Abstract
The nematode parasite Neoascarophis mariae was collected from the intestine of Parupeneus forsskali and Mulloidichthys flavolineatus from the Red Sea in Egypt and was described using both light and scanning electron microscopy. It is mainly characterized by the presence of four submedian labia surrounded the oral aperture in the outer side, two sublabia and two pseudolabia in the inner side, rounded cephalic end, and short vestibule in both sexes. The females vulva is located near the posterior end of the body, their oval uterine eggs have a smooth surface devoid of filaments. Males have bifurcated deirids, a large left spicule (85–425 µm) with a tapered tip, and a short right spicule (50–181 µm) with a broad and rounded tip.
Keywords: Neoascarophis mariae, Nematode, Parupeneus forsskali, Mulloidichthys flavolineatus, Red Sea, Egypt
Introduction
Goatfish, of the family Mullidae are widely distributed throughout the Pacific and Indo-Pacific regions. Goatfish has three common genera, that exists in Hurghada, Red Sea. They are Upeneus, Parupeneus and Mulloidichthys (Kuronuma and Abe 1986). Goatfishes are an important component of demersal fish assemblages and artisanal fisheries that are established around coral reefs in shallow water of the Red Sea (Kumaran and Randall 1984). Goat fishes are considered to be the most economically and commercially important species inhabiting the northern Egyptian Red Sea sector). Mulloidichthys flavolineatus feeds mostly on polychaetes, tanaids and harpacticoid (Kolasinski et al. 2009). Nematodes of the cystidicolid Neoascarophis Machida, 1976, are all parasites of macrourid fishes, nematodes referable to Neascarophis were found in ulcers in the gastric mucosa (Moravec and Klimpel 2009).
Nematodes of the family Cystidicolidae Skrjabin 1946 are common parasites of mullids, with six species of the genus Ascarophis Van Beneden 1870 reported in mullets. The classification of this family is problematic (Moravec 2007), with several genera closely related to Ascarophis being erected or re-erected based on differences in cephalic structure (Moravec et al. 2007).
Neoascarophis Machida, 1976 is distinguished from Ascarophis by having a very short vestibule (Moravec et al. 2006). Although Machida (1976) pointed out differences in the mouth structure, this is practically identical in both genera (Ko 1986; Moravec et al. 2006). However, Ascarophis upeneichthys also has a short vestibule (Johnston and Mawson 1945). Due to this characteristic, Machida (1976) suggested that this species may belong to Neoascarophis; however, it has been considered within Ascarophis in recent systematic studies (Ferrer et al. 2005; Brugni and Viozzi 2008). At the same time, the specimens were described as Ascarophis upeneichthys were transferred to Neoascarophis upeneichthys (Johnston and Mawson 1945). Neoascarophis mariae was described from Mullus argentinae (Family Mullidae) from Brazil (Pereira et al. 2012). Neoascarophis macrouri, which was discovered in the stomach of a Macrourus berglax individual from the North Atlantic Ocean's marine waters, was recorded as a new species by Moravec et al. (2006). A Neoascarophis sphaerocaudata specimen was identified inside a Macrourus carinatus in Argentine water (Rossin et al. 2012). And a Neoascarophis sinensis was described as a new species following its discovery in a Conger myriaster in the Yellow and East China Seas. This paper denoted the first report of Neoascarophis mariae (Nematoda: Cystidicolidae) parasitic in Parupeneus forsskali and Mulloidichthys flavolineatus (Perciformes: Mullidae) from the Red Sea in Egypt using LM and SEM.
Materials and methods
Sample collection
A total of 179 fish samples were collected from the coast of Hurghada and Safaga in the Red Sea of Egypt from February 2016 to January 2017. The samples belonged to three orders: Beloniformes, Beryciformes, and Perciformes. Fishes were captured and freshly transported to the Parasitology Laboratory, Zoology Department, Faculty of Science, South Valley University, at the Qena Governorate, Egypt. Fishes were identified according to the criteria (Randall 1983; Lieske and Myers 2004; Lieske et al. 2004) and confirmed based on the information on the FishBase website (http://www.fishbase.org).
Macroscopic examination
The gastrointestinal tract was untangled using fingers (Justine et al. 2012). The collected nematodes were cleaned and preserved in bottles containing a mixture of 70% alcohol and 5% glycerin.
Light microscope examination
For microscopical examination, nematodes were mounted, photographed, and drawn using a camera lucida (Hussein et al. 2020). The samples were identified using keys for vertebrate nematode parasites (Yorke et al. 1926; Yamaguti 1963; Anderson et al. 2009).
Scanning electron microscope (SEM) examination
For SEM studies, the specimens were fixed for six hours at 4 °C in 3% buffered glutaraldehyde, washed several times in 0.1 M sodium cacodylate buffer, dehydrated in ascending ethanol concentrations, and transferred to pure acetone. Samples were then processed in a BOMER–900 critical point dryer with Freon 13. The dried samples were sputter-coated with gold in a Technics Hummer V (Lee 1993) and examined with a JEOL JSM5400LV SEM operated at 15 kV in the Electron Microscopy Unit at Assiut University.
Results
We examined 179 marine fish specimens for Neoascarophis mariae parasites and found that only fishes of the Perciformes order were infected with Neoascarophis mariae nematodes. The nematodes were found in 12 of 29 specimens of Parupeneus forsskali Fourmanoir Guézé, 1976, and in one of 24 specimens of Mulloidichthys flavolineatus Lacepède, 1801 (Mullidae), (worm burden: 2–5 and 2, respectively).
Morphology
(Based on 15 mature specimens; 6♂ and 9♀), (Figs. 1, 2 and 3).
Fig. 1.
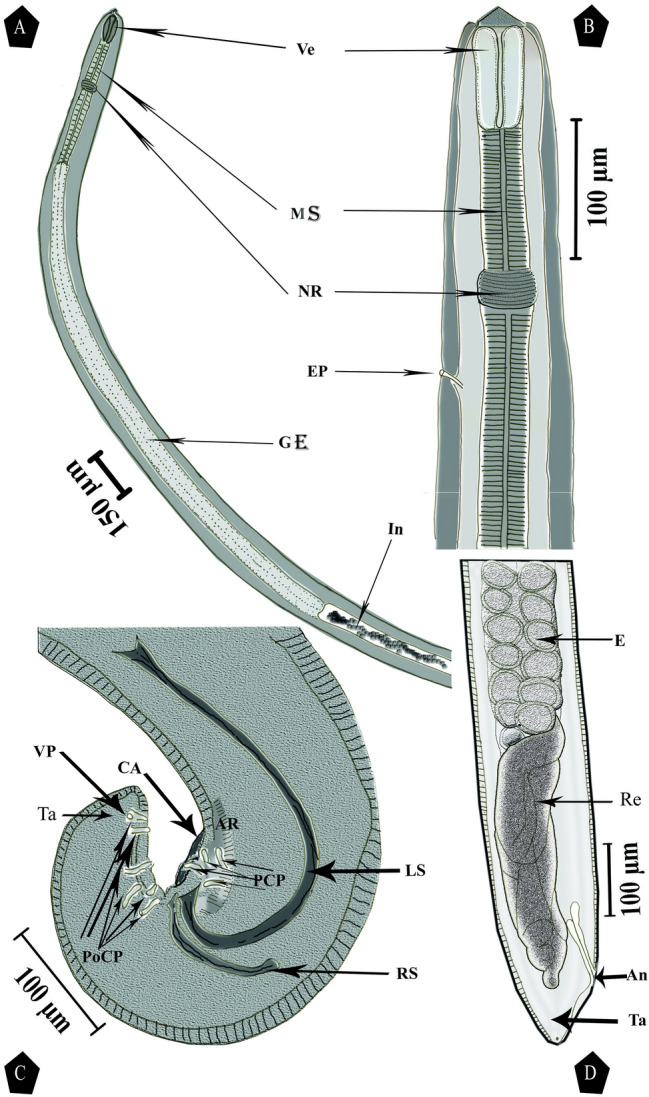
Camera lucida drawing of Neoascarophis mariae infecting Parupeneus forsskali and Mulloidichthys flavolineatus showing: a Lateral view of male anterior extremity. b Ventrolateral view of enlarged female anterior extremity. c Lateral view of enlarged male posterior extremity. d Lateral view of enlarged female posterior extremity. Ve, vestibule; MS, muscular esophagus portion; NR, nerve ring; EP, excretory pores; GE, glandular esophagus portion; In, intestine; Re, rectum; An, anus; E, egg; Ta, tail; CA, caudal alae; RS, right spicule; LS, left spicule; PCP, pre-cloacal papillae; PoCP, post-cloacal papillae; VP, ventral papillae; AR, area rugosa
Fig. 2.
SEM micrographs of Neoascarophis mariae male infecting Parupeneus forsskali and Mulloidichthys flavolineatus showing: a Lateral view of male anterior extremity. b Lateral high-magnification view of male anterior extremity, showing cephalic papillae (CP), amphaid (AM), deirid (D), and excretory pore (EP). c Subapical view of male cephalic end showing cephalic papillae (CP), amphaid (AM), pseudolabium (P), and sublabium (Sl). d Lateral view of male posterior extremity showing four pairs pre-cloacal papillae (PCP) and post-cloacal papillae (PoCP). e Lateral view of male posterior extremity showing caudal alae (CA), area rugosa (AR), and a pair of ventral papilla (VP). f Lateral view of male posterior extremity showing pre-cloacal papillae (PCP), post-cloacal papillae (PoCP), and area rugosa (AR)
Fig. 3.

SEM micrographs of Neoascarophis mariae female infecting Parupeneus forsskali and Mulloidichthys flavolineatus showing: a Apical view of the female cephalic end showing cephalic papillae (CP), amphaid (AM), pseudolabium (P), and sublabium (Sl). b Ventral view of the middle part of the female showing vulval opening (VO). c Ventral view of female posterior extremity showing anus (An) and a pair of phasmids (Ph)
The collected specimens were small-sized nematodes. Live specimens are whitish, filiform, females larger than males, with rounded cephalic end in both sexes (Fig. 1a, b). The vestibule is short with an indistinct prostom visible in the lateral view, glandular part of the esophagus more than twice as long as the muscular portion (Fig. 1a, b). Nerve ring encircles the muscular part of the esophagus near its posterior third. The excretory pore lies posterior to the nerve ring (Figs. 1b, 2b).
Male
Body 3.7–6.4 mm in length, 88–208 µm with a maximum width, vestibule, including a very short prostome, 32–65 µm in length, muscular part of the esophagus measuring 163–474 µm in length 20–47 µm in width and glandular part of the esophagus 1.462–1.858 mm in length, 47–70 µm in width. The length ratio of the muscular and glandular parts of the esophagus is 1:3.5–5; length of the entire esophagus and vestibule representing 36–51 (43)% of total body length. Nerve ring is located approximately 78–274 µm from the anterior extremity. Excretory pore is located approximately 89–408 µm from the anterior extremity (Table 1 and Fig. 1a), posterior end of the body is spirally coiled (Fig. 1c). Left spicule 85–425 µm in length, with tapered distal tip and sub-terminal barb providing a fish hook-like tip; right spicule 50–181 µm in length with a broad and rounded distal tip (Fig. 1c). The length ratio of the spicules 1:1.3–2.7, conical tail with rounded tip measuring 71–180 µm in length.
Table 1.
Comparative measurements (in millimeters) of the collected Neoascarophis mariae specimens with the previously described forms
| Reference | Moravec et al. (2006) | Moravec and Klimpel (2009) | Rossin et al. (2012) | Pereira et al. (2012) | Chen et al. (2017) | Present study |
|---|---|---|---|---|---|---|
| Related species | Neoascarophis macrouri | Neoascarophis longispicula | Neoascarophis sphaerocaudata | Neoascarophis mariae | Neoascarophis sinensis | Neoascarophis mariae |
| Fish host (s) |
Macrourus berglax (Family: Macrouridae) |
Coryphaenoides mediterraneus (Family: Macrouridae) |
Macrourus carinatus (Family: Macrouridae) |
Mullus argentinae (Perciformes: Mullidae) |
Conger myriaster (Perciformes: Congridae) |
Parupeneus forsskali and Mulloidichthys flavolineatus (Perciformes: Mullidae) |
| Locality | Eastern Greenland Sea | Mid-Atlantic Ridge | Argentinean | Coastal zone of Rio de Janeiro, Brazil | Yellow and East China Seas | Hurghada and Safaga, Egypt in Red Sea |
| Site of infection | Stomach | Digestive tract | Stomach | Stomach and intestine | Stomach | Intestine |
| Parameters | ||||||
| Body length | ||||||
| Male | 5.83–6.92 | 8.43–15.78 | 7.4–8.9 | 3.6–5.9 | 8.5–10.5 | 3.7–6.4 |
| Female | 8.92–12.57 | Unknown | 18.8–22.7 | 5.1–9.9 | 9.5–14.0 | 6.4–8.3 |
| Maximum body width | ||||||
| Male | 0.204–0.245 | 0.517–0.558 | 0.270–0.330 | 0.79 − 0.150 | 0.129–0.178 | 0.088–0.208 |
| Female | 0.313–0.585 | Unknown | 1.100–1.360 | 0.112–0.222 | 0.178–0.297 | 0.120–0.250 |
| Vestibule length | ||||||
| Male | 0.54–0.60 | 0.60–0.75 | 0.50–0.52 | 0.45–0.64 | 0.40–0.50 | 0.32–0.65 |
| Female | 0.68–0.95 | Unknown | 0.90–0.112 | 0.30–0.69 | 0.30–0.59 | 0.21–0.54 |
| Muscular esophagus length | ||||||
| Male | 0.339–0.408 | 1.44–1.97 | 1.0–1.2 | 0.300–0.380 | 0.426–0.475 | 0.163–0.474 |
| Female | 0.558–0.653 | Unknown | 1.1–2.8 | 0.315–0.494 | 0.328–0.465 | 0.93–0.428 |
| Muscular esophagus width | ||||||
| Male | 0.27–0.36 | 0.42–0.109 | 0.50–0.75 | 0.20–0.45 | 0.40 | 0.20–0.47 |
| Female | 0.54–0.82 | Unknown | 0.75–0.135 | 0.24–0.44 | 0.30–0.69 | 0.22–0.53 |
| Glandular esophagus length | ||||||
| Male | 2.30–2.80 | 3.22–4.94 | 2.4–3.3 | 0.963–1.587 | 2.5–3.1 | 1.462–1.858 |
| Female | 3.18–3.51 | Unknown | 6.5–8.0 | 1.027–2.223 | 3.1–3.5 | 335–1.800 |
| Glandular esophagus width | ||||||
| Male | 0.75–0.109 | 0.204–0.258 | 0.150–0.180 | Not mentioned | 0.99–0.109 | 0.47–0.70 |
| Female | 0.190–0.204 | Unknown | 0.300–0.500 | 0.61–0.94 | 0.99–0.139 | 0.51–0.69 |
| Length ratio of muscular and glandular parts of esophagus | ||||||
| Male | 1:5.6–7.6 | 1:1.68–2.83 | 1:2.3–2.7 | 1:2.9–5.2 | 1: 5.8–6.6 | 1:3.5–5 |
| Female | 1:5.4–5.7 | Unknown | 2.8–3.5 | 1:3.0–6.6 | 1:7.0–9.4 | 1:2.2–5.5 |
| Length of entire esophagus and vestibule% of body length | ||||||
| Male | 42–51% | 35–63% | 44–53% | 26–43% | 34.7–42.7% | 36–51% |
| Female | 34–46% | Unknown | 48–52% | 22–32% | 27.1–35.2% | 19–33% |
| Nerve ring from anterior extremity | ||||||
| Male | 0.228–0.313 | 0.313–0.476 | 0.255–0.317 | 0.183–0.227 | 0.238–0.267 | 0.78–0.274 |
| Female | 0.340–0.490 | Unknown | 0.450–0.550 | 0.166 –0.271 | 0.149–0.277 | 0.53–0.263 |
| Excretory pore from anterior extremity | ||||||
| Male | 0.326–0.408 | 0.462–0.639 | 0.387–0.437 | 0.232–0.313 | 0.287–0.327 | 0.89–0.408 |
| Female | 0.554–0.680 | Unknown | 0.700–0.850 | 0.183–0.375 | 0.168–0.356 | 0.88–0.402 |
| Right spicule length | ||||||
| Male | 0.153–0.204 | 0.267–0.351 | 0.187–0.200 | 0.137–0.198 | 0.25–0.145 | 0.50–0.181 |
| Female | – | – | – | – | – | |
| Left spicule length | ||||||
| Male | 0.567–0.615 | 0.960–1.149 | 0.537–0.650 | 0.272–0.390 | 0.400–0.413 | 0.85–0.425 |
| Female | – | – | – | – | – | – |
| Length ratio of spicules | ||||||
| Male | 1:2.8–3.9 | 1:3.25–5.32 | 1:3.2 | 1:1.7–2.5 | 1:2.8–3.2 | 1:1.3–2.7 |
| Female | – | – | – | – | – | – |
| Tail length | ||||||
| Male | 0.180–0.222 | 0.177–0.279 | 0.182–0.192 | 0.105–0.167 | 0.198 | 0.71–0.180 |
| Female | 0.95–0.122 | Unknown | 0.100–0.162 | 0.49 –0.79 | 0.119–0.188 | 0.49–0.115 |
| Vulva from posterior extremity | ||||||
| Male | – | – | – | – | – | – |
| Female | 0.585–0.762 | Unknown | 0.800–1.180 | 2.75–5.29 | 4.9–7.4 | 1.75–4.73 |
| Vulva from posterior extremity% body length | ||||||
| Male | – | – | – | – | – | – |
| Female | 93–94% | Unknown | 95–96% | 50–63% | 48.0–52.7% | 0.37–0.62 |
Female
Body tapering slightly anteriorly not posteriorly, widest at its posterior end, at the level of the anus, measuring 6.4–8.3 mm in length, 0.113–0.149 mm in width at the level of the esophagus-intestine junction and the maximum width is 0.120–0.250 mm. Vestibule, including prostome, is 21–54 µm, the muscular portion of the esophagus is 93–428 µm × 22–53 µm, and the glandular part of the esophagus is 335–1800 µm × 51–69 µm. The length ratio of the muscular and glandular parts of the esophagus is 1:2.2–5.5; length of the entire esophagus and vestibule represents 19–33% of the total body length. Nerve ring is located approximately 53–263 µm from the anterior extremity. Excretory pore is located approximately 88–402 µm from the anterior extremity (Fig. 1b). Vulva equatorial and situated approximately 1.75–4.73 mm from the posterior end of the body, approximately 37–62% of the body length, the uterus occupies a major part of the body and is filled with numerous eggs. Uterine eggs oval, thick-shelled, with a smooth surface, devoid of filaments, and measuring 23–46 µm × 12–25 µm (Table 1 and Fig. 1d). Tail with rounded tip measuring 49–115 µm (Fig. 1d).
Ultrastructure
The cephalic end with an oval oral aperture, laterally depressed and surrounded by four sub-median labia (two dorsolateral and two ventrolateral; each lateral pair partially fused), each bearing narrow sublabium on its inner side; further, sublabia was interrupted by a notch near the medial end. Lateral pseudolabia as long as wide, each provided with an oval apical protrusion with the inner parts of pseudolabia covering the mouth. There are four sub-median cephalic papillae and a pair of lateral amphids (Figs. 2a–c, 3a), small bifurcated deirids situated immediately anterior to the nerve ring, and excretory pore posterior to the nerve ring (Fig. 2a, b). Cuticle is thick with fine transverse striations and inflated in the cephalic region, forming a cephalic vesicle from the anterior end to the level of the deirids (Fig. 2a, b). The male posterior extremity provided with narrow vesicular caudal alae (Fig. 2e). Pre-cloacal papillae: four pairs of sub-ventral pedunculate papillae present, of which first and third lateral and anterior to second and fourth, respectively. Post-cloacal papillae: six pairs including five pairs of pedunculate sub-ventral papillae and a pair of minute ventral sessile papillae located at the level of the last sub-ventral pair and the first, second, fifth, and sixth at the same level but shifted laterally in relation to each other. Additionally, a pair of very small ventral papilla-like phasmids located posterior to the caudal papillae with anterior serrate ventral cuticular ridges (area rugosa) (Fig. 2d–f). The female posterior extremity flanked by a pair of minute phasmids (Fig. 3c). Vulval lips are not elevated (Fig. 3b).
Discussion
The collected specimens were identified as belonging to the family Cystidicolidae Skrjabin, 1946 according to the following criteria: Cephalic papillae reduced to four at the base of the pseudolabia, esophagus divided into a short anterior muscular portion and a long posterior glandular portion, presence of caudal alae, oval and larvated eggs, parasites of alimentary tract and swim bladder of fishes (Arai and Smith 2016).
The present specimens were assigned to the genus Neoascarophis Machida, 1976 based on the comparatively short vestibule than is found in members of Ascarophis upeneichthys.
Neoascarophis Machida, 1976 is currently comprised of nine species; six species parasitic in macrourid fishes as follows: Neoascarophis yarihige and Neoascarophis bathygadi was described from Coelorhynchus multispinulosus Katayama and Bathygadus garretti Gilbert and Hubbs both from Suruga Bay off the coast of Japan (Machida 1976); Neoascarophis insulana was described from Coryphaenoides acrolepis (Bean) and Albatrossia pectoralis (Gilbert) off the northern Kuril Islands in the Pacific Ocean (Solovjeva 1991); Neoascarophis macrouri was described from Macrourus berglax Lace´pe`de from the eastern Greenland Sea, North Atlantic Ocean (Moravec et al. 2006); Neoascarophis longispicula was described from Coryphaenoides mediterraneus (Giglioli) from the mid-Atlantic Ridge (Moravec and Klimpel 2009); and Neoascarophis sphaerocaudata was described from Macroourus carinatus (Gu¨nther) from Patagonian waters in Argentina (Rossin et al. 2012).
On the other hand, Neoascarophis upeneichthys was described from Upeneichthys lineatus (Family Mullidae) in Australian waters (Johnston and Mawson 1945), and Neoascarophis mariae was described from Mullus argentinae (Family Mullidae) in Brazil (Pereira et al. 2012).
However, Neoascarophis sinensis was described from the white-spotted conger Conger myriaster (Brevoort) (Anguilliformes: Congridae) in the Yellow and East China Seas (Chen et al. 2017). Among these species were N. yarihige and N. bathygadi (Machida 1976), whose vulvas are near the equatorial region. However, both male and female N. yarihige are longer (9–11.7 mm and 13.7–14.6 mm, respectively) than the present specimens. N. bathygadi differs from the present specimens in having a distinctly shorter vestibule (15–18 and 15–20 µm long in males and females, respectively). Additionally, both males and females are longer (9.8–135 mm and 14.1–26.7 mm, respectively).
Among the remaining six species parasitizing macrourid fish, N. insulana, N. macrouri, and N. sphaerocaudata were distinguished from the present specimens by females with vulva near the posterior end of the body (Solovjeva 1991; Moravec et al. 2006; Rossin et al. 2012). Furthermore, N. sphaerocaudata males and females are much longer (7.4–8.9 mm and 18.8–22.7 mm, respectively). Additionally, they have much longer muscular and glandular parts of the esophagus and a long (left) spicule than the present specimens. In N. longispicula, only the male is known, which could be distinguished from the present specimens by its larger body (8.43–15.78 mm versus 3.7–6.4 mm), much longer muscular and glandular parts of the esophagus, and larger spicules [left spicule 960–1,149 µm versus 85–425 µm; right spicule 216–243 µm versus 50–181 µm] (Moravec and Klimpel 2009).
In addition to all these differences among the six species compared with the present specimens, the fish hosts are also different.
In N. sinensis, the vulva is near the equatorial region. However, both males and females are longer (8.5–10.5 mm and 9.5–14.0 mm, respectively) with a different ratio of muscular and glandular parts of the esophagus, and a spiracle length ratio longer than those of the present specimens. Additionally, the fish hosts are different (Chen et al. 2017).
Neoascarophis upeneichthys (Johnston and Mawson 1945) and Neoascarophis mariae (Pereira et al. 2012) were described from a mulled host. However, the original description by Johnston and Mawson (1945) is inadequate and lacks important morphometric features, such as the size of the vestibules and the esophagus in males.
Among Neoascarophis mariae (Pereira et al. 2012), most of the measurements and the body shape were in agreement with those of the present specimens. Despite this, the uterine eggs were thin-shelled compared with thick-shelled uterine eggs in the present specimens.
The present SEM study is similar to that of Pereira et al. (2012) in that it also noted the presence of four sub-median labia, lateral pseudolabia, and sublabia surrounding the oval mouth. The study also noted the presence of four cephalic papillae and a pair of amphids, the male posterior end provided with vesicular caudal alae, four pairs of pre-cloacal papillae, six pairs of post-cloacal papillae, a pair of ventral papillae and area rugose, and the female tail was provided with a pair of phasmids.
In the current study, the specimens were recorded from other fish hosts from the same family (Perciformes: Mullidae) from Hurghada and Safaga, Egypt in the Red Sea as new hosts and new locality; they were mainly Parupeneus forsskali (in 12 out of 29 examined fish), had a higher worm burden (2–5), and were rarely from Mulloidichthys flavolineatus (only two worms in one fish from the 24 examined fishes) meaning that Parupeneus forsskali is the main fish host for that parasite in Egypt.
It is worth mentioning that the nematode parasite Neoascarophis mariae was redescribed for the first time from new host records (Parupeneus forsskali and Mulloidichthys flavolineatus from the Red Sea at Hurghada, Egypt). Moreover, the parasite was fully redescribed with the addition of several light microscopical morphometric and SEM ultrastructural details documented by clear photomicrographs and camera lucida drawings as well as SEM pictures. Moreover, the present nematode was clearly differentiated from all previously described spp. of the genus Neoascarophis Machida 1976.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Anderson RC, Chabaud AG, Willmott S. Keys to the nematode parasites of vertebrates: archival volume CABI. Oxon: Wallingford; 2009. [Google Scholar]
- Arai HP, Smith JW. Guide to the parasites of fishes of Canada part V: nematoda. Zootaxa. 2016;4185(1):21. doi: 10.11646/zootaxa.4185.1.1. [DOI] [PubMed] [Google Scholar]
- Brugni NL, Viozzi GP. New cystidicolid species (Nematoda) from Galaxias platei (Osmeriformes: Galaxiidae) in Patagonian freshwater environments. J Parasitol. 2008;94:841–846. doi: 10.1645/GE-1367.1. [DOI] [PubMed] [Google Scholar]
- Chen HX, Zhang LP, Du X, Li L. Neoascarophis sinensis n. sp. (Nematoda: Cystidicolidae), a parasite of Conger myriaster (Brevoort) (Anguilliformes: Congridae) in Chinese waters. Syst Parasitol. 2017;94:499–504. doi: 10.1007/s11230-017-9715-7. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Aznar FJ, Balbuena JA, Kostadinova A, Raga JA, Moravec F. A new cystidicolid nematode from Mullus surmuletus (Perciformes: Mullidae) from the western Mediterranean. J Parasitol. 2005;91:335–344. doi: 10.1645/GE-366R. [DOI] [PubMed] [Google Scholar]
- Hussein NM, Khalifa RMA, Abdel-Ghaffar ZTM. recording of Procamallanus (Procamallanus) annulatus and Procamallanus (Procamallanus) elatensis from Red Sea Fishes in Egypt. Egypt J Aquat Biol Fish. 2020;24:341–360. doi: 10.21608/ejabf.2020.100407. [DOI] [Google Scholar]
- Johnston TH, Mawson PM. Parasitic nematodes. Antarctic Res Exp. 1945;5:73–159. [Google Scholar]
- Justine JL, Briand MJ, Bray RA. A quick and simple method, usable in the field, for collecting parasites in suitable condition for both morphological and molecular studies. Parasitol Res. 2012;111:341–351. doi: 10.1007/s00436-012-2845-6. [DOI] [PubMed] [Google Scholar]
- Ko RC (1986) A preliminary review of the genus Ascarophis van Beneden, 1871 (Nematoda: Cystidicolidae) of the gastrointestinal tract of fishes. Department of Zoology, University of Hong Kong, Hong Kong, China, 54 pp
- Kolasinski J, Frouin P, Sallon A, Rogers K, Bruggemann HJ, Potier M. Feeding ecology and ontogenetic dietary shift of yellowstripe goatfish Mulloidichthys flavolineatus (Mullidae) at Reunion Island, SW Indian Ocean. Mar Ecol Prog Ser. 2009;386:181–195. doi: 10.3354/meps08081. [DOI] [Google Scholar]
- Kumaran M, Randall JE (1984) Mullidae. In Fischer W, Bianchi G (Eds), FAO species identification sheets for fishery purposes. Western Indian Ocean fishing area 51. vol 3. http://www.fao.org/3/ad468e/ad468e00.htm
- Kuronuma K, Abe Y. Fishes of the Arabian Gulf. Kuwait: Kuwait Institute for Scientific Research; 1986. p. 356. [Google Scholar]
- Lee MR. The petrography, mineralogy and origins of calcium sulphate within the Cold Bokkeveld CM carbonaceous chondrite. Meteoritics. 1993;28:53–62. doi: 10.1111/j.1945-5100.1993.tb00248.x. [DOI] [Google Scholar]
- Lieske L, Myers RF. Coral reef guide of Red Sea. London: Fulham Palace Road; 2004. [Google Scholar]
- Lieske E, Fiedler KE, Myers RF. Coral Reef Guide: Red Sea to Gulf of Aden, South Oman; (Definitive Guide to Over 1200 Species of Underwater Life) London: Collins; 2004. [Google Scholar]
- Machida M. Nematodes from the deep-sea fishes of Suruga Bay. II. Two new rhabdochonid nematodes from the macrouroid fishes. Bull Natl Museum Nat Sci Ser A. 1976;2:1–6. [Google Scholar]
- Moravec F. Some aspects of the taxonomy and biology of adult spirurine nematodes parasitic in fishes: a review. Folia Parasitol. 2007;54:239–257. doi: 10.14411/fp.2007.033. [DOI] [PubMed] [Google Scholar]
- Moravec F, Klimpel S. Two new species of cystidicolid nematodes from the digestive tract of the deep-sea fish Coryphaenoides mediterraneus (Giglioli) (Macrouridae) from the Mid-Atlantic Ridge. Syst Parasitol. 2009;73:37–47. doi: 10.1007/s11230-009-9182-x. [DOI] [PubMed] [Google Scholar]
- Moravec F, Klimpel S, Kara E. Neoascarophis macrouri n. sp. (Nematoda: Cystidicolidae) from the stomach of Macrourus berglax (Macrouridae) in the eastern Greenland Sea. Syst Parasitol. 2006;63:231–237. doi: 10.1007/s11230-005-9019-1. [DOI] [PubMed] [Google Scholar]
- Moravec F, Hanzelová V, Gerdeaux D. New data on the morphology of Comephoronema oschmarini (Nematoda, Cystidicolidae), a little-known gastrointestinal parasite of Lota lota (Teleostei) in Palaearctic Eurasia. Acta Parasitol. 2007;52(2):135–141. doi: 10.2478/s11686-007-0018-z. [DOI] [Google Scholar]
- Pereira AN, Timi JT, Vieira FM, Luque JL. A new species of Neoascarophis (Nematoda: Cystidicolidae) parasitic in Mullus argentinae (Perciformes: Mullidae) from the Atlantic coast of South America. Folia Parasitol. 2012;59:64–70. doi: 10.14411/fp.2012.010. [DOI] [PubMed] [Google Scholar]
- Randall JE (1983) Red Sea Reef Fishes. IMMEL Publishing. 20 Berkeley Street, Berkeley Square, London
- Rossin MA, Incorvaia IS, Timi JT. A new species of Neoascarophis (Nematoda: Cystidicolidae) parasitic in Macrourus carinatus (Macrouridae) from Argentinean waters. J Parasitol. 2012;98:643–647. doi: 10.1645/JP-GE-2947.1. [DOI] [PubMed] [Google Scholar]
- Solovjeva GF. Metabronema insulanum sp. n. (Nematoda: Spirurina), a parasite of deep-sea fishes of the Pacific Ocean. Parazytologiya. 1991;25:556–558. [Google Scholar]
- Yamaguti S (1963) Systema Helminthum. Vol. III The Nematodes of Vertebrates Part I, LTD. Interscience Publishers, London
- Yorke W, Maplestone PA, Stiles CW. The nematode parasites of vertebrates. New York: Hafner Publishing Company; 1926. [Google Scholar]



