Abstract
Malaria still is the most fatal parasitic disease affecting 50% of the world's population. Although annual deaths attributed to malaria has reduced, crucial importance of its prevention and treatment remains a priority for health care systems and researchers. The worldwide increase in resistance to most common antimalarial drugs such as chloroquine, their unpleasant side effects and low efficiencies persuade researchers to prioritize finding alternative drugs including herbal medication from plant roots. The present study aimed to examine in vitro and in vivo effects of hydroalcoholic extract of herbal medicinal plant, Allium paradoxum, on growth rate in Plasmodium falciparum and Plasmodium berghei. The cytotoxicity assay was performed for hydroalcoholic extract of A. paradoxum. The 3D7 strain of P. falciparum was cultured. The IC50 assay and enzymatic activity of lactate dehydrogenase were performed. BALB/c mice were infected with P. berghei in vivo. Toxicity and histopathological changes in the tissues of liver and kidney were also examined. The highest efficacy of A. paradoxum extract was observed at 80 μg/mL in P. falciparum culture resulting in 60.43% growth inhibition compared to control groups. The significantly highest parasite growth inhibition with 88.71% was seen in the mice infected with P. berghei when administered with 400 mg/kg extract compared to control groups. No significant changes in the liver and kidney cells were observed between experimental and control groups. The study showed that A. paradoxum extract exhibited significant antimalarial properties in vitro on P. falciparum and in vivo in mice infected with P. berghei. There was no significant toxicity in the liver and kidney of the treated mice.
Keywords: Malaria, Plasmodium falciparum, Plasmodium berghei, Hydroalcohol ic extract, Allium paradoxum, IC 50
Introduction
Malaria is known as the deadliest parasitic disease with more than 400,000 annual deaths and the prevalence of 228 million worldwide as of 2018. The natural and dominant transmission route is through the bite of an infected female anopheles mosquito as the biological vector. The most prevalent human malaria parasites are Plasmodium falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi of which P. falciparum is responsible for almost all deaths.
The most prescribed anti-malaria drugs include chloroquine, artemisinin, primaquine, mefloquine and lumefantrine, or selected appropriate combination of these. However, continuous expanding resistance in P. falciparum and P. vivax to chloroquine has imposed major global health problems particularly in developing countries to combat the disease. The resistance to chloroquine in P. falciparum was first reported in 1910 and later on during 1950s and in early 1960s. Since then, chloroquine-resistant P. falciparum has expanded to almost all endemic areas and even to other malaria parasites such as P. vivax (Elmi et al. 2019; Hajialiani et al. 2020). This major health problem which could cause higher morbidity and mortality has urged many prominent researchers to focus on developing alternative new drugs to overcome antimalarial resistance. In addition to chloroquine resistance resulting in unsuccessful treatment, many unpleasant side effects have arisen and may suffer different organs such as skin and heart, as well as hearing and vision and the gastrointestinal tract. Regarding these undesirable facts, possibility of utilising herbal medicines and discovering effective safe herbal drugs have always been in the centre of researchers' priorities and is well documented in medical history. The vast diversity in the geographical and environmental conditions in Iran provides rich different sources of flora and fauna including herbal plants like many species of allium genus such as A. paradoxum. It is fortunately abundant across the country and has been historically known as an herbal plant. Allium genus species belong to flowering plants and incorporate many chemical compounds. There is no report of any toxic or deleterious effect for any of the plant's components when used at normal dosage. Allium paradoxum as a native herb in Mazandaran province is known as an effective remedy in ameliorating digestive problems and has anti-cholesterol properties (Nateghpour and Miahipour 2008). Also, some degrees of anti-bacterial, anti-viral and anti-parasitic properties have been evident for allium genus members. Moreover, this herb has been shown to be an antioxidant and anticancer agent (Bhattacharjee and Shivaprakash 2016; Ebrahimzadeh et al. 2010; Radwan et al. 2012). Therefore, considering the global impact of malaria and resistance to its routine synthetic drugs, tremendous researches are in place to find safe and effective substitute herbal medications with fewer side effects.
To address this critical global health issue, the present study aimed to examine the anti-malarial effects of hydroalcoholic extract of Allium paradoxum on P. falciparum and P. berghei in vitro and in vivo, respectively.
Materials and Methods
Preparation of Allium paradoxum extract
Collection of A. paradoxum
The plants were collected from different regions of Mazandaran province, north of Iran, with 200–800 m altitude above sea level during spring. The identification of the genus and species of gathered plants were approved by the experts at Department of Pharmacognosy in Mazandaran University of Medical Sciences (Voucher No: E2/2/154). The bulbs and parts of the plant above ground level were separated and dried under environmental airflow and normal temperature with no direct sunlight. They were then grounded and weighed (5 kg) in the laboratory located in the faculty of Pharmacy and extracted using percolation method at 4:1 ratio of ethanol to water solution. The final solution was dried with a rotary evaporator device and the resultant powder was collected after lyophilization. The stock standard solution was obtained from hydroalcoholic extract powder to normal saline with the same ratio of 1:1 (Elmi et al. 2019).
It is noteworthy to say that selectivity index (SI) ratio was calculated because it indicates the antiviral activity to the cytotoxicity effect of any compound. The higher the selectivity index (SI ratio, the more efficacy and safety would be expected during the treatment of an infection. The SI of A. paradoxum was previously measured at 0.33 by Ebrahimzadeh and his colleagues (2017).
In vitro anti-plasmodial assay
P. falciparum cultures
The 3D7 strain of P. falciparum was obtained from Pasteur Institute of Iran, Tehran, Iran and cultured using Trager and Jensen’s (1976) technique. Parasites were cultured in 7 mL of RPMI1640 medium with 5% serum obtained from Iranian Blood Transfusion Organisation, 10% haematocrit, hypoxanthine (13.6 mg/L) and gentamicin (25 mg/L) in 75 mL flasks and incubated at 37 °C with 5% O2, 5% CO2 and 90% N2. The culture medium was refreshed every 48 h. When parasitaemia reached to 5%, the parasites were diluted with washed blood and complete medium to 2% haematocrit. The prepared suspensions were then separately added to ELISA plate's wells (BioTek ELX800) at 150 µL per well. Different concentrations of A. paradoxum extract were prepared at 0.025, 0.05, 0.1, 1, 10, 20, 40 and 80 µg/mL and were then added to each well. The control wells were designated as normal saline, negative and positive control including the one treated with chloroquine. The microplates were then transferred to a candle jar and incubated for 48 h at 37 °C to verify the obtained results (Trager and Jensen 1976). Each test was conducted with triplicate wells. The parasites were then counted by microscopy and with parasite enzymatic lactate dehydrogenase assay (pLDH).
Microscopic study
The thin smears were prepared from each well and stained with 5% Giemsa. For more accuracy in parasitaemia calculation, all infected red blood cells (pRBC) containing asexual stages of the parasites were counted at least in 500 scopes and the mean percentages of all observed scopes were measured.
Parasite lactate dehydrogenase assay (pLDH)
pLDH assay was performed according to the procedure described by D'Alessandro (2013). Briefly, 100 µL of Malstate solution was added in duplicate to each well of a 96-well plate. The medium suspension at 20 μL containing 0.5% parasitaemia and 1.5% haematocrit was then added. Different concentrations of the A. paradoxum extract and positive and negative controls were also added. Finally, 25 μL solution of phenazine auto sulphate and nitro blue tetrazolium chloride was added; the absorbance was then read at 620 nm (Parvazi et al. 2016; D'alessandro et al. 2013).
Percentage of growth inhibition in parasite was calculated using the following formula:
In vivo anti-plasmodial assay
Mice
The experiments were conducted on male BALB/c mice weighing 22 ± 2 g and aged 7–9 weeks provided from the Pasteur Institute of Iran. Mice were accommodated and acclimatised at conditions of 12 h light and dark circles at 22–25 °C. They were properly fed and watered with standard diet.
Parasite inoculation
P. berghei cryopreserved in liquid nitrogen was provided by the Pasteur Institute of Iran. An amount of 0.2 mL of blood containing the parasite was intraperitoneally injected into three normal mice. After three consecutive passages, parasitaemia usually reached up to 14% within 3 to 4 days. The blood was collected using the cardiac puncture under general anaesthesia. Throughout the study, inoculation dose was 106 pRBC which was prepared in physiological saline solution and diluted to 0.2 mL before intraperitoneal injection (Garedaghi and Khaki 2014).
Antimalarial activity
After observation of parasites in the blood, treatment was carried out using the method suggested by Knight and Peters (1980). Fifty-five mice were divided into 11 groups of five. The first group was the negative control consisting of infected mice which received only normal saline. The second group was set as the positive control including infected mice treated with 20 mg/kg chloroquine. The other eight experimental groups were mice infected with 106 parasitised erythrocytes of P. berghei and intraperitoneally administrated with different concentrations of A. paradoxum extract. The extracts included 10, 20, 40, 50, 80, 100, 200 and 400 mg/kg at 0.2 mL volume. The last group was left uninfected as a lifespan control. The treatment course was set for 4 days. The blood samples were collected from the tails at the end of the 4th day. Parasitaemia was determined with microscopy after preparing blood smear and Giemsa's stain. The most efficient dose was defined as the one that showed the lowest parasitaemia in comparison to the control groups while there was no sign of toxicity on the affected mice (Tang et al. 2012) The EC50 assay was calculated by linear regression using SPSS 24.0.
Toxicity test
Cytotoxicity assay
The 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyltetrazolium bromide (MTT) assay was performed to evaluate the effects of A. paradoxum extract on PC12 cells so as to examine and observe their proliferation and viability. Mitochondrial dehydrogenase cleaved MTT salt in the metabolic active cells. It was then reduced to an insoluble formazan crystal with purple colour. Spectrophotometric means were utilized to solubilize and quantify the resulting intracellular purple formazan. PC12 cells were obtained from Iran University of Medical Sciences. Briefly, all cell lines were seeded in 96-well plates at a density of 6 × 103 cells per well and incubated at 37 °C in 5% CO2 to allow cell attachment. Different concentrations of A. paradoxum extract including 0.1, 1, 10, 100, 200, 400, 800 and 1000 µg/mL which were added to wells containing cell lines for 48 h. The supernatant of each well was then removed and 100 μL of RPMI 1640 (sans phenol red) and 50 μL of MTT solution were added and kept at 37 C° under 5% CO2 for 4 h. Isopropanol solution at 50 μL was then added to the plate and mixed in the dark for 15 min. The absorbance of all samples was read at 540 nm and recorded (Jo et al. 2015).
In vivo toxicity assay
The number of 50 male mice were divided into 10 groups. The two first control groups were negative control and positive received only normal saline and were treated with chloroquine. The experimental groups were set as 8 groups which were treated with different concentrations of A. paradoxum including 10, 20, 40, 50, 80, 100, 200 and 400 mg/kg. The weight of mice was measured after 4 days and compared with the weight of mice in control groups. Mice were anesthetized and sacrificed and their blood samples were collected by the heart puncture. The blood samples were assayed for level of alanine transaminase (ALT), aspartate transaminase (AST) and Alkaline phosphatase (ALP) enzymes (Elmi et al. 2019). Tissues of brain, spleen, kidney and liver were also prepared and washed in physiologic serum and then fixed in formalin. For microscopic studies, the paraffinised samples were then cut to 5 μm sections using a microtome and stained with haematoxylin and eosin (H&E) (Khodaparast et al. 2014). The data from experimental samples were then compared with the control groups.
Statistical analysis
The normal distribution of data was evaluated using the Kolmogorov–Smirnov test. The significance of the differences among various groups either experimental or control was examined using one-way ANOVA test and thereafter, post-hoc Tukey test. The comparison between categorical variables was performed using chi-square test. The IC50, ED50 and CC50 values were obtained by nonlinear regression analysis. The statistical differences were regarded as significant when p ≤ 0.05.
Results
In vitro anti-plasmodial activity
The results demonstrated that the highest significant (p ≤ 0.05) efficacy of A. paradoxum extract was related to 80 μg/mL dosage which led to a 60.43% -growth inhibition of parasites in cultures compared to the control groups. The growth inhibition was also statistically significant with 52.48% (p ≤ 0.05) when 40 μg/mL was administrated in comparison with the control groups. For doses of 0.025, 0.05, 0.1, 1 and 10 μg/mL, the growth inhibition percentage was not significantly (p ≥ 0/05) different between experimental and control groups (Fig. 1). IC50 was calculated to be 38 µg/mL by linear regression equation (Fig. 2).
Fig. 1.
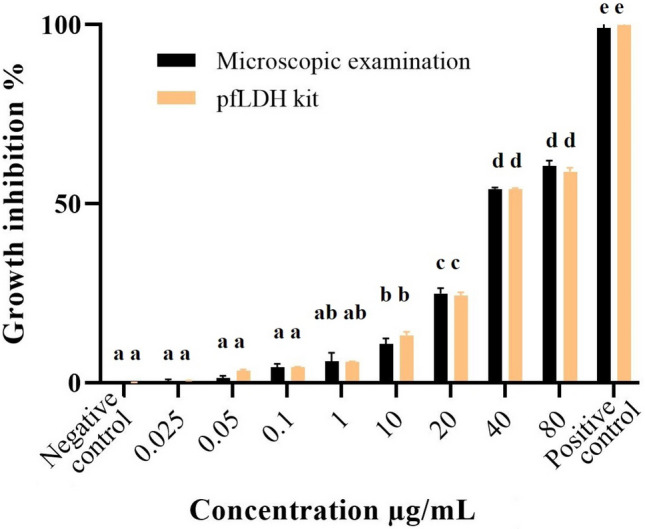
Growth inhibition of A. paradoxum extracts on P. falciparum in vitro. Different letters indicate significant statistical differences between groups (p < 0.05)
Fig. 2.
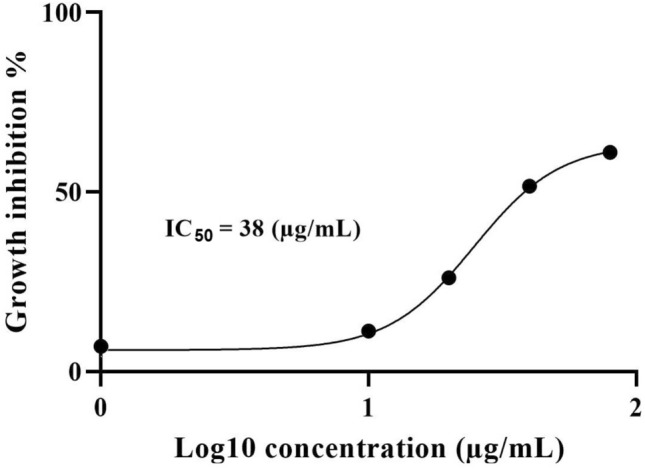
Determination of IC50 values of A. paradoxum on P. falciparum in vitro with nonlinear regression analysis
In vivo anti-plasmodial activity
As depicted in Fig. 3, the highest significant (p ≤ 0.05) parasite growth inhibition (88.71%) was observed in mice infected with P. berghei when they received A. paradoxum extract at 400 mg/kg compared to the control group. For the other doses of 10 and 20 mg/kg, the differences between the treated and control groups were not significant (p > 0.05).
Fig. 3.
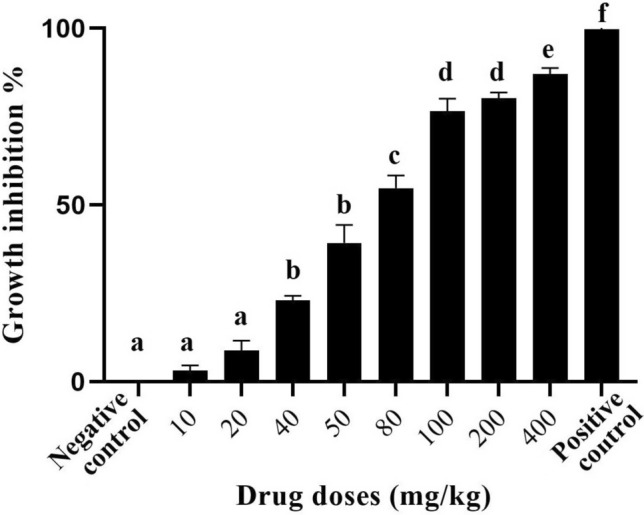
Growth inhibition of A. paradoxum extracts on P. berghei in vivo. Different letters indicate significant statistical differences between groups (p < 0.05)
Figure 4 shows the effective dose of ED50 which was related to 68 mg/kg of hydroalcoholic extract of A. paradoxum. The present study also showed that there was 98 ± 1.23% of the parasite growth inhibition in the chloroquine treated group (drug control) compared to other groups including controls.
Fig. 4.
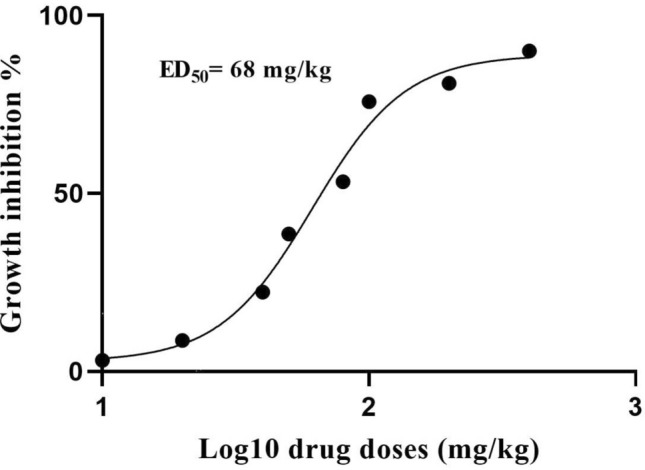
Determination of ED50 values of A. paradoxum on P. berghei in vivo with nonlinear regression analysis
The longest survival time in experimental groups was related to mice treated with 200 mg/kg extract with an average of 26 ± 1 day while the mean survival time for the negative control group was 11 ± 2 days (Table 1). Mice in uninfected control group survived up to 30 days. The difference for survival rate was significant (p < 0.05) between the control and treated groups.
Table 1.
The average life span of mice receiving different doses of A. paradoxum extract( p < 0.05)
| Groups | Dose (mg/kg) | life span (days) (Mean ± SD) | *p value |
|---|---|---|---|
| A. paradoxum | 10 | 12.3 ± 1.5 | 0.277 |
| 20 | 11.6 ± 1.1 | 0.710 | |
| 40 | 17.2 ± 1.2 | 0.031 | |
| 50 | 18 ± 1 | 0.008 | |
| 80 | 23.3 ± 1.5 | < 0.001 | |
| 100 | 26 ± 1 | < 0.001 | |
| 200 | 26.3 ± 0.5 | < 0.001 | |
| 400 | 24 ± 1 | < 0.001 | |
| + Control | 25 | 29 ± 1 | < 0.001 |
| − Control | – | 10 ± 1.6 | 1.000 |
| Uninfected | – | 30 | < 0.001 |
Cytotoxicity activity
The cytotoxicity of A. paradoxum was determined by MTT assay. Data analysis indicated that cytotoxicity was a dose-dependent phenomenon in which cell viability decreased with increasing concentrations of A. paradoxum extract compared to the control group (Fig. 5). The selectivity index (SI) of this extract was calculated at 19.73.
Fig. 5.
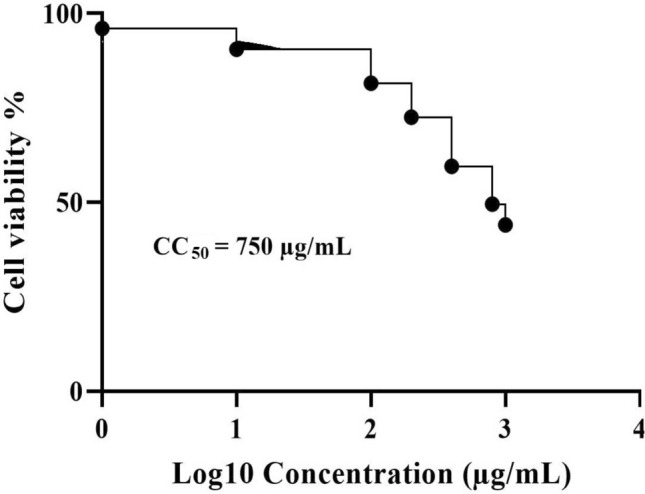
Cytotoxic study of A. paradoxum extract against PC12 cell line on a dose dependent manner
In vivo Toxicity of A. paradoxum
The mean weight of the mice in the treated group with 400 mg/kg of A. paradoxum extract was decreased compared to the weight of mice in the negative control group. However, no significant differences were observed across all groups (p ≥ 0.05). Comparison of the weight between the mice treated with other concentrations and control groups did not show any significant (p ≥ 0.05) differences (Table 2).
Table 2.
Weight and serum ALT, AST, ALP levels in the experimental and control groups of mice infected with P. berghei
| Variables (Mean ± SEM) | ||||
|---|---|---|---|---|
| Drug doses (mg/kg) | Weight (g) | ALT (U/L) | AST (U/L) | ALP (U/L) |
| 10 | 26 ± 6.22 | 77.60 ± 3.64 | 117.14 ± 3.36 | 159 ± 3.96 |
| 20 | 27.31 ± 0.64 | 69 ± 3.13 | 130 ± 14.29 | 151 ± 15.22 |
| 40 | 26.62 ± 0.24 | 71.64 ± 2.34 | 126.61 ± 13.27 | 157.32 ± 7.11 |
| 50 | 27.12 ± 0.69 | 70.31 ± 7.23 | 145.60 ± 12.75 | 158.61 ± 11.90 |
| 80 | 26.31 ± 0.73 | 81 ± 7.48a | 159 ± 17.21a | 173 ± 5.17 |
| 100 | 27 ± 0.37 | 79 ± 7.81a | 126.64 ± 12.56 | 172 ± 7.22 |
| 200 | 27.62 ± 0.68 | 99.31 ± 4.12a | 168.31 ± 12.37a | 209.64 ± 12a |
| 400 | 24 ± 0.44 | 267 ± 11.46a | 301 ± 63a | 323.34 ± 13.22a |
| Positive control | 26.31 ± 0.53 | 99 ± 5.65a | 166.34 ± 20a | 189 ± 19.10a |
| Negative control | 27.32 ± 0.61 | 67.32 ± 1.88 | 122 ± 8.94 | 155 ± 13.14 |
"a" indicate significant statistical differences between negative control and experimental groups (p < 0.05)
When different doses of 10, 20, 40, 50, 80 and 100 mg/kg were administered (Table 2) comparison of means for ALT, AST and ALP between the sample and the negative control groups did not show any differences. However, when doses of 200 and 400 mg/kg were used, ALT, AST and ALP increased significantly (p < 0.05) compared to the control group.
Histopathology studies
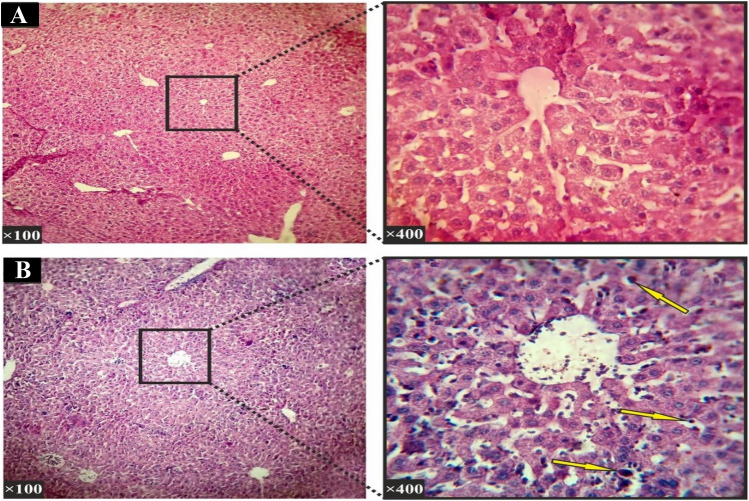
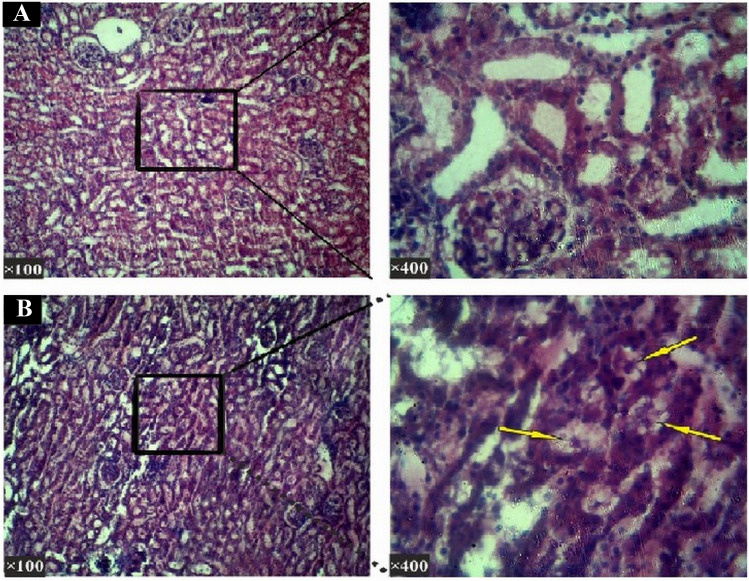
When the therapeutic doses of 10, 20, 40, 50, 80, 100, 200 mg/kg were used, histopathological studies of hepatic tissues showed no significant (p value > 0.05) differences between experimental and control groups. For these studies, parameters such as apoptosis, cell necrosis, local inflammation and hyperaemia (Fig. 6) were also considered. For kidneys, normal tissues were also observed like those seen in the hepatic tissues (Fig. 7). However, when the liver and kidney cells were investigated, significant (p < 0.05) histopathological changes were observed between the experimental group receiving 400 mg/kg and the control groups. For example, these significant changes were found in parenchymal disruption and cellular degradation in the kidney in the treated group compared to the control group. Similarly, necrosis and inflammatory cells were also observed in the liver compared to controls.
Fig. 6.
Histopathological section of the liver (Hematoxylin–Eosin staining). a The negative control group: In the histological study of the liver in the control group, Hepatocytes, their dispersion and liver sinusoids were normal and there was no hyperemia, edema and swelling. b A sample section for the group received 400 mg/kg: The study of liver histopathological sections showed visible inflammatory cells in the liver parenchyma (shown with arrows)
Fig. 7.
Histopathological section of the kidneys (Hematoxylin—Eosin staining). a In the histological study in the negative control group of the kidneys in the control group, renal corpuscle, proximal and distal tubules and the collecting ducts were normal. b In the group received 400 mg/kg dosage: The kidney histopathological studies of the sections in this group showed visible degradation in the lumen of the proximal and distal tubules. The cell wall of the proximal and distal tubules seen to be peeling off and dropping into the inner lumen (shown with arrows) and renal parenchyma disrupted too
Discussion
Using herbal medicine is rooted in human medicine history. In the present study, we showed anti-parasitic properties of the extract of A. paradoxum to inhibit growth rate of P. falciparum in vitro and P. berghei in vivo. A. paradoxum, a member of allium genus, has been widely utilised as a medicinal herb. They are known as antioxidants with anti-bacterial, anti-parasitic and anti-fungal properties. While comparing among studies, it is worthy to mention that the chosen herb and the extraction method were the main factors to be considered. These two factors are also the main elements for finding the most optimised and effective administration dose. Among other medicinal herbs, cinnamon, a member of lauraceae family, has different therapeutic properties. Parvazi and colleagues (2016) showed that the aqueous extract of cinnamon was able to inhibit P. falciparum growth when administrated at 1.25 mg/mL. Studies showed that, allicin, a naturally occurring compound generated when garlic cloves are crushed, can inhibit malaria infection. In line with our results, it has been shown that the garlic extract at 100 mg/kg inhibited 83% of P. berghei growth (Elmi et al. 2019; Parvazi et al. 2016; Nabavi et al. 2012a, b).
The present results showed that the parasitaemia in the infected positive control mice increased by 5% post 48 h of the inoculation. Chloroquine as a standard anti-malarial drug reduced parasitaemia to zero in the cured control group both in vitro and in vivo, enhancing the life expectancy. In P. falciparum culture, the experiments showed that IC50 was observed when 38 µg/mL administration dose of A. paradoxum extract was used. Various studies have reported that A. paradoxum, a member of the alliaceae family, has different effects including antioxidant activity against liver toxicity induced by CCl4 (Ebrahimzadeh et al. 2010; Baba 2018; Coppi et al. 2006; Nabavi et al. 2012a, b). Our results demonstrated that A. paradoxum had no toxic effects on the liver and kidney cells when a common therapeutic dose was used. Moreover, while we showed that this extract was safer than chloroquine in the control group, the difference was not statistically significant (p > 0.05).
There are some effective substances for liver and kidney cells in Allium genus members such as diallyl disulfide, S-allyl cysteine sulfoxide and allyl propyl disulfide. In the present study, investigations on the affected liver showed that in mice receiving 80 mg/kg of the extract necrosis was less than (EC50) that in the infected control group. However, when the extract concentration increased to 400 mg/kg, necrosis of the liver cells was significantly (p < 0.05) higher than that in the control group. Also, when the experimental group received 400 mg/kg, the levels of ALT, AST and ALP significantly (p < 0.05) increased compared to the control group. These results indicated that the extract of A. paradoxum at large therapeutic doses may have undesirable side effects for both liver and kidney. However, further investigations are required to prove such conclusions due to study limitations.
We have shown that the best therapeutic dosage of A. paradoxum was 200 mg/kg in vivo. This dosage demonstrated the best results with no toxic outcome in the liver and kidney. Histopathological examinations of both liver and kidney in the experimental groups, except those receiving 400 mg/kg, showed that the dispersion of hepatocytes and liver sinusoids were normal. Moreover, hyperemia, edema and swelling had similar status in both experimental and the negative control groups. Kidney body, proximal and distal tubules and the collecting ducts were also observed to be normal. Although the ALT and AST levels did not have a significant increase compared to the control group, the ALP level was significantly (p < 0.05) higher compared to the control group. This finding may be another question which remains to be answered and deserves more intense investigations.
Previous studies on A. paradoxum showed that this plant can be used as a natural herbal medicine for the treatment of leishmania parasites, Giardia lamblia and Toxoplasma gondii (Hezarjaribi et al. 2015; Ebrahimzadeh et al. 2017). Although the present results proved anti-plasmodial effects of A. paradoxum extract, it may have the same effect on other parasitic infections. These observations encourage us to claim that more intense investigations should be carried out to examine the effect of A. paradoxum extract against other virulent parasites.
Several studies have shown that extraction methods including aquatic, alcoholic or chloroform alter the efficacy of the prepared herbal drugs. For example, Elmi and co-workers (2019) reported that the chloroform extract of A. paradoxum, compared to hydroalcoholic extract, had higher effectiveness in treating giardiasis by reducing the parasite's cysts in BALB/c mice. Another study showed that alcoholic extract compared to its aquatic extract had better effectivity on Giardia lamblia growth (Hezarjaribi 2015). In the present study, only the effect of the hydroalcoholic extract of allium plant on plasmodium parasites was investigated; therefore, further comparative studies should be performed with extended samples size to evaluate the effects of aqueous and chloroform extracts of A. paradoxum.
We only used the crude extract of the bulb and grounded portions of A. paradoxum. Other studies (Ebrahimzadeh et al. 2017; Rezaee et al. 2018) have proved that when 0.1 mg/kg and 0.07 mg/kg of Cichorium intybus were administrated, they effectively reduced parasitaemia in mice infected with P. berghei. They demonstrated that, as we did in A. paradoxum against malaria parasites, increased dosages of C. intybus extracts were more effective in reducing parasitaemia compared to lower concentrations. However, in agreement with our results, it has been shown that very high dosages caused inverse effects such as toxicity in the liver and kidney cells. These findings suggested that optimising the administration dose at its best effectiveness needs to be considered in all experimental settings when evaluation of a given herbal medicine as an alternative drug is investigated.
It has been known that increased prescription of chloroquine, primaquine and other anti-malarial drugs have rendered resistance against them (Adebayo and Krettli 2011). Therefore, these observations optimistically motivate researchers to find more effective herbal medicinal substitute drugs with the least unpleasant side effects as one of the prioritised purposes in clinic.
Conclusion
The present study showed that A. paradoxum extract exhibited significant antimalarial properties on P. falciparum in vitro. Also, interestingly our in vivo studies demonstrated that along with its therapeutic effect, there was no significant toxicity in the liver and kidney cells of mice infected with P. berghei when they received A. paradoxum extract. Our findings proved that A. paradoxum extract may be added as a new natural herbal antimalarial drug against one of the most fatal infectious diseases. Taken together, further investigations are required to determine the optimised dosages and extraction methods to obtain the best fraction(s) or even molecules of A. paradoxum with more effective bioactivity against malaria parasites and even to other prevalent parasitic diseases.
Acknowledgements
The authors would like to acknowledge the valuable contribution of the Pasteur Institute of Iran for their donating parasites and providing experimental in vitro works including culture of Plasmodium falciparum. We would also like to thank Iran University of Medical Sciences for providing the necessary funding for this research.
Abbreviations
- PLDH
Parasite Lactate Dehydrogenase assay
- MTT
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- H&E
Haematoxylin and eosin
Authors’ Contributions
Taher Elmi, Fateme Hajialiani, Sedigheh Sadeghi and Mohamad Reza Asadi performed experiments and analysed the data. Fatemeh Tabatabaie and Zahra Zamani searched the literature and designed it. All authors have participated in drafting the manuscript and supervised the research. Fatemeh Tabatabaie, Taher Elmi and Mohammad Javad Namazi wrote the final manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Iran University of Medical Sciences [Grant Code: 97–02-30–33789].
Compliance with ethical standards
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
The national law on the care and use of laboratory animals was followed. The ethics committee at Iran University of Medical Sciences approved performing the present study and issued an ethical code of: IR.IUMS.FMD.REC.1397.025 to the study. The approval license was in accordance with Helsinki Declaration guidelines.
Consent for publication
The authors declare that they have no competing interests regarding the publication of this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Taher Elmi, Email: elmi1364@yahoo.com.
Fateme Hajialiani, Email: fateme.aliani82@gmail.com.
Mohamad Reza Asadi, Email: Asadimohammadreza@yahoo.com.
Sedigheh Sadeghi, Email: sedisadeghi@yahoo.com.
Mohammad Javad Namazi, Email: mjnamazi@gmail.com.
Fatemeh Tabatabaie, Email: tabatabaei.f@iums.ac.ir.
Zahra Zamani, Email: zamani@pasteur.ac.ir.
References
- Adebayo JO, Krettli AU. Potential antimalarials from Nigerian plants: a review. J Ethnopharmacol. 2011;133(2):289–302. doi: 10.1016/j.jep.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Baba MSh. In vivo antimalarial assessment and toxicity evaluation of garlic (Allium sativum) in plasmodium berghei NK65-induced mice. Malays Appl Biol. 2018;47:17–24. [Google Scholar]
- Bhattacharjee D, Shivaprakash G. Drug resistance in Malaria-in a nutshell. J Appl Pharm. 2016;6(03):137–143. [Google Scholar]
- Coppi A, Cabinian M, Mirelman D, Sinnis Ph. Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents chemother. 2006;50(5):1731–1737. doi: 10.1128/AAC.50.5.1731-1737.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, et al. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemoth. 2013;68(9):2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- Ebrahimzadeh MA, Nabavi SF, Nabavi SM, Eslami B. Antihemolytic and antioxidant activities of Allium paradoxum. Cent Eur J Biol. 2010;5(3):338–345. [PubMed] [Google Scholar]
- Ebrahimzadeh MA, Taheri M, Ahmadpour E, Montazeri M, Sarvi Sh, Akbari MJ, et al. Anti-toxoplasma effects of methanol extracts of Feijoa sellowiana, Quercus castaneifolia and Allium paradoxum. J Pharmacopuncture. 2017;20(3):220. doi: 10.3831/KPI.2017.20.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmi T, Hajialiani F, Asadi MR, Orujzadeh F, Kalantari Hesari A, Rahimi Esboe B, et al. A Study on the effect of zingiber officinale hydroalcoholic extract on plasmodium berghei in infected mice: an experimental study. JRUMS. 2019;18(4):353–364. [Google Scholar]
- Elmi T, Shafiee Ardestani M, Hajialiani F, Motevalian M, Mohamadi M, Sadeghi S, et al. Novel chloroquine loaded curcumin based anionic linear globular dendrimer G2: a metabolomics study on Plasmodium falciparum in vitro using 1H NMR spectroscopy. Parasitology. 2020;147(7):747–759. doi: 10.1017/S0031182020000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garedaghi Y, Khaki A. Evaluation of the effectiveness of ethanolic extract of Solanum surattense against Plasmodium berghei in comparison with chloroquine in Sourian Mice using in Vivo tests. CJMB. 2014;1(3):76–79. [Google Scholar]
- Hajialiani F, Elmi T, Mohamadi M, Sadeghi S, Shahbazzadeh D, Ghaffarifar F, et al. Analysis of the active fraction of Iranian Naja naja oxiana snake venom on the metabolite profiles of the malaria parasite by 1HNMR in vitro. Iran J Basic Med Sci. 2020;23(4):534–543. doi: 10.22038/IJBMS.2020.39386.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezarjaribi HZ, Elmi T, Dayer MS, Gholami S, Fakhar M, Akbariqomi F, A, et al. Systematic review of the effects of Iranian pharmaceutical plant extracts on Giardia lamblia. Asian Pac J Trop Dis. 2015;5(12):925–929. doi: 10.1016/S2222-1808(15)60959-8. [DOI] [Google Scholar]
- Jo HY, Kim Y, Woo Park H, Eun Moon H, Bae S, Kim J, et al. The Unreliability of MTT assay in the cytotoxic test of primary cultured glioblastoma cells. Exp Neurobiol. 2015;24(3):235–245. doi: 10.5607/en.2015.24.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodaparast Z, Yousofi A, Khoshvaghti A. Investigation of curcumin effects on liver tissue in adult male rats treated with cyclophosphamide. J F UM S/Majallah-i Danishgah-i Ulum-i Pizishki-i Fasa. 2014;4(3):344–352. [Google Scholar]
- Knight DJ, Peters W. Theantimalarial action of N-benzyloxydihydrotrazines. The action of clocigunail (BRL50216) against rodent malaria and studies on its mode of action. Annals Trop Med Parasitol. 1980;74:393–404. doi: 10.1080/00034983.1980.11687360. [DOI] [PubMed] [Google Scholar]
- Nabavi SM, Moghaddam H, Fazli M, Bigdellou R, Mohammadzadeh S, et al. Hepatoprotective activity of Allium paradoxum. Eur Rev Med Pharmacol Sci. 2012;16(3):43–46. [PubMed] [Google Scholar]
- Nabavi SF, Nabavi SM, Hajizadeh Moghaddam A, Naqinezhad A, Bigdellou R, Mohammadzadeh S. Protective effects of Allium paradoxum against gentamicin-induced nephrotoxicity in mice. Food Funct. 2012;3(1):28–29. doi: 10.1039/C1FO10173K. [DOI] [PubMed] [Google Scholar]
- Nateghpour M, Miahipour A. Effectiveness of ethanolic extract of Otostegia Persica against Plasmodium Berghei in comparison with chloriquine in white mice using in vivo tests. J Sch Public Health Inst Public Health Res. 2008;6(1):57–63. [Google Scholar]
- Parvazi Sh, Sadeghi S, Azadi M, Mohammadi M, Arjmand M, Vahabi F, et al. The effect of aqueous extract of cinnamon on the metabolome of Plasmodium falciparum using 1HNMR spectroscopy. J Trop Med. 2016 doi: 10.1155/2016/3174841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwan N, Khalil AI, Wahdan AE. In vitro evaluation of anthelminthic activity of Allium sativum against adult Cotylophoron cotylophorum (Paramphistomidae) PUJ. 2012;5:135–146. [Google Scholar]
- Rezaee F, Zolfaghari B, Dinani MS. Isolation of dioscin-related steroidal saponin from the bulbs of Allium paradoxum L with leishmanicidal activity. RPS. 2018;13(5):469. doi: 10.4103/1735-5362.236875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Cui Y, Luo F, Liu X, Wang X, Wu A, et al. Cell viability and dopamine secretion of 6-hydroxydopamine-treated PC12 cells co-cultured with bone marrow-derived mesenchymal stem cells. Neural Regen Res. 2012;7(14):1101. doi: 10.3969/j.issn.1673-5374.2012.14.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]