Abstract
The application of a no-touch technique to create an autologous radiocephalic arteriovenous fistula might improve the patency rate. In the present report, we have expanded the concept of the no-touch technique by introducing a modified no-touch technique in which we preserve the perivenous vascular tissue, followed by a functional end-to-side anastomosis to create a radiocephalic arteriovenous fistula with early maturation for hemodialysis.
Keywords: Functional end-to-side anastomosis, Hemodialysis, Maturation, MNTT, Modified no-touch technique, Radiocephalic arteriovenous fistula
A meta-analysis showed that the patency rate of arteriovenous fistulas (AVFs) is only 60% at 1 year after surgery and 51% at 2 years postoperatively.1 AVF vein stenosis in the juxta-anastomotic region is one of the main reasons for such low maturation rates.2 The tissues surrounding the cephalic vein will be stripped off during conventional AVF surgery, which can cause surgical damage to the vessels and result in intimal hyperplasia and vein stenosis. Furthermore, end-to-side anastomosis is a highly recommended and often used type of anastomosis but also plays a role in the development of venous stenosis.3 Thus, a functional end-to-side anastomosis (using a side-to-side anastomosis with distal vein ligation) would be a better choice.4
Souza et al5 proposed the no-touch technique (NTT) to separate the great saphenous vein during coronary artery bypass surgery, in which 3 to 5 mm of connective tissue around the vein is reserved without direct contact with the vein. Subsequently, Hörer et al6 successfully applied the NTT to the construction of radiocephalic (RC)-AVFs.
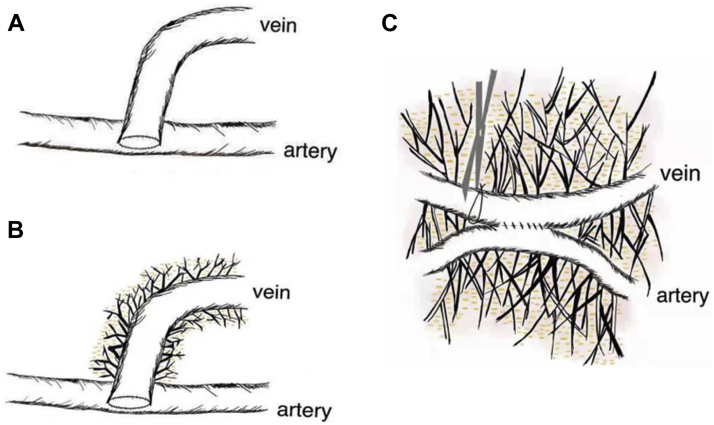
In the present report, we have expanded the concept of the NTT by introducing a modified NTT (MNTT) to create RC-AVFs, in which we preserve the perivenous vascular tissue. In contrast to the NTT, the purpose of the MNTT is to minimize blood vessel damage (Fig 1).
Fig 1.
A, Traditional arteriovenous fistula (AVF) surgery. B, AVF created using no-touch technique (NTT). C, AVF created using modified NTT (MNTT) and functional end-to-side anastomosis.
Surgical technique
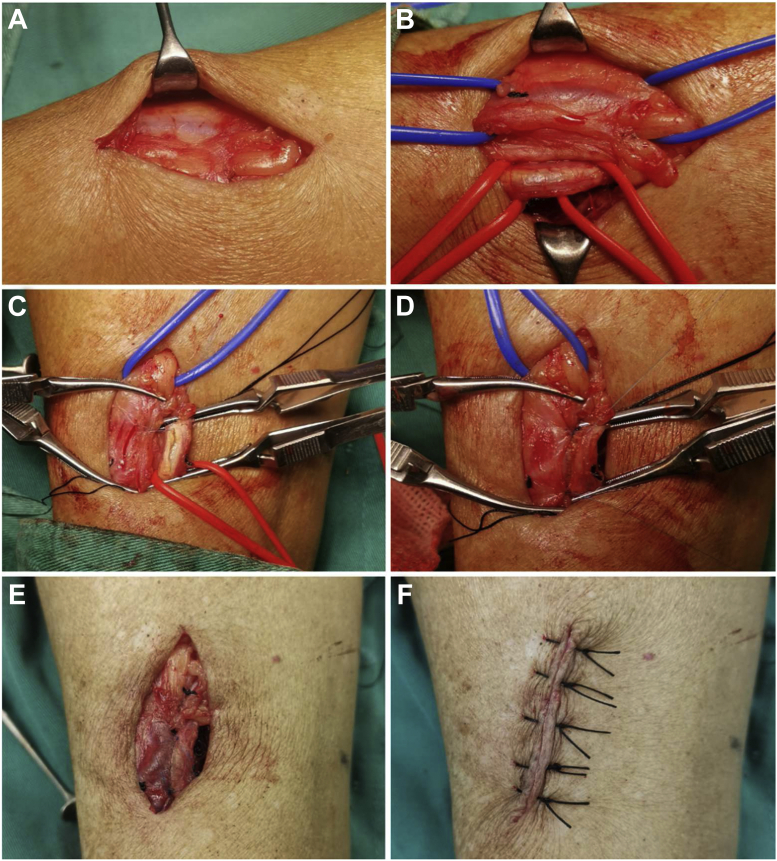
The MNTT was used to separate the cephalic vein, and a functional end-to-side anastomosis was performed. First, after a 4-cm skin incision was made in the radial artery near the cephalic vein of the forearm, the subcutaneous adipose tissue was isolated using curved hemostatic forceps, and the perivenous vascular tissue remained intact. If branches of the cephalic vein were present, ligation was performed 10 mm from the main cephalic vein (Fig 2, A).
Fig 2.
Photographs showing arteriovenous fistula (AVF) created using the modified no-touch technique (MNTT). A, The cephalic vein exposed with the surrounding tissue in situ. B, The cephalic vein (blue vessel loop) and radial artery (red vessel loop) dissected using the MNTT. C and D, Anastomosis using the Kunlin technique. E, The distal cephalic vein was ligated to form a functional end-to-side anastomosis. F, The surgical incision was sutured with a mattress suture.
Second, the tissue was dissected layer by layer and the radial artery sheath identified. The radial artery and companion vein were isolated using red vessel loops. Bulldog clamps were used to control the blood flow in the proximal and distal radial arteries. The same method was applied using blue vessel loops and bulldog clamps to isolate the cephalic vein and control the blood flow. The vessel loop crossing point should be a minimum of 10 mm from the edge of the cephalic vein (Fig 2, B).
Third, the artery was opened using a 3.0-mm Beaver knife (Beaver-Visitec International, Frenchs Forest, Australia). An 8-mm longitudinal anterior arteriotomy was performed using Potts scissors. Both vessels were prepared for anastomosis, with preservation of the surrounding pedicle (Fig 2, C).
Fourth, a vein-to-artery anastomosis (side-to-side) was created with 7-0 nonabsorbable, monofilament, continuous suture using the Kunlin technique. At this stage, although both ends of the cephalic vein vessels were completely blocked, blood was still escaping from the inner wall of the vessel. Thus, the vessels required intermittent rinsing with heparin saline (Fig 2, C and D).
Fifth, the distal cephalic vein was ligated to form a functional end-to-side anastomosis (Fig 2, E).
Finally, the surgical incision was sutured using a mattress suture (Fig 2, F).
Methods
From January 2021 to June 2021, 19 patients had had RC-AVFs established using the MNTT, and all the patients participated voluntarily in the present study after providing written informed consent. The patient characteristics are listed in Table I. We evaluated the forearm vessels using color Doppler ultrasound before surgery (Table II). At 4 weeks postoperatively, all the patients were examined clinically by an experienced nurse, underwent an ultrasound examination with flow measurements, and a doctor evaluated the maturation of the AVF (vessel diameter, 4-5 mm; blood flow, 400-500 mL/min).7 The physical examination of the 19 patients at 4 weeks postoperatively showed that the cephalic vein vessels were filled, had good elasticity, and could be compressed throughout the whole process. During the arm lifting test, the vessels could collapse, with palpable thrills along the length and the presence of persistent noises. The ultrasound examination at 4 weeks after surgery showed obvious dilation of the radial artery and the inner diameter of the cephalic vein. The blood flow spectrum of the cephalic vein 1.5 cm from the anastomosis was spiral laminar flow (Fig 3). The blood flow of the brachial artery was >500 mL/min in all 19 patients except for 1. That patient had had substandard flow owing to extensive plaques in the radial artery (Table II). Of the 19 patients, 14 had been treated with hemodialysis, and two-needle punctures had been started within 4 weeks postoperatively. The 4-hour hemodialysis was completed successfully with extracorporeal blood flow of ≥200 mL/min.
Table I.
Patient characteristics
| Variable | No. or mean ± standard deviation |
|---|---|
| Sex | |
| Male | 11 |
| Female | 8 |
| Age, years | 56.95 ± 15.55 |
| Primary disease | |
| Glomerulonephritis | 9 |
| Diabetic nephropathy | 7 |
| Multiple myeloma | 1 |
| Systemic vasculitis | 1 |
| Obstructive nephropathy | 1 |
| Procedure location | |
| Left forearm | 13 |
| Right forearm | 6 |
| Hemodialysis | |
| Yes | 14 |
| No | 5 |
| Diabetes | |
| Yes | 10 |
| No | 9 |
Table II.
Ultrasound results of 19 cases before and 4 weeks after RC-AVF surgery
| Pt. No. | Preoperative diameter, mm |
At 4 weeks postoperatively |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radial artery | Cephalic vein | Cephalic vein 1.5 cm from anastomosis |
Cephalic vein 5 cm from anastomosis |
Radial artery |
Brachial artery |
|||||||
| SLF | Diameter, mm | SLF | Diameter, mm | Vessel wall thickness, mm | Subcutaneous depth, mm | Flow, mL/min | Diameter, mm | Diameter, mm | Flow, mL/min | |||
| 1 | 2.70 | 2.10 | Yes | 4.13 | Yes | 5.17 | 0.85 | 0.89 | 594.00 | 4.56 | 7.05 | 632.00 |
| 2 | 1.71 | 1.84 | Yes | 4.52 | Yes | 4.98 | 0.55 | 4.39 | 586.00 | 2.59 | 3.81 | 735.00 |
| 3 | 1.95 | 2.62 | Yes | 4.80 | Yes | 6.60 | 0.79 | 1.40 | 669.16 | 4.01 | 6.70 | 808.00 |
| 4 | – | 3.50 | Yes | 5.10 | Yes | 3.77 | 0.66 | 4.38 | 337.00 | 3.41 | 5.54 | 601.00 |
| 5 | 2.20 | 2.30 | Yes | 4.56 | Yes | 5.04 | 0.71 | 2.01 | 814.00 | 4.61 | 5.96 | 1274.00 |
| 6 | 2.70 | 1.90 | Yes | 6.15 | Yes | 5.59 | 0.85 | 2.19 | 623.00 | 3.59 | 5.05 | 1003.00 |
| 7 | 2.40 | 3.40 | Yes | 6.44 | Yes | 5.47 | 0.61 | 2.58 | 521.00 | 3.55 | 6.62 | 749.00 |
| 8 | – | – | Yes | 6.59 | Yes | 7.63 | 0.45 | 4.81 | 1995.00 | 5.37 | 7.12 | 1681.00 |
| 9 | 2.98 | 3.46 | Yes | 6.80 | Yes | 7.29 | 0.97 | 1.34 | 670.00 | 4.13 | 6.03 | 1545.00 |
| 10 | 3.22 | 3.28 | Yes | 5.05 | Yes | 6.01 | 0.71 | 3.34 | 528.00 | 3.58 | 5.29 | 904.00 |
| 11 | 1.88 | 2.73 | Yes | 4.60 | Yes | 4.38 | 0.71 | 3.10 | 222.00 | 2.00 | 4.99 | 323.00 |
| 12 | 3.10 | 1.82 | Yes | 5.71 | Yes | 5.41 | 0.67 | 2.13 | 656.00 | 4.13 | 6.76 | 1195.00 |
| 13 | 2.19 | 3.22 | Yes | 5.05 | Yes | 4.34 | 0.77 | 2.45 | 493.00 | 3.23 | 4.56 | 602.00 |
| 14 | 3.40 | – | Yes | 7.11 | Yes | 6.02 | 0.79 | 2.61 | 1545.00 | 4.61 | 7.54 | 2742.00 |
| 15 | 2.30 | 2.01 | Yes | 7.05 | Yes | 6.44 | 0.79 | 1.28 | 855.00 | 5.41 | 5.46 | 1457.00 |
| 16 | 2.06 | 1.70 | Yes | 4.68 | Yes | 4.80 | 0.61 | 2.73 | 1033.00 | 3.76 | 5.89 | 1798.00 |
| 17 | – | – | Yes | 4.43 | Yes | 5.34 | 1.04 | 2.86 | 690.00 | 3.95 | 6.25 | 666.00 |
| 18 | 1.80 | 1.80 | Yes | 4.49 | Yes | 4.74 | 0.73 | 4.74 | 605.00 | 3.83 | 5.65 | 676.00 |
| 19 | 1.90 | 1.90 | Yes | 4.13 | Yes | 4.61 | 0.79 | 2.00 | 581.00 | 2.61 | 4.98 | 539.00 |
Pt. No., Patient number; RC-AVF, radiocephalic arteriovenous fistula; SLF, spiral laminar flow.
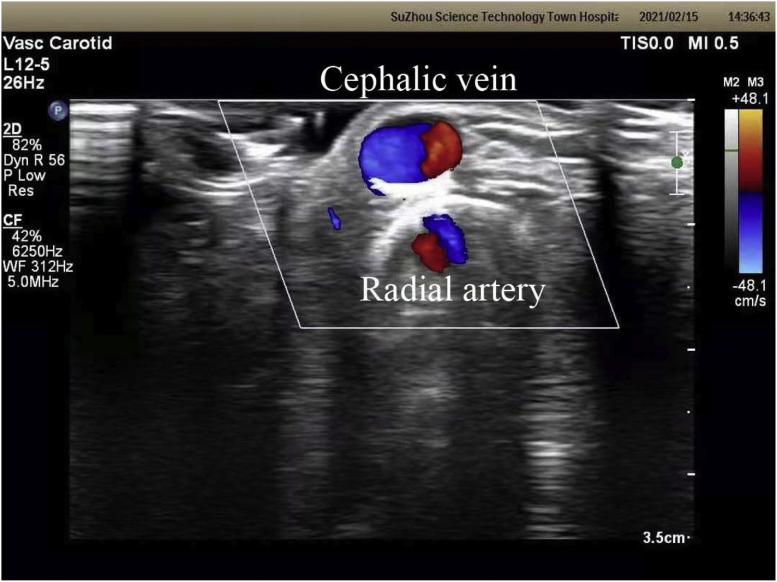
Fig 3.
The cephalic vein and radial artery are demonstrated showing spiral laminar flow (SLF).
Discussion
In our study, we preserved the perivenous vascular tissue during RC-AVF anastomosis. In contrast to the coronary artery bypass surgery performed by Souza et al,5 the establishment of an RC-AVF does not require venous displacement. We were able to perform RC-AVF anastomosis without separating the cephalic vein, which we have demonstrated to be feasible in our practice. During the operation, the fat and connective tissues around the veins were entirely preserved, and injury to the cephalic vein was fully avoided.8 Several studies have suggested that early intimal hyperplasia and inadequate outward remodeling are important causes of cephalic vein stenosis in the juxta-anastomotic region.9 Factors contributing to intimal hyperplasia and inadequate outward remodeling include inflammation, uremia, hypoxia, and shear stress.10 The MNTT minimizes contact with blood vessels during surgery, reducing the risk of spasms and kinking of blood vessels.11 Our strategy results in better preservation of the supplying blood vessels to maintain the oxygen concentration and nutrient levels in the vessel wall and reduces the inflammation due to biochemical factors related to the perivenous adipose tissue.12,13
Sadaghianloo et al14 developed the radial artery deviation and reimplantation (RADAR) method, which has been associated with an increased prevalence of maturation, a decreased prevalence of juxta-anastomotic venous stenosis, and increased primary and secondary patency. Compared with RADAR, the MNTT is indicated for the construction of an AVF at any place in the upper arm where the arteries and veins are in close proximity. The MNTT requires a smaller vessel diameter, has a wide range of indications, and does not require the dissection of the perivascular tissue of the vein. Thus, the MNTT will result in a lower prevalence of vascular stenosis than will RADAR, especially in arterial vessels.
Marie et al15 proposed spiral laminar flow as a potential predictor of the maturity of newly constructed AVFs and also found that spiral laminar flow was present in the nonoperated segment of AVFs. In our study, it was encouraging that the blood flow spectrum of the cephalic vein 1.5 cm from the anastomosis was spiral laminar flow in the operated segment of the AVFs. The reason for such a phenomenon might be closely associated with the use of the MNTT or the functional end-to-side anastomosis, an area for further study.
One disadvantage of the present study was the short follow-up time. Therefore, a randomized controlled study is needed to confirm whether the MNTT is superior to traditional AVF surgery.
Conclusions
The MNTT and functional end-to-side anastomosis for construction of AVFs are feasible, and the short-term results are encouraging. We believe that this technique is more conducive to the early maturity of an AVF and has the potential to be the most common surgical procedure in the future.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Wang Xiaohe and Zhang Yuanyuan contributed equally to the present study.
References
- 1.Al-Jaishi A.A., Oliver M.J., Thomas S.M., Lok C.E., Zhang J.C., Garg A.X. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:464–478. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Noordzij M., Jager K.J., Veer S.N., Kramar R., Collart F., Heaf J.G. Use of vascular access for haemodialysis in Europe: a report from the ERA-EDTA registry. Nephrol Dial Transplant. 2014;29:1956–1964. doi: 10.1093/ndt/gfu253. [DOI] [PubMed] [Google Scholar]
- 3.Cheung A.K., Imrey P.B., Alpers C.E., Robbin M.L., Radeva M., Larive B. Intimal hyperplasia, stenosis, and arteriovenous fistula maturation failure in the hemodialysis fistula maturation study. J Am Soc Nephrol. 2017;28:3005–3013. doi: 10.1681/ASN.2016121355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weigang T., Wei X., Lifeng G., Jingkui L., Yani L., Huaqin J. A meta-analysis of traditional and functional end-to-side anastomosis in radiocephalic fistula for dialysis access. Int Urol Nephrol. 2021;2:1–10. doi: 10.1007/s11255-020-02691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souza D.S., Christofferson R.H., Bomfim V., Filbey D. "No-touch" technique using saphenous vein harvested with its surrounding tissue for coronary artery bypass grafting maintains an intact endothelium. Scand Cardiovasc J. 1999;33:323–329. doi: 10.1080/14017439950141362. [DOI] [PubMed] [Google Scholar]
- 6.Hörer T.M., Skoog P., Quell R., Nilsson K.F., Larzon T., Souza D.R. No-touch technique for radiocephalic arteriovenous fistula: surgical technique and preliminary results. J Vasc Access. 2016;17:6–12. doi: 10.5301/jva.5000456. [DOI] [PubMed] [Google Scholar]
- 7.Lok C.E., Huber T.S., Lee T., Shenoy S., Yevzlin A.S., Abreo K. KDOQI clinical practice guideline for vascular access: 2019 update. Am J Kidney Dis. 2020;75:S1–S164. doi: 10.1053/j.ajkd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Loesch A., Dashwood M.R. Vasa vasorum inside out/outside in communication: a potential role in the patency of saphenous vein coronary artery bypass grafts. J Cell Commun Signal. 2018;12:631–643. doi: 10.1007/s12079-018-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee T., Misra S. New insights into dialysis vascular access: molecular targets in arteriovenous fistula and arteriovenous graft failure and their potential to improve vascular access outcomes. Clin J Am Soc Nephrol. 2016;11:1504–1512. doi: 10.2215/CJN.02030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmbhatt A., Remuzzi A., Franzoni M., Misra S. The molecular mechanisms of hemodialysis vascular access failure. Kidney Int. 2016;89:303–316. doi: 10.1016/j.kint.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dashwood M.R., Tsui J.C. “No-touch” saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: a journey from bedside to bench. Vascul Pharmacol. 2013;58:240–250. doi: 10.1016/j.vph.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Johansson B.L., Souza D.S.R., Bodin L., Filbey D., Loesch A., Geijer H. Slower progression of atherosclerosis in vein grafts harvested with “no touch” technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg. 2010;38:414–419. doi: 10.1016/j.ejcts.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed S.R., Johansson B.L., Karlsson M.G., Souza D.S.R., Dashwood M.R., Loesch A. Human saphenous vein and coronary bypass surgery: ultrastructural aspects of conventional and "no-touch" vein graft preparations. Histol Histopathol. 2004;19:421–433. doi: 10.14670/HH-19.421. [DOI] [PubMed] [Google Scholar]
- 14.Sadaghianloo N., Declemy S., Jean-Baptiste E., Haudebourg P., Robino C., Shariful Islam M. Radial artery deviation and reimplantation inhibits venous juxta-anastomotic stenosis and increases primary patency of radial-cephalic fistulas for hemodialysis. J Vasc Surg. 2016;64:698–706. doi: 10.1016/j.jvs.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 15.Marie Y., Guy A., Tullett K., Krishnan H., Jones R.G., Inston N.G. Patterns of blood flow as a predictor of maturation of arteriovenous fistula for haemodialysis. J Vasc Access. 2014;15:169–174. doi: 10.5301/jva.5000214. [DOI] [PubMed] [Google Scholar]