Abstract
The mortality rate of leishmaniasis is increasing at an alarming rate and is currently second to malaria amongst the other neglected tropical diseases. Unfortunately, many governments and key stakeholders are not investing enough in the development of new therapeutic interventions. The available treatment options targeting different pathways of the parasite have seen inefficiencies, drug resistance, and toxic side effects coupled with longer treatment durations. Numerous studies to understand the biochemistry of leishmaniasis and its pathogenesis have identified druggable targets including ornithine decarboxylase, trypanothione reductase, and pteridine reductase, which are relevant for the survival and growth of the parasites. Another plausible target is the sterol biosynthetic pathway; however, this has not been fully investigated. Sterol biosynthesis is essential for the survival of the Leishmania species because its inhibition could lead to the death of the parasites. This review seeks to evaluate how critical the enzymes involved in sterol biosynthetic pathway are to the survival of the leishmania parasite. The review also highlights both synthetic and natural product compounds with their IC50 values against selected enzymes. Finally, recent advancements in drug design strategies targeting the sterol biosynthesis pathway of Leishmania are discussed.
Keywords: Leishmaniasis, Ornithine decarboxylase, Trypanothione reductase, Sterol biosynthesis, Synthetic compounds, Natural product compounds
Introduction
Leishmaniasis is endemic in more than 98 countries worldwide including Brazil, Algeria, Ethiopia, Sudan, South Sudan, Bangladesh, Kenya, Somalia, and India. It affects over 350 million people worldwide (WHO 2020; Jain and Jain 2015; Leta et al. 2014) and it is estimated that 700,000 to 1 million new cases of leishmaniasis occur annually with a mortality rate of about 10% representing 20,000 to 40,000 cases (WHO 2020). The rise in the number of reported cases as well as the increase in the number of geographical areas has sparked concerns (Kholoud et al. 2018; Costa et al. 2011). Furthermore, the increase in the number of leishmania coinfection with HIV in Brazil, Ethiopia, India, and more recently in Nigeria is worrying (Távora et al. 2015; Kone et al. 2019). Visceral, cutaneous, and mucocutaneous leishmaniasis are the three endemic forms that present as ulcers of the skin, mouth, and nose. It mostly affects people living in poverty-stricken regions around the world (WHO 2020). Presently, there are no vaccines for leishmaniasis, and the only treatment option is also besieged with long term treatment course, toxicity and in some instances severe side effects (Ghorbani and Farhoudi 2018; Alvar et al. 2012).
The first-line drug used is the repurposed organometallic prodrug called pentavalent antimony (SbV) sodium stibogluconate which works by inhibiting the trypanothione reductase (Nagle et al. 2014). However, there are growing concerns about its efficacy, chemotoxicity, and drug resistance (Nagle et al. 2014). The second-line treatment includes pentamidine (PTM) and amphotericin B (Amp B) which works by inhibiting the DNA and sterol biosynthetic pathways, respectively (Bhargava and Singh 2012; Rodrigues et al. 2015). Currently, PTM appears to be no longer useful because of serious toxic side effects and concerns of drug resistance. While the use of Amp B requires hospitalization because of toxicity, it is also expensive and most of those affected by the disease cannot afford it, for example, a powder of 50 mg intravenous injection cost about $43 and one requires about $200 for complete treatment (Singh et al. 2016; https://www.drugs.com/price-guide/amphotericin-b).
Another medication used to treat leishmaniasis is Miltefosine (Milt), which works by inhibiting the phosphatidylcholine biosynthesis and is the only available oral drug which provides a relatively short treatment course (Pape 2008; Vijayakumar and Das 2017). However, the treatment of leishmaniasis using Milt causes teratogenicity effect in pregnant women (Sundar et al. 2011; Tiwari et al. 2017). Paromomycin in combination with benzethodium chloride has also been used in treating leishmaniasis by inhibiting PTEN-induced putative kinase 1 (PINK1) leading to a decrease in parasite mitochondrion membrane potential. Liposomal Amp B, an inhibitor of the sterol biosynthetic pathway leading to a destruction of the parasite cell membrane has also been useful as leishmaniasis treatment (Shakya et al. 2011; Wortmann et al. 2010; Tamiru et al. 2016).
Chemotherapeutic options targeting various enzymes in combating leishmaniasis are not only expensive but are also associated with drug resistance and toxic side effects (Mohapatra 2014; Oryan and Akbari 2016). With the increasing rate of leishmaniasis infections and the bottlenecks associated with the various chemotherapeutic options, combination therapy is possibly the game changer (Ponte-Sucre et al. 2017). A combination of 15 mg/kg/day paromomycin and 20 mg/kg/day sodium stibogluconate required only 17 days for effective treatment representing 91.4% efficacy in East Africa (Abongomera et al. 2016). The same can be said of Amp B in combination with Milt used in treating leishmaniasis coinfection with HIV in India and Ethiopia (Diro et al. 2019). Although, combination therapy is helpful, there are still challenges relating to longer duration of the treatment course, toxic side effects, and drug efficacy (Ghorbani and Farhoudi 2018; Menezes et al. 2015). It is therefore imperative to employ novel approaches in drug design on the validated pathways (Table 1) to overcome these challenges.
Table 1.
Validated pathways comprising plausible targets essential for parasite survival that are necessary for drug design against Leishmania parasites
| Category | Pathway/Enzyme | References |
|---|---|---|
| Glycolysis | Glycolytic pathway and glyoxalase enzymes | Silva et al. (2005) and Padmanabhan et al. (2005) |
| Isoprenoid and sterol synthesis | Squalene synthase, sterol 14α-demethylase | Kumar Saha et al. (1986) and Pomel et al. (2015) |
| Folate metabolism | Thymidylate synthase, dihydrofolate reductase and pteridine reductase | Nare et al. (1997) |
| Polyamine metabolism | Hypusine pathway, arginase, and ornithine decarboxylase | Jiang et al. (1999) |
| Antioxidant metabolism and Detoxification | Thiol pathway, superoxide dismutase and Thyperdoxin peroxidase | Leroux and Krauth-Siegel (2016) |
| Nucleotide metabolism | Purine salvage, nucleotide transporters | Jain and Jain 2018) |
| Cell division and DNA replication | Peptidase, topoisomerase, kinases | Merritt et al. (2014) and Brumlik et al. (2011) |
Modulation of the sterol biosynthetic pathway has been extensively studied as a putative drug target against most fungal and kinetoplastids (Borba-Santos et al. 2016; Singh and Babu 2018; Zulfiqar et al. 2017). Novel sterol inhibitors that target various enzymes in this pathway have also been exhaustively reviewed (Vijayakumar and Das 2017; Kumar et al. 2016; Yardley et al. 2002; Lepesheva and Waterman 2011; Rodrigues et al. 2008; Mukherjee et al. 2019; Bhargava et al. 2010; Brand et al. 2012; Yao and Wilson 2016). Even though sterol biosynthesis has been described as an essential drug target (Mukherjee et al. 2019; de Souza et al. 2009), it has also been considered as not being critical for extracellular survival but for heat resistance (Xu et al. 2014). The role of each of the enzymes in the sterol biosynthetic pathway to the survival of Leishmania parasites and hence their importance for drug design against leishmaniasis are discussed in this review. The review also highlights both synthetic and natural products compounds with their inhibitory IC50 against enzymes involved in the biosynthetic pathway. It discusses recent advancement in combination chemotherapy involving azoles, quinuclidine and terbinafine, inhibiting two or more of the enzymes in the sterol biosynthetic pathway with a synergistic effect leading to the disruption of cell membrane and mitochondrion of the Leishmania parasite. Finally, emerging and innovative approaches of drug design strategies targeting Leishmania sterol pathway such as drug repurposing, multi-target drug design, and conjugation of transitional metal complexes to two or more known inhibitors of the sterol biosynthesis are proposed.
Structural features of different sterol compositions in mammalian host cells and leishmania species
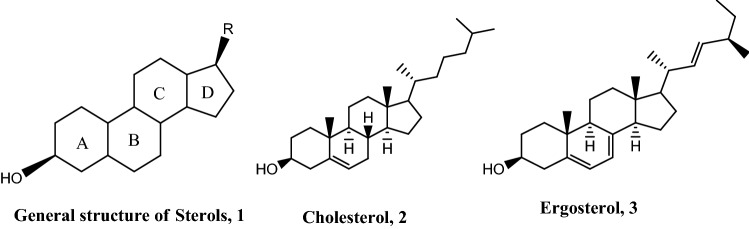
Sterols are constituents of the cellular membranes which are essential for normal structure and function. Ergosterol and cholesterol are known sterols which are important membrane components and precursors for the synthesis of critical biomolecules, including steroid hormones in mammals. Cholesterol is the main sterol found in membranes of mammalian cells while ergosterol is the main sterol in the cell membranes of Leishmania parasite (Yao and Wilson 2016). Sterols have a general structure consisting of a fused tetracyclic ring system of which there are three six-membered rings and one five-membered ring (1) with difference arising from the alkyl (R) substituent at the C-17 position (Fig. 1). Both cholesterol (2) and ergosterol (3) have a tetracyclic nucleus with 3β-OH which is important for cell growth (Souza and Rodrigues 2009).
Fig. 1.
Tetracyclic nature of the general structure of sterols, 1 and the structural difference between cholesterol, 2 and ergosterol, 3, biosynthesized in humans and Leishmania parasites, respectively
The 3β-OH of ergosterol is important for parasite survival as acetylation of the −OH group decreased sterol levels thereby retarding the growth of the Leishmania parasite (Souza and Rodrigues 2009). The methyl groups at C4 and C14 have virtually no effect on parasite growth as demethylation at the two positions leads to the formation of 4α and 14α-carboxysterols by oxidation followed by C–C cleavage to generate ketosterols (Blosser et al. 2014; Darnet and Schaller 2019; Ryley 1990). Ergosterol is different from cholesterol due to the presence of two double bonds in ring B of the steroid nucleus, a β-methyl group at position 24, and a double bond at C22 in the side chain. These double bonds and β-methyl substitution at C24 are needed for ergosterol biosynthesis in leishmania and hence are necessary for supporting growth and are therefore important drug targets for consideration against leishmaniasis (Souza and Rodrigues 2009; Darnet and Schaller 2019). The main sterols present in Leishmania amastigotes and promastigotes are ergosta-5,7,24(28)-triene-3β-ol, ergosta-7,22-diene-3β-ol, stigmasta-7,24(28)-diene-3β-ol, cholesterol, zimasterol and lanosterol with the first three constituting the majority (Giner and Zhao 2004). Even though, Leishmania parasites are known for not synthesizing cholesterol, the small amount absorbed in humans to support their growth (Semini et al. 2017) has raised concerns about the importance of essential sterols for Leishmania survival.
Sterol biosynthetic pathway in Leishmania species
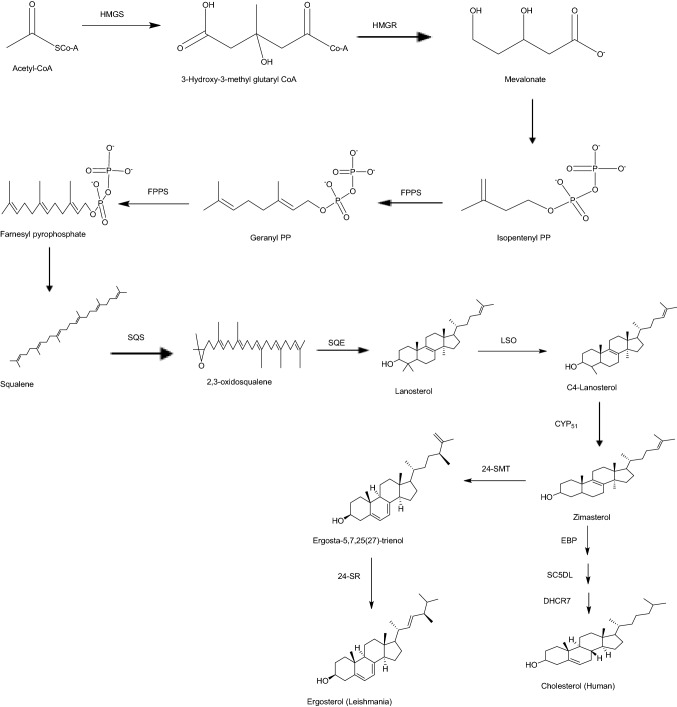
The identification of drug targets is essential in drug discovery and understanding the disease mechanisms. Therefore, the development of methods to identify drug targets has become a critical issue (Kwofie et al. 2019,2020a; Lionta et al. 2014; Cosconati et al. 2010; Lage et al. 2018; Hughes et al. 2010). Ergosterol, the product of leishmania sterol biosynthesis is a major component of the cell membrane and is useful to produce energy in the mitochondrion of the parasite cell. However, some enzymes involved in the biosynthesis have homologues in the human host cell making them unattractive targets. The sterol biosynthetic pathway of the Leishmania species were compared with that of the mammalian host cell (Fig. 2) in identifying putative targets in the said pathway for designing inhibitors with leishmanicidal properties (Yao and Wilson 2016; McCall et al. 2015).
Fig. 2.
Enzyme catalyzed sterol biosynthetic pathway reactions leading to the formation of ergosterol and cholesterol in Leishmania and human, respectively. Enzymes required in each step of the reaction comprised HMGS, 3-Hydroxy-3-methyl-glutaryl-coenzyme A synthase; HMGR, 3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase; FPPS, Farnesyl-pyrophosphate synthase; SQS, Squalene synthase; SQE, Squalene epoxidase; LSO, Lanosterol oxidase; CYP51, Sterol 14alpha-demethylase Cytochrome P450; 24-SMT, Δ24 Sterol methyltransferase; 24-SR, Sterol C-24 reductase; Emopamil-Binding Protein (EBP), Sterol Δ8(7) – isomerase; SC5DL, Sterol C-5 desaturase; and DHCR7, Sterol- Δ7-reductase
Both cholesterol and ergosterol biosynthesis involves mevalonate and isoprenoid pathways to produce farnesyl diphosphate. Squalene synthase then catalyzes the head-to-head condensation of two farnesyl diphosphate to form squalene. The 30-carbon squalene is then oxidized by squalene epoxidase to a tetracyclic sterol skeleton called 2,3-oxidosqualene. The 2,3-oxidosqualene undergoes rearrangement catalyzed by 2,2-oxidosqualene cyclase to form lanosterol. One of the important steps in the ergosterol biosynthesis but absent in the cholesterol biosynthetic pathway is the addition of a methyl group at the C24 position of the side chain. The sterol methyltransferase (24-SMT) catalyzes the transfer of a methyl group from S- adenosyl-L-methionine (SAM) to C24 of sterol which undergoes further reactions to afford ergosterol.
Essential and validated enzymes in leishmania sterol biosynthetic pathway
3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase) (HMGR)
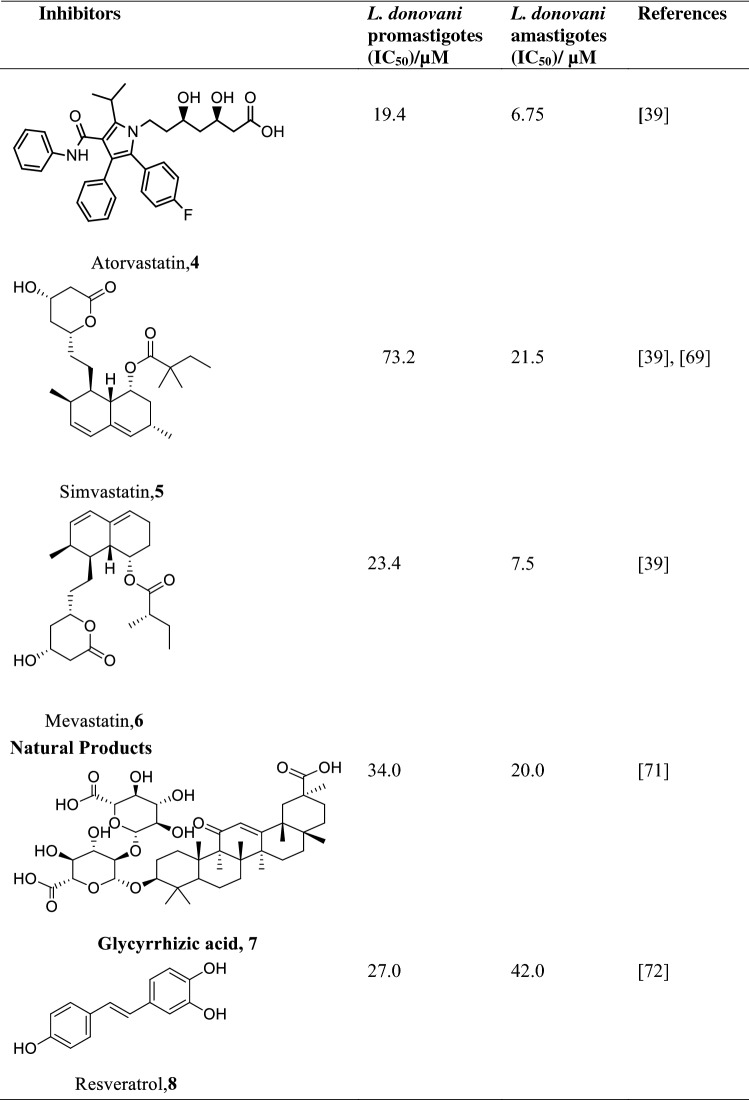
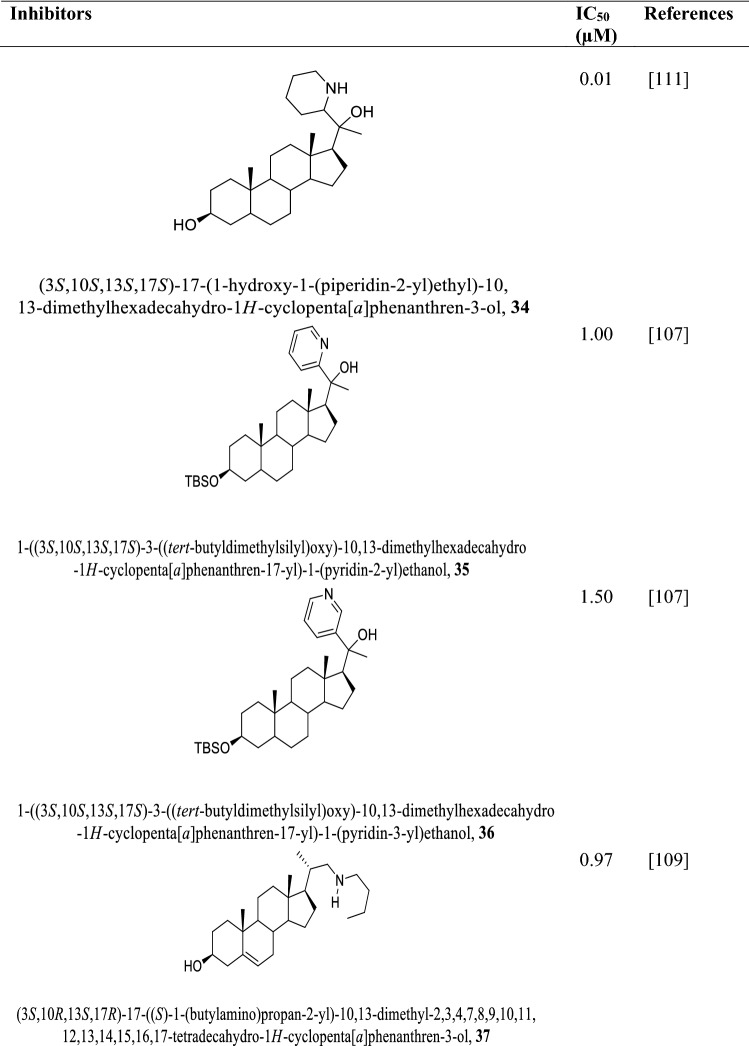
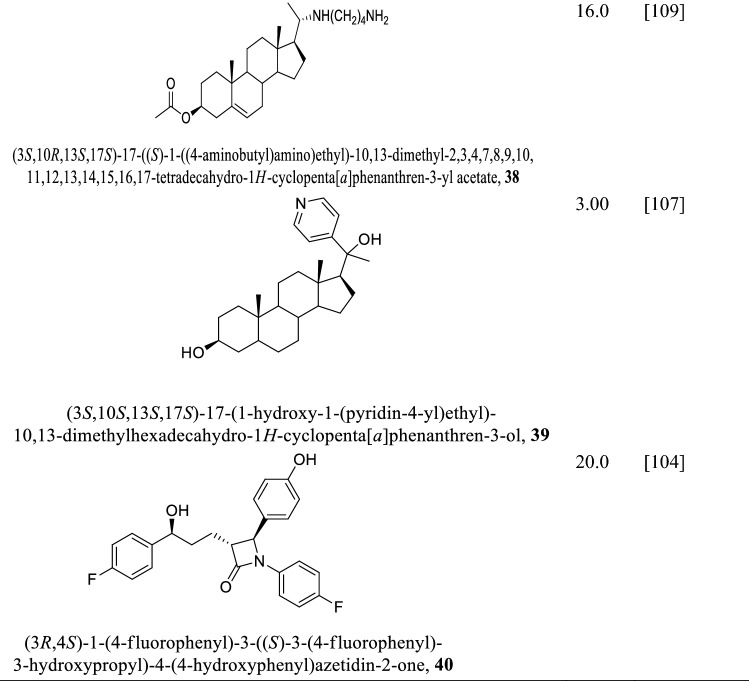
3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase, HMGR) is the rate-limiting enzyme involved in ergosterol biosynthesis by catalyzing the NADPH synthesis of mevalonate from HMG-CoA (Singh and Babu 2018). Ergosterol and the other ergosterol-related sterols required for maintaining the cell membrane and production of energy in the mitochondrion depend on HMGR. HMG-CoA reductase of Leishmania major was the first highly purified protein of the Leishmania family by ammonium sulphate precipitation followed by chromatography on hydroxyapatite (Montalvetti et al. 2000). HMG-CoA reductase of Leishmania major with molecular mass of 46 kDa encoding for 434 amino acids was used as a template for HMG-CoA reductase of Leishmania donovani (LdHMGR) via basic alignment search tool (BLAST) (Singh and Babu 2018; Montalvetti et al. 2000; Dinesh et al. 2014). The kinetic study of the enzyme revealed Km value of 35.7 ± 2.5 μM for the (R,S)-HMG-CoA and 81.4 ± 5.3 μM for the cofactor NADPH and Vmax 33.55 units/mg (Dinesh et al. 2014), while the pH for optimal activity was 7.2 at the temperature of 37 °C. Their use as a drug target is challenged by the fact that the LdHMGR shares 35% similarity with the human HMGR, which could lead to potential off-target effects (Singh and Babu 2018). Notwithstanding, the variations in their sequences can be exploited in the design of inhibitors against HMGR target. HMGR promote growth in both the promastigote and amastigote phases of the parasite life cycle and aid in infecting THP-1 macrophages (Dinesh et al. 2014; Alcolea et al. 2010). Statin and other natural products (Table 2) inhibit HMGR killing both amastigotes and promastigotes of the parasites (Singh and Babu 2018; Kumar et al. 2016). However, statins deplete cholesterol levels of the host cells and exhibit cytotoxicity, neurological and other side effects such as muscle pain, liver damage, and increased blood sugar (Reid et al. 2007; Gabor and Fessler 2016; Ward et al. 2019; Parihar et al. 2016). To address these limitations, lovastatin administered in combination with chromium chloride was found to kill amastigotes without appreciable cytotoxicity to the host (Verma et al. 2017).
Table 2.
Structures of various statins and natural products inhibitors of HMB-CoA reductase of L. donovani amastigotes and promastigotes and their IC50
Farnesyl-pyrophosphate synthase (FPPS)
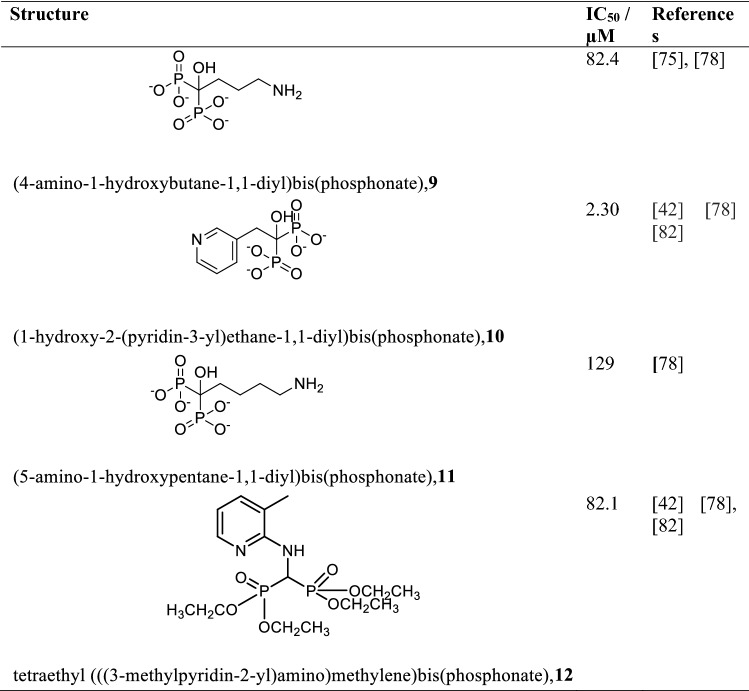
Farnesyl-Pyrophosphate Synthase (FPPS) is one of the prominent enzymes in the isoprenoid pathway that supplies precursors such as isopentenyl pyrophosphate in the biosynthesis of ergosterol and the other 24-alkylated sterols needed for the growth of the parasite as well as protein prenylation (Aripirala et al. 2014; Dhar et al. 2013). FPPS catalyzes the head to tail condensational reaction of 2 mol of isopentenyl pyrophosphate with a mole of dimethylallyl diphosphate to produce an elongated farnesyl pyrophosphate product. The kinetic study of Leishmania major co-crystallized with Guanosine-5′-diphosphate-alpha-D-mannose (GDD) and isopentenyl diphosphate (IPP) revealed Km values of 13.77 μM and 30.57 μM, respectively, with Vmax value of 3225 units/mg (Aripirala et al. 2014; Gabelli et al. 2006; Navabi and Soleimanifard 2015). The pH for maximum activity at 37 °C of this enzyme is 7.4 with a molecular mass of 40.9 kDa composed of 362 amino acids. FPPS shows 46% similarity with the human homologue with a potential of causing off-target effects (Ortiz-Gómez et al. 2006). FPPS has however been exploited to develop inhibitors in treating leishmaniasis with minimal off-target effects. Bisphosphonate, originally developed for the treatment of osteoporosis is reported to inhibit FPPS and is applied in the treatment of leishmaniasis (Dhar et al. 2013; Martin et al. 2001; Docampo and Moreno 2001). In vivo and in vitro studies of bisphosphonates (Table 3) especially those of aromatic analogues containing nitrogen, have been found to show strong activity by inhibiting the growth of Leishmania donovani (Yardley et al. 2002; Aripirala et al. 2014; Martin et al. 2001). In vivo analysis of alendronate, a derivative of bisphosphate on Leishmania donovani was found to be inactive when used as a monotherapy but a vanadium/alendronate complex [V(Ale)2] retarded cell growth better than most of the bisphosphonates used alone (Christensen et al. 2016). Other metals such as copper, cobalt, manganese, and nickel complexed with bisphosphonates have also been reported to inhibit Leishmania FPPS (Aripirala et al. 2014; Christensen et al. 2016; Ong et al. 2019).
Table 3.
Some bisphosphonate derivatives as inhibitors of Leishmania FPPS and their IC50
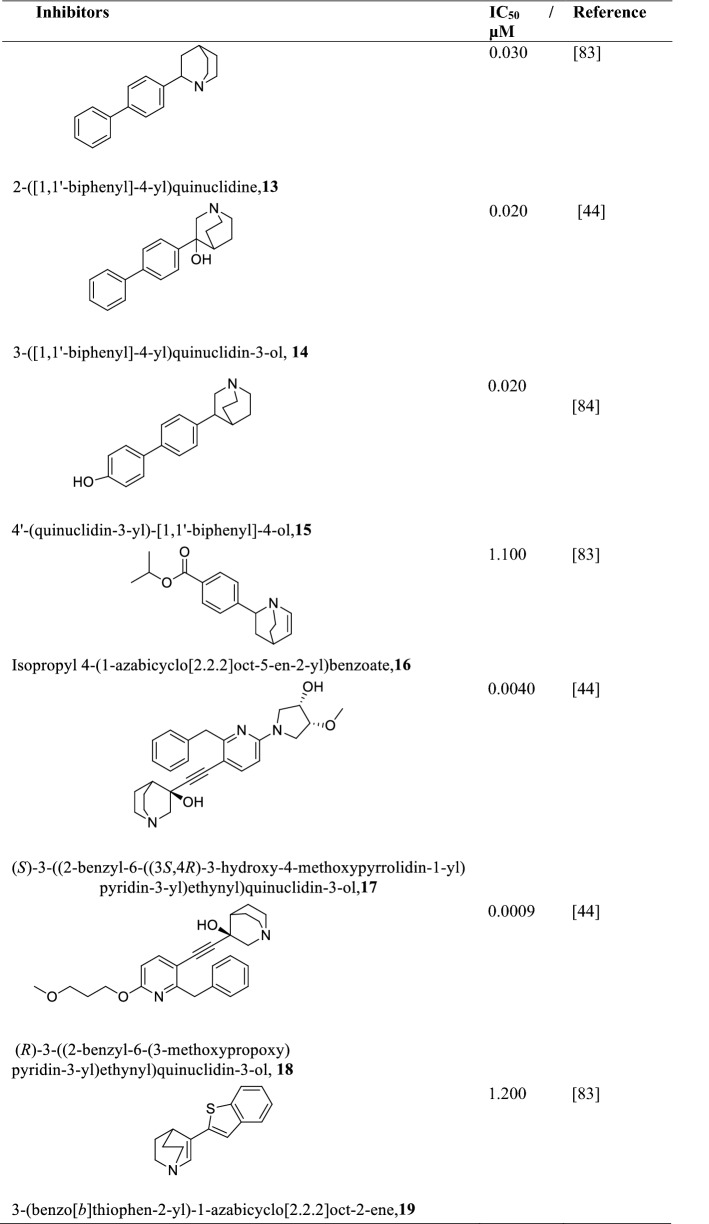
Squalene synthase (SQS)
Squalene Synthase (SQS) catalyze the head-to-head reductive dimerization of two molecules of farnesyl pyrophosphate to form squalene. SQS is essential for the survival and growth of most Leishmania species, especially L. donovani (Granthon et al. 2007). The pH and temperature of expressed and purified L. donovani squalene synthase is 7.4 and 37 °C, respectively, with a molecular mass of 47.3 kDa composed of 414 amino acids. The Km and Vm of SQS obtained from biochemical studies of the substrate FPP were 3.8 μM and 0.59 nM min−1 mg−1, and that of NADPH was 43.23 μM and 0.56 nM min−1 mg−1 (Bhargava et al. 2010), respectively. The activity of SQS is greatly influenced by denaturants such as urea or guanidine hydrochloride, for example, a 2 M urea or 0.2 M guanidine hydrochloride solution causes the activity of SQS to be lost by as much as 81% and 86%, respectively (Bhargava et al. 2010). Quinuclidine and its derivatives (Table 4) are known inhibitors of SQS with IC50 values of less than 30 μM (Cammerer et al. 2007). The inhibition of SQS by quinuclidine leads to a complete disruption of the mitochondrion eventually leading to the death of the parasite (Rodrigues et al. 2008). On the other hand, zaragozic acid, a known inhibitor of the human squalene synthase competitively inhibits L. donovani SQS with a Ki of 74 nM (Bhargava et al. 2010). This can be explored in developing exclusive inhibitors of zaragozic acid against Leishmania SQS. One significant advantage of targeting SQS is that it has low similarity to the human homologues hence any potential inhibitors could be highly selective to the parasite. (Rodrigues et al. 2008; Urbina et al. 2002).
Table 4.
Chemical structures of various quinuclidine derivatives as Leishmania SQS inhibitors and their IC50
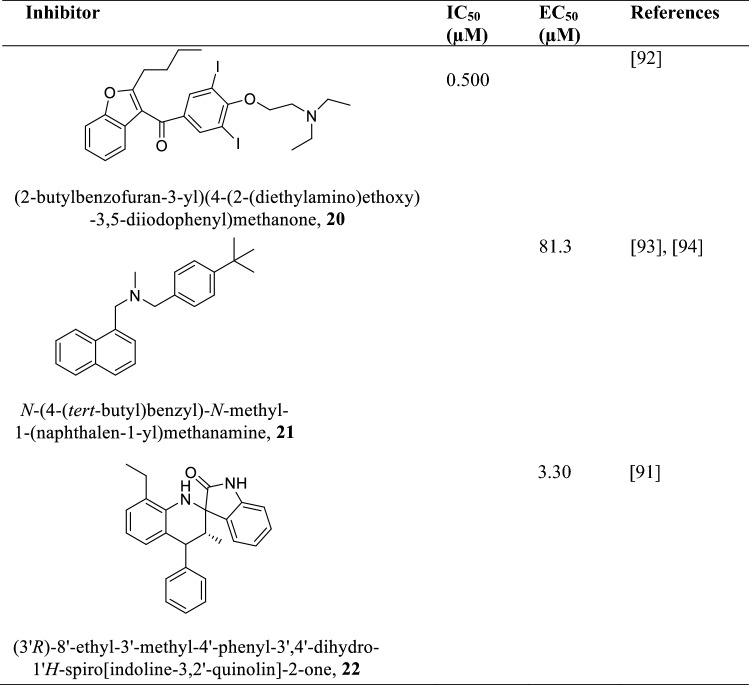
Squalene epoxidase (SQE)
Squalene Epoxidase (SQE) is an enzyme in the parasite sterol biosynthetic pathway that utilizes molecular oxygen to oxidize squalene to 2,3-oxidosqualene (Souza and Rodrigues 2009; Kaneshiro et al. 2000). The insertion of the hydroxyl group (-OH) in sterols begins with SQE and hence its absence means the essential sterols needed for parasite survival and growth would virtually be nonexistent (Nowosielski et al. 2011). SQE is a 63.5 kDa protein composed of 569 amino acids which is known to be inhibited by terbinafine, an allylamine (Beach et al. 1989; Hart 1989). Terbinafine inhibits SQE of parasite promastigotes and intracellular amastigotes with IC50 of 1 μM and 100 nM, respectively (Nowosielski et al. 2011; Beach et al. 1989). Inhibition of SQE leads to growth arrest due to the accumulation of high levels of squalene thereby reducing endogenous sterol levels (Beach et al. 1989; Hart 1989). Amiodarone and Spiro dihydroquinoline-oxindoles (Table 5) are also squalene epoxidase inhibitors depleting the sterol levels of the parasite leading to their death with IC50 values in the nanomolar range (Serrano-Martín et al. 2009; Leañez et al. 2019).
Table 5.
Allylamine, 1-benzofuran and Spiro quinoline-oxindole class of compounds as squalene epoxidase inhibitors and their IC50/ µM and EC50/ µM
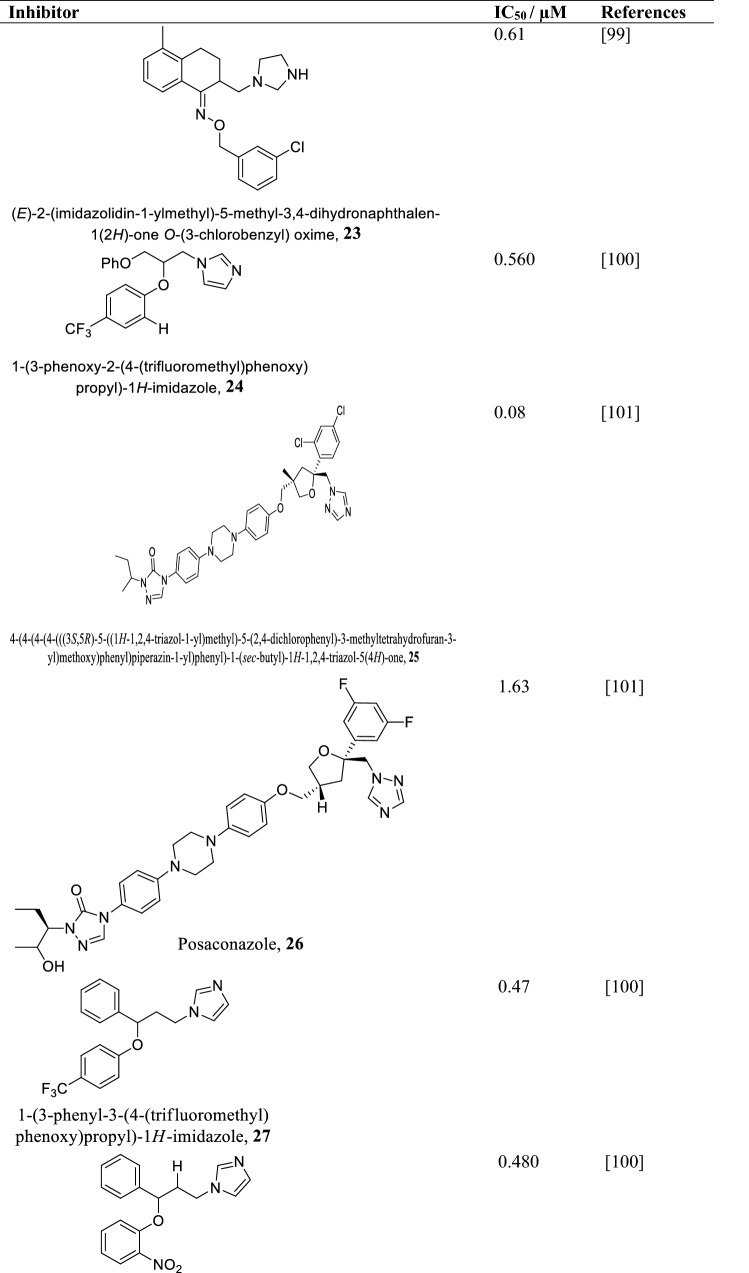
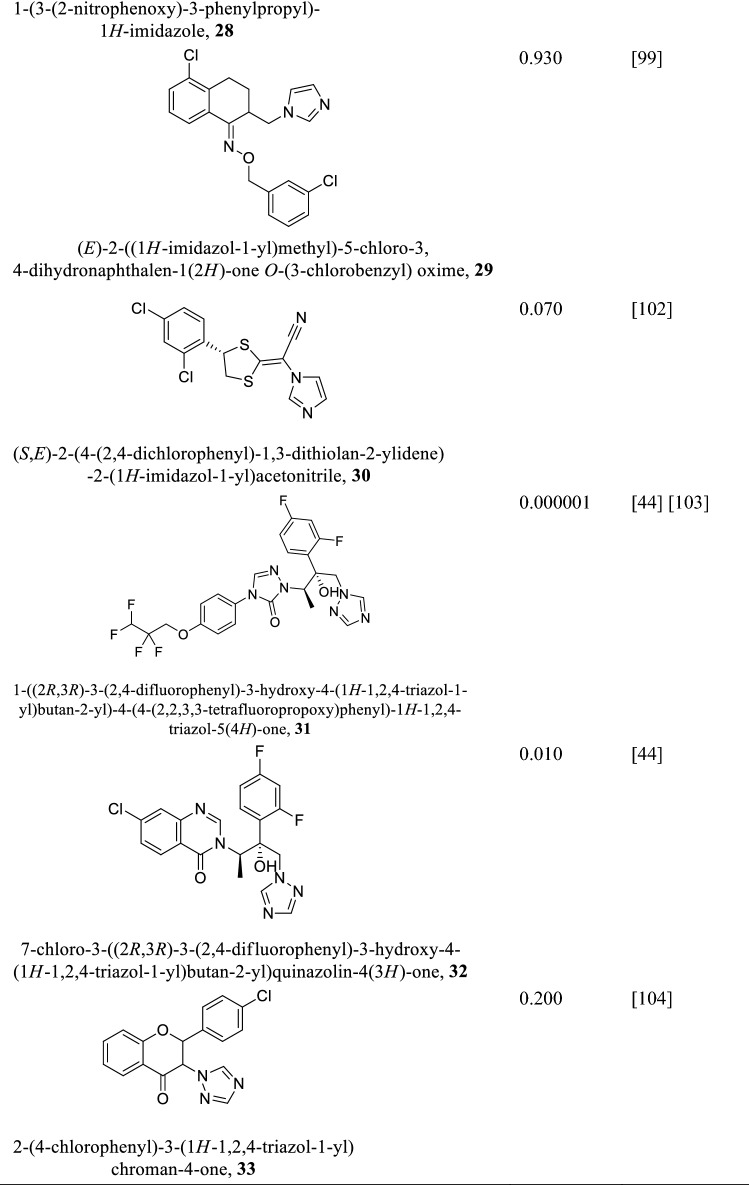
Sterol 14alpha-demethylase (CYP51)
Sterol 14alpha-demethylase (CYP51) also called lanosterol 14alpha-demethylase is a cytochrome P450 monooxygenase that catalyzes the formation of 4,4-dimethylcholesta-8(9),14,24-triene-3β-ol from lanosterol. The molecular mass of Leishmania infantum CYP51 was found to be 54 kDa composed of 480 amino acids (Keighobadi et al. 2018). It shows 95% similarity with Leishmania braziliensis, 96% with Leishmania major, and 97% with Leishmania mexicana and Leishmania amazonensis (Hargrove et al. 2011). However, its similarity with the human CYP51 is only about 26% meaning inhibitors against Leishmania CYP51 are less likely to have any effect on host homologue, making CYP51 a plausible target for drug design against leishmaniasis (Lepesheva and Waterman 2011). Leishmania CYP51 essentiality based on survival was unknown until the application of gene knockout approach and parasites CYP51 inhibition led to growth retardation and eventual death of Leishmania donovani (McCall et al. 2015). Azoles and its derivatives (Table 6) are effective inhibitors of Leishmania CYP51 (McCall et al. 2015; Choi et al. 2014). During azole inhibition, toxic methylated sterols build up and damage the cell membrane affecting its division and multiplication and eventually leading to parasite death (Lepesheva and Waterman 2011). Notwithstanding the effectiveness of azoles, drug resistance and side effects have been reported (Barrett and Croft 2012). On the other hand, non-azole compounds (N-(3-(1H-indol-3-yl)-1-oxo-1(pyridin-4-ylamino)propan-2-yl)-4-methyl cyclohexanecarboxamide and 2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)-N-(2-(pyridin-3- yl)ethyl)acetamide) have also been found to inhibit Leishmania CYP51 with IC50 of 20 µM much lower compared to azoles (Lepesheva and Waterman 2011).
Table 6.
Some azole and triazole compounds as CYP51 inhibitors and their IC50/µM
Δ24 Sterol methyltransferase (24-SMT)
All the previously discussed enzymes have human homologues implying inhibiting these enzymes of Leishmania parasites may potentially cause off-target effects to the host cells. The sterol methyltransferase (24-SMT) catalyzes the transfer of methyl group from S-adenosyl-L-methionine (SAM) to C24 of the sterol. Since 24-SMT does not have any homologue in the human cell, it is an attractive target for drug design for leishmania. The choice of 24-SMT as a drug target was not in doubt but its importance to parasite survival was unknown but later found to be essential for optimal mitochondrion function and parasite virulence (Borba-Santos et al. 2016; Mukherjee et al. 2019; Rodrigues et al. 2002). This observation is contrary to an earlier held view that sterols were not necessary for survival of Leishmania species (Yao and Wilson 2016). Even though, cell fractionation studies suggest that 24-SMT was located in the glycosomes and mitochondrion (Pérez-Moreno et al. 2012), immunofluorescence and electron microscope observations using antibodies showed that the enzyme could be found in the endoplasmic reticulum and in translucent vesicles (Souza and Rodrigues 2009). Despite the differences in the exact location of 24-SMT in the cell, it has been found that growth depends on it, and hence inhibiting it would have effects on the sterol composition and may lead to parasite death (Mukherjee et al. 2019). 24-SMT is therefore one of the plausible targets in the search for inhibitors against Leishmania parasites. Derivatives of azasterol and non-azasterol (Table 7) have been found to be efficient inhibitors against 24-SMT affecting sterol composition and proliferation of the parasite (Rodrigues et al. 2002; Magaraci et al. 2003; Chawla and Madhubala 2010). The activity of the azasterol depend on the basicity and stereochemical position of the nitrogen as well as the presence of 3β-OH group (Pinto and Tempone 2018; Lorente et al. 2004). Toxic side effects and drug resistance of azasterol have been reported (Bezerra-Souza et al. 2019; Gros et al. 2006).
Table 7.
Inhibitors of 24-SMT and their IC50 (µM)
Lanosterol oxidase (LSO)
Unlike cholesterol which has been extensively studied and very well characterized in literature, the functions of ergosterol the main lipid component of membranes and its interactions with other pathways is not fully understood. Recent efforts in probing the biological role of LSO and the resulting consequences of its absence have brought to light a new biological understanding that holds a future in Leishmania drug discovery (Ning et al. 2020). Lanosterol oxidase (LSO) also called C-5 sterol desaturase catalyzes the double bond formation between C5 and C6 in the formation of ergosterol.
Recent advancements in chemotherapy targeting leishmania sterol biosynthetic pathway (the multifunctional compound approach)
To date no approved “one-drug-one-target” antileishmanial drug has been able to completely relieve patients suffering from severe leishmanial conditions. This suggest that singly mode of action drugs targeting only one enzyme of the Leishmania parasite may possibly not completely eliminate the parasite. Renewed interest in using multi-target strategies to improve leishmaniasis treatment offer some hope. Here the paradigms to increase therapeutic efficiencies in inhibiting multiple targets of the sterol biosynthetic pathway are described.
Combinational therapy inhibiting leishmania sterol biosynthesis
Combination chemotherapy is the use of more than one chemotherapeutic agent at a time in combating a disease. Isoniazid in combination with rifampin, sulfadoxine with pyrimethamine, and lamivudine with zidovudine are some of the known combination chemotherapeutic regimens administered for effective treatment of tuberculosis, malaria, and HIV/AIDS infections, respectively. Combination chemotherapy targeting Leishmania sterol biosynthesis has been shown to have high therapeutic efficacy (Singh et al. 2016; Chakravarty and Sundar 2019).
E5700, an aryl-quinuclidine, a known Leishmania SQS inhibitor exhibits a synergistic effect when used in combination with Itraconazole (ITZ) and Posaconazole (POSA) (Table 6) (Macedo-Silva et al. 2015). While E5700 in combination with ITZ and then POSA showed amastigotes antiproliferative effect with fractional inhibitory concentration (FIC) of 0.175 μM and 0.1125 μM, respectively, that of the same pair gave FIC of 0.0525 μM and 0.0162 μM, respectively (Macedo-Silva et al. 2015). There was a complete destruction of the mitochondrion in both cases and an increase in the production of reactive oxygen species eventually led to growth cessation. Additionally, azasterols when used in combination with azoles was more effective than when used separately. Azoles used in combination with azasterol produced a synergistic effect with reduced treatment time and FIC in the nanomolar range (Rodrigues et al. 2007). Posaconazole used in combination with Amiodarone led to growth retardation and acidocalcisome with an FIC around 0.42 μM (Macedo-Silva et al. 2015; Veiga-Santos et al. 2012). Ezetimibe in combination with ketoconazole and miconazole produced an antiproliferation effect synergistically with an IC50 of 4.14 μM, and 8.25 μM, respectively (Andrade-Neto et al. 2016). Terbinafine, a known inhibitor of squalene epoxidase inhibited promastigotes and amastigotes with IC50 of 1 μM and 100 nM, respectively. However, a combination of terbinafine and ketoconazole produced a much drastic antiproliferative effects on intracellular amastigotes with FIC of 1 nM (Vannier-Santos et al. 1995). There was a great change in the structural morphology of the mitochondrion leading to parasite death.
Mevinolin, a lovastatin derivative and a known inhibitor of HMGR exhibits antiproliferative effect on promastigotes and amastigotes with IC50 of 25 μM and 0.75 μM, respectively. A combination of mevinolin and ketoconazole, however, produced a more pronounced antiproliferative effect with a much lower FIC (Urbina et al. 1993). A more aggressive antiproliferative and synergistic effect was observed when mevinolin, ketoconazole, and terbinafine were combined (Urbina et al. 1993) resulting in loss of mitochondrion shape with the production of acidocalcisome organelles. From the aforementioned, modulating more than one stage of the post squalene biosynthetic pathway appears to increase therapeutic efficacy. This affords an effective means of killing these pathogens whilst at the same time restoring physiological balance, but key is the selectivity of drugs with synergism and reduced toxicity.
Multi-node drug target as a new focus on targeting sterol inhibition in leishmaniasis
Drug combinations have been extensively investigated for increasing the therapeutic window of potent inhibitors with a coordinated mechanism against Leishmania parasite, but the associated sophistication in design coupled with synergistic toxicity, threats of multi-drug resistance, and lengthy treatment periods makes this approach partly unfavorable (Chen et al. 2016). This underpins the need for alternative drug design techniques to reduce drug-drug interactions and cytotoxicity, improve pharmacokinetics, and increase synergism. Like the adopted strategies currently used in treating multifactorial diseases including neurological disorders, malaria, cancers, and cardiovascular diseases, multitarget drugs are significant because simplified dosage regimens are needed to minimize complications associated with leishmaniasis. In this respect, a drug that can modulate multiple proteins with high selectivity like the conventional magic bullet avoiding off-targets will be a welcome one.
Multi-target drugs can be designed by conjugating multicomponent ligands which are known to pertinently inhibit the aimed targets with a biodegradable linker or fusing the inhibitors. On the other hand, a multi-target ligand designed based on small molecules known as warheads of the various single regimens inhibiting targeted enzymes is also plausible. Notably, due to the complexity of biological systems, selectivity and synergism represent a complex hurdle but since the sterol biosynthesis is just a single pathway, the likelihood of strong coordinated action is possible (Proschak et al. 2019). A critical examination of the positive synergistic effects from some combination drugs including posaconazole, itraconazole, and aryl quinuclidine suggest they might possess good selectivity and reduced doses during administration. On the other hand, azoles and azasterols which have also shown remarkable in vivo activity in nano molar concentrations represent promising compounds to be fused. Additionally, recent studies on luliconazole showed strong inhibition activity but were found to be very toxic. The luliconazole scaffold represents a potential template that can be optimized and conjugated or fused to promote its recognition against intended targets (Shokri et al. 2018).
Drug repurposing targeting leishmania sterol biosynthetic pathway
Drug repurposing also sometimes termed as drug repositioning, reprofiling, or retasking involves the reuse of an approved drug for the treatment of disease outside the domain of the original medication. Sildenafil, a phosphodiesterase type 5 (PDE5) inhibitor, was marketed for the treatment of hypertension but is now a repurposed drug for treating erectile dysfunction. Zidovudine, an anticancer drug is now a repurposed drug for treating HIV/AIDS infections (Dhir et al. 2020). There are several repurposed drugs available for treating various diseases including leishmaniasis (Dhir et al. 2020; Pushpakom et al. 2018). Old drugs including pentavalent antimony, amphotericin B, pentamidine, and more recently miltefosine are repurposed drugs available for leishmaniasis treatment. Potent antileishmanials are very few and considering the uncertainty and laborious nature of de novo drug discovery, drug repurposing is essential. Notwithstanding, numerous drugs have been explored from diverse classes for combating leishmaniasis. To highlight a few, repurposed sterol inhibitors are mainly antifungals including amiodarone, dronedarone, fluconazole, terbinafine, posaconazole, and more recently, butenafine which has been optimized for oral use (Nagle et al. 2014; Sundar et al. 2011; Bhargava et al. 2010; Verma et al. 2017; Kasam 2009). Azoles inhibit P450-CYP51 by first water breaking away from the axial position of the enzyme forming a pentacoordinate active site. The basic nitrogen group in the azole then forms a dative covalent bond at the sixth position of the pentacoordinate activate site by donating a lone pair of electrons. Though the Fe–N bond in the azole-CYP51 complex is not very strong compared to Fe–O, the hydrophobic interaction between the two coordinating molecules increases the binding energy so that the azole can stay long enough at the active site of CYP51 and cause the inhibition of sterol biosynthesis. On the other hand, terbinafine a squalene epoxidase inhibitor binds at the active site of squalene epoxidase through hydrogen bonding interaction between the hydroxyl group of Tyr90 and amine nitrogen of terbinafine. Additionally, azasterols with electron-withdrawing groups at C(25) of the sterol side chain form a suicide inhibition with 24-SMT by a nucleophilic attack of the enzyme at the C(25) electrophilic position.. Therefore azoles, allylamines, polyenes, and azasterols form part of important drug scaffolds that can be repurposed against sterol biosynthesis for the treatment of leishmaniasis. Whilst the focus has solely been on inhibiting essential pathways necessary for survival, drugs which are known to activate macrophages and promote Th1 immune response are also viable alternatives that should not be neglected.
Designing multi-target transition metal complexes as inhibitors of leishmania sterol biosynthesis
Despite the known medicinal properties of transition metals and the recent upsurge of interest, there is little application in the treatment of leishmaniasis. Indeed, after the discovery of pentavalent antimony for the treatment of leishmaniasis, virtually no new metallodrug has found prominent clinical use. On the other hand, the design of multi-target metal coordinated compounds has been under exploited for the treatment of leishmaniasis. Nevertheless, several transition metals containing organic compounds have been shown to exhibit high therapeutic efficacy against several diseases such as malaria, cancer, and diabetics (Iniguez et al. 2013; Kwofie et al. 2020b). The recent improvement in structural-based drug design techniques and the availability of modern drug discovery tools have presented the opportunity to iterate millions of organometallic compounds against multiple targets. The new paradigm in drug discovery should focus on designing multi-target metallodrugs inhibiting two or more enzymes in the sterol biosynthetic pathway of Leishmania parasites.
Conclusion
Sterol biosynthetic pathway of Leishmania form major components of the cell membrane and serve as a source of energy in the form of ATP in the mitochondrion of the parasite cell. Several enzymes including, hydroxy-3-methyl-glutaryl-coenzyme A reductase, farnesyl-pyrophosphate synthase, sterol 14alpha-demethylase, sterol methyltransferase, squalene epoxidase and lanosterol oxidase have been validated as potential drug targets for the treatment of leishmaniasis. However, most of these enzymes in the Leishmania sterol biosynthetic pathway have homologues in the mammalian host cell making it challenging to design a highly selective inhibitor without encountering potential off-target activity. Fortunately, enzymes such as 24-SMT do not have a homologue in the host cell and therefore present a unique target for Leishmania drug design. It is increasingly becoming difficult to secure safe drugs since there is rise in toxic side effects, concerns about drug resistance, and lengthy treatment durations. To overcome these challenges, multi-target drugs, drug repurposing and multi-target transition metal complexes are plausible alternatives worthy of exploration as antileishmanial molecules.
Abbreviations
- PTM
Pentamidine
- Amp B
Amphotericin B
- Milt
Miltefosine
- 24-SMT
Sterol methyltransferase
- SAM
S-Adenosyl-l-methionine
- HMG-CoA reductase, HMGR
3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase
- FPPS
Farnesyl-pyrophosphate synthase
- SQS
Squalene synthase
- SQE
Squalene epoxidase
- CYP51
Sterol 14alpha-demethylase
- ITZ
Itraconazole
- POSA
Posaconazole
- FIC
Fractional inhibitory concentration
- LSO
Lanosterol oxidase
Author contributions
P.O.S., R.K.A. and S.K.K. conceptualized the review, whilst P.O.S. wrote the first draft. S.K.K. and P.O.S. supervised the work, with all authors making contributions to the manuscript. All authors read and accepted the final draft manuscript.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Patrick O. Sakyi, Email: patrick.sakyi@uenr.edu.gh, Email: opsakyi@st.ug.edu.gh
Richard K. Amewu, Email: ramewu@ug.edu.gh
Robert N. O. A. Devine, Email: robert.devine@stu.uenr.edu.gh
Alfred K. Bienibuor, Email: alfred.bienibuor@uenr.edu.gh
Whelton A. Miller, III, Email: wmiller6@luc.edu.
Samuel K. Kwofie, Email: skkwofie@ug.edu.gh
References
- Abongomera C, Gatluak F, Buyze J, Ritmeijer K. A comparison of the effectiveness of sodium stibogluconate monotherapy to sodium stibogluconate and paromomycin combination for the treatment of severe post kala azar dermal leishmaniasis in south Sudan—a retrospective cohort study. PLoS ONE. 2016;11(9):e0163047. doi: 10.1371/journal.pone.0163047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcolea PJ, Alonso A, Gómez MJ, Sánchez-Gorostiaga A, Moreno-Paz M, González-Pastor E, et al. Temperature increase prevails over acidification in gene expression modulation of amastigote differentiation in Leishmania infantum. BMC Genomics. 2010;11(1):31. doi: 10.1186/1471-2164-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7:e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphotericin b Prices, Coupons & Patient Assistance Programs - Drugs.com. https://www.drugs.com/price-guide/amphotericin-b. Cited 2020 Jun 1
- Andrade-Neto VV, Cunha-Júnior EF, Do Canto-Cavalheiro MM, Atella GC, De Almeida FT, Costa PRR, et al. Antileishmanial activity of ezetimibe: inhibition of sterol biosynthesis, in vitro synergy with azoles, and efficacy in experimental cutaneous leishmaniasis. Antimicrob Agents Chemother. 2016;60(11):6844–6852. doi: 10.1128/AAC.01545-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aripirala S, Gonzalez-Pacanowska D, Oldfield E, Kaiser M, Amzel LM, Gabelli SB. Structural and thermodynamic basis of the inhibition of Leishmania major farnesyl diphosphate synthase by nitrogen-containing bisphosphonates. Acta Crystallogr Sect D Biol Crystallogr. 2014;70(3):802–810. doi: 10.1107/S1399004713033221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104(1):175–196. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach DH, Goad LJ, Berman JD, Ellenberger TE, Beverley SM, Holz GG (1989) Effects of a squalene-2,3-epoxidase inhibitor on propagation and sterol biosynthesis of leishmania promastigotes and amastigotes. In: Leishmaniasis. Springer US, pp 885–890
- Bezerra-Souza A, Yamamoto ES, Laurenti MD, Ribeiro SP, Passero LFD, Passero FD. The antifungal compound butenafine eliminates promastigote and amastigote forms of Leishmania (Leishmania) amazonensis and Leishmania (Viannia) braziliensis. Parasitol Int. 2016;65(6):702–707. doi: 10.1016/j.parint.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Bezerra-Souza A, Fernandez-Garcia R, Rodrigues GF, Bolas-Fernandez F, Laurenti MD, Passero LF, et al. Repurposing butenafine as an oral nanomedicine for visceral leishmaniasis. Pharmaceutics. 2019;11(7):353. doi: 10.3390/pharmaceutics11070353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari K, Srinivas N, Marrapu VK, Verma A, Srivastava S, Gupta S. Synthesis of substituted aryloxy alkyl and aryloxy aryl alkyl imidazoles as antileishmanial agents. Bioorganic Med Chem Lett. 2010;20(1):291–293. doi: 10.1016/j.bmcl.2009.10.117. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Singh R. Developments in diagnosis and antileishmanial drugs. Interdiscip Perspect Infect Dis. 2012;2012:626838. doi: 10.1155/2012/626838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava P, Kumar K, Chaudhaery SS, Saxena AK, Roy U. Cloning, overexpression and characterization of Leishmania donovani squalene synthase. FEMS Microbiol Lett. 2010;311(1):82–92. doi: 10.1111/j.1574-6968.2010.02071.x. [DOI] [PubMed] [Google Scholar]
- Blosser SJ, Merriman B, Grahl N, Chung D, Cramer RA. Two C4-sterol methyl oxidases (Erg25) catalyse ergosterol intermediate demethylation and impact environmental stress adaptation in Aspergillus fumigatus. Microbiology (United Kingdom) 2014;160(11):2492–2506. doi: 10.1099/mic.0.080440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borba-Santos LP, Visbal G, Gagini T, Rodrigues AM, De Camargo ZP, Lopes-Bezerra LM, et al. Δ24-sterol methyltransferase plays an important role in the growth and development of Sporothrix schenckii and Sporothrix brasiliensis. Front Microbiol. 2016;7(Mar):1–13. doi: 10.3389/fmicb.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand S, Cleghorn LAT, McElroy SP, Robinson DA, Smith VC, Hallyburton I, et al. Discovery of a novel class of orally active trypanocidal N-Myristoyltransferase inhibitors. J Med Chem. 2012;55(1):140–152. doi: 10.1021/jm201091t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik MJ, Pandeswara S, Ludwig SM, Murthy K, Curiel TJ. Parasite mitogen-activated protein kinases as drug discovery targets to treat human protozoan pathogens. J Signal Transduct. 2011;2011:1–16. doi: 10.1155/2011/971968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammerer SB, Jimenez C, Jones S, Gros L, Lorente SO, Rodrigues C, et al. Quinuclidine derivatives as potential antiparasitics. Antimicrob Agents Chemother. 2007;51(11):4049–4061. doi: 10.1128/AAC.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo J, Santos B, Melo JA De, Maheshwari S, Marina W (2020) Bisphosphonate-based molecules as potential new antiparasitic drugs, pp 1–22 [DOI] [PMC free article] [PubMed]
- Chakravarty J, Sundar S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin Pharmacother. 2019;20(10):1251–1265. doi: 10.1080/14656566.2019.1609940. [DOI] [PubMed] [Google Scholar]
- Chawla B, Madhubala R. Drug targets in Leishmania. J Parasit Dis. 2010;34(1):1–13. doi: 10.1007/s12639-010-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Ren B, Chen M, Wang Q, Zhang L, Yan G. NLLSS: predicting synergistic drug combinations based on semi-supervised learning. PLoS Comput Biol. 2016;12(7):e1004975. doi: 10.1371/journal.pcbi.1004975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Podust LM, Roush WR. Drug strategies targeting CYP51 in neglected tropical diseases. Chem Rev. 2014;114:11242–11271. doi: 10.1021/cr5003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AT, McLauchlan CC, Dolbecq A, Mialane P, Jones MA (2016) Studies of the effectiveness of bisphosphonate and vanadium-bisphosphonate compounds in vitro against axenic leishmania tarentolae. Oxid Med Cell Longev 2016 [DOI] [PMC free article] [PubMed]
- Cosconati S, Forli S, Perryman AL, Harris R, Goodsell DS, Olson AJ. Virtual screening with AutoDock: theory and practice. Expert Opin Drug Discov. 2010;5(6):597–607. doi: 10.1517/17460441.2010.484460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa CHN, Peters NC, Maruyama SR, de Brito EC, de Miranda Santos IKF. Vaccines for the leishmaniases: proposals for a research agenda. PLoS Negl Trop Dis. 2011;5(3):e943. doi: 10.1371/journal.pntd.0000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnet S, Schaller H. Metabolism and biological activities of 4-methyl-sterols. Molecules. 2019;24(3):451. doi: 10.3390/molecules24030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Macedo-Silva ST, Urbina JA, De Souza W, Rodrigues JCF. In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS ONE. 2013;8(12):e83247. doi: 10.1371/journal.pone.0083247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Macedo-Silva ST, Visbal G, Urbina JA, De Souza W, Rodrigues JCF. Potent in vitro antiproliferative synergism of combinations of ergosterol biosynthesis inhibitors against Leishmania amazonensis. Antimicrob Agents Chemother. 2015;59(10):6402–6418. doi: 10.1128/AAC.01150-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Menezes JPB, Guedes CES, De Oliveira Almeida Petersen AL, Fraga DBM, Veras PST. Advances in development of new treatment for leishmaniasis. Biomed Res Int. 2015;2015:15–18. doi: 10.1155/2015/815023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza W, Rodrigues JCF. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip Perspect Infect Dis. 2009;2009:1–19. doi: 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar MK, Koul A, Kaul S. Farnesyl pyrophosphate synthase: a key enzyme in isoprenoid biosynthetic pathway and potential molecular target for drug development. N Biotechnol. 2013;30(2):114–123. doi: 10.1016/j.nbt.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Dhir N, Jain A, Mahendru D, Prakash A, Medhi B (2020) Drug Repurposing and Orphan Disease Therapeutics. In: Drug Repurposing [Working Title]. IntechOpen
- Dinesh N, Pallerla DSR, Kaur PK, Kishore Babu N, Singh S. Exploring Leishmania donovani 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) as a potential drug target by biochemical, biophysical and inhibition studies. Microb Pathog. 2014;66:14–23. doi: 10.1016/j.micpath.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Dinesh N, Neelagiri S, Kumar V, Singh S. Glycyrrhizic acid attenuates growth of Leishmania donovani by depleting ergosterol levels. Exp Parasitol. 2017;176:21–29. doi: 10.1016/j.exppara.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diro E, Blesson S, Edwards T, Ritmeijer K, Fikre H, Admassu H, et al. A randomized trial of Am Bisome monotherapy and Am Bisome and miltefosine combination to treat visceral leishmaniasis in HIV co-infected patients in Ethiopia. PLoS Negl Trop Dis. 2019;13(1):e0006988. doi: 10.1371/journal.pntd.0006988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Bisphosphonates as chemotherapeutic agents against trypanosomatid and apicomplexan parasites. Current drug targets. Infect Disorders. 2001;1:51–61. doi: 10.2174/1568005013343191. [DOI] [PubMed] [Google Scholar]
- Emami S, Tavangar P, Keighobadi M. An overview of azoles targeting sterol 14α-demethylase for antileishmanial therapy. Eur J Med Chem. 2017;135:241–259. doi: 10.1016/j.ejmech.2017.04.044. [DOI] [PubMed] [Google Scholar]
- Ferreira C, Soares DC, Cunha Do Nascimento MT, Pinto-da-Silva LH, Sarzedas CG, Tinoco LW, et al. Resveratrol is active against Leishmania amazonensis: in vitro effect of its association with amphotericin B. Antimicrob Agents Chemother. 2014;58(10):6197–6208. doi: 10.1128/AAC.00093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: implications for drug design. Proteins Struct Funct Genet. 2006;62(1):80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- Gabor K, Fessler M. Roles of the mevalonate pathway and cholesterol trafficking in pulmonary host defense. Curr Mol Pharmacol. 2016;10(1):27–45. doi: 10.2174/1874467209666160112123603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M, Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des Devel Ther. 2018;12:25–40. doi: 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giner J-L, Zhao H. Detailed sterol compositions of two pathogenic rust fungi. Lipids. 2004;39(8):763–767. doi: 10.1007/s11745-004-1293-4. [DOI] [PubMed] [Google Scholar]
- Granthon AC, Braga MV, Rodrigues JCF, Cammerer S, Lorente SO, Gilbert IH, et al. Alterations on the growth and ultrastructure of Leishmania chagasi induced by squalene synthase inhibitors. Vet Parasitol. 2007;146(1–2):25–34. doi: 10.1016/j.vetpar.2006.12.022. [DOI] [PubMed] [Google Scholar]
- Gros L, Castillo-Acosta VM, Jiménez CJ, Sealey-Cardona M, Vargas S, Estévez AM, et al. New azasterols against Trypanosoma brucei: role of 24-sterol methyltransferase in inhibitor action. Antimicrob Agents Chemother. 2006;50(8):2595–2601. doi: 10.1128/AAC.01508-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Liu J, Nes WD, Waterman MR, Lepesheva GI. Substrate preferences and catalytic parameters determined by structural characteristics of sterol 14α-demethylase (CYP51) from Leishmania infantum. J Biol Chem. 2011;286(30):26838–26848. doi: 10.1074/jbc.M111.237099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart DT (ed) (1989) Status TC. Leishmaniasis. Springer, Boston. 10.1007/978-1-4613-1575-9
- Hughes JP, Rees SS, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2010;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez E, Sánchez A, Vasquez MA, Martínez A, Olivas J, Sattler A, et al. Metal-drug synergy: New ruthenium(II) complexes of ketoconazole are highly active against Leishmania major and Trypanosoma cruzi and nontoxic to human or murine normal cells. J Biol Inorg Chem. 2013;18(7):779–790. doi: 10.1007/s00775-013-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K, Jain NK. Vaccines for visceral leishmaniasis: a review. J Immunol Methods. 2015;422:1–12. doi: 10.1016/j.jim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Jain V, Jain K. Molecular targets and pathways for the treatment of visceral leishmaniasis. Drug Discov Today. 2018;23(1):161–170. doi: 10.1016/j.drudis.2017.09.006. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Roberts SC, Jardim A, Carter NS, Shin S, Ariyanayagam M, et al. Ornithine decarboxylase gene deletion mutants of Leishmania donovani. J Biol Chem. 1999;274(6):3781–3788. doi: 10.1074/jbc.274.6.3781. [DOI] [PubMed] [Google Scholar]
- Kaneshiro ES, Collins MS, Cushion MT. Inhibitors of sterol biosynthesis and amphotericin B reduce the viability of Pneumocystis carinii f. sp. carinii. Antimicrob Agents Chemother. 2000;44(6):1630–1638. doi: 10.1128/AAC.44.6.1630-1638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasam VK (2009) In silico drug discovery on computational Grids for finding novel drugs against neglected diseases (September)
- Keighobadi M, Emami S, Lagzian M, Fakhar M, Rafiei A, Valadan R. Molecular modeling and structural stability of wild-type and mutant CYP51 from leishmania major: In vitro and in silico analysis of a laboratory strain. Molecules. 2018;23(3):696. doi: 10.3390/molecules23030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholoud K, Denis S, Lahouari B, El Hidan MA, Souad B. Management of leishmaniases in the era of climate change in Morocco. Int J Environ Res Public Health. 2018;15(7):1542. doi: 10.3390/ijerph15071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kone AK, Niaré DS, Piarroux M, Izri A, Marty P, Laurens MB, et al. Visceral leishmaniasis in West Africa: clinical characteristics, vectors, and reservoirs. J Parasitol Res. 2019;2019:9282690. doi: 10.1155/2019/9282690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GA, Roy S, Jafurulla M, Mandal C, Chattopadhyay A. Statin-induced chronic cholesterol depletion inhibits Leishmania donovani infection: relevance of optimum host membrane cholesterol. Biochim Biophys Acta Biomembr. 2016;1858(9):2088–2096. doi: 10.1016/j.bbamem.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Kumar Saha A, Mukherjee T, Bhaduri A. Mechanism of action of amphotericin B on Leishmania donovani promastigotes. Mol Biochem Parasitol. 1986;19(3):195–200. doi: 10.1016/0166-6851(86)90001-0. [DOI] [PubMed] [Google Scholar]
- Kwofie SK, Broni E, Teye J, Quansah E, Issah I, Wilson MD, et al. Pharmacoinformatics-based identification of potential bioactive compounds against Ebola virus protein VP24. Comput Biol Med. 2019;113(August):103414. doi: 10.1016/j.compbiomed.2019.103414. [DOI] [PubMed] [Google Scholar]
- Kwofie SK, Broni E, Dankwa B, Enninful KS, Kwarko GB, Darko L, et al. Outwitting an old neglected nemesis: a review on leveraging integrated data-driven approaches to aid in unraveling of leishmanicides of therapeutic potential. Curr Top Med Chem. 2020;20(5):349–366. doi: 10.2174/1568026620666200128160454. [DOI] [PubMed] [Google Scholar]
- Kwofie SK, Broni E, Dankwa B, Enninful KS, Teye J, Davidson CR et al (2020) Review of atypical organometallic compounds as antimalarial drugs. 10.1155/2020/9414093
- Lage OM, Ramos MC, Calisto R, Almeida E, Vasconcelos V, Vicente F. Current screening methodologies in drug discovery for selected human diseases. Mar Drugs. 2018;16:279. doi: 10.3390/md16080279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pape P. Development of new antileishmanial drugs—current knowledge and future prospects. J Enzyme Inhib Med Chem. 2008;23(5):708–718. doi: 10.1080/14756360802208137. [DOI] [PubMed] [Google Scholar]
- Leañez J, Nuñez J, García-Marchan Y, Sojo F, Arvelo F, Rodriguez D, et al. Anti-leishmanial effect of spiro dihydroquinoline-oxindoles on volume regulation decrease and sterol biosynthesis of Leishmania braziliensis. Exp Parasitol. 2019;198:31–38. doi: 10.1016/j.exppara.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. Sterol 14alpha-demethylase (CYP51) as a therapeutic target for human trypanosomiasis and leishmaniasis. Curr Top Med Chem. 2011;11(16):2060–2071. doi: 10.2174/156802611796575902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux AE, Krauth-Siegel RL. Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol Biochem Parasitol. 2016;206(1–2):67–74. doi: 10.1016/j.molbiopara.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Leta S, Dao THT, Mesele F, Alemayehu G. Visceral Leishmaniasis in Ethiopia: an evolving disease. PLoS Negl Trop Dis. 2014;8(9):e3131. doi: 10.1371/journal.pntd.0003131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionta E, Spyrou G, Vassilatis D, Cournia Z. Structure-based virtual screening for drug discovery: principles, applications and recent advances. Curr Top Med Chem. 2014;14(16):1923–1938. doi: 10.2174/1568026614666140929124445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente SO, Rodrigues JCF, Jiménez CJ, Joyce-Menekse M, Rodrigues C, Croft SL, et al. Novel azasterols as potential agents for treatment of leishmaniasis and trypanosomiasis. Antimicrob Agents Chemother. 2004;48(8):2937–2950. doi: 10.1128/AAC.48.8.2937-2950.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaraci F, Jimenez Jimenez C, Rodrigues C, Rodrigues JCF, Vianna Braga M, Yardley V, et al. Azasterols as Inhibitors of Sterol 24-Methyltransferase in Leishmania Species and Trypanosoma cruzi. J Med Chem. 2003;46(22):4714–4727. doi: 10.1021/jm021114j. [DOI] [PubMed] [Google Scholar]
- Martin MB, Grimley JS, Lewis JC, Heath HT, Bailey BN, Kendrick H, et al. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. J Med Chem. 2001;44(6):909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- McCall LI, El Aroussi A, Choi JY, Vieira DF, De Muylder G, Johnston JB, et al. Targeting ergosterol biosynthesis in Leishmania donovani: essentiality of Sterol 14alpha-demethylase. PLoS Negl Trop Dis. 2015;9(3):1–17. doi: 10.1371/journal.pntd.0003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C, Silva LE, Tanner AL, Stuart K, Pollastri MP. Kinases as druggable targets in trypanosomatid protozoan parasites. Chem Rev. 2014;114:11280–11304. doi: 10.1021/cr500197d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. Drug resistance in leishmaniasis: newer developments. Trop Parasitol. 2014;4(1):4. doi: 10.4103/2229-5070.129142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvetti A, Peña-Díaz J, Hurtado R, Ruiz-Pérez LM, González-Pacanowska D. Characterization and regulation of Leishmania major 3-hydroxy-3-methylglutaryl-CoR reductase. Biochem J. 2000;349(1):27–34. doi: 10.1042/bj3490027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Xu W, Hsu FF, Patel J, Huang J, Zhang K. Sterol methyltransferase is required for optimal mitochondrial function and virulence in Leishmania major. Mol Microbiol. 2019;111(1):65–81. doi: 10.1111/mmi.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJN, et al. Recent developments in drug discovery for leishmaniasis and human african trypanosomiasis. Chem Rev. 2014;114:11305–11347. doi: 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nare B, Hardy LW, Beverley SM. The roles of pteridine reductase 1 and dihydrofolate reductase- thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J Biol Chem. 1997;272(21):13883–13891. doi: 10.1074/jbc.272.21.13883. [DOI] [PubMed] [Google Scholar]
- Navabi A, Soleimanifard S. Enzymatic characterization of acid phosphatase in the logarithmic and stationary phase of leishmania major promastigotes. Shiraz E Med J. 2015;16(1):1–4. doi: 10.17795/semj26246. [DOI] [Google Scholar]
- Ning Y, Frankfater C, Hsu F-F, Soares RP, Cardoso CA, Nogueira PM et al (2020) Lathosterol oxidase (sterol C5-desaturase) deletion confers resistance to amphotericin B and sensitivity to acidic stress in Leishmania major. bioRxiv; 2020.04.20.051540 [DOI] [PMC free article] [PubMed]
- Nowosielski M, Hoffmann M, Wyrwicz LS, Stepniak P, Plewczynski DM, Lazniewski M, et al. Detailed mechanism of squalene epoxidase inhibition by terbinafine. J Chem Inf Model. 2011;51(2):455–462. doi: 10.1021/ci100403b. [DOI] [PubMed] [Google Scholar]
- Ong YC, Roy S, Andrews PC, Gasser G. Metal compounds against neglected tropical diseases. Chem Rev. 2019;119:730–796. doi: 10.1021/acs.chemrev.8b00338. [DOI] [PubMed] [Google Scholar]
- Ortiz-Gómez A, Jiménez C, Estévez AM, Carrero-Lérida J, Ruiz-Pérez LM, González-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate. Eukaryot Cell. 2006;5(7):1057–1064. doi: 10.1128/EC.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oryan A, Akbari M. Worldwide risk factors in leishmaniasis. Asian Pac J Trop Med. 2016;9:925–932. doi: 10.1016/j.apjtm.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Padmanabhan PK, Mukherjee A, Singh S, Chattopadhyaya S, Gowri VS, Myler PJ, et al. Glyoxalase I from Leishmania donovani: a potential target for anti-parasite drug. Biochem Biophys Res Commun. 2005;337(4):1237–1248. doi: 10.1016/j.bbrc.2005.09.179. [DOI] [PubMed] [Google Scholar]
- Parihar SP, Hartley MA, Hurdayal R, Guler R, Brombacher F. Topical simvastatin as host-directed therapy against severity of cutaneous leishmaniasis in mice. Sci Rep. 2016;6(1):33458. doi: 10.1038/srep33458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Moreno G, Sealey-Cardona M, Rodrigues-Poveda C, Gelb MH, Ruiz-Pérez LM, Castillo-Acosta V, et al. Endogenous sterol biosynthesis is important for mitochondrial function and cell morphology in procyclic forms of Trypanosoma brucei. Int J Parasitol. 2012;42(11):975–989. doi: 10.1016/j.ijpara.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Pinto EG, Tempone AG. Activity of the antiarrhythmic drug amiodarone against Leishmania (L.) infantum: an in vitro and in vivo approach. J Venom Anim Toxins Incl Trop Dis. 2018;24(1):29. doi: 10.1186/s40409-018-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomel S, Cojean S, Loiseau PM. Targeting sterol metabolism for the development of antileishmanials. Trends Parasitol. 2015;31:5–7. doi: 10.1016/j.pt.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):1–24. doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschak E, Stark H, Merk D. Polypharmacology by design: a medicinal chemist’s perspective on multitargeting compounds. J Med Chem. 2019;62:420–444. doi: 10.1021/acs.jmedchem.8b00760. [DOI] [PubMed] [Google Scholar]
- Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, et al. Drug repurposing: Progress, challenges and recommendations. Nat Rev Drug Discov. 2018;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- Reid PC, Urano Y, Kodama T, Hamakubo T. Alzheimer’s disease: Cholesterol, membrane rafts, isoprenoids and statins. J Cell Mol Med. 2007;11(3):383–392. doi: 10.1111/j.1582-4934.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JCF, Attias M, Rodriguez C, Urbina JA, De Souza W. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a Δ24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrob Agents Chemother. 2002;46(2):487–499. doi: 10.1128/AAC.46.2.487-499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JCF, Bernardes CF, Visbal G, Urbina JA, Vercesi AE, de Souza W. Sterol methenyl transferase inhibitors alter the ultrastructure and function of the leishmania amazonensis mitochondrion leading to potent growth inhibition. Protist. 2007;158(4):447–456. doi: 10.1016/j.protis.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Rodrigues JCF, Concepcion JL, Rodrigues C, Caldera A, Urbina JA, De Souza W. In vitro activities of ER-119884 and E5700, two potent squalene synthase inhibitors, against Leishmania amazonensis: antiproliferative, biochemical, and ultrastructural effects. Antimicrob Agents Chemother. 2008;52(11):4098–4114. doi: 10.1128/AAC.01616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues IA, Mazotto AM, Cardoso V, Alves RL, Amaral ACF, Silva JRDA, et al. Natural products: insights into leishmaniasis inflammatory response. Mediators Inflamm. 2015;2015:835910. doi: 10.1155/2015/835910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryley JF (ed) (1990) Chemotherapy of fungal diseases. Springer, Berlin (Handbook of Experimental Pharmacology; vol 96). 10.1007/978-3-642-75458-6
- Semini G, Paape D, Paterou A, Schroeder J, Barrios-Llerena M, Aebischer T. Changes to cholesterol trafficking in macrophages by Leishmania parasites infection. Microbiologyopen. 2017;6(4):e00469. doi: 10.1002/mbo3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Martín X, García-Marchan Y, Fernandez A, Rodriguez N, Rojas H, Visbal G, et al. Amiodarone destabilizes intracellular Ca2+ homeostasis and biosynthesis of sterols in leishmania mexicana. Antimicrob Agents Chemother. 2009;53(4):1403–1410. doi: 10.1128/AAC.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya N, Bajpai P, Gupta S. Therapeutic switching in leishmania chemotherapy: a distinct approach towards unsatisfied treatment needs. J Parasit Dis. 2011;35:104–112. doi: 10.1007/s12639-011-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri A, Emami S, Fakhar M, Teshnizi SH, Keighobadi M. In vitro antileishmanial activity of novel azoles (3-imidazolylflavanones) against promastigote and amastigote stages of Leishmania major. Acta Trop. 2017;167:73–78. doi: 10.1016/j.actatropica.2016.12.027. [DOI] [PubMed] [Google Scholar]
- Shokri A, Abastabar M, Keighobadi M, Emami S, Fakhar M, Teshnizi SH, et al. Promising antileishmanial activity of novel imidazole antifungal drug luliconazole against Leishmania major: in vitro and in silico studies. J Glob Antimicrob Resist. 2018;14:260–265. doi: 10.1016/j.jgar.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Silva MS, Ferreira AEN, Tomás AM, Cordeiro C, Freire AP. Quantitative assessment of the glyoxalase pathway in Leishmania infantum as a therapeutic target by modelling and computer simulation. FEBS J. 2005;272(10):2388–2398. doi: 10.1111/j.1742-4658.2005.04632.x. [DOI] [PubMed] [Google Scholar]
- Singh S, Babu NK (2018) 3-Hydroxy-3-methylglutaryl-CoA Reductase (HMGR) enzyme of the sterol biosynthetic pathway: a potential target against visceral leishmaniasis. Leishmaniases as Re-emerging Dis
- Singh OP, Singh B, Chakravarty J, Sundar S. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect Dis Poverty. 2016 doi: 10.1186/s40249-016-0112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: An open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377(9764):477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- Tamiru A, Tigabu B, Yifru S, Diro E, Hailu A. Safety and efficacy of liposomal amphotericin B for treatment of complicated visceral leishmaniasis in patients without HIV, North-West Ethiopia. BMC Infect Dis. 2016;16(1):548. doi: 10.1186/s12879-016-1746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Távora LGF, Nogueira MB, Gomes ST. Visceral Leishmaniasis/HIV co-infection in northeast Brazil: evaluation of outcome. Braz J Infect Dis. 2015;19(6):651–656. doi: 10.1016/j.bjid.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari N, Gedda MR, Tiwari VK, Singh SP, Singh RK. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini-Reviews Med Chem. 2017;18(1):26–41. doi: 10.2174/1389557517666170425105129. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Lazardi K, Marchan E, Visbal G, Aguirre T, Piras MM, et al. Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob Agents Chemother. 1993;37(3):580–591. doi: 10.1128/AAC.37.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R. Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana. Mol Biochem Parasitol. 2002;125(1–2):35–45. doi: 10.1016/S0166-6851(02)00206-2. [DOI] [PubMed] [Google Scholar]
- Vannier-Santos MA, Urbina JA, Martiny A, Neves A, de Souza W. Alterations induced by the antifungal compounds ketoconazole and terbinafine in leishmania. J Eukaryot Microbiol. 1995;42(4):337–346. doi: 10.1111/j.1550-7408.1995.tb01591.x. [DOI] [PubMed] [Google Scholar]
- Veiga-Santos P, Barrias ES, Santos JFC, De Barros Moreira TL, De Carvalho TMU, Urbina JA, et al. Effects of amiodarone and posaconazole on the growth and ultrastructure of Trypanosoma cruzi. Int J Antimicrob Agents. 2012;40(1):61–71. doi: 10.1016/j.ijantimicag.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Verma A, Srivastava S, Sane SA, Marrapu VK, Srinivas N, Yadav M, et al. Antileishmanial activity of benzocycloalkyl azole oximino ethers: the conformationally constraint analogues of oxiconazole. Acta Trop. 2011;117(2):157–160. doi: 10.1016/j.actatropica.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Verma AK, Laha B, Pandey M, Pal U, Ghosh M. Cholesterol-lowering drug, in combination with chromium chloride, induces early apoptotic signals in intracellular L. donovani amastigotes, leading to death. J Biosci. 2017;42(3):427–438. doi: 10.1007/s12038-017-9690-9. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Das P. Recent progress in drug targets and inhibitors towards combating leishmaniasis. Acta Trop. 2017;2018(181):95–104. doi: 10.1016/j.actatropica.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Ward NC, Watts GF, Eckel RH. Statin toxicity: mechanistic insights and clinical implications. Circ Res. 2019;124:328–350. doi: 10.1161/CIRCRESAHA.118.312782. [DOI] [PubMed] [Google Scholar]
- WHO (2020) Leishmaniasis. WHO
- Wortmann G, Zapor M, Ressner R, Fraser S, Hartzell J, Pierson J, et al. Lipsosomal amphotericin B for treatment of cutaneous leishmaniasis. Am J Trop Med Hyg. 2010;83(5):1028–1033. doi: 10.4269/ajtmh.2010.10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Hsu FF, Baykal E, Huang J, Zhang K. Sterol biosynthesis is required for heat resistance but not extracellular survival in leishmania. PLoS Pathog. 2014;10(10):e1004427. doi: 10.1371/journal.ppat.1004427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Wilson ME. Dynamics of sterol synthesis during development of Leishmania spp. parasites to their virulent form. Parasites Vectors. 2016;9(1):1–12. doi: 10.1186/s13071-016-1470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley V, Khan AA, Martin MB, Slifer TR, Araujo FG, Moreno SNJ, et al. In vivo activities of farnesyl pyrophosphate synthase inhibitors against Leishmania donovani and Toxoplasma gondii. Antimicrob Agents Chemother. 2002;46(3):929–931. doi: 10.1128/AAC.46.3.929-931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar B, Jones AJ, Sykes ML, Shelper TB, Davis RA, Avery VM. Screening a natural product-based library against kinetoplastid parasites. Molecules. 2017;22(10):1–19. doi: 10.3390/molecules22101715. [DOI] [PMC free article] [PubMed] [Google Scholar]