Abstract
Liposomal amphotericin B (LAmB) is the drug of choice in Bangladesh to eliminate the burden of visceral leishmaniasis, also known as kala-azar, a fatal protozoan parasitic disease if left untreated. We aimed to assess efficacy and safety of a single-dose (10 mg/kg) LAmB in visceral leishmaniasis (VL) treatment among the visiting children and adults in a tertiary care setting. This prospective study includes 11 children and 19 adults with a confirmed diagnosis of kala-azar (total 30 cases). Intravenous infusion of LAmB (10 mg/kg body weight) was given to all of the patients. Clinical assessments were conducted during treatment, before hospital discharge, and on days 30 and 180 after treatment. Efficacy was estimated in terms of initial cure (at day 30) and the final cure (at 180 days). All information was recorded in a preformed case record form and analysis was performed in SPSS 22. The mean age was 27.13 ± 18.04 years (3–65) with male predominance (60%). Significant regression of spleen size was found following treatment with LAmB at 30 days and 180 days follow up visit (p < 0.05 for all). Overall, rate of initial cure was 90% (n = 27) (child 90.9% vs 89.47% adult) and final cure was 96.66% (n = 29) (child 100% vs 94.73% adult). Fourteen adverse events were recorded mostly including fever and/or shivering (85.71%). No case relapsed or were referred either due to management or Severe Adverse Event (SAE). In real-life experience, the LAmB treatment for visceral leishmaniasis is as safe and effective for treatment of kala-azar patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12639-021-01379-w.
Keywords: Kala-azar, LAmB, Efficacy, Leishmaniasis, Real life experience
Background
Visceral leishmaniasis (VL), also known as kala-azar is one of the seven most important tropical diseases, often being neglected in the tropics (Sundar and Jaya 2010; Georgiadou et al. 2015; Arenas et al. 2017; Zahra et al. 2018). At times, case-fatality can exceed up to 10% in VL-endemic areas such as Asia and Africa even with appropriate treatment (Bern et al. 2005). Despite steady decline in the number of kala-azar cases worldwide, nearly about 0.7–1 million new cases of leishmaniasis were identified yearly (Burza et al. 2018; Rijal et al. 2019). More than ninety percent of the cases were came from six countries (Bangladesh, Brazil, Ethiopia, India, South Sudan & Sudan) and Bangladesh, India and Nepal alone contribute 60% of the world's annual VL burden (Huda et al. 2014; Torres-guerrero et al. 2017). As a consequence, government of Bangladesh, India and Nepal along with Bhutan & Thailand committed to eliminating this public health problem by 2020 (World Health Organization (WHO) 2017; Hasnain et al. 2018) Since starting of the national kala-azar elimination program from 2008, Bangladesh has made significant progress and only 18 new cases was reported by 25th June 2020 (Ghalib 2014; Kalacore 2020; Kala-Azar National Program 2020). While case reduction is significant, to achieve the elimination threshold, the reduction must be upheld for several years and retain its development and maintenance phases to avoid the incidence of recurrent VL epidemic peaks as is happening over the past decades (Gibson 1983; Bern and Chowdhury 2006; Kalacore 2020).
There are only a few treatment options available for kala-azar. These are; sodium stibogluconate, amphotericin B deoxycholate, liposomal amphotericin B, paromomycin and miltefosine (Tamiru et al. 2016). Life-threatening toxicity or loss of efficacy or intolerable side effects profile limits the use of Miltefosine, Paromomycin or amphotericin B deoxycholate (Sundar et al. 2004, 2012; Pandey et al. 2017). Another available drug for VL is liposomal amphotericin B (LAmB). It has the same formulation as amphotericin B but added with cholesterol and other phospholipids within a small unilamellar liposome. It acts by binding to parasite ergosterol precursors disrupting the parasite membrane (Sundar and Jaya 2010). This modified drug specifically reaches the targeted organs such as the liver and therefore limits the toxicity to other organs, unlike the conventional amphotericin B (Balasegaram et al. 2012; Pandey et al. 2017). Evidence suggests a single dose of LAmB at 10 mg/kg IV has shown excellent results of safety and efficacy and it has gained popularity in the Indian subcontinent including Bangladesh (Sundar et al. 2010; Sundar et al. 2010; Mondal et al. 2014; Tamiru et al. 2016; Pandey et al. 2017). Therefore, Bangladesh government has recommended LAmB for treatment of kala-azar in their national guideline (National Guideline for Kala-azar Case Management 2013). Sadly, a few reports were published from Bangladesh about the safety and efficacy of this drug in this indication. Therefore, this study was conducted to assess the efficacy and safety of 10 mg/kg LAmB (manufactured by Gilead pharmaceuticals) in VL among the children and adults attending in Dhaka Medical College Hospital, Bangladesh.
Materials and methods
Design, subjects and procedure of the study
This prospective study was conducted in the Department of Medicine and Department of Paediatrics in Dhaka Medical College Hospital (DMCH), one of the largest capacity, tertiary care hospitals situated in the capital (Health Bulletin 2018). The study duration was from January 2017 to December 2018. After taking consent either from the patients or parents (or guardians) of the child, all consecutive adult and paediatric cases (age range 3–65 years) suspected of VL were approached for inclusion into the study. During final inclusion, parasites found on microscopy of a splenic aspirate smear or bone marrow was considered the prime selection criteria. Exclusion criteria included: patients who were seropositive for HIV, and had a serious concurrent infection (e.g. TB or bacterial pneumonia) (Zahra et al. 2018), a serum creatinine level of > 1.5 times the upper limit of the normal range, or have a history of allergy or hypersensitivity to amphotericin B. Moreover, anti-leishmanial or unlicensed investigational treatments within 180 days, pregnant women and patient and/or attendant refusing to give consent to take part in the study were also excluded. Confirmed cases were awaited up to the correction of the blood picture and then included. Written consent was obtained in separate forms for each invasive procedure (needle aspirate from splenic, bone marrow or any other tissue of involvement) undertaken.
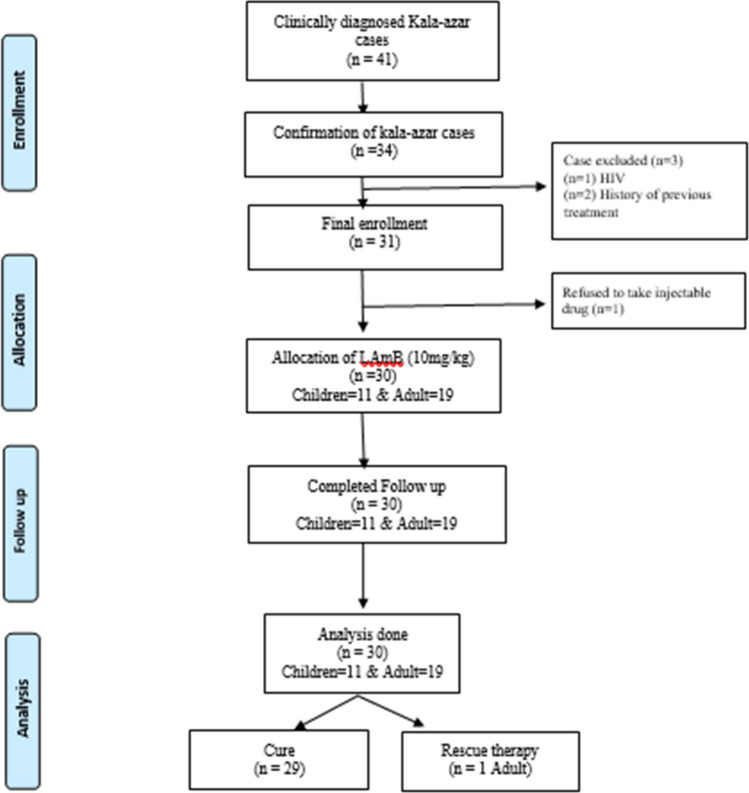
In Bangladesh, complicated kala-azar cases were referred and managed in Surja Kanta Kala Azar Research Centre (SKKRC), which is a specialized center to treat VL relapse cases, VL cases with complications, and patients with post-kala-azar dermal leishmaniasis (PKDL) (Ahmed et al. 2014). DMCH deals with small numbers of cases, particularly new cases. A total of 41 cases were approached and 34 cases were confirmed by parasitological evidence. Four cases were excluded, due to either concomitant infection of HIV (n = 1), previous history of treatment (n = 2) or due to refusal of injectable treatment (n = 1). Kala-azar with HIV case was notified to National AIDS/STD Control program in Bangladesh and the patients who refused the injectable treatment was managed by oral Miltefosine. Another two patients were managed to according to the national guideline. Finally, a total of 30 kala-azar patients were included and prospectively evaluated. All patients underwent follow up at the end of 30 days and at the end of 180 days after completion of treatment (Fig. 1). For understating the efficacy and safety of the test drug in according to the age variation, study population were divided into two groups based on chronological age cut off < 18 years. Here, patients age < 18 were defined as children and ≥ 18 years were considered an adult. As a result, a total of 11 children and 19 adult cases were included during the final analysis.
Fig. 1.
Flow chart of patient selection
Structured questionnaire was used to collect the patient details including adverse events. An adverse event was defined as any untoward medical occurrence which follows drug administration and which does not necessarily have a causal relationship with the usage of the drugs that occurred within period of hospital stay. The reported Adverse Events (AEs) were further graded according to the Common Terminology Criteria for Adverse Events (CTCAE) grading system (Supplementary 1). To assess the adverse effect necessary investigations such as ECG for arrhythmia, serum electrolytes for hypokalemia, and others were checked and recorded. Before administration of the test drug, baseline investigations including CBC, ECG, chest X-ray, routine electrolyte, serum creatinine, serum urea, urine RME etc. were performed for each patient. Initial cure was defined as an improvement of all clinical parameters, including an absence of fever, regression of enlarged spleen, and return to appetite at day 30. Definitive cure was defined as no fever along with not palpable spleen or reduction of spleen size compared with day 30 (if spleen size was huge at baseline), weight gain. Both definitions comply with the National Guideline Guidelines for Kala-azar case management (National Guideline for Kala-azar Case Management 2013). However, in this study, presence of parasitological evidence was not sought mostly due to regression of spleen size.
Primary safety endpoint was death while the secondary endpoint was occurrence of serious adverse event. All data were recorded into the case record form (CRF) and kept in a secure and safe place with maintaining confidentiality. During the study period, there was no dietary restriction for participants or any other restriction that prevented access to healthcare.
Detailed activities of the study
Before administration of the liposomal amphotericin B, hospital staffs were trained about management, treatment of patients with visceral leishmaniasis, possible side effects and adverse drug reaction and reporting system. During enrollment, a complete medical history and physical examination were performed (by MRK, RN and PKM). Bodyweight (measured by Salter scale, 200 WHGYDR), Height (with a locally made Tape height scale), was measured for each person. BMI was calculated as weight (kg) divided by height (m) squared. Haemoglobin concentrations were measured in the biochemistry lab of DMCH and an rK39 test (with kala-azar detect; InBios, Seattle, WA, USA) was also conducted during enrollment. Screening test to confirm pregnancy was done for women in child-bearing age and excluded if positive. All participants admitted to the study sites were followed up for a minimum of 5 days by lead author MRK. Before giving LAmB, potential allergies to the drug were screened and were tested by administering 1 mg of liposomal amphotericin B (diluted in 12.5 mL of dextrose 5% in water with infused upon within 15–20 min). Participants who did not show any allergic reactions during the test were administered a dose of 10 mg/kg of liposomal amphotericin B. The intravenous infusion was administered over 2 h. Measurements of vital parameters were recorded before their treatment medication, every 60 min during the drug infusion, at 2 h and 24 h after treatment, also at 5th day before discharging of the patient. In case of appearance of rash and or fever, the cases were managed by paracetamol (500 mg for adults and 10 mg/kg for children younger than 12 years) and chlorphenamine (4 mg for adults and 1–2 mg for children younger than 12 years) either alone or concurrently.
Statistical analyses
Continuous data was expressed as mean with standard deviation and categorical data was expressed as frequency with percentage. The safety analysis included calculation of the incidence of all adverse events. Chi-square test (χ2) test and Yate’s correction were performed (where applicable) during comparison of groups. p ≤ 0.0.05 was defined as statistically significant.
Results
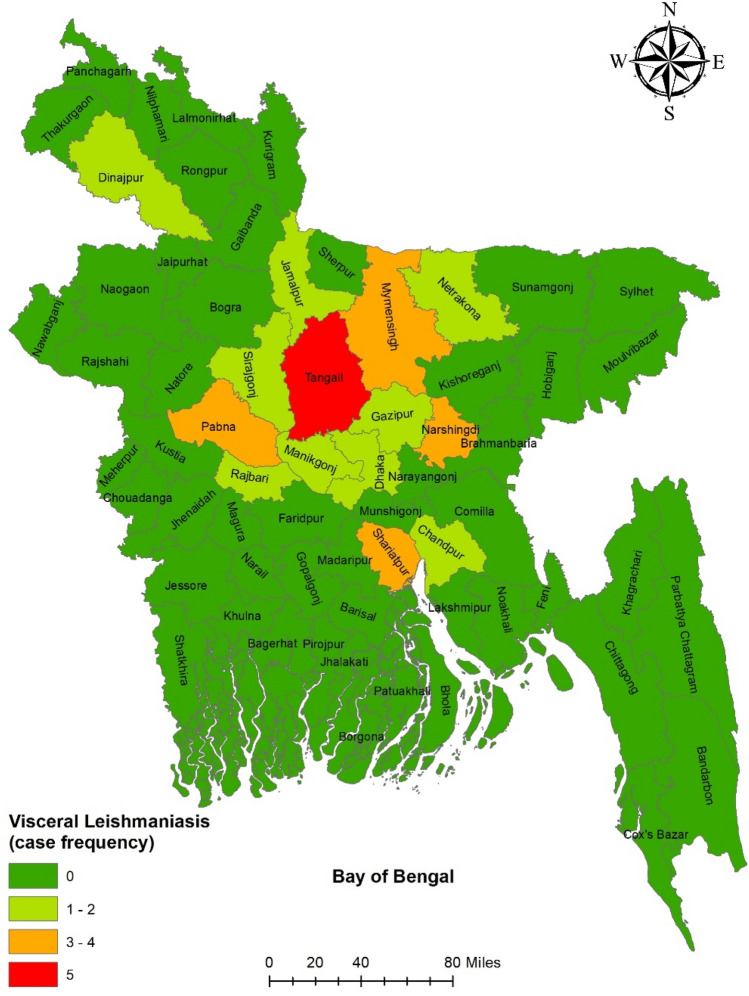
A total of 30 cases (11 children and 19 adults) were included in this study and were managed with 10 mg/kg liposomal amphotericin B. Patients were referred from all over the country. The district-wise distribution of the patients is illustrated in Fig. 2.
Fig. 2.
District wise distribution of the patients (n = 30)
Only 6.9% of cases lived in concrete buildings, while remainder lived in either kacha-ghor (mud-plastered house) or tin shed houses. Gross underweight (measured by BMI) were present in both children and adults in 72.7% and 73.7% cases, respectively. Other baseline characteristics are described in (Table 1). Physical signs and clinical characteristics of the newly detected kala-azar patients are described in Table 2.
Table 1.
Base line characteristics of the newly detected kala-azar patients (n = 30)
| Baseline characteristics | Children (< 18 yrs) (n = 11) | Adults (≥ 18 yrs) (n = 19) | Overall (n = 30) |
|---|---|---|---|
| Age (years) | |||
| Mean ± SD | 11.4 ± 3.4 | 33.2 ± 11.4 | 21 ± 13.42 |
| Median (IQR) | 10 (8–13) | 31 (23–44) | 14(8–28) |
| Sex | |||
| Male | 6 (54.5%) | 12 (63.2%) | 18 (60.0%) |
| Female | 5 (45.5%) | 7 (36.8%) | 12 (40.0%) |
| House type | |||
| Kacha ghar | 6 (54.54%) | 12 (63.15%) | 18 (60.0%) |
| Tinshed | 3 (27.28%) | 6 (31.57%) | 9 (30.0%) |
| Building | 2 (18.18%) | 1 (5.28%) | 3 (10.0%) |
| BMI (kg/m2) | |||
| Mean ± SD | 13.8 ± 1.8 | 17.5 ± 2.3 | 16.3 ± 2.6 |
| Underweight | 8 (72.7%) | 14 (73.7%) | 22 (73.3%) |
| Pulse (beat/min) | 97 (66–128) | 85 (60–120) | 92(60–128) |
Table 2.
Clinical characteristics of the study population (n = 30)
| Children (< 18 yrs) (n = 11) | Adults (≥ 18 yrs) (n = 19) | Overall (n = 30) | |
|---|---|---|---|
| Physical Signs* | |||
| Fever | 11 (100%) | 18 (94.73%) | 29 (96.66%) |
| Anaemia | 11 (100%) | 18 (94.73%) | 29 (96.66%) |
| Jaundice | 1 (9.09%) | 4 (21.05%) | 5 (16.66%) |
| Lymphadenopathy | 0 | 1 (5.26%) | 1 (3.33%) |
| Spleen size enlarged (cm) | |||
| Mean ± SD | 6.8 ± 3.4 | 6.3 ± 3.2 | 6.5 ± 3.3 |
| Palpable | |||
| ≤ 5 cm | 5 (45.5%) | 3 (15.8%) | 8 (26.7%) |
| 5- < 10 cm | 4 (36.4%) | 9 (47.4%) | 13 (43.3%) |
| ≥ 10 cm | 2 (18.2%) | 6 (31.6%) | 8 (26.7%) |
| Not palpable | 0 | 1(5.3%) | 1 (3.3%) |
| Hepatic enlargement (cm) | |||
| Palpable | |||
| < 4 cm | 5 (45.46%) | 4 (21.05%) | 9 (30.0%) |
| 4–8 cm | 3 (27.27%) | 7 (36.84%) | 10 (33.3%) |
| > 8 cm | 1 (9.09%) | 4 (21.05%) | 5 (16.7%) |
| Not palpable | 2 (18.18%) | 4 (21.05%) | 6 (20.0%) |
*Multiple response
Efficacy
After completion of the treatment, there was a remarkable improvement of body weight, as well as significant regression of spleen size at the 30 days and 180 days after the treatment. The clinical parameters that indicate the treatment effectiveness are presented in (Table 3). At 30 days, the initial cure rate was 90.9% (10 cases out of 11) in children and 89.47% in adults (17 out of 19 cases). However, the final cure was estimated 100% in children and 94.73% in adults. Only one case remained positive clinically and was successfully managed by a total dose of 15 mg/kg bodyweight injectable amphotericin B (three injections of 5 mg/kg each over 3 days) (Fig. 3).
Table 3.
Comparison of clinical and lab parameters before and after treatment (n = 30)
| Parameters | Before treatment (BT) | 1st visit (30 d) | P* (BT vs 30 d) | 2nd visit (180 d) | P** (BT vs 180 d) |
|---|---|---|---|---|---|
| Weight (kg) | 38.82 ± 12.54 | 41.89 ± 12.30 | < 0.05 | 44.93 ± 12.03 | < 0.001 |
| Spleen size (cm below left costal margin) | 6.5 ± 3.3 | 2.78 ± 2.55 | < 0.001 | 0.53 ± 1.30 | < 0.001 |
*p value determined by paired t test (before treatment vs 30 days)
**p value determined by paired t test (before treatment vs 180 days)
Fig. 3.
Proportion of initial cure and final cure among the kala-azar cases (n = 30)
Safety
A total of 14 adverse events (AEs) were reported in the study. Fever and shivering were the most commonly reported AEs followed by a single event of anuria and back pain. None of the patient developed nephrotoxicity, bleeding, or any other serious AEs. All the AEs were mild in severity (CTCAE grade 1) and resolved with assurance and symptomatic management. No patient developed cardiac arrhythmia or anaphylaxis. Only one patient was febrile at 30 days follow up and only one in 6 months follow up who was later diagnosed as enteric fever. A total of 96% of patients improved appetite in both follow-up reviews. The summary of AEs reported in the study is shown in (Table 4).
Table 4.
Adverse events and Fever and appetite during the follow up (n = 30)
| Adverse effects | Number of positive patients | CTCAE Grade | ||
|---|---|---|---|---|
| Children (< 18 yrs) (n = 11) | Adults (≥ 18 yrs) (n = 19) | Overall (n = 30) | ||
| Fever | 4 | 3 | 7(23.3%) | 1 |
| Shivering | 2 | 3 | 5 (16.7%) | 1 |
| Anuria | 0 | 1 | 1(3.3%) | 1 |
| Back pain | 0 | 1 | 1(3.3%) | 1 |
| Fever | ||||
| Before treatment | 29 | 96.66 | ||
| 1st visit (after 1 month) | 1 | 3.33 | ||
| 2nd visit (after 6 months) | 1 | 3.33 | ||
| Appetite improvement | ||||
| 1st visit (after 1 month) | 29 | 96.66 | ||
| 2nd visit (after 6 months) | 29 | 96.66 | ||
Discussion
Visceral leishmaniasis (VL), also known as kala-azar, is a neglected tropical disease that is still an important public health problem in Bangladesh, India, and Nepal, as these three countries contribute a significant amount of the annual VL world burden (Hasnain et al. 2018). Contrary to the severity, few drugs are available for its treatment and are further limited by safety, efficacy, cost-effectiveness, using at the peripheral level, and challenges in administration (Sinha and Bhattacharya 2014). We conducted this study to examine the efficacy and safety of single-dose (10 mg/kg) liposomal amphotericin B (LAmB) in visceral leishmaniasis treatment among the visiting children and adults in a tertiary care setting. A total of 30 cases with VL, comprising 11 children and 19 adults, were included in this study. In real-life experience, our study revealed that single-dose (10 mg/kg) liposomal amphotericin B is an effective (overall 96.67% cure rate) and safe (no case with severe adverse effect) treatment option for primary VL in Bangladesh, in contrast to internationally accepted parameters for effectiveness ≥ 95% and safety (SAE < 5%) for VL treatment (World Health Organization 2010). Furthermore, the cure rate effectiveness was found more among children compared to adults (100% vs 94.7%). Our finding is corroborated with the study done by Mondal et al. (2014), who observed that LAmB could be used in visceral leishmaniasis with high cure rate (97% in the intention-to-treat analysis) and good safety profile, assessing the feasibility of administering single-dose liposomal amphotericin B close to endemic villages at a rural hospital primary health-care level. Although the findings are in favor of the liposomal amphotericin B in the present study, a limitation was the small sample size, therefore caution is needed when generalizing the findings. Kala-azar elimination program has been ongoing since 2007 and achieved tremendous progress. A gradual decline in the number of overall cases and referral of only a small number of cases to the study site is responsible for the small number of samples we found in our study.
Despite a good safety profile, a total of 14 Adverse Events (AEs) were reported in our study, wherein fever and/or shivering were the most common (85.71%, 12 out of 14 AEs). None of the patients developed nephrotoxicity, bleeding, or any other serious AEs. All the AEs were mild in severity (CTCAE grade 1) and resolved with assurance and symptomatic management. No patient developed cardiac arrhythmia or anaphylaxis. No case was referred due to severe adverse events (SAEs). Only one patient developed anuria who was successfully managed by IV fluid and returned to normal at 30 days follow up. Detailed investigations were not done as the patients recovered quickly. Patient complaints of low back pain were managed by paracetamol and physiotherapy. The common side effects of fever and shivering responded to oral or per rectal paracetamol. Only one patient was febrile at 30 days follow up and only one at 6 months follow up which was later proved as enteric fever. The patients came with high grade temperature and we investigate the case considering it could be a case of relapse or co-infection. Later, the isolation of the Salmonella typhi in blood culture proved the case as typhoid fever.
However, medical staff should be skilled in the management of reported adverse effects before the start of treatment. As any reaction of Liposomal amphotericin B needs to be label as severe reaction according to WHO SEARO guideline (World Health Organization 2010), these adverse reactions need to be taken seriously. Hence the allergic tests before giving the intravenous infusion are recommended and should be made as early as possible as it can also help to predict the anaphylaxis.
Though we did not get any case of relapse, yet previous reports from Bangladesh (Lucero et al. 2015) and India (Burza et al. 2014) found spleen size on discharge as a risk factor for VL relapse. We found a marked reduction of spleen size of VL patients after 1 and 6 months of treatment (mean size 2.78 and 0.53 cm respectively) compared to enrollment measurements (6.5 cm) and the spleens became non-palpable. This is concordant with that of Sinha et al. (2010).
Therefore, spleen size may be a good indicator of cure as severe VL disease (Veress et al. 1977; Malla and Mahajan 2006; Stanley and Engwerda 2007). Hence, a follow-up period of 6 months is required to capture the majority of VL relapse cases based on these easily-measured clinical parameters which could be incorporated in VL treatment protocol in limited-resource settings.
However, our study has some limitations. Firstly, a small sample size due to a decreasing number of kala-azar patients in Bangladesh with very few referrals to the study site. Secondly, long term follow-up data regarding safety and efficacy was not recorded. Lastly, we have no scope to evaluate the effectiveness of single-dose LAmB for relapsed cases of VL.
Conclusion
In real-life settings, treatment response of single-dose liposomal amphotericin B against visceral leishmaniasis is as effective and safe as in controlled settings. Therefore, the present recommendation of WHO for its use as a first-line drug for visceral leishmaniasis in Southeast Asia is supported by these results.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank NKEP for giving the investigations facility including rK39 and ensuring on demand supply of standard liposomal amphotericin B to carry out the management and research. We greatly appreciate the kind support of the Disease Control Unit, Directorate General of Health Services, and the Government of Bangladesh in conducting this study in the DMCH. We are grateful to the management team- doctors, and nurses of the DMCH for diagnosis and treatment of study patients. We are also grateful to the study participants who gave us consent to conduct the study. We are also thankful to DSMB boards and teams who carried out GCP training to carry out the research. The authors also showed their gratitude to the Pi Research Consultancy Center, Bangladesh (www.pircc.org) for their constant support during journal selection and overall, the study process. Also, thanks Dr Noor-E-Ambia and Dr Tamanna Tabassum for language help.
Author contributions
MRE and RA conceive and developed the concept of the study. Conception and design of this Research were made by RA, MJH, and RN and PKM. Data collection was done MRE, RN and PKM. MJH wrote the first draft of the manuscript and ASK, AB, RA, MR, AL, reviewed the draft. Data analysis was done by conjointly MRR, MJH and ASK. All authors read and revised the article and MJH works as a corresponding author.
Funding
This trial received no external fund or grant. However, LAmB (Gilead pharmaceuticals) was collected from the National Kala-azar elimination program (NKEP) which was also provided by World Health Organization.
Availability of data and material
Data and materials supporting our findings in the manuscript will be shared on request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Ethics approval
The study was approved by the ethical review committee of Dhaka Medical College Hospital (Memo no. MEU-DMC/ECC/2016/53-D) and the study conformed to the current Declaration of Helsinki. Moreover, confidentiality and anonymity were maintained in all over the study.
Ethical consideration
The researcher was duly concerned about the ethical issues related to the study. Formal ethical clearance was taken from the Ethical Review Committee (ERC) of the DMC for conducting the study as well as formal permission was taken from the responders. Confidentiality was maintained properly.
Informed consent
Informed written consent was taken from the subject informing the nature & purpose of the study, the procedure of the study, the right to refuse, accept & withdraw to participate in the study as well as the participants didn't gain financial benefit from this study.
Footnotes
Md. Rezaul Ekram, Mohammad Robed Amin and Mohammad Jahid Hasan Joint first authors.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Robed Amin, Email: robedamin@yahoo.com.
Mohammad Jahid Hasan, Email: dr.jahid61@gmail.com, Email: jahid@pircc.org.
Md. Abdullah Saeed Khan, Email: abdullahdmc@gmail.com.
References
- Ahmed BN, Nabi SG, Rahman M, Selim S, Bashar A, Rashid MM, Lira FY, Choudhury TH, Mondal D. Kala-azar (Visceral Leishmaniasis) elimination in Bangladesh: successes and challenges. Curr Trop Med Rep. 2014;1(3):163–169. doi: 10.1007/s40475-014-0027-6. [DOI] [Google Scholar]
- Arenas R, Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J. Leishmaniasis: a review. F1000Research. 2017;6(5):1–15. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasegaram M, Ritmeijer K, Lima MA, Burza S, Genovese GO, Milani B, Gaspani Y, Potet J, Chappuis C. Liposomal amphotericin B as a treatment for human leishmaniasis. Expert Opin Emerg Drugs. 2012;17(4):493–510. doi: 10.1517/14728214.2012.748036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Haque R, Kurkjian K, Vaz EL, Begum M, Akter T, Cetre-Sossah CB, Ahluwalia IB, Dotson E, Secor WE, Breiman RF, Maguire JH. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11(5):655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: Prospects for improved control. Indian J Med Res. 2006;123(3):275–288. [PubMed] [Google Scholar]
- Burza S, Sinha PK, Mahajan R, Lima MA, Mitra G, Verma N, Balasegaram M, Das P. Risk factors for visceral leishmaniasis relapse in immunocompetent patients following treatment with 20 mg/kg liposomal amphotericin B (Ambisome) in Bihar, India. PLoS Negl Trop Dis. 2014;8(1):e2536. doi: 10.1371/journal.pntd.0002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–970. doi: 10.1016/S01406736(18)31204-3. [DOI] [PubMed] [Google Scholar]
- Georgiadou SP, Makaritsis KP, Dalekos GN. Leishmaniasis revisited: current aspects on epidemiology, diagnosis and treatment. J Transl Intern Med. 2015;3(2):43–50. doi: 10.1515/jtim-2015-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghalib CM (2014) Update on the Kala-Azar elimination program in Bangladesh. Health Sci Bullet 12(4):7–13
- Gibson M. The identification of kala-azar and the discovery of Leishmania Donovani. Med Hist. 1983;27:203–213. doi: 10.1017/s0025727300042691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain MG, Nath P, Maruf S, Nabi SG, Hossain AFMA, Ahmed BN, Mondal D, Basher A. Amphotericin B deoxycholate for relapse visceral leishmaniasis in Bangladesh: a cross-sectional study. BMC Res Notes. 2018;11(1):1–5. doi: 10.1186/s13104-018-4036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Bulletin 2018. Government of the People's Republic of Bangladesh, Ministry of the Health and Family welfare
- Huda MM, Chowdhury R, Ghosh D, Dash AP, Bhattacharya SK, Mondal D. Visceral leishmaniasis-associated mortality in Bangladesh: a retrospective cross-sectional study. BMJ Open. 2014;4(7):e005408. doi: 10.1136/bmjopen-2014-005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala-azar program 2020, DHis2 online database, People's Republic of Bangladesh: Bangladesh National Portal
- KalaCORE: control and elimination of visceral leishmaniasis, Bangladesh 2020. http://www.kalacore.org/. Acccesed 17 Aug 2020
- Lucero E, Collin SM, Gomes S, Akter F, Asad A, Das AK, Ritmeijer K. Effectiveness and safety of short course liposomal amphotericin B (AmBisome) as first line treatment for visceral leishmaniasis in Bangladesh. PLoS Negl Trop Dis. 2015;9(4):1–11. doi: 10.1371/journal.pntd.0003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malla N, Mahajan RC. Pathophysiology of visceral leishmaniasis—some recent concepts. Indian J Med Res. 2006;123(3):267–274. [PubMed] [Google Scholar]
- Mondal D, Alvar J, Hasnain MG, Hossain MS, Ghosh D, Huda MM, Nabi SG, Sundar S, Matlashewski G, Arana B. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. Lancet Glob Heal. 2014;2(1):e51–e57. doi: 10.1016/S2214-109X(13)70118-9. [DOI] [PubMed] [Google Scholar]
- National Guideline for Kala-azar Case Management (2013) Kala-azar Elimination Program Communicable Disease Control (CDC) and Directorate General of Health Services, Ministry of Health and Family Welfare Government of the People’s Republic of Bangladesh. https://www.who.int/leishmaniasis/burden/National_Guideline_for_Kala-azar_Case_Management_Bangladesh_2013.pdf?ua=1
- Pandey K, Pal B, Das BNR, Murti K, Lal CS, Verma N, Bimal S, Ali V, Verma RB, Topno RK, Siddiqi NA, Das P. Safety and efficacy of a combination of paromomycin and miltefosine for two vs. three courses in patients with post-kala-azar dermal leishmaniasis: an observational pilot study. Br J Dermatol. 2017;177(2):557–559. doi: 10.1111/bjd.15119. [DOI] [PubMed] [Google Scholar]
- Rijal S, Sundar S, Mondal D, Das P, Alvar J, Boelaert M. Eliminating visceral leishmaniasis in South Asia: the road ahead. BMJ. 2019 doi: 10.1136/bmj.k5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha PK, Roddy P, Palma PP, Kociejowski A, María Lima AN, Das VNR, Gupta J, Kumar N, Mitra G, Saint-Sauveur JF, SeenaS BM, Parreño F, Pandey K. Effectiveness and safety of liposomal amphotericin B for visceral leishmaniasis under routine program conditions in Bihar, India. Am J Trop Med Hyg. 2010;83(2):357–364. doi: 10.4269/ajtmh.2010.10-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha PK, Bhattacharya S. Single-dose liposomal amphotericin B: an effective treatment for visceral leishmaniasis. Lancet Glob Heal. 2014;2(1):e7. doi: 10.1016/S2214-109X(13)70141-7. [DOI] [PubMed] [Google Scholar]
- Stanley AC, Engwerda CR. Balancing immunity and pathology in visceral leishmaniasis. Immunol Cell Biol. 2007;85(2):138–147. doi: 10.1038/sj.icb7100011. [DOI] [PubMed] [Google Scholar]
- Sundar S, Singh A, Rai M, Prajapati KV, Singh AK, Ostyn B, Boelaert M, Dujardin Chakravarty J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis Off Publ Infect Dis Soc Am. 2012;55(4):543–550. doi: 10.1093/cid/cis474. [DOI] [PubMed] [Google Scholar]
- Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362(6):504–512. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- Sundar S, Jaya J. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis. 2010;2(2):159. doi: 10.4103/0974-777X.62886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Mehta H, Suresh AV, Singh SP, Rai M, Murray HW. Amphotericin B treatment for Indian visceral leishmaniasis: conventional versus lipid formulations. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38(3):377–383. doi: 10.1086/380971. [DOI] [PubMed] [Google Scholar]
- Tamiru A, Tigabu B, Yifru S, Diro E, Hailu A. Safety and efficacy of liposomal amphotericin B for treatment of complicated visceral leishmaniasis in patients without HIV, North-West Ethiopia. BMC Infect Dis. 2016;16(1):1–7. doi: 10.1186/s12879-016-1746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-guerrero E, Quintanilla-cedillo MR, Ruiz-esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Research. 2017;6(6):1–15. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veress B, Omer A, Satir AA, El Hassan AM. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977;33(5):605–610. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Control of the leishmaniases. World Health Organ Tech Rep Ser. 2010;949:22–26. [PubMed] [Google Scholar]
- World Health Organization (WHO) (2017). Leishmaniasis country profiles. WHO
- Zahra N, Davood K, Morteza A, Zahra S. Epidemiological aspects of visceral leishmaniasis in Larestan and Ghiro-Karzin Counties, Southwest of Iran. Osong Public Heal Res Perspect. 2018;9(2):81–85. doi: 10.24171/j.phrp.2018.9.2.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials supporting our findings in the manuscript will be shared on request.