum) to detect the eimerian endogenous stages by histopathological examination. The tissues were processed using a method previously described by Bancroft and Gamble (2008). For histopathology, small pieces of each part of the intestine were collected in 10% buffered formalin. The fixed tissues were washed overnight in running tap water, dehydrated, and infiltrated with paraffin wax. Serial paraffin Sects. (5 µm thicknesses) were obtained, and the sections were deparaffinized in three, consecutive washings in xylol for 5 min, and rehydrated with five, successive washings with alcohol in descending order of 100, 95, 80, 70, and 50% in deionized water. The histological sections were then subjected to conventional Hematoxylin and Eosin (H and E) staining procedures.
Results and discussion
Sporulated oocysts of E. labbeana observed in this study were ovoid or subspherical in shape, lacked an oocyst or sporocyst residual body, and had an average size 15–18.9 µm × 14–17.5 μm) (Fig. 1). Field isolated E. labbeana sporulated oocysts caused clinical signs in squabs on day 5 PI. On the sixth day PI, the infected squabs developed rough feathers, greenish watery diarrhea, an arched back, and a decrease in food intake, resulting in squab weakness. These findings are comparable to those of Stewart (1957), who documented these clinical signs. The pre-patent period began with infection and ended with oocyst shedding in feces on day 6 PI. Oocyst shedding increased gradually until it peaked on day 8 PI, then began to decline beginning on day 9 PI, reaching its lowest count on day 14 PI (Table 1). This finding is consistent with that of Saikia et al. (2017), who observed the highest concentration of oocysts shed in feces after eight days of oral infection.
Fig. 1.
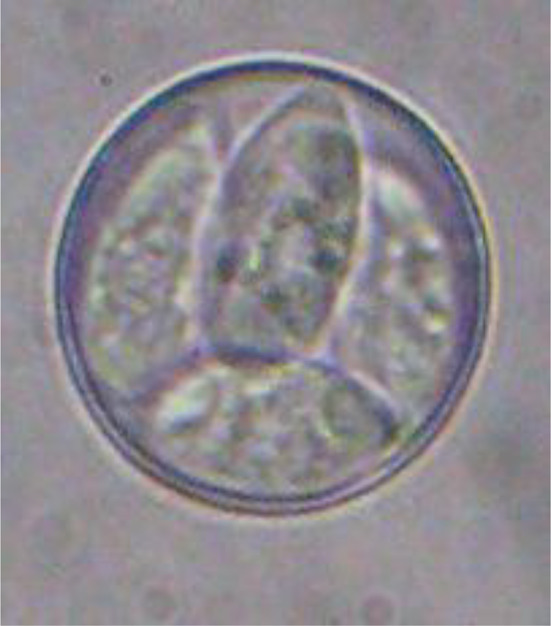
E. labbeana sporulated oocysts (average size 15–18.9 urn × 14–17.5 μm) (X1000)
Table 1.
E. labbeana daily oocyst count per gram feces detected in experimentally infected domestic pigeons
| Days post-infection | Oocyst counts per gram feces (OPG) Mean + SD |
|---|---|
| 6 dpi | 2.541.67 ± 226.84 |
| 7 dpi | 200.458.30 ± 2444.20 |
| 8 dpi | 405.583.30 ± 954.70 |
| 9 dpi | 128.250.00 ± 2459.04 |
| 10 dpi | 34.908.33 ± 212.62 |
| 11 dpi | 32.541.67 ± 162.66 |
| 12 dpi | 20.000 ± 100 |
| 13 dpi | 12.100 ± 200 |
| 14 dpi | 1.008.33 ± 162.66 |
The patent period began with the shedding of oocysts in feces and ended when the oocyst count reached a minimum on day 14 PI (Table 1). This finding corroborated those of Nieschulz (1925), Stewart (1957), Srivastava (1966), Varghese (1977), and Krautwald-Junghanns et al (2009). Bondois (1936), Morini (1950), and Aleksandra and Pilarczyk (2014); however, documented the pre-patent period on the 7-8th day PI. This discrepancy is common due to the study's geographical location and the species of pigeons used. The mean patent period was extended to 14 days PI in our study. This is agreeing with Stewart's (1957) findings that the patent period of E. labbeana in its acute stage was less than two weeks. Additionally, the various parasitic stages were identified in sectioned stained tissues. E. labbeana first demonstrated a parasitic vacuole, followed by multinucleated cells, schizonts, macro and microgametocytes, and finally the formation of oocysts in the duodenal and jejunal intestinal epithelium. On the first and second days PI, parasitic vacuoles and trophozoites were observed. In the duodenal epithelium, the parasite vacuole measured 12.12 ± 1.08 µm × 9.5 ± 0.82 µm (Fig. 2a). On day two PI, the first-generation schizont appeared. It contained up to 32 merozoites with an average length of 5.25 ± 0.74 µm in the duodenum (Fig. 2a, b, c). Second-generation schizonts were observed on day 3 PI, and third-generation schizonts were observed on days 4 and 5 PI in duodenal epithelium (Fig. 3a, b, c).
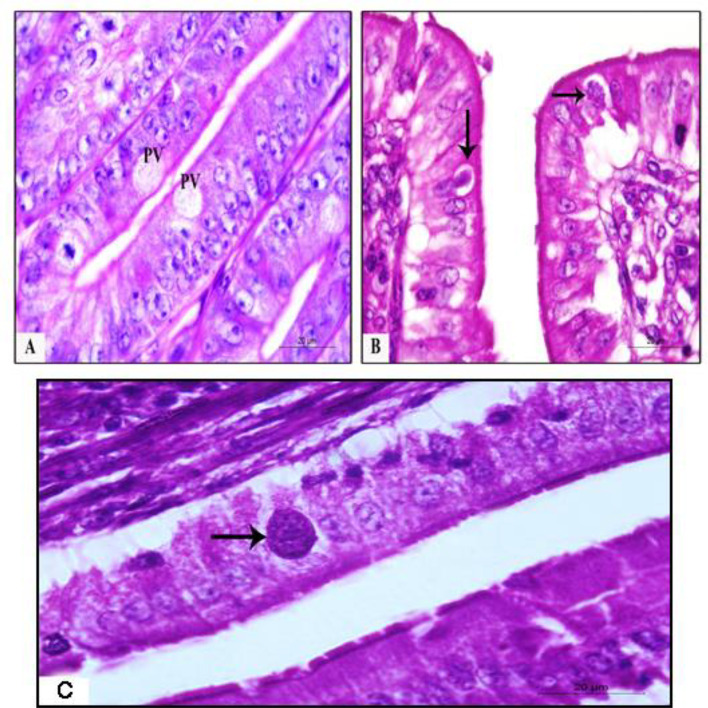
Fig. 2.
Histopathological findings in pigeon’s duodenum experimentally infected with E. labbeana. a. Parasitophorous vacuole (PV) at 1st day PI (H&E stain, X1000). b Trophozoite (vertical arrow) in PV at 2nd day PI. c First-generation schizont (horizontal arrow) at day 2 PI (H&E stain, X1000)
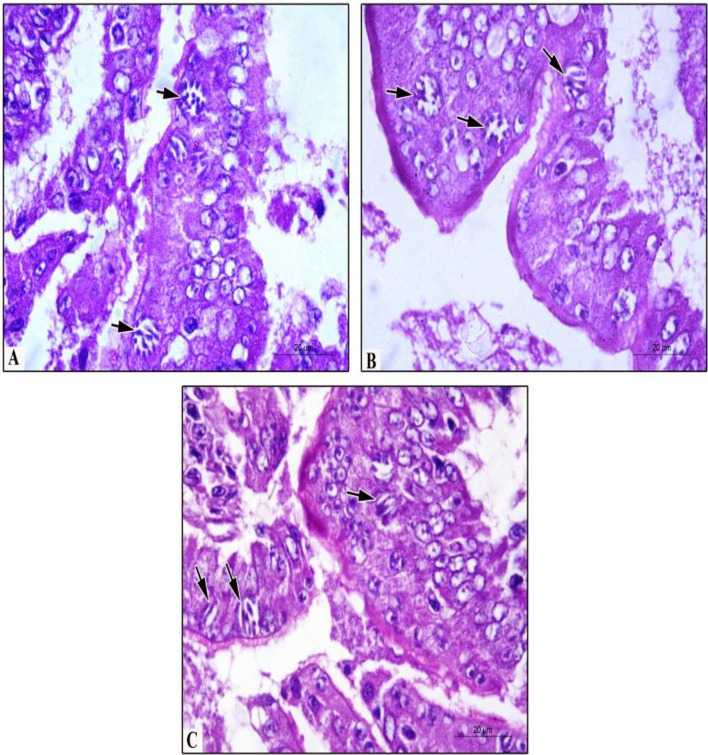
Fig. 3.
Histopathological findings in pigeon’s duodenum experimentally infected with E. labbeana. a First generation schizont (arrow) with numerous merozoites at day 3 PI. b Mature schizonts of second generation (arrows) contain mature merozoites at day 4 PI. c Mature schizonts of third generation (arrows) with few mature merozoites at day 5 PI (H&E stain, ×1000)
The macro and microgametocytes appeared on day 5 PI; the macrogametocytes were ovoid in shape and measured 12.09 ± 0.20 um × 8.5 ± 0.12 µm in diameter, while the microgametocytes were 8.80 ± 0.82 µm in diameter. These findings were similar to those of Nieschulz 1925; Stewart 1957; Srivastava 1966; and Varghese 1977, who reported that it takes nearly six days for E. labbeana to develop in pigeons up to the appearance of the first oocysts in the feces. Varghese's measurements and duration of each stage of different endogenous stages were nearly identical (1975). Merozoites were found in schizonts in numbers ranging from 20 in first-generation schizonts to 10–18 in second-generation schizonts to 4–8 in third-generation schizonts (Fig. 3). These findings are consistent with previous research that found a variation in the number of merozoites (6–30) in first-generation schizonts (Varghese 1977 and Mennemeier 1985). Additionally, the macro and microgametocytes recorded were similar to those previously described (Varghese 1975, 1976). Meanwhile, macro and microgametes, as well as zygote (early oocyst), were found in the intestinal epithelia of the duodenum and jejunum on days 6 and 7 PI (Fig. 4c). In the ileum and large intestine; however, no parasitic stages were found. Stewart (1957) reported that E. labbeana life cycle stages occurred in the duodenum and jejunum, which was consistent with our findings. Varghese (1977) and Mennemeier (19,855); however, found parasitic stages of E. labbeana throughout the small intestine, primarily in the midsection.
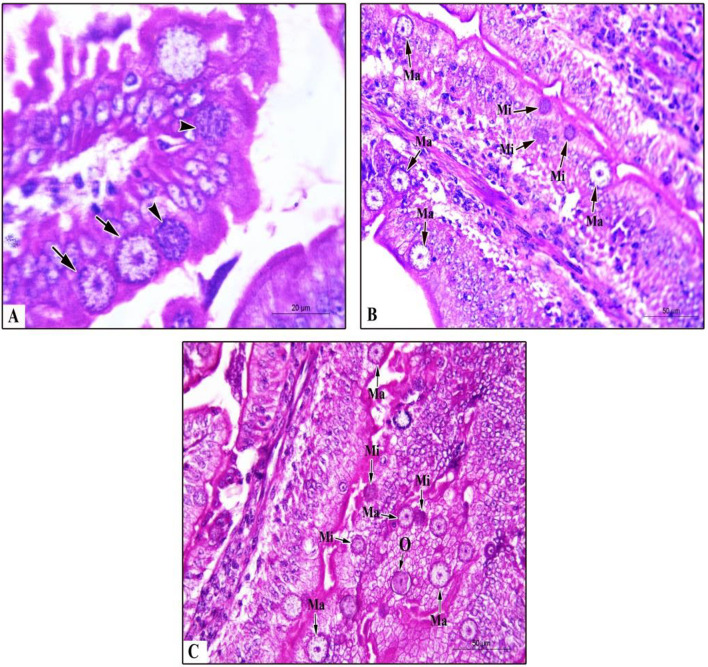
Fig. 4.
Histopathological findings in pigeon’s duodenum and and jejunum experimentally infected with E. labbeana. a. Different macrogametocytes (Ma, arrows) and microgametocyte (Mi, arrowheads) of Eimeria labbeana. in the intestinal epithelia of duodenum at day 5 PI. b Numerous macrogametocytes and microgametocytes in the intestinal epithelia of jejunum at day 6 PI. c Numerous macrogametocytes, microgametocytes, and zygot (early oocyst) in the intestinal epithelia of jejunum at day 7 PI (H&E stain; ×1000, ×400, and ×400, respectively)
Conclusion
The course of E. labbeana in experimentally infected domestic pigeons Egyptian field isolates is described in this study. According to these findings, the average pre-patent period was 6 days, and the average patent period lasted 14 days PI. The peak of oocyst shedding occurred on day 8 PI. Before oocysts shed, there were three generations of schizonts. The current study may contribute to a better understanding of the course of E. labbeana infection in particular, and coccidiosis in pigeons in general. Additional molecular studies are necessary to address this point.
Acknowledgements
The authors would like to thank Dr. Abdelazeem S Abdelbaki, Professor of parasitology at Beni-Suef University's Faculty of Science, for his assistance with this study.
Author's contribution
All authors contributed equally in the study and approved this manuscript.
Funding
None.
Availability of data and material
All the data was analyzed during this study and included in this manuscript.
Declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdul Latif A, Fazal S, Manzoor F, Maqbool A, Asghar S, Wajid I, Ashraf A. A comparative study on prevalence of coccidian parasites in broiler chicken (Gallus gallus domesticus), Japanese Quail (Coturnix coturnix japonica) and Wild Pigeon (Columba livia) Pakistan J Zool. 2016;48:295–297. [Google Scholar]
- Aboelhadid SM, El-Ashram S, Hassan KM, Arafa WM, Darwish AB. Hepato-protective effect of curcumin and silymarin against Eimeria stiedae in experimentally infected rabbits. Livest Sci. 2019;221:33–38. doi: 10.1016/j.livsci.2019.01.011. [DOI] [Google Scholar]
- Aleksandra B, Pilarczyk B. Occurrence of coccidia infection in pigeons in amateur husbandry.Diagnosis and prevention. Annals of Parasitol J. 2014;60:93–97. [PubMed] [Google Scholar]
- Ali JK, Alewi HH, Sawdi HA. Treatment of natural infection in pigeons birds with coccidiosis by using ginger extract in Babylon province. J for Vet Med Sci. 2015;6:15–21. [Google Scholar]
- Bancroft J, Gamble A (2008) Theory and Practice of Histological Techniques, 6th ed. Churchill-Livingstone, Edinburgh, UK London, UK Melbourne, Australia; New York, NY, USA.
- Bondois CM (1936) Contribution a 1T etude de la coc cidiose intestinale du pigeon~voyageur. Trav. Lab. Zool. et Parasitol. Fac. Med. Lille, (thesis).
- Dong H, Zhao Q, Zhu S, Han H, Huang B. Prevalence of Eimeria infection in domestic pigeons (Columba livia domestica) in Shanghai, China. J Vet Med Res. 2018;5:1155–1157. [Google Scholar]
- Elseify MA, Metwally AM, Mahmoud SZ, Abdelrheem EH. Prevalence of coccidia infection among Domestic Pigeons (Columba livia domestica) and Quails (Coturnix Ypsilophora) in QenaProvince. Southern Egypte Kafrelsheikh Vet Med J. 2018;16:1–21. doi: 10.21608/kvmj.2018.110173. [DOI] [Google Scholar]
- Gadelhaq SM, Abdelaty AH. The occurrence and distribution pattern of Eimeria species among domestic pigeons in Minia. Egypt J of Vet Med Res. 2019;26:164–173. doi: 10.21608/jvmr.2019.66098. [DOI] [Google Scholar]
- Krautwald-Junghanns M, Zebisch R, Schmidt V. Relevance and Treatment of Coccidiosis in Domestic Pigeons {Columba livia forma domestica) With Particular Emphasis on Toltrazuril. J Avian Med Surgery. 2009;23:1–5. doi: 10.1647/2007-049R.1. [DOI] [PubMed] [Google Scholar]
- McDougald LR. Coccidiosis. In: Saif YM, editor. Diseases of Poultry. 11. Ames: Iowa State Press; 2003. pp. 974–991. [Google Scholar]
- Mennemeier G (1985) Experimentelle Untersuchungen u¨ber die Pathogenita¨t der Kokzidien der Taube [dissertation]. Hannover, Germany: Tiera¨ rztliche Hochschule Hannover
- Morini EG. La coccidiosis de la paloma. Rev Med Vet Buenos Aires. 1950;32:207–228. [Google Scholar]
- Nieschulz OC. Ueber die Entwicklung des Taubencoccids Eimeri a Tf)ifferi (Labbe 1896) Arch Protistenk. 1925;51:479–494. [Google Scholar]
- Radfar MH, Fathi S, As EN, Dehaghi MM, Seghinsara HR. A survey of parasites of domestic pigeons (Columba liviadomestica) in South Khorasan. Iran Vet Res. 2011;4:18–23. [Google Scholar]
- Saikia M, Bhattacharjee K, Sarmah PC, Deka DK, Kakati P, Konch P. Prevalence of coccidia in domestic pigeon (Columba livia domestica) of Assam. India Int J Chem St. 2017;5:453–457. [Google Scholar]
- Sood NK, Singh H, Kaur S, Kumar A, Singh RS. A note on mixed coccidian and Capillaria infection in pigeons. J Parasit Dis. 2017 doi: 10.1007/s12639-017-0961-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulsby EJ. Helminths, arthropods and protozoa of domesticated animals. 6. London: Bailliere Tindall and Cassel; 1968. pp. 615–682. [Google Scholar]
- Soulsby EJL. Helminths, arthropods and protozoan’s of domesticated animals. 7. London: Bailliere, Tindall; 1982. [Google Scholar]
- Srivastava HK. The morphology of the schizogonous stages of Eimeria labbeana Pinto 1928, a parasite of the common pigeon, Columba livia. Ind J Vet Sei. 1966;36:221–256. [Google Scholar]
- Stewart D (1957) Some aspects of the biology of the piogean coccidium, Eimeria labbeana Pinto, 1928. In: PhD thesis Boston Univ. https://hdl.handle.net/2144/5748
- Tenter AM, Barta JR, Beveridge I, Duszynski DW, Mehlhorn H, Morrison Thompson DA, Conrad PA. The conceptual basis for a new classification of the coccidia. Int J Parasitol. 2002;32:595–616. doi: 10.1016/S0020-7519(02)00021-8. [DOI] [PubMed] [Google Scholar]
- Varghese T. Fine structure of the endogenous stages of Eimeria labbeana. 1 the first generation Merozoites. J Protozool. 1975;22:66–71. doi: 10.1111/j.1550-7408.1975.tb00945.x. [DOI] [PubMed] [Google Scholar]
- Varghese T. The fine structure of the endogenous stages of Eimeria labbeana. 4. Microgametogenesis Z Parasitenkd. 1976;50:227–235. doi: 10.1007/BF02462967. [DOI] [PubMed] [Google Scholar]
- Varghese T. Fine structure of the endogenous stages of Eimeria labbeana. 5. Schizonts and merogony, with special reference to the rhoptrymicroneme system. J Protozool. 1977;24:376–382. doi: 10.1111/j.1550-7408.1977.tb04754.x. [DOI] [PubMed] [Google Scholar]
- Yabsley M J (2008) Eimeria In: Parasitic diseases of wild birds. In: Atkinson CT, Thomas NJ, Hunter DB (ed). Willey-Black well, Ames, pp 162–180.
- Yang R, Brice B, Elloit A, Ryan U. Morphological and molecular characterization of Eimeria labbeana-like (Apicomplexa:Eimeriidae) in a domestic pigeon (Columba livia domestica, Gmelin, 1789) in Australia. Exp Parasitol. 2016;166:124–130. doi: 10.1016/j.exppara.2016.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data was analyzed during this study and included in this manuscript.