Abstract
Toxoplasmosis is one of the widest spread parasitic infections which is caused by Toxoplasma gondii protozoon. Many experimental studies have evaluated the effect of aminoguanidine upon parasitic load and inflammatory process. However, few reports have illustrated the impact of combining aminoguanidine with spiramycin in the treatment of toxoplasmosis. Therefore, our study aimed to explore the possible effects of spiramycin used alone and combined with aminoguanidine against the avirulent (ME49) Toxoplasma gondii strain in experimental toxoplasmosis. Fifty-five Swiss albino mice were included in the study and were divided into five groups: (GI): non-infected control group; (GII): infected untreated control group; (GIII): infected- spiramycin treated group; (GIV): infected-aminoguanidine treated group; (GV): infected and received combination of spiramycin and aminoguanidine. Obtained results exhibited a significant increase in brain cysts numbers in aminoguanidine treated groups compared to infected untreated control groups. Histopathological studies denoted that combination between spiramycin and aminoguanidine improved the pathological features only in liver and heart tissues of the studied groups. Moreover, it was noticed that spiramycin administered alone had no effect on nitric oxide expression, whereas its combination with aminoguanidine had an inhibitory effect on inducible nitric oxide synthase enzyme in brain, liver and heart tissues of different study groups. In conclusion, the combination of spiramycin and aminoguanidine significantly reduced the parasitic burden, yet, it failed to resolve the pathological sequels in brain tissues of Toxoplasma gondii infected mice.
Keywords: Toxoplasmosis, Nitric oxide, Aminoguanidine, Spiramycin
Introduction
Toxoplasma gondii (T. gondii) is an opportunistic coccidian parasite which infects a wide range of hosts, including humans (Tagel et al. 2019). The parasite affects nearly one-third of the world’s population (Okada et al. 2013). Transmission of toxoplasmosis occurs either through ingestion of tissue cysts in undercooked meat or by accidental ingestion of mature oocysts in contaminated food and drink. Organ transplantation, congenital transmission and blood transfusion are other important routes of the infection (Ahmed et al. 1996). T. gondii spreads through the lymphatics and the hepatic portal area to reach different orangs like; the liver, spleen, lung, brain and eyes (Remington and Klein 2010).
Treatment of human toxoplasmosis remains a true challenge. Many therapeutic drugs are used but, they are usually accompanied by numerous side effects. The standard chemical agents for treatment of T.gondii infection are dihydrofolate reductase (DHFR) inhibitors as pyrimethamine (PYR). These drugs are only active against tachyzoites and not bradyzoites. Moreover, they are not effective when used as single agents. So, they are commonly used in combination with sulfa drugs (Rosowsky et al. 1998).
Poor brain penetration of the available drugs is one of the main problems of the treatment of chronic T. gondii infection. The combined administration of sulfadiazine and pyrimethamine has shown efficacy against acute toxoplasmosis (Israelski and Remington 1993), but was unsuccessful against chronic cerebral toxoplasmosis (Faucher et al. 2011). Besides, prolonged use of these drugs may cause hematological and renal toxicity (Crespo et al. 2000).
Spiramycin is a macrolide antibiotic with anti-parasitic properties. It’s produced by Streptomyces ambofaciens and is considered the drug of choice against T. gondii in pregnancy. The drug reduces the risk of congenital toxoplasmosis and vertical transmission (Peyron et al. 2017). Spiramycin combination was evaluated by Valentini et al. (2015) who found that the association Spiramycin/Cotrimoxazole (Sp/C) has significant efficacy in reducing maternal–fetal transmission of toxoplasmosis. Spiramycin, when co-administered with other drugs like metronidazole, (Chew et al. 2012) was shown to be effective in the treatment of chronic toxoplasmosis in a mouse model. The combined therapy of spiramycin and metronidazole led to increased brain uptake of spiramycin and accomplished almost complete eradication of brain cysts.
Aminoguanidine (AG) is a selective inhibitor of inducible nitric oxide synthase (iNOS) enzyme which was first described by Corbett et al. (1992). It’s a bi-functional molecule comprising the guanido group from L-arginine linked to hydrazine. Aminoguanidine has been used in several experimental T. gondii infections to assess the role of nitric oxide in toxoplasmosis. Treatment of T. gondii infected mice with aminoguanidine, prevented early death, hepatic degeneration and small bowel necrosis in these mice despite the high parasitic burden (Khan et al. 1997). However, Hayashi et al. (1996) reported the effect of aminoguanidine in exacerbating ocular inflammation and pathology in mice experimentally infected with T. gondii.
Nitric oxide (NO) is a nitrogenous free radical which is produced from L-arginine amino acid by the enzymatic action of nitric oxide synthase (NOS). There are three different isoforms of NOS; inducible ‘i’NOS, endothelial ‘e’NOS and neuronal ‘n’NOS (Bredt and Snyder1990). Inducible NOS is an immune-inflammatory factor that’s found mainly in macrophages, glial cells and hepatocytes (Bogdan et al. 2000). Nitric oxide has immunoregulatory and anti-parasitic roles that directly influence the control of the parasite (Rayan et al. 2011). In toxoplasmosis, NO triggers the conversion from tachyzoite to bradyzoite forms, in addition to its reported parasiticidal effects (Ibrahim et al. 2009).
Various studies have used (NOS) inhibitors to demonstrate the consequences of nitric oxide production on inflammation (Tahir et al. 2015). However, to the best of our knowledge, nothing is known about the efficacy of combining the traditional drug for toxoplasmosis (spiramycin) with one of the inhibitors of nitric oxide synthesis (aminoguanidine). Therefore, in the present study, we evaluated spiramycin used alone and in combination with aminoguanidine in experimental T. gondii infection.
Materials and methods
T. gondii strain
The avirulent strain of T. gondii (ME49) was provided by the Medical Parasitology Department, Faculty of Medicine, Zagazig University. Strain maintenance was performed by serial intraperitoneal injection of Swiss albino mice every 8 weeks with 0.1 ml of brain suspension of previously infected mice containing approximately, 1 × 102 cysts /ml (El-Sayed and Aly 2014).
Mice and experimental design
Fifty-five laboratory-bred male Swiss albino mice were included in the study. The selected mice were 10- weeks old and each weighing about 20–25 g. All mice were supplied with water and standard pellet food and were placed in well-ventilated cages (El-Fakhry et al. 1998). Mice of different study groups were maintained under controlled conditions of lighting (12 h light/12 h dark cycle) and temperature (25 ± 2 °C). Stool examination was performed to exclude any parasitic infections (Giarcia and Bruckner 1977). Mice were divided into five groups (11 mice each) as follows:
(GI): Non- infected control group.
(GII): Infected untreated control group.
(GIII): Infected- spiramycin treated group. Spiramycin (Rovamycin, Sanofi-aventis, Egypt), was given at a dose of 100 mg/ kg/day (Filice and Pomeroy 1991). It was administrated at a fixed hour daily for 10 days.
(GIV): Infected- aminoguanidine treated group. Aminoguanidine (Sigma-Aldrich, St Louis, MO, USA) was given at a dose of 50 mg/kg/ day orally for 2 weeks (Mahmoud et al. 2015). Both spiramycin and aminoguanidine were administrated 6 weeks post-infection. They were provided in powder form and were administered orally to mice as liquid suspensions via tube feeding.
(GV): Infected and received a combination of spiramycin and aminoguanidine, each drug was given in the same dose and schedule of administration as in (GIII) and (GIV).
Nine weeks post-infection, mice have fasted for overnight with free access to water. Animals were then anaesthetized and sacrificed to perform the intended assessment procedures which included parasitological, histopathological and immunohistochemical measures.
Parasitological evaluation
Quantification of brain cysts
Brains of the sacrificed mice were individually crushed in a mortar. Then, 5 ml of normal saline were added to obtain homogenates of brain emulsion. The total number of cysts per mouse brain was determined by adding 2 drops (20 µl each) of brain homogenate onto microscopic slides and counted under light microscopy using X40 objective. The count was then multiplied by 25 to obtain the number of tissue cysts per 1 ml (1000 µl) of brain suspension (Djurkovic´-Djakovic´et al. 2002). Afterwards, the mean cysts number in each group was calculated.
Histopathological evaluation
Specimens from the brain, liver and heart tissues of different study groups were collected, fixed in 10% formalin, dehydrated in ascending grades of ethyl alcohol and then embedded in paraffin for further processing. Microtomy was performed to obtain serial sections of 5- micron thick. Staining was then conducted using haematoxylin and eosin (H&E) stain (Drury and Wallington 1980). Standard light microscopy was used for histopathological examination. The inflammatory cellular infiltrates were assessed according to Nickel et al. (2001) who graded the typical inflammatory cellular density (cells/mm2) as follow; grade ‘1′(mild) denoted individual inflammatory cells, most of which are separated by distinct intervening spaces (< 100), grade ‘2′ (moderate) which defined confluent sheets of inflammatory cells with no tissue destruction (100 ± 500) and grade’3′ (severe) denoted confluent sheets of inflammatory cells with tissue destruction (> 500).
Immunohistochemical evaluation
Serial sections from the brain, liver and heart tissues were collected, fixed in 10% formalin and processed for paraffin embedding. Expression of iNOS level was evaluated using the biotin–peroxidase-ABC method (Czarnewski et al. 2017). For blocking endogenous peroxidase activity, 3% hydrogen peroxide was used. The sections were incubated with phosphate-buffered saline (PBS) for 5 min and subsequently put in citrate buffered saline (pH 6.0) in a microwave oven for 20 min for antigen retrieval. Tissue sections were incubated with rabbit anti-iNOS antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer’s recommended procedures. After washing, the sections were incubated with biotin-labelled goat anti-rabbit antibody (Dako A/S, Glostrup, Denmark) at a dilution of 1:200. All sections were then incubated with avidin–biotin-peroxidase complex (ABC kit, PK-4000). Visualization was conducted using 3, 5 diaminobenzidine (DAB) substrate (DAKO Corp, Fremont, CA, USA). Marker expression was recorded by a semi-quantitative grading scheme based upon the percentage of the immune-stained area in relation to the total area; negative = 0%, low = 1%–6%, mild = 7%–10%; moderate = 11%–59% and severe ≥ 60% (Goldstein and Bosler 2007).
Statistical methods
Data were analyzed using Statistical Package for the Social Sciences "SPSS version 22 (Armonk 2013). Quantitative data were described using mean, range and standard deviation. ANOVA F-test was performed to calculate the difference between quantitative variables among different study groups (Chan 2003). Abnormally distributed data and comparison between two independent groups were made using the Mann Whitney test (Conover 1999). According to Mantas (2002), Z score or the standard score is the number of standard deviations by which the value of a raw score is above or below the mean value of what is being observed or measured. P value < 0.05 indicates significant results (Leslie et al. 1991).
Ethical considerations
The study was conducted and approved by the Ethical Committee of Zagazig University in accordance with the international regulations and guidelines of animal experiments.
Results
Parasitological results
According to Table 1, there was a high statistically significant difference (P < 0.001) in the mean values of brain cysts numbers in different study groups. While there was a significant reduction in the mean cysts numbers in the brain sections of (GIII), aminoguanidine induced a notable increase in the Toxoplasma cysts in (GIV).
Table 1.
Comparison between mean values of brain cysts numbers in different study groups
| GI | GII | GIII | GIV | GV | F | P value | |
|---|---|---|---|---|---|---|---|
|
Mean Mean ± SD Range |
0 0 0 |
240 240 ± 20 200–280 |
120 120 ± 50 80–140 |
350 350 ± 40 300–380 |
280 280 ± 20 290–320 |
45 | 0.000* |
GI: Non−infected control group, GII: infected untreated control group, (GIII): infected− spiramycin treated group, GIV: infected− aminoguanidine treated group, GV: infected and received combination of spiramycin and aminoguanidine
F: F test (ANOVA)
*P < 0.05 (statistically significant)
Histopathological results
Brain
Brain sections from non- infected control group (GI) showed normal glial tissue (Fig. 1a), while those of infected untreated mice (GII) showed severe inflammatory infiltration along with Toxoplasma cyst (Fig. 1b). In (GIV), after AG treatment, marked increase in brain cysts number was observed (Fig. 1c). The combination between both drugs in (GV), didn’t resolve the degree of inflammatory infiltration (Fig. 1d). After treatment with spiramycin in (GIII), improvement of numerous pathological changes was reported. The drug induced degeneration of existing tissue cysts. In addition, there was a marked reduction in the degree of brain gliosis in spiramycin treated group (GIII) when compared to infected untreated control group (GII) with highly statistically significant difference (P < 0.001) (Table 2).
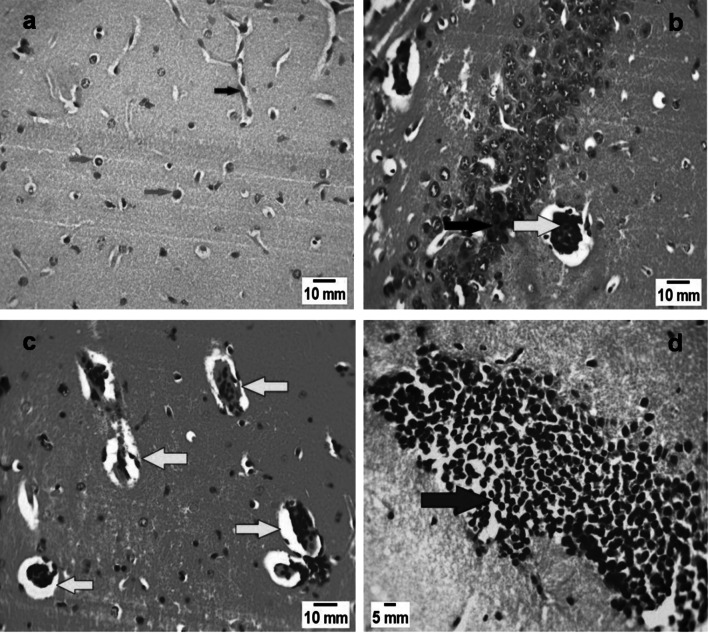
Fig. 1.
Brain sections of mice of different study groups. a Non-infected control group (GI), showing normal architecture: normal blood vessels (black arrow) and normal astrocytes (red arrows) (H&E X 400). b Brain section in infected untreated group (GII) showing marked lymphocytic inflammatory infiltration (black arrow) and Toxoplasma gondii cyst (yellow arrow) (H&E X 400). c Brain section of infected—aminoguanidine treated group (GIV) showing multiple Toxoplasma cysts (yellow arrows) (H&E X 400). d Sections in the brain of the mice group infected and received combination of spiramycin and aminoguanidine (GV) showing marked lymphocytic inflammatory infiltration (red arrow) (H&E stain, X 400)
Table 2.
Comparison between degrees of brain gliosis in (GII) and (GIII)
| Group | N Mean | Rank | Z score | P value | |
|---|---|---|---|---|---|
| Brain gliosis |
GII GIII Total |
11 11 22 |
16.32 6.68 |
− 3.59 | 0.000* |
GII: Infected untreated control group, (GIII): infected −spiramycin treated group
*P < 0.05(statistically significant)
• Mann−Whitney U testLiver
Liver
Liver sections of the infected untreated control group (GII) showed several pathological changes which resulted from the toxic effects of the parasite on the liver tissues. These changes included; lymphocytic inflammatory cellular infiltration and hepatic degeneration (Fig. 2a). Toxoplasma gondii cyst as well as karyorrhexis of nuclei were also demonstrated in (GII) (Fig. 2b). There was a marked reduction in the degree of inflammatory infiltration in both spiramycin (GIII) and aminoguanidine (GIV) treated groups (Fig. 2c, d). The effect of spiramycin in reducing the degree of inflammatory infiltration was demonstrated in Fig. 3 which showed that more than 80% of spiramycin treated mice had only mild degrees of hepatic inflammatory inflammation. The hepatic sections of (GV), showed recovery of most pathological changes. The degrees of hepatic degeneration and inflammatory infiltrations significantly improved (P < 0.05) in (GV) when compared to the infected untreated control group (GII).
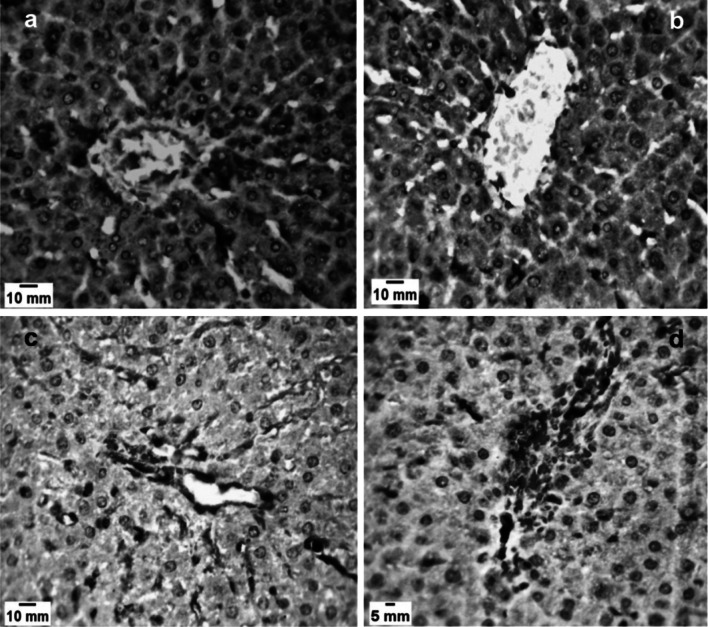
Fig. 2.
Liver sections of Toxoplasma gondii infected mice. a,b Infected untreated group (GII). a Lymphocytic infiltration (black arrow) and hepatic degeneration (red arrows) (H&E X 400). b Toxoplasma gondii cyst (black arrow) and Karyorrhexis in nuclei (red arrows) (H&E X 400). c Liver sections of infected-spiramycin treated group (GIII) showing restoration of normal architecture with mild inflammatory infiltration around areas of hepatic necrosis (black arrows) (H&E X 400). d Liver section of infected- aminoguanidine treated group (GIV) showing showed mild inflammatory infiltration (black arrows) (H&E X 400)
Fig. 3.
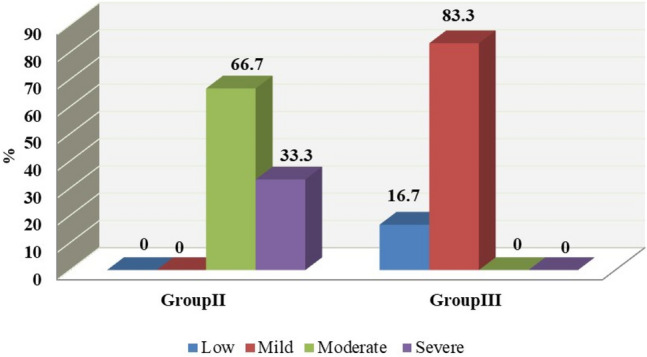
Comparison between inflammatory infiltration changes in liver sections of infected untreated (GII) and infected-spiramycin treated (GIII) groups
Heart
Heart sections of non-infected control mice (GI), showed normal tissue architecture (Fig. 4a). Interfibrillar haemorrhage and inflammatory infiltrations were demonstrated in the cardiac sections of the infected untreated control group (GII) (Fig. 4b). Improvement of vasculature and inflammatory infiltration were observed in (GV) (Fig. 4c). The degree of inflammatory infiltration reduced in both (GIII) and (GIV) after administration of spiramycin and AG respectively.
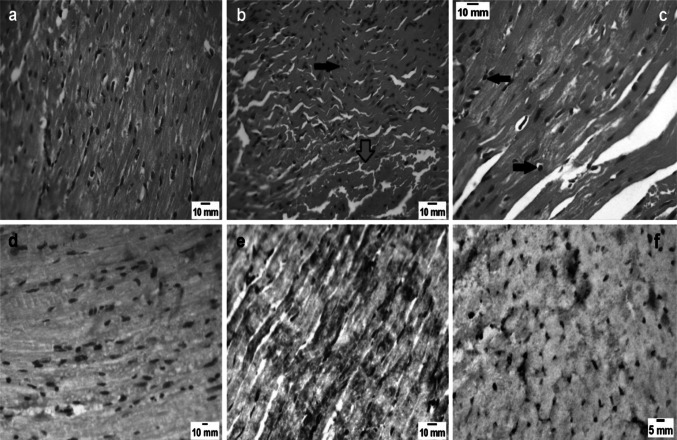
Fig. 4.
Heart sections of mice of different study groups. a Non-infected control group (GI), showing normal cardiac tissue (H&E X400). b Heart sections of infected untreated group (GII) showing inter-fibrillar haemorrhage (red arrows) and inflammatory cellular infiltration (black arrow) (H&E X 400). c Sections in the heart of the mice group infected and received combination of spiramycin and aminoguanidine (GV) showing few inflammatory cellular infiltrates (black arrows) with restoration of normal cardiac tissue architecture (H&E X 400). d Heart sections of non-infected control group (GI) showing negative iNOS expression (IHC X 400). e Heart sections of infected- spiramycin treated group (GIII) showing strong iNOS expression (IHC X 400). f Heart sections of infected- aminoguanidine treated group (GIV) displaying weak iNOS expression (IHC X 400)
Immunohistochemical results
Inducible nitric oxide synthase (iNOS) expression was observed with different intensities in brain, liver and heart tissues of different study groups. Brain sections of infected untreated control mice (GII) showed strong iNOS expression (Fig. 5a). After administration of spiramycin in (GIII), iNOS expression was similar to that of infected untreated control group (GII) (Fig. 5b), with no statistically significant difference (P > 0.05).
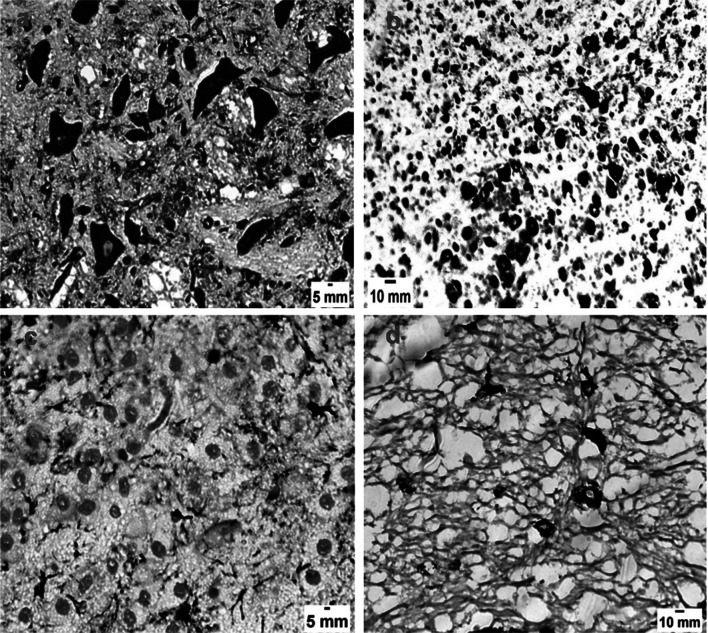
Fig. 5.
Brain sections of Toxoplasma gondii infected mice. a Infected untreated group (GII) showing strong iNOS expression with brown coloration (IHC X 400). b Brain sections of infected- spiramycin treated group (GIII) exhibiting strong iNOS expression (IHC X 400). c Brain sections of infected-aminoguanidine treated group (GIV) showing weak iNOS expression (IHC X 400). d Sections in the brain of the mice group infected and received combination of spiramycin and aminoguanidine (GV) showing low intensity of the iNOS marker (IHC X 400)
However, after AG administration in (GIV), iNOS expression was markedly lowered (Fig. 5c). Weak expression of the marker was also demonstrated in (GV) (Fig. 5d). Liver tissues from both infected untreated group (GII) as well as spiramycin-treated group (GIII), revealed strong iNOS expression (Fig. 6a, b). On the other hand, weak iNOS expression was detected in both (GIV) and (GV) (Fig. 6c, d), with no statistically significant difference (P > 0.05).
Fig. 6.
Liver sections of Toxoplasma gondii infected mice. a Infected untreated group (GII) showing strong iNOS expression with brown coloration (IHC X 400). b Liver sections of infected-spiramycin treated group (GIII) displaying strong iNOS expression (IHC X 400). c Liver sections of infected-aminoguanidine treated group (GIV) showing low intensity of the iNOS marker (IHC X 400). d Sections in the liver of the mice group infected and received combination of spiramycin and aminoguanidine (GV) showing weak iNOS expression (IHC X 400)
Distribution of iNOS enzyme was not observed in heart tissues from the non-infected control mice (GI) (Fig. 4d). Spiramycin treated group (GIII) exhibited strong expression of the marker which was similar to that of the infected untreated group (GII) (Fig. 4e). AG caused marked reduction in the expression of iNOS enzyme in (GIV) (Fig. 4f). According to Table 3, AG induced a significant (P < 0.05) reduction in the distribution of iNOS enzyme when compared to the infected untreated control group (GII).
Table 3.
Distribution of iNOS marker in heart sections of (GII) and (GIV)
| Group | N | Mean Rank | Z score | P value | |
|---|---|---|---|---|---|
| iNOS distribution |
GII GIV Total |
11 11 22 |
15.27 7.73 |
− 2.83 | 0.005* |
GII: Infected untreated control group, GIV: infected− aminoguanidine treated group
iNOS: inducible nitric oxide synthase
*P < 0.05 (statistically significant)
Mann−Whitney U test
Discussion
Successful treatment of toxoplasmosis is hard to achieve. Current therapeutics don’t clear latent infection of the parasite. The most commonly used drugs for the treatment of asymptomatic T. gondii infection are spiramycin, azithromycin and traditional Chinese medicine. However, the low bioavailability of these drugs hinders their full therapeutic potential (Wei et al. 2015). In this respect, the search for an alternative drug or drug combinations with new or complementary mechanisms of action should be pursued (Ma et al. 2013). It’s worth mentioning that a number of compounds have been investigated for effective treatment of latent infection. Ducournau et al. (2020) have developed a new nanoparticle-based vaccine which has been shown to induce a robust immune response against acute, latent and congenital toxoplasmosis in experimentally infected mice.
Over the last decade, a significant progress has been achieved in identifying and developing new compounds for the treatment of toxoplasmosis. More than 20 preclinical drug development projects have been described. These projects have included different classes of bisphosphonates, artemisinin and fluoroquinolone derivatives. Unlike the clinically used medicines, these compounds have been optimized for efficacy against toxoplasmosis during preclinical development (Alday and Doggett 2017).
AG has antioxidant characters as it protects against the harmful effects of oxygen-free radicals. Yet, the exact mechanism underlying this remains unclear (Couderot- Masuyer et al. 1999). Inhibitors of nitric oxide synthesis have been used in experimental filariasis (Rajan et al. 1996), toxocariasis (Espinoza et al. 2002) and trichinellosis (Kołodziej-Sobocin´ska et al. 2006).
The exact mechanism of action of spiramycin in toxoplasmosis is not well clarified. However, the drug could act through inhibition of protein synthesis and subsequent cell growth (McCarthy et al. 2014) or modulation of the inflammatory cascade (Tamaoki et al. 2004). Despite the reported anti-inflammatory effect of spiramycin during T. gondii infection (Franco et al. 2011), there’s a lack of reports which described the efficacy of the drug on nitric oxide production. Accordingly, in the present study, we aimed to evaluate spiramycin used alone and in combination with aminoguanidine on nitric oxide expression in mice experimentally infected with T. gondii.
The current work demonstrated highly significant (P < 0.001) difference in the mean number of Toxoplasma gondii cysts in brain sections of different study groups (Table 1). In (GIII), spiramycin induced a remarkable reduction in brain cysts number when compared to the infected untreated control group (GII). Also, Farag et al. (2019) denoted that spiramycin decreased cyst burden in murine toxoplasmosis. However, in (GIV) after intake of aminoguanidine, the mean number of brain cysts significantly (P < 0.001) increased. These observations come in accordance with those of Kang et al. (2004) who found that AG induced a marked increase in the number of brain cysts in T. gondii-infected mice in both acute and chronic infections. After combining aminoguanidine and spiramycin in (GV), a notable reduction in the mean cysts number was reported when compared to aminoguanidine treated group (GIV) (Table 1). Combination therapy for toxoplasmosis was elucidated in Etewa et al. (2017) who denoted that combination of spiramycin and methotrexate increased the number of brain cysts in mice experimentally infected with Toxoplasma gondii. Also, FarahatAllam et al. (2020) have reported that the spiramycin-metronidazole drug combinations gave the best results in acute experimental toxoplasmosis. Not only did the used drugs succeed to prolong the survival time of mice, but also they reduced the parasitic load in the brain and liver sections of the infected animals.
Histopathological changes were evaluated in this study to detect the pathological alterations associated with Toxoplasma gondii infection. Brain tissues of non-infected control mice (GI), showed normal tissue architecture (Fig. 1a). While Toxoplasma gondii cyst along with severe inflammatory infiltration were demonstrated in brain sections of the infected untreated group (GII) (Fig. 1b). Atmaca et al. (2014) showed that T. gondii infection causes an intense inflammatory reaction in the brain tissues with infiltration of mononuclear cells in the perivascular and meningeal regions. A marked increase in the number of brain cysts was observed in (GIV), after aminoguanidine administration (Fig. 1c), in addition to a notable increase in the degree of inflammatory infiltration. Conversely, Scharton-Kersten et al. (1997) reported that iNOS deficient mice survived acute toxoplasmosis and controlled the growth of the parasite at the site of inoculation. Improvement of pathological changes was noticed in (GIII), after spiramycin administration. The study showed that the drug produced a significant (P < 0.001) reduction in the degree of brain gliosis in (GIII) when compared to the infected untreated group (GII) (Table 2). Etewa et al. (2018) also demonstrated that spiramycin loaded nanoparticles caused a marked reduction in cyst numbers with an improvement of pathological pictures of brain tissues in both acute and chronic stages of Toxoplasma gondii infection.
In this study, liver sections of the infected untreated group (GII) exhibited different pathological changes like; hepatic degeneration and Karyorrhexis in nuclei (Fig. 2a, b). Hepatic vacuolar degeneration, portal fibrosis and necrosis were also recorded by Silva et al. (2013). Unlike brain tissues, aminoguanidine generated an improvement of inflammatory infiltration (Fig. 2d) and other pathological features in the liver sections of (GIV). Moreover, Khan et al. (1997) reported the protective effect of AG in preventing hepatic degeneration in T.gondii infected mice. Our study demonstrated improvement of the pathological features in the liver sections of (GV). Combination between aminoguanidine and spiramycin resulted in a significant (P < 0.05) improvement of hepatic degeneration and inflammatory infiltrations in (GV) when compared to the infected untreated control group (GII).
In the present study, Toxoplasma gondii infection induced inter-fibrillar haemorrhage in the heart sections of the infected untreated group (GII) (Fig. 4b). Also, Pereira et al. (2017) demonstrated inter-fibrillar oedema, pericarditis and endocarditis in cardiac sections of mice experimentally infected with toxoplasmosis. Our study denoted the beneficial effects of combining spiramycin and aminoguanidine in resolving the pathological changes in the heart sections of (GV) (Fig. 4c).
In the current study, immunohistochemical labelling of inducible nitric oxide synthase (iNOS), has been demonstrated in brain, liver and heart sections of different study groups. Strong expression of the enzyme was observed in the brain and liver sections of both infected untreated (GII) and spiramycin treated (GIII) groups (Figs. 5a, b, 6a, b respectively). Dincel and Atmaca (2015) observed that iNOS expression in mice infected with T. gondii at 10 and 30 days post-infection was higher than its expression at 60 days post-infection. On the other hand, low intensity of the marker was noticed in both brain and liver sections of (GIV) and (GV) (Figs. 5c, d, 6c, d respectively). The effect of combining both spiramycin and aminoguanidine in (GV) had a suppressive impact on iNOS expression. There was no statistically significant difference (P > 0.05) in the marker expression in the liver sections of both (GIV) and (GV). According to Table 3, there was a significant (P < 0.05) reduction in iNOS expression in heart sections of aminoguanidine treated group (GIV) compared to the infected untreated group (GII). The suppressive effect of aminoguanidine on iNOS expression has been demonstrated in different tissues of various parasites; in the eyes of T. gondii infected mice (Hayashi et al. 1996); muscle tissues of Trichinella spiralis infected mice (Zeromski et al. 2005); brain tissues of Toxocara canis infected mice (Nassef et al. 2014) and liver tissues of mice experimentally infected with Leishmania major (Mahmoud et al. 2016).
Conclusions
To conclude, our study evaluated for the first time a novel combination between spiramycin, as anti-parasitic, and aminoguanidine, as an anti-inflammatory, for the treatment of toxoplasmosis. The drug combination succeeded to reduce the number of Toxoplasma gondii cysts in brain tissues. While aminoguanidine alone failed to eliminate the parasite. Spiramycin alone had no effect on inducible nitric oxide synthase expression, whereas combining both drugs had an inhibitory impact on the enzyme. Moreover, it improved both liver and heart tissue pathology of the infected animals.
Acknowledgements
We would like to thank Dr Heba O. Abdelal, Research associate and Data innovation Coordinator at LIS cross- national data centre in Luxembourg, for her assistance in the statistical analysis.
Author contributions
Marwa Omar conceptualized the idea. Study design and material preparation were performed by Marwa Omar, Beessa E. Abaza, Esraa Mousa, Shereen M. Ibrahim and Tahani I. Farag. All authors participated in the experimental work. Hayam E. Rashed evaluated the pathological and immunohistochemical results. The first draft of the manuscript was written by Marwa Omar and all authors commented of previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data and materials used to support the findings of this study are included within this article.
Declarations
Conflict of interest
The authors report no conflicts of interest regarding the publication of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed JH, Safar EH, Omar SH, Khattab HM, el-Kholy HS, Toxoplasma antibodies in clinically suspected cases of toxoplasmosis. J Egypt Soc Parasitol. 1996;26:653–659. [PubMed] [Google Scholar]
- Alday PH, Doggett JS. Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des Devel Ther. 2017;11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armonk N (2013) IBM SPSS Statistic for windows version 22.0. 2013
- Atmaca HT, Kul O, Karakuş E, Terzi OS, Canpolat S, Anteplioğlu T. Astrocytes, microglia/macrophages, and neurons expressing Toll-like receptor 11 contribute to innate immunity against encephalitic Toxoplasma gondii infection. Neuroscience. 2014;269:184–191. doi: 10.1016/j.neuroscience.2014.03.049. [DOI] [PubMed] [Google Scholar]
- Bogdan C, Rollinghoff M, Diefenbach A. The role of nitric oxide in innate immunity. Immunol Rev. 2000;173:17–26. doi: 10.1034/j.1600-065X.2000.917307.x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YH. Biostatistics 102: quantitative data—parametric & non-parametric tests. Singap Med J. 2003;44(8):391–396. [PubMed] [Google Scholar]
- Chew WK, Segarra I, Ambu S, Mak JW. Significant reduction of brain cysts caused by Toxoplasma gondii after treatment with spiramycin coadministered with metronidazole in a mouse model of chronic toxoplasmosis. Antimicrob Agents Chemother. 2012;56(4):1762–1768. doi: 10.1128/AAC.05183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover WJ. Practical Nonparametric Statistics. 3. New York: John Wiley & Sons Inc; 1999. pp. 428–433. [Google Scholar]
- Corbett JA, Tilton RG, Chang K, Hasan KS, Ido Y, Wang JL, Sweetland MA, Lancaster JR, Jr, Williamson JR, McDaniel ML. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Courderot-Masuyer C, Dalloz F, Rochette MV, L, Antioxidant properties of aminoguanidine. Fundam Clin Pharmacol. 1999;13:535–540. doi: 10.1111/j.1472-8206.1999.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Crespo M, Quereda C, Pascual J, Rivera M, Clemente L, Cano T. Patterns of sulfadiazine acute nephrotoxicity. Clin Nephrol. 2000;54(1):68–72. [PubMed] [Google Scholar]
- Czarnewski P, Araujo ECB, Oliveira MC, Mineo TWP, Silva NM. Recombinant TgHSP70 Immunization Protects against Toxoplasma gondii Brain Cyst Formation by Enhancing Inducible Nitric Oxide Expression. Front Cell Infect Microbiol. 2017;7:142. doi: 10.3389/fcimb.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dincel GC, Atmaca HT. Nitric oxide production increases during Toxoplasma gondii encephalitis in mice. Exp Parasitol. 2015;156:104–112. doi: 10.1016/j.exppara.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chemother. 2002;50(6):981–987. doi: 10.1093/jac/dkf251. [DOI] [PubMed] [Google Scholar]
- Drury RAB, Wallington EA. Carleton’s histological technique. 5. Oxford, New York, Toronto: Oxford University Press; 1980. [Google Scholar]
- Ducournau C, Moiré N, Carpentier R, Cantin P, Herkt C, Lantier I, Betbeder D, Dimier-Poisson I. Effective nanoparticle-based nasal vaccine against latent and congenital toxoplasmosis in sheep. Front Immunol. 2020;11:2183. doi: 10.3389/fimmu.2020.02183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fakhry Y, Achbarou A, Desportes I, Mazier D. Encephalitozoon intestinalis: humoral responses in interferon-c receptor knockout mice infected with a microsporidium pathogenic in AIDS patients. Exp Parasitol. 1998;89:113–121. doi: 10.1006/expr.1998.4267. [DOI] [PubMed] [Google Scholar]
- El-Sayed NM, Aly EM. Toxoplasma gondii infection can induce retinal DNA damage: an experimental study. Int J Ophthalmol. 2014;7(3):431–436. doi: 10.3980/j.issn.2222-3959.2014.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinoza EY, P´erez-Arellano JL, Carranza C, Coll´ıa F, Muro A, In vivo inhibition of inducible nitric oxide synthase decreases lung injury induced by Toxocara canis in experimentally infected rats. Parasite Immunol. 2002;24(11–12):511–520. doi: 10.1046/j.1365-3024.2002.00598.x. [DOI] [PubMed] [Google Scholar]
- Etewa SE, Abo El-Maaty DA, El-Azeem A, Mai E, El-Shafey MA, Sarhan MH, Saad E. In vivo assessment of the effects of methotrexate on latent toxoplasmosis. J Egypt Soc Parasitol. 2017;47(3):589–598. [Google Scholar]
- Etewa SE, El-Maaty DAA, Hamza RS, Metwaly AS, Sarhan MH, Abdel-Rahman SA, El-Shafey MA. Assessment of spiramycin-loaded chitosan nanoparticles treatment on acute and chronic toxoplasmosis in mice. J Parasit Dis. 2018;42(1):102–113. doi: 10.1007/s12639-017-0973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag TI, Salama MA, Yahia SH, Elfeqy RA. Therapeutic efficacy of Thymus vulgaris and Myristica fragrance houtt (Nutmeg) ethanolic extract against toxoplasmosis in murine model. J Egypt Soc Parasitol. 2019;49(1):73–79. doi: 10.21608/jesp.2019.68288. [DOI] [Google Scholar]
- FarahatAllam A, Shehab AY, Fawzy HMNM, Farag HF, Elsayed Y, Abd El-Latif NF. Effect of nitazoxanide and spiramycin metronidazole combination in acute experimental toxoplasmosis. Heliyon. 2020;6(4):e03661. doi: 10.1016/j.heliyon.2020.e03661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucher B, Moreau J, Zaegel O, Frank J, Piarroux P. Failure of conventional treatment with pyrimethamine and sulfadiazine for secondary prophylaxis of cerebral toxoplasmosis in a patient with AIDS. J Antimicrob Chemother. 2011;66:1654–1656. doi: 10.1093/jac/dkr147. [DOI] [PubMed] [Google Scholar]
- Filice GA, Pomeroy C. Effect of clindamycin on pneumonia from reactivation of toxoplasma gondii infection in mice. Antimicrob Agents Chemother. 1991;35:780–782. doi: 10.1128/AAC.35.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco PS, Gomes AO, Barbosa BF, Angeloni MB, Silva NM, Teixeira-Carvalho A, Martins-Filho OA, Silva DAO, Mineo JR, Ferro EAV. Azithromycin and spiramycin induce anti-inflammatory response in human trophoblastic (BeWo) cells infected by Toxoplasma gondii but are able to control infection. Placenta. 2011;32(11):838–844. doi: 10.1016/j.placenta.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Giarcia LS, Bruckner DA. Macroscopic and microscopic examination of fecal specimens. In: Giboda MN, Vokurkova P, Kopacek O, editors. Diagnostic medical parasitology. 3. Washington: ASM Press; 1977. pp. 608–649. [Google Scholar]
- Goldstein NS, Bosler D. An approach to interpreting immunohistochemical stains of adenocarcinoma in small needle core biopsy specimens: the impact of limited specimen size. Am J Clin Pathol. 2007;2:273–281. doi: 10.1309/ATHVF5R4CQUKB7LX. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Bannai H, Xuan X, Nishikawa Y. Toxoplasma gondii cyclophilin 18-mediated production of nitric oxide induces bradyzoite conversion in a CCR5-dependent manner. Infect Immun. 2009;77(9):3686–3695. doi: 10.1128/IAI.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelski DM, Remington JS. Toxoplasma gondii is an intracellular protozoan parasite: toxoplasmosis in the non-AIDS immunocompromised host. Curr Clin Top Infect. 1993;13:322–356. [PubMed] [Google Scholar]
- Hayashi S, Chan CC, Gazzinelli RT, Pham NT, Cheung MK, Roberge FG. Protective role of nitric oxide in ocular toxoplasmosis. Br J Ophthalmol. 1996;80(7):644–648. doi: 10.1136/bjo.80.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KM, Lee GS, Lee JH, Choi IW, Shin DW, Lee YH (2004) Effects of iNOS inhibitor on IFN-γ production and apoptosis of splenocytes in genetically different strains of mice infected with Toxoplasma gondii. Korean J Parasitol 42(4): 175- –183 [DOI] [PMC free article] [PubMed]
- Khan IA, Schwartzman JD, Matsuura T. Dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proceed Nat Acad Sci USA. 1997;25:13955–13960. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołodziej-Sobocińska M, Dziemian E, Machnicka-Rowińska B. Inhibition of nitric oxide production by aminoguanidine influences the number of Trichinella spiralis parasites in infected "low responders" (C57BL/6) and "high responders" (BALB/c) mice. Parasitol Res. 2006;99(2):194–196. doi: 10.1007/s00436-006-0144-9. [DOI] [PubMed] [Google Scholar]
- Leslie E, Geoffrey J, James M. Statistical analysis. In: Kirkpatrick LA, Feeney BC, editors. Interpretation and uses of medical statistics. 4. Oxford: Oxford Scientific Publications; 1991. pp. 411–416. [Google Scholar]
- Ma CI, Diraviyam K, Maier ME, Sept D, Sibley LD. Synthetic chondramide A analogues stabilize filamentous actin and block invasion by Toxoplasma gondii. J Nat Prod. 2013;76:1565–1572. doi: 10.1021/np400196w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AE, Attia RA, Eldeek HE, Farrag HMM, Makboul R. Polymerase chain reaction detection and inducible nitric-oxide synthase expression of Leishmania major in mice inoculated by two different routes. Trop Parasitol. 2016;6(1):42–50. doi: 10.4103/2229-5070.175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud MF, Zakaria S, Fahmy A. Can Chronic Nitric Oxide Inhibition Improve Liver and Renal Dysfunction in Bile Duct Ligated Rats? Adv Pharmacol Sci. 2015;2015:298792. doi: 10.1155/2015/298792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantas J. Statistical methods. Stud Health Technol Inform. 2002;65:136–147. [PubMed] [Google Scholar]
- McCarthy JS, Wortmann GW, Kirchhoff LV (2014) Drugs for protozoal infections other than malaria. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th edn, by John E. Bennett, Raphael, Dolin, Martin, J. Blaser, Amesterdam, the Netherlanda: Elservier
- Nassef NA, El-Kersh WM, El-Nahas NS, El-Din SAS, Oshiba SF, Nosseir MM. Parasitological, histopathological, and immunohistochemical assessment of nitric oxide synthase inhibitor: aminoguanidine versus albendazole in the treatment of experimental murine toxocariasis. Menoufia Med J. 2014;27(1):103. doi: 10.4103/1110-2098.132778. [DOI] [Google Scholar]
- Nickel JC, True LD, Krieger JN, Berger RE, Boag AH, Young ID. Consensus development of a histopathological classification system for chronic prostatic inflammation. BJU Int. 2001;87(9):797–805. doi: 10.1046/j.1464-410x.2001.02193.x. [DOI] [PubMed] [Google Scholar]
- Okada T, Marmansari D, Li ZM, Adilbish A, Canko S, Ueno A, Shono H, Furuoka H, Igarashi M. A novel dense granule protein, GRA22, is involved in regulating parasite egress in Toxoplasma gondii. Mol Biochem Parasit. 2013;189(1–2):5–13. doi: 10.1016/j.molbiopara.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Pereira AV, Góis MB, Lera KRJL, Falkowski-Temporini GJ, Massini PF, Drozino RN, Pavanelli WR. Histopathological lesions in encephalon and heart of mice infected with Toxoplasma gondii increase after Lycopodium clavatum 200dH treatment. Patholo Res Pract. 2017;213(1):50–57. doi: 10.1016/j.prp.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Peyron F, Mc Leod R, Ajzenberg D, Contopoulos- Ioannidis D, Kieffer F, Mandelbrot L, Sibley LD, Pelloux H, Villena I, Wallon M, Montoya GJ. Congenital toxoplasmosis in France and the United States: one parasite, two diverging approaches. PLoS Negl Trop Dis. 2017;11(2):e0005222. doi: 10.1371/journal.pntd.0005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan TV, Porte P, Keefer YJA, L, Shultz, LD, Role of nitric oxide in host defense against an extracellular, metazoan parasite. Brugia malayi Infect Immun. 1996;64(8):3351–3353. doi: 10.1128/iai.64.8.3351-3353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayan HZ, Wagih HM, Atwa MM. Efficacy of Black Seed Oil from Nigella sativa against Murine Infection with Cysts of Me49 Strain of Toxoplasma gondii. PUJ. 2011;4(2):165–176. [Google Scholar]
- Remington JS, Klein JO. Infectious diseases of the fetus and newborn infant. Philadelphia, PA: Saunders; 2010. pp. 918–1041. [Google Scholar]
- Rosowsky A, Papoulis AT, Queener SF. 2,4-Diamino-67-dihydro-5H-cyclopenta[d]pyrimidine analogues of trimethoprim as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1998;41(6):913–918. doi: 10.1021/jm970614n. [DOI] [PubMed] [Google Scholar]
- Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185(7):1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AF, Oliveira FCR, Leite JS, Mello MFV, Brandão FZ, Leite RIJCK, FrazãoTeixeira E, Lilenbaum W, Fonseca ABM, Ferreira AMR. Immunohistochemical identification of Toxoplasma gondii in tissues from Modified Agglutination Test positive sheep. Vet Parasitol. 2013;191(4):347–352. doi: 10.1016/j.vetpar.2012.09.022. [DOI] [PubMed] [Google Scholar]
- Tagel M, Lassen B, Viltrop A, Jokelainen P. Large-scale epidemiological study on Toxoplasma gondii seroprevalence and risk factors in sheep in Estonia: Age, farm location, and breed associated with seropositivity. Vector Borne Zoonotic Dis. 2019;19:421–429. doi: 10.1089/vbz.2018.2343. [DOI] [PubMed] [Google Scholar]
- Tahir S, Badshah A, Hussain RA. Guanidines from 'toxic substances' to compounds with multiple biological applications–detailed outlook on synthetic procedures employed for the synthesis of guanidines. Bioorg Chem. 2015;59:39–79. doi: 10.1016/j.bioorg.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(9):5–11. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Valentini P, Buonsenso D, Barone G, Serranti D, Calzedda R, Ceccarelli M, Speziale D, Ricci R, Masini L. Spiramycin/cotrimoxazole versus pyrimethamine/sulfonamide and spiramycin alone for the treatment of toxoplasmosis in pregnancy. J Perinatol. 2015;35(2):90–94. doi: 10.1038/jp.2014.161. [DOI] [PubMed] [Google Scholar]
- Wei HX, Wei SS, Lindsay DS, Peng HJ. A Systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS ONE. 2015;10(9):e0138204. doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeromski J, Boczoń K, Wandurska-Nowak E, Mozer-Lisewska I (2005) Effect of aminoguanidine and albendazole on inducible nitric oxide synthase (iNOS) activity in T.spiralis-infected mice muscles. Folia Histochem Cytobiol 43(3):157:159 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials used to support the findings of this study are included within this article.