Abstract
The unique structure of the stomach, including the rumen, reticulum, omasum, and abomasum, indicates the differences between the ruminant and monogastric animals in the digestion of nutrients. This difference is reflected in the majority of dietary nutrients that may be fermented in the rumen. Significant proteins and a certain amount of starch can flow to the small intestine apart from rumen. The initial phase of small intestinal digestion requires pancreatic digestive enzymes. In theory, the enzymatic digestion and utilization efficiency of starch in the small intestine are considerably higher than that in the rumen, but the starch digestibility in the small intestine is quite low in ruminants. Therefore, improving the digestion of nutrients, especially starch in the small intestine is more urgent for high-yield ruminants. Although the pancreas plays a central role in nutrient digestion, the progress of research investigating pancreatic exocrine regulation in the ruminant is slow due to some factors, such as the complex structure of the pancreas, the selection of experimental model and duration, and internal (hormones or ages) and external (diet) influences. The present review is based on the research findings of pancreatic exocrine regulation of dairy animals and expounded from the physiological structure of the ruminant pancreas, the factors affecting the digestion and exocrine processing of carbohydrates, and the regulatory mechanism governing this process. The review aims to better understand the characteristics of enzymatic digestion, thereby advancing pancreatic exocrine research and improving the digestion and utilization of nutrients in ruminants. Additionally, this review provides the theoretical basis for improving nutrient utilization efficiency, reducing wastage of feed resources, and promoting the efficient development of the dairy industry.
Keywords: Ruminant, Pancreas, Exocrine function, Starch
1. Introduction
Digestion of feed represents a decisive process of nutrient utilization for productive purposes such as the production of meat or milk (Harmon and Swanson, 2020). Different from monogastric animals, the rumen plays more fermentative role during nutrients digestion in ruminants. Also, the digestive function of the small intestine cannot be ignored. Pancreatic exocrine secretion is an essential step of nutrients digestion, which provides an efficient supply model. The exocrine pancreas consists of the acinus, which secretes pancreatic juice, and the pancreatic duct, which transports pancreatic juice. The exocrine pancreas synthesizes and secretes several digestive enzymes (including amylase, lipase, and proteases) to digest feedstuff, supplying nutrients (such as starch, fat, and protein) for ruminants.
In the small intestine of ruminants, starch digestion into dextrins and maltose depends on amylase secreted by the pancreas. The digestion and utilization of starch may be limited and these limitations could be caused by inadequate amylase activity in the small intestine (Swanson, 2019). The evidence is that approximately 40% of rumen bypass starch cannot be digested and utilized by ruminants (Moharrery et al., 2014). This lack of digestion is an important reason why many studies have focused on the secretion and regulation of pancreatic amylase. Theoretically, the type of energy supplied from the glucose absorbed in the small intestine is more efficient than that supplied from volatile fatty acids (VFA) in the rumen. However, the limited starch digestibility in the small intestine restricts total-tract energy yield in comparison to energy yield from ruminal fermentation of starch (Harmon, 2009). A recent summary for ruminant intestinal starch and protein assimilation (Harmon and Swanson, 2020) reported the crucial function of pancreatic α-amylase in small intestinal starch digestion. Improving pancreatic exocrine secretion, especially amylase, may strengthen energy supply performance, feed efficiency, and reduce environmental pollution.
Because of the ruminal microbial influence on the nutrient profile, a complete description of the macronutrients like rumen bypass starch digestion and absorption in ruminant small intestine remains elusive (Harmon and Swanson, 2020). Furthermore, the molecular mechanism that regulates pancreatic secretions in ruminants remains unclear. The objective of the present review is to bring together the current information on pancreatic regulation with consideration of the regulation mechanism of pancreatic exocrine. The primary focus of this review is to assess the regulatory factors of ruminants and to examine the involved potential mechanisms.
2. Process of carbohydrate digestion in ruminant
Ruminants have a complex stomach with 4 compartments consisting of the rumen, reticulum, omasum, and abomasum. Numerous bacteria, protozoa, and anaerobic fungi are found in the rumen. Feeds are fermented in the rumen and VFA and microbial proteins are produced. These digestive processes are different from those in nonruminants. A large proportion of the total metabolizable energy (50% to 85%) made available to the animal is provided by the VFA (Harmon and Swanson, 2020). Although there is no digestive gland in the rumen, the rumen and microorganisms play essential roles in the digestion process.
A characteristic of ruminants is that they can utilize both structural and non-structural carbohydrates, which results in a more complex utilization of carbohydrates than nonruminants. The proportion of carbohydrates in ruminant diets is usually up to 60% to 70%, which is the main energy source. The structural carbohydrates such as cellulose and hemicellulose are degraded by ruminal microorganisms to produce acetic acid (mainly), while the non-structural carbohydrates, especially the starch, are fermented to produce propionic acid and butyric acid. These VFA are absorbed and used by the peripheral tissue to synthesize glucose or milk components. It is beneficial to nitrogen balance and higher microbial yields to ensure a certain proportion of starch fermentation in the rumen. However, some types of microbes could use acetic acid to generate methane, and this provides less net energy as a result of heat production from fermentation. One study found that the energy loss of rumen microbial fermentation of starch is approximately 3% to 12% of the digestive energy of the diet (Huhtanen and Sveinbjörnsson, 2006). Therefore, changing the carbohydrates fermentation types from acetic acids type to propionic acids type in the rumen could increase the overall energy supply efficiency.
Regulating the digestion of carbohydrates such as starch to glucose in the intestine is a direct energy supply model (Harmon and Swanson, 2020). In the digestive process of starch in the small intestine, ruminants are similar to monogastric animals. Small intestinal starch digestion in ruminants requires the following processes: hydrolysis of starch into small molecular oligosaccharides by amylase, decomposition of oligosaccharide into glucose by α-saccharide hydrolase, and transport of glucose in the intestinal lumen to the intestinal epithelial cells by glucose transporters (Huntington, 1997). Starch digestion in the small intestine needs the amylase secreted by pancreatic acinar cells. Amylase is activated in the intestinal lumen and hydrolyses α-1,4 glycosidic bonds that break down starch into glucose, or small molecule oligosaccharides (such as maltose and maltotriose). Mcleod et al. (2001) reported that gastric-perfusion starch is 25% more efficiently converted to metabolic energy than starch through the rumen fermentation process. In the process of digesting starch in the small intestine, although there is no fermentation and heat production, under the action of digestive enzymes, breaking glycosidic bonds also releases thermal energy. The heat released by starch in the small intestine digestion is equivalent to 0.6% of the digestive energy of the diet, far less than the energy loss in the rumen. Starch can theoretically be digested in the small intestine and efficiently absorbed and directly supplied in the form of glucose. The efficiency is considerably higher than the efficiency of microbial degradation in the rumen to produce VFA. Dairy animals have limited digestion capacity of nutrients such as starch in the small intestine and only 40% to 62% rumen escape starch can be digested (Harmon et al., 2004), which causes energy supply shortage during the peripartum period and feed resource waste.
3. Physiological structure of the ruminant pancreas
As a digestive gland, the pancreas is composed of the endocrine part secreting hormones such as insulin and glucagon (Jenstad and Chaudhry, 2013), and the exocrine part secreting digestive enzymes such as amylase, protease, and lipase, which play an important role in the growth, development, reproduction, and production processes of animals. Small intestinal digestion in ruminants is similar to that of monogastric animals, which mainly relies on digestive enzymes secreted by the pancreas. However, because rumen fermentation degrades a major part of the ingested feed, the pancreas function and regulation may differ between ruminants and monogastric animals.
The pancreas is divided into 4 structures, namely, the head, neck, body, and tail, but there is no boundary among each section. The surface of the pancreas is covered by thin connective tissue. The pancreas has endocrine and exocrine functions. The exocrine pancreas consists of a multi-branched lobular, acinus that secretes pancreatic enzymes and electrolytes, as well as other fluids. Pancreatic juice is composed of inorganic salts, zymogen particles, and water, and flows into the duodenum through a duct to digest starch, protein, and fat in the chyme. The endocrine part of the pancreas is tightly globular, embedded in the exocrine section, and composed of islet cells. Islet cells are divided into α-, β-, δ- and PP-cells and secrete glucagon, insulin, somatostatin, and pancreatic polypeptides (inhibition of gastrointestinal motility, pancreatic secretion, and gallbladder contraction), respectively (Brereton et al., 2015). Endocrine cells only account for a small fraction of the total number of pancreatic cells, but the hormones secreted by them have essential regulatory functions, especially in terms of blood glucose balance (Moullé and Parnet, 2019).
The acinus is the basic functional structure of the pancreas that exercises the exocrine function. The acinus consists of conical serous gland cells, which are primarily used for the synthesis, storage, and secretion of various digestive enzymes. Acinar cells are typical secretory cells whose structures are shown in Fig. 1. This figure presents the ultrastructural map of freshly isolated cow pancreatic acinar cells taken with a transmission microscope. The elliptical nucleus is in the middle of the acinar cells. A large number of zymogen particles are distributed at the base of the cell, whose numbers vary by function and state of the cells. Such organelles as ribosomes and endoplasmic reticulum are near the zymogen granules. The endoplasmic reticulum ribosomes synthesize proteins while the Golgi apparatus fabricates these proteins to zymogen-secreting granules. The acinar cells release the zymogen granules into the acinar cavity by exocytosis and then these zymogen granules enter the duodenum through the pancreatic duct.
Fig. 1.
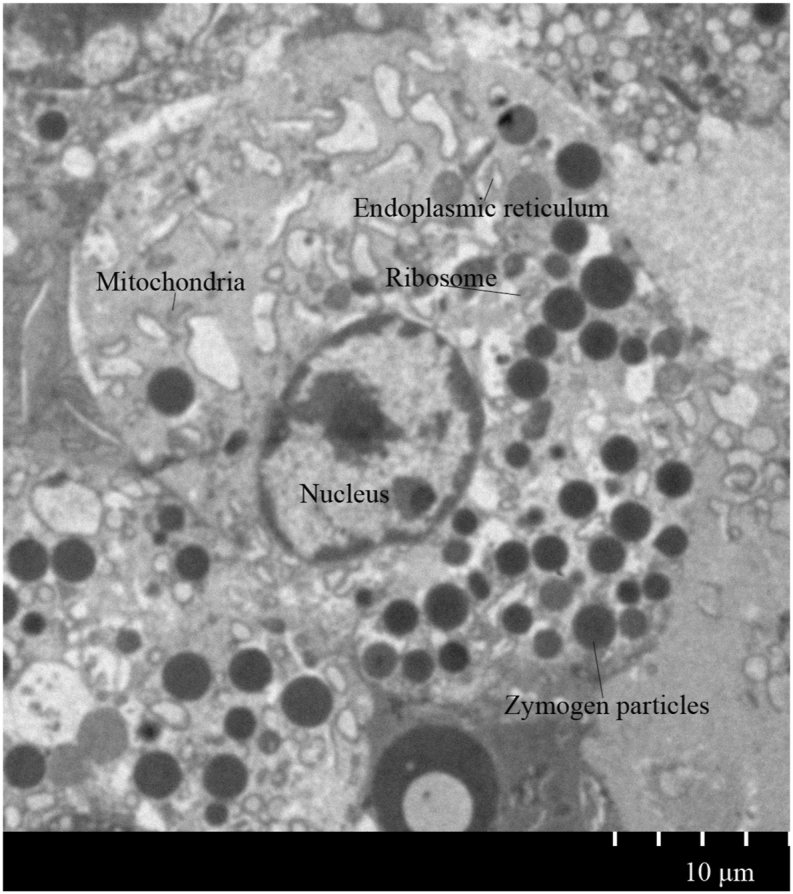
Ultrastructure of pancreatic acinar cells in dairy cows. Organelles, such as mitochondria, endoplasmic reticulum, and nucleus are shown. The brown circles are zymogen particles.
4. Factors affecting the exocrine function of the pancreas
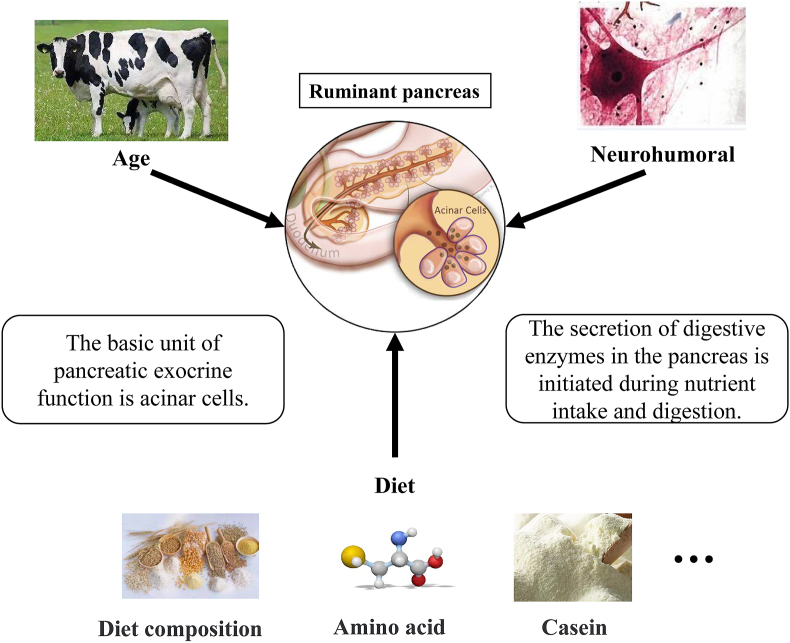
The secretion of digestive enzymes in the pancreas is affected by a variety of factors, such as animal age, neurohumoral regulation, and dietary components (Fig. 2).
Fig. 2.
Factors affecting the exocrine function of the pancreas. Age, neurohumoral properties and diet affected pancreatic secretion. The basic unit of pancreatic exocrine function is the acinar cell, and the secretion of digestive enzymes in the pancreas is initiated during nutrient intake and digestion.
4.1. Animal age
The age of an animal is the main factor that affects the pancreatic exocrine function of ruminants. As age increases, the development of the animal pancreas gradually matures, the total amount of pancreatic juice increases, and the number of digestive enzymes contained in the pancreas is also increased (Naranjo et al., 1997). The flow rate of pancreatic juice secreted by a 4-day-old calf is only 150 mL/24 h, while a 100-day-old calf secreted over 1, 000 mL/24 h (Mccormick and Stewart, 1967). It was also reported that the daily pancreatic secretion increased from 7.9 mL/kg body weight in 3-week-old calves up to 14.2 mL/kg body weight 2 months later (Zabielski et al., 2001). A more substantial increase (3-fold) was also observed in the daily pancreatic protein output during that period. Moreover, the activity of digestive enzymes that contain amylase, protease, and lipase in the small intestine of newborn calves is low, but as age increases, the activity of these enzymes is significantly higher than that at the time of birth.
4.2. Neurohumoral regulation
The central nervous system regulates pancreatic secretion through the vagus nerve. After blocking the vagus nerve, the secretion of pancreatic juice, the activity of trypsin, and the concentration of protein in pancreatic juice were reduced (Konturek et al., 2003). There is also strong evidence suggesting the importance of the brain-gut axis in regulating pancreatic secretion (Jaworek et al., 2010). In addition, stimulation of cholinergic nerves can increase pancreatic exocrine secretion and increase secretion of endogenous secretin and cholecystokinin (CCK). However, early research found that local neural reflexes and secretin-mediated exocrine responses may be more important than stimulation by CCK (Croom et al., 1992). These experimental results indicate that nerves play an essential regulatory role in regulating the exocrine function of the pancreas.
CCK is an important brain-gut peptide that regulates pancreatic enzyme release. CCK is produced and secreted by intestinal mucosal type I cells when stimulated by amino acids (AA) and fatty acids. Feeding intact casein also increased CCK secretion in steers (Lee et al., 2016). The addition of exogenous CCK to mice increased pancreatic weight, individual acinar cell volume, and DNA, RNA, and protein content (Yu et al., 2011). CCK directly stimulates pancreatic acinar cells to secrete pancreatic amylase (Wang and Cui, 2007), a process that relies on CCK and CCK receptor binding on the surface of acinar cell membranes. There are 2 subtypes of CCK receptors, CCK1 and CCK2, both of which belong to the G-protein coupled receptor family (Williams, 2001). By locating CCK receptors in the calf, it was found that both receptors were expressed in the calf pancreas, but in adulthood, CCK2 was the main receptor in the pancreas and was primarily distributed in islet cells and ducts. However, the membrane of the pancreatic acinar cells does not find these 2 receptors, which is primarily observed because with the development of calves, the pancreatic function is transformed and degraded, causing some functions to be lost. Additionally, the effect of imaging methods of immunofluorescence assays and selection of the receptor protein's antibody cannot be ignored. Determining whether the CCK2 receptor is present on the membrane of pancreatic acinar cells in adult cows requires further investigation.
Insulin is a hormone secreted by islet β cells, which regulates the body's blood glucose concentration and acts as a signalling molecule to participate in other physiological metabolic processes of the body, such as promoting the mammals' target of rapamycin (mTOR) signalling pathway to regulate protein synthesis (Tremblay and Marette, 2001). Insulin regulates blood glucose levels by promoting tissue uptake and glucose utilization and inhibiting glycogenolysis and gluconeogenesis (Yea et al., 2009). There may be differences in the role of insulin in regulating pancreatic exocrine function in monogastric and ruminant animals. Insulin can improve the exocrine function of the pancreas in diabetic mice (Otsuki and Williams, 1982), but the regulation of pancreatic α-amylase mRNA expression in the duodenal perfusion of leucine in dairy goats does not depend on changes in serum insulin (Yu et al., 2014).
4.3. Animal diet
The initiation of digestive enzyme secretion is before feed intake, which means that when ruminants smell or see feeds, this could stimulate the release of the digestive enzymes by the pancreas. Different types of feed can affect the secretion of pancreatic enzymes.
4.3.1. Carbohydrate
The proportion and type of dietary carbohydrates can affect the exocrine function of the pancreas, especially the secretion of amylase. Yu et al. (2013, 2014) reported a significant linear relationship between starch digestibility and chyme amylase in dairy goats, which indicated that dietary rumen-protected starch affects pancreatic amylase secretion. Sheep were fed with different types of carbohydrates, including hay and crushed corn, and it was found that the concentration and viability of pancreatic amylase in the smashed corn diet group were greater than those in the hay feeding group (Janes et al., 1985). Secretion of α-amylase of the pancreas in dairy goats increased with the amount of rumen escape starch, peaked at 113 g/h, and then decreased (Xu et al., 2010). Dairy goats fed with a diet containing 20% starch showed lower secretion of pancreatic α-amylase compared with goats fed with 30%, 40%, and 50% dietary starch; increasing rumen escape starch resulted in a quadratic increase in pancreatic amylase and lipase secretion (Xu et al., 2010). However, Holstein steers fed hydrolyzed starch had reduced pancreatic α-amylase concentration and secretion rate (Swanson et al., 2002). Those results show that the amount of starch bypassing the rumen and entering the small intestine was a key factor that affected the exocrine function of the pancreas and these responses disappear with time and dose.
4.3.2. Functional AA
Leucine can be used as a nutrient signal to stimulate the secretion of α-amylase, trypsin, chymotrypsin, and lipase in a dose- and time-dependent manner. In goats, Yu et al. (2014) found duodenal leucine perfusion did not affect pancreatic juice secretion, protein output, trypsin and lipase secretion, and plasma insulin concentration. While amylase release linearly increased in a 14-d experimental period, pancreatic juice and α-amylase secretion responded quadratically with the greatest values being observed in responses to provisions of 3 and 6 g/d leucine, respectively (Yu et al., 2014). The research also suggested that duodenal leucine perfusion dose- and time-dependently regulated pancreatic enzyme secretion and it was not associated with the change in plasma insulin concentration. Cao et al. (2018a) used 16 healthy yearling dairy goats to duodenal infuse leucine and found that an appropriate amount of leucine can effectively improve the expression and secretion of pancreatic amylase, which is important for improving intestinal starch digestion in ruminant animals. Duodenal perfusion of leucine can also regulate the development of the pancreas as well as the expression of digestive enzyme genes in dairy goats and plays a significant role in the digestion of starch and other nutrients in the intestinal tract of ruminants (Cao et al., 2018a). Liu et al. (2015) poured different amounts of leucine into pancreatic cannulas fitted to 4 Holstein heifers, and found that 10 g of leucine significantly increased the secretion of pancreatic fluid as well as the concentration and secretion rate of amylase. Analysis of the results of a 20 male Holstein calves feeding experiment showed that leucine tended to increase the concentration of total pancreatic protein (mg/kg of body weight), serum glucose and essential amino acids (EAA), body index, and the average daily gain without amylase secretion (Cao et al., 2018b).
Isoleucine is a branched-chain amino acid (BCAA) and has a similar structure and function to leucine (Nair and Short, 2005). In Holstein heifers, duodenal isoleucine perfusion increased pancreatic exocrine function, especially α-amylase, and the increases appeared to be dose- and time-dependent. Also, the protein concentration in the pancreatic secretions (mg/mL), the pancreatic secretion rate (mg/h), and the CCK concentration were all increased. However, the pH of pancreatic secretions, the activity of chymotrypsin and lipase, the concentrations of plasma glucose and insulin were not affected (Liu et al., 2017b).
Phenylalanine belongs to the aromatic AA and has a complex metabolic process in animal organisms (Wu et al., 2014). Studies have shown that this AA regulates the exocrine secretion of the pancreas through the CCK pathway (Liddle et al., 1986). The continuous perfusion of phenylalanine into the duodenum of dairy goats for 14 days increased the amount of pancreatic bile secretion, amylase secretion, and serum CCK concentration quadratically, with the greatest values at 2 g/d; the trypsin secretion also showed a quadratic curve with a maximum at 4 g/d; however, the release of lipase decreased by phenylalanine addition (Yu et al., 2013). Phenylalanine increased amylase, trypsin synthesis, secretion, and mRNA expression in cow pancreas cultured in vitro (Guo et al., 2018a). It has been reported that phenylalanine could inhibit the effects of leucine in promoting intestinal development and affects pancreas growth (Cao et al., 2018c).
Ruminal proteins and other AA also affect the exocrine ability of the ruminant pancreas. Richards et al. (2002) noted that starch digestibility and pancreatic enzyme secretion are linearly related to casein content infused into the small intestine of steers. There is a dynamic relationship between the amount of ruminal protein and the secretion of amylase. The ratio of EAA to starch in the small intestine could influence pancreatic amylase secretion of ruminants (Swanson et al., 2004). Swanson et al. (2008) also speculated that the higher the protein content in the small intestine, the greater would be the secretion of pancreatic enzymes. Dairy cows with a duodenal infusion of non-essential amino acids (NEAA) and glutamate also show increased intestinal digestibility (Brake et al., 2014; Blom et al., 2016).
5. Mechanism of pancreatic exocrine regulation
5.1. Intracellular calcium signal
Calcium ions are principally deposited in the endoplasmic reticulum in acinar cells (Van de Put and Elliott 1997). Within the cytoplasm, calcium ions act as a coupling center from stimulation to secretion. When intracellular CCK or acetylcholine hormones reach physiological stimulating concentrations, the cells produce 2 secondary messengers, IP3 and nicotinic acid adenine dinucleotide phosphate. These 2 secondary messengers combine with the receptors of ryanodine and inositol 1,4,5-trisphosphate, which releases the calcium ions stored in the endoplasmic reticulum (Petersen and Tepikin, 2008). The concentration of calcium ions in the cytoplasm is slightly oscillated, thereby increasing the release of zymogen particles from intracellular to extracellular (Petersen, 2005).
5.2. Proteasome
The proteasome is an essential protein complex that functions to degrade proteins in cells. The ubiquitin-proteasome is a crucial regulator of cell life processes and has received intensive attention in disease model cells due to its function and role. However, further research is needed on secretory cells with dynamic protein metabolism activities (Ristic et al., 2014). Some studies have shown that the anti-factor 4 of the 26S proteasome in the mouse pancreas can significantly regulate the secretion of digestive enzymes (Coux et al., 1996). The super-physiological concentration of leucine (1.35 mmol/L) significantly inhibited proteasome activity and the secretion of amylase, which indicated that the proteasome is a possible key factor in digestive enzyme secretion in the pancreatic acinar cells of the cow. After using the specific inhibitor of proteasome MG132, the results also showed that the proteasome plays a critical role in the secretion of amylase (Guo et al., 2018b). Using label-free proteomics analysis technology, we also found that leucine significantly affects the expression of proteasome-associated protein subunits in acinar cells (Guo et al., 2020). In pancreatic β-type cells, insulin secretion is affected by proteasome activity (Liu et al., 2017a).
5.3. Molecular signalling pathway
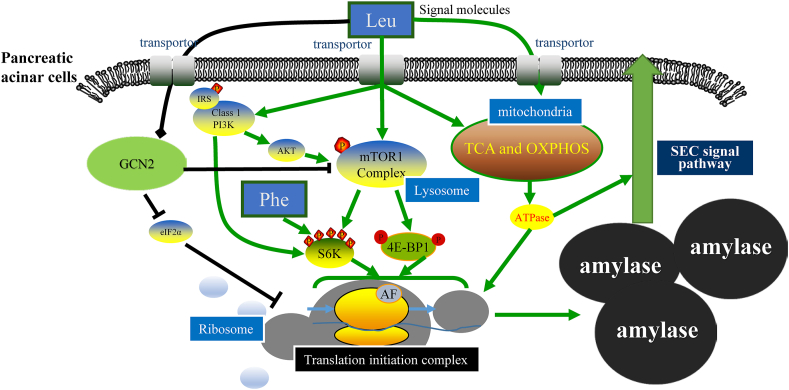
The translational control of protein synthesis must regulate the growth and the synthesis of digestive enzymes of the pancreas. Stimulation of protein synthesis in acinar cells is primarily mediated by mTOR, the phosphatidylinositol-3 kinase (PI3K)-RAC-alpha serine/threonine-protein kinase (Akt), and the general AA control repressor 2 (GCN2) signalling pathways (Guo et al., 2018c). The mTOR signalling pathway is central to regulate intracellular protein synthesis and degradation, cell growth and proliferation (Ma and Blenis, 2009). Leucine and phenylalanine can activate Akt-mTOR and ribosomal protein S6 kinase beta-1 (S6K1), respectively, to increase the formation of translation initiation complexes and increase the synthesis of digestive enzymes (Guo et al., 2018a, 2019). We also demonstrated that isoleucine could also phosphorylate the mTOR signalling pathway factors, which increases the efficiency of protein synthesis (Cao et al., 2019). In an experiment incubating pancreatic tissue of dairy goats in vitro, we found that isoleucine increased the phosphorylation level of eukaryotic translation initiation factor 4E-binding protein 1γ (4EBP1γ), decreased the phosphorylation level of eukaryotic elongation factor 2 (eEF2), increased the tissue concentration of amylase, trypsin, and chymotrypsin, and increased the concentration of the released buffer (Cao et al., 2019). AA regulation of signal transduction pathways in protein turnover and metabolism in acinar cells has been reviewed in detail by Gallinetti et al. (2013). The specific regulatory network is shown in Fig. 3.
Fig. 3.
Amino acid regulation signalling network for digestive enzyme secretion in pancreatic acinar cells of dairy cows. The green arrow represents the promotion, and the black line represents the inhibition. IRS = insulin receptor; P = phosphorylation; PI3K = phosphatidylinositol 3 kinase; GCN2 = general AA control repressor 2; Akt = RAC-alpha serine/threonine-protein kinase; mTOR1 = mammalian target of rapamycin complex 1; TCA = tricarboxylic acid cycle; OXPHOS = oxidative phosphorylation; SEC = secretory; S6K = S6 kinase; 4E-BP1 = eukaryotic translation initiation factor 4E-binding protein 1; AF = action factor.
The general secretory (SEC) signalling pathway regulates protein export and is widely present in various cell types (Van den Berg et al., 2004). SEC YEG channel protein and SEC61 complex are the key protein complexes in the SEC system of prokaryotes and eukaryotes, respectively (Park et al., 2012). Our previous study reported that the SEC signalling pathway regulates pancreatic α-amylase release in pancreatic acinar cells of dairy calves (Guo et al., 2020). We used the primary pancreatic acinar cells to study the function of exogenous leucine on protein synthesis and enzyme secretion. The main effects of leucine supplementation were increased citrate synthase and ATPase activity, which enlarged the cytosolic ATP pool, and the upregulation of Sec61 expression. Further results showed that ATPase mediates this molecular process. The detailed mechanism of the Sec signalling pathway that controls the enzyme release needs further research.
Currently, the study of digestive physiology has focused on the role of small intestinal microbes, the regulation of intestinal glucose transporters, and the barrier function of the small intestine. The regulation of enzymatic digestive ability in the small intestine should receive more attention, which would allow us to target the pancreatic exocrine. We should note that the pancreatic exocrine ability of ruminants is complex and affected by many factors. Although the application of dietary carbohydrate structure and the addition of single functional AA have achieved positive effects, the balance and functionality of AA and the role of AA integration warrant further research. The addition of dietary digestive enzymes can also improve the digestion of intestinal nutrients, but the fundamental indicators such as the type and quantity of enzymes merit further investigation. Intestinal microorganisms also play a crucial role in the digestion process of nutrients and may also influence the secretion of the digestive enzymes. To increase our knowledge in this complex field, further study must be undertaken on the colonization and metabolism of microorganisms in the intestine.
Author contributions
Long Guo: Conceptualization; Data curation; Visualization; Writing-Original draft preparation. Junhu Yao: Writing-review and editing. Yangchun Cao: Supervision; Writing-reviewing and editing; Funding acquisition.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Award Numbers: 2018YFD0501600 and 2017YFD0500500) and the National Natural Science Foundation of China (Award Numbers: 31672451 and 31472122).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Blom E.J., Anderson D.E., Brake D.W. Increases in duodenal glutamic acid supply linearly increase small intestinal starch digestion but not nitrogen balance in cattle. J Anim Sci. 2016;94:5332–5340. doi: 10.2527/jas.2016-0783. [DOI] [PubMed] [Google Scholar]
- Brake D.W., Titgemeyer E.C., Anderson D.E. Duodenal supply of glutamate and casein both improve intestinal starch digestion in cattle but by apparently different mechanisms. J Anim Sci. 2014;92:4057–4067. doi: 10.2527/jas.2014-7909. [DOI] [PubMed] [Google Scholar]
- Brereton M.F., Vergari E., Zhang Q., Clark A. Alpha-, delta- and PP-cells: are they the architectural cornerstones of islet structure and co-ordination? J Histochem Cytochem. 2015;63:575–591. doi: 10.1369/0022155415583535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.C., Yang X.J., Guo L., Zheng C., Wang D.D., Cai C.J., Yao J.H. Regulation of pancreas development and enzymatic gene expression by duodenal infusion of leucine and phenylalanine in dairy goats. Livest Sci. 2018;216:9–15. [Google Scholar]
- Cao Y.C., Yang X.J., Guo L., Zheng C., Wang D.D., Cai C.J., Liu S.M., Yao J.H. Effects of dietary leucine and phenylalanine on pancreas development, enzyme activity, and relative gene expression in milk-fed Holstein dairy calves. J Dairy Sci. 2018;101:4235–4244. doi: 10.3168/jds.2017-13987. [DOI] [PubMed] [Google Scholar]
- Cao Y.C., Liu K., Liu S.M., Guo L., Cai C.J., Yao J.H. Isoleucine regulates the synthesis of pancreatic enzymes via the activation of mRNA expression and phosphorylation in the mammalian target of rapamycin signaling pathways in pancreatic tissues. BioMed Res Int. 2019 doi: 10.1155/2019/6302950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.C., Liu S.M., Yang X.J., Guo L., Cai C.J., Yao J.H. Effects of dietary leucine and phenylalanine on gastrointestinal development and small intestinal enzyme activities in milk-fed holstein dairy calves. Biosci Rep. 2018;39 doi: 10.1042/BSR20181733. BSR20171733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coux O., Tanaka K., Goldberg A.L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Croom W.J., Leonard S.B., Taylor I.L. Regulation of pancreatic exocrine secretion in ruminants: a review. J Nutr. 1992;122:191–202. doi: 10.1093/jn/122.1.191. [DOI] [PubMed] [Google Scholar]
- Gallinetti J., Harputlugil E., Mitchell J.R. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J. 2013;449:1–10. doi: 10.1042/BJ20121098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Tian H.B., Jing S., Chen Z., Liu S.M., Cao Y.C., Cai C.J., Yao J.H. Phenylalanine regulates initiation of digestive enzyme mRNA translation in pancreatic acinar cells and tissue segments in dairy calves. Biosci Rep. 2018;38 doi: 10.1042/BSR20171189. BSR20171189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Liu B.L., Zheng C., Bai H.X., Ren H., Yao J.H., Xu X.R. Inhibitory effect of high leucine concentration on α-amylase secretion by pancreatic acinar cells: possible key factor of proteasome. Biosci Rep. 2018;38 doi: 10.1042/BSR20181455. BSR20181455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Liang Z.Q., Zheng C., Liu B.L., Yin Q.Y., Cao Y.C., Yao J.H. Leucine affects α-amylase synthesis through PI3K/Akt-mTOR signaling pathways in pancreatic acinar cells of dairy calves. J Agric Food Chem. 2018;66:5149–5156. doi: 10.1021/acs.jafc.8b01111. [DOI] [PubMed] [Google Scholar]
- Guo L., Yao J.H., Zheng C., Tian H.B., Liu Y.L., Liu S.M., Cai C.J., Xu X.R., Cao Y.C. Leucine regulates α-amylase and trypsin synthesis in dairy calf pancreatic tissue in vitro via the mammalian target of rapamycin signalling pathway. Animal. 2019;13:1–8. doi: 10.1017/S1751731118003683. [DOI] [PubMed] [Google Scholar]
- Guo L., Tian H.B., Yao J.H., Ren H., Yin Q.Y., Cao Y.C. Leucine improves α-amylase secretion through the general secretory signaling pathway in pancreatic acinar cells of the dairy calves. Am J Physiol Cell Physiol. 2020;318:1284–1293. doi: 10.1152/ajpcell.00396.2019. [DOI] [PubMed] [Google Scholar]
- Harmon D.L. Understanding starch utilization in the small intestine of cattle. AJAS (Asian-Australas J Anim Sci) 2009;22:915–922. [Google Scholar]
- Harmon D.L., Swanson K.C. Review: nutritional regulation of intestinal starch and protein assimilation in ruminants. Animal. 2020;14:17–28. doi: 10.1017/S1751731119003136. [DOI] [PubMed] [Google Scholar]
- Harmon D.L., Yamka R.M., Elam N.A. Factors affecting intestinal starch digestion in ruminants: a review. Can J Anim Sci. 2004;84:309–318. [Google Scholar]
- Huhtanen P., Sveinbjörnsson J. Evaluation of methods for estimating starch digestibility and digestion kinetics in ruminants. Anim Feed Sci Technol. 2006;130:95–113. [Google Scholar]
- Huntington G.B. Starch utilization by ruminants: from basics to the bunk. J Anim Sci. 1997;75:852–867. doi: 10.2527/1997.753852x. [DOI] [PubMed] [Google Scholar]
- Janes A.N., Weekes T.E.C., Armstrong D.G. Carbohydrase activity in the pancreatic tissue and small intestine mucosa of sheep fed dried-grass or ground maize-based diets. J Agric Sci. 1985;104:435–443. [Google Scholar]
- Jaworek J., Nawrot-Porabka K., Leja-Szpak A., Konturek S.J. Brain-gut axis in the modulation of pancreatic enzyme secretion. J Physiol Pharmacol. 2010;61:523–531. [PubMed] [Google Scholar]
- Jenstad M., Chaudhry F.A. The amino acid transporters of the glutamate/GABA-glutamine cycle and their impact on insulin and glucagon secretion. Front Endocrinol (Lausanne) 2013;31:199. doi: 10.3389/fendo.2013.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S.J., Zabielski R., Konturek J.W., Czarnecki J. Neuroendocrinology of the pancreas; role of brain–gut axis in pancreatic secretion. Eur J Pharmacol. 2003;481:1–14. doi: 10.1016/j.ejphar.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Lee B.H., Rose D.R., Lin A.H., Quezada-Calvillo R., Nichols B.L., Hamaker B.R. Contribution of the individual small intestinal alpha-glucosidases to digestion of unusual alpha-linked glycemic disaccharides. J Agric Food Chem. 2016;64:6487–6494. doi: 10.1021/acs.jafc.6b01816. [DOI] [PubMed] [Google Scholar]
- Liddle R.A., Green G.M., Conrad C.K., Williams J.A. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol-Gastr L. 1986;251:243–248. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- Liu K., Liu Y., Liu S.M., Xu M., Yu Z.P., Wang X., Cao Y.C., Yao J.H. Relationships between leucine and the pancreatic exocrine function for improving starch digestibility in ruminants. J Dairy Sci. 2015;98:2576–2582. doi: 10.3168/jds.2014-8404. [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Hao Y.N., Yin F., Zhang Y.L., Liu J.H. Geniposide accelerates proteasome degradation of Txnip to inhibit insulin secretion in pancreatic β-cells. J Endocrinol Invest. 2017;40:505–512. doi: 10.1007/s40618-016-0591-9. [DOI] [PubMed] [Google Scholar]
- Liu K., Shen J., Cao Y.C., Cai C.J., Yao J.H. Duodenal infusions of isoleucine influence pancreatic exocrine function in dairy heifers. Arch Anim Nutr. 2017;72:1–11. doi: 10.1080/1745039X.2017.1396144. [DOI] [PubMed] [Google Scholar]
- Ma X.M., Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Mccormick R.J., Stewart W.E. Pancreatic secretion in the bovine calf. J Dairy Sci. 1967;50:568–571. doi: 10.3168/jds.S0022-0302(67)87467-8. [DOI] [PubMed] [Google Scholar]
- Mcleod K.R., Baldwin R.L., Harmon D.L., Richards C.J., Rumpler W.V. In: Energy metabolism in animals. Chwalibog A., Jakobsen K., editors. Wageningen Press; Wageningen, The Netherlands: 2001. Influence of ruminal and postruminal starch infusion on energy balance in growing steers; p. 385. [Google Scholar]
- Moharrery A., Larsen M., Weisbjerg M.R. Starch digestion in the rumen, small intestine, and hind gut of dairy cows – a meta-analysis. Anim Feed Sci Technol. 2014;192:1–14. [Google Scholar]
- Moullé V.S., Parnet P. Effects of nutrient intake during pregnancy and lactation on the endocrine pancreas of the offspring. Nutrients. 2019;11:2708. doi: 10.3390/nu11112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K.S., Short K.R. Hormonal and signaling role of branched-chain amino acids. J Nutr. 2005;135:1547–1552. doi: 10.1093/jn/135.6.1547S. [DOI] [PubMed] [Google Scholar]
- Naranjo J.A., Martínez-Victoria E., Valverde A., Yago M.D., Mañas M. Effect of age on the exocrine pancreatic secretion of the preruminant milk-fed goat. Arch Physiol Biochem. 1997;105:144–150. doi: 10.1076/apab.105.2.144.12930. [DOI] [PubMed] [Google Scholar]
- Otsuki M., Williams J.A. Effect of diabetes mellitus on the regulation of enzyme secretion by isolated rat pancreatic acini. J Clin Invest. 1982;70:148–156. doi: 10.1172/JCI110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Rapoport T.A. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys. 2012;41:21–40. doi: 10.1146/annurev-biophys-050511-102312. [DOI] [PubMed] [Google Scholar]
- Petersen O.H. Ca2+ signalling and Ca2+-activated ion channels in exocrine acinar cells. Cell Calcium. 2005;38:171–200. doi: 10.1016/j.ceca.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Tepikin A.V. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- Richards C.J., Branco A.F., Bohnert D.W., Huntington G.B., Macari M., Harmon D.L. Intestinal starch disappearance increased in steers abomasally infused with starch and protein. J Anim Sci. 2002;80:3361–3368. doi: 10.2527/2002.80123361x. [DOI] [PubMed] [Google Scholar]
- Ristic G., Tsou W.L., Todi S.V. An optimal ubiquitin-proteasome pathway in the nervous system: the role of deubiquitinating enzymes. Front Mol Neurosci. 2014;7:72. doi: 10.3389/fnmol.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson K.C. In: Reference module in food science. Smithers G., Knoerzer K., editors. Elsevier; Amsterdam, NL: 2019. Small intestinal anatomy, physiology, and digestion in ruminants; pp. 1–7. [Google Scholar]
- Swanson K.C., Richards C.J., Harmon D.L. Influence of abomasal infusion of glucose or partially hydrolyzed starch on pancreatic exocrine secretion in beef steers. J Anim Sci. 2002;80:1112–1116. doi: 10.2527/2002.8041112x. [DOI] [PubMed] [Google Scholar]
- Swanson K.C., Benson J.A., Matthews J.C., Harmon D.L. Pancreatic exocrine secretion and plasma concentration of some gastrointestinal hormones in response to abomasal infusion of starch hydrolyzate and/or casein. J Anim Sci. 2004;82:1781–1787. doi: 10.2527/2004.8261781x. [DOI] [PubMed] [Google Scholar]
- Swanson K.C., Kelly N., Salim H., Wang Y.J., Holligan S., Fan M.Z., Mcbride B.W. Pancreatic mass, cellularity, and alpha-amylase and trypsin activity in feedlot steers fed diets differing in crude protein concentration. J Anim Sci. 2008;86:909–915. doi: 10.2527/jas.2007-0514. [DOI] [PubMed] [Google Scholar]
- Tremblay F., Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem. 2001;276:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- Van de Put F.H., Elliott A.C. The endoplasmic reticulum can act as a functional Ca2+ store in all subcellular regions of the pancreatic acinar cell. J Biol Chem. 1997;272:27764–27770. doi: 10.1074/jbc.272.44.27764. [DOI] [PubMed] [Google Scholar]
- Van den Berg B., Clemons W.M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S.C., Rapoport T.A. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- Wang B.J., Cui Z.J. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am J Physiol Reg I. 2007;292:666–678. doi: 10.1152/ajpregu.00131.2006. [DOI] [PubMed] [Google Scholar]
- Williams J. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F.W., Dai Z., Li D., Wang J., Wu Z. Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci. 2014;2:387–417. doi: 10.1146/annurev-animal-022513-114113. [DOI] [PubMed] [Google Scholar]
- Xu M., Du S., Wang J., Yu Z.P., Harmon D.L., Yao J.H. Influence of rumen escape starch on pancreatic exocrine secretion of goats. J Anim Physiol An N. 2010;93:122–129. doi: 10.1111/j.1439-0396.2007.00792.x. [DOI] [PubMed] [Google Scholar]
- Yea K., Kim J., Yoon J.H., Kwon T., Kim J.H., Lee B.D., Lee H.J., Lee S.J., Kim J.I., Lee T.G. Lysophosphatidylcholine activates adipocyte glucose uptake and lowers blood glucose levels in murine models of diabetes. J Biol Chem. 2009;284:33833–33840. doi: 10.1074/jbc.M109.024869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Rashmi C., Leigh Ann S., Barry G., Fee B.E., J Michael C., Vigna S.R., Grant A.O., Liddle R.A. Amino acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol-Gastr L. 2011;300:528–537. doi: 10.1152/ajpgi.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.P., Xu M., Yao J.H., Liu K., Li F., Liu Y., Wang F., Sun F.F., Liu N.N. Regulation of pancreatic exocrine secretion in goats: differential effects of short- and long-term duodenal phenylalanine treatment. J Anim Physiol An N. 2013;97:431–438. doi: 10.1111/j.1439-0396.2012.01276.x. [DOI] [PubMed] [Google Scholar]
- Yu Z.P., Xu M., Liu K., Yao J.H., Yu H.X., Wang F. Leucine markedly regulates pancreatic exocrine secretion in goats. J Anim Physiol An N. 2014;98:169–177. doi: 10.1111/jpn.12069. [DOI] [PubMed] [Google Scholar]
- Zabielski R., Pierzynowski S.G. Development and regulation of pancreatic juice secretion in cattle. State-of-the-art. J Anim Feed Sci. 2001;10:25–45. [Google Scholar]