Abstract
Manipulation of perinatal diets, such as supplementing feed with rumen-protected glucose (RPG), has been positively regarded as a strategy to improve milking performance. This study was conducted to assess the effects of RPG on the fermentation profiles, resident microbiota and mucosal immunity in the cecum. Ten Holstein dairy cows were randomly assigned to either a 25 g/kg RPG diet (DM basis) or a 11 g/kg coating fat diet (control, CON). Compared with the CON group, the acetate-to-propionate ratio was lower in the RPG group. Gene expression analysis indicated that RPG supplementation tended to upregulate the expression of Na+/H+ hydrogen exchanger 3 (NHE3) (P = 0.076). RPG supplementation downregulated the expression of genes involved in self-rehabilitation such as matrix metalloproteinase 1 (MMP1), MMP3, MMP9 and MMP13. Additionally, the mRNA expression of genes involved in immunity including Toll-like receptors (TLR4, TLR6 and TLR7) and proinflammatory cytokines (immune interferon gamma [IFNG] and interleukins interleukin 17A [IL17F], IL17A, IL22), was downregulated by RPG supplementation. Nonetheless, no differences existed in the bacterial copy number and beta diversity between the 2 groups. Overall, supplementation with RPG would probably cause a shift towards propionate production in the cecal digesta, and promote the immune homeostasis of the cecal mucosa in transition dairy cows. Our results extended the basic understanding of RPG supplementation and utilization in transition dairy cows in terms of host microbe interplay in the cecum.
Keywords: Cecum, Rumen-protected glucose, Fermentation, Fatty acid metabolism, Microbiota
1. Introduction
The transition period is a stressful stage for dairy cows because of negative energy balance (NEB), which is induced by the sharp decline of voluntary feed intake and drastic rise in energy demand (Agrawal et al., 2017). During this period, endocrine and metabolic disorders sharply decrease the productivity of dairy cows (Janovick and Drackley, 2010). Several research efforts have been devoted to dietary manipulation to relieve the detrimental effects of NEB, including regulating dietary energy levels (Janovick and Drackley, 2010) and adding rumen-protected methionine to the diet (Batistel et al., 2017, 2018). Our previous study demonstrated that supplementation with rumen-protected glucose (RPG) increases milk production and reduces blood proinflammatory marker levels in transition dairy cows (Li et al., 2019). Moreover, dietary RPG supplementation can improve immune homeostasis, and promote epithelial metabolism in the ileum of transition dairy cows (Zhang et al., 2019).
It is acknowledged that the gut environment is different between the small intestine and hindgut in terms of physiology, substrate availability, microbial biogeography, and compartmentalized host immune system (Mowat and Agace, 2014; Donaldson et al., 2015). Dietary glucose is directly absorbed in the small intestine, and acts as a glucogenic precursor in the form of propionate in the hindgut of ruminants (Rigout et al., 2003; Rodriguez et al., 2017). In addition, short chain fatty acids produced by microbial fermentation in the hindgut are recognized as key signals in intestinal homeostasis maintenance (Zhang et al., 2016; Kobayashi et al., 2017). Herein, we hypothesized that a proportion of RPG bypassed the small intestine and was then released into the closest cecum. Therefore, the present study aimed to profile the adaptation to RPG supplementation in the transition dairy cows cecum by sequencing techniques, qPCR and traditional nutritional technology.
2. Materials and methods
All procedures were carried out in accordance with guidelines approved by the Animal Care Committee, Institute of Subtropical Agriculture, Chinese Academy of Science (permit no. ISA000259).
2.1. Animals and experimental design
Animal and experimental designs have been described in detail previously (Zhang et al., 2019). Briefly, ten dairy cows (4.7 ± 0.5 years of age; 515 ± 42 kg; 16.1 ± 3.7 kg/d milk yield) were randomly allocated to 2 dietary treatments: a control diet (CON, a basal diet supplemented with coating fat at 11 g/kg DM [90 g/cow per day]) and an RPG diet (a basal diet supplemented with 25 g/kg DM [200 g/cow per day]). The RPG was comprised of 450 g/kg glucose and 450 g/kg fat.
All cows were individually fed and received the same close-up diet from −21 d to expected parturition, and lactation diet from parturition through to 14 d in milk (DIM). The close-up diet was formulated to meet the requirements of a dry cow close (about 3 weeks) to calving. After parturition, all cows were fed a lactation diet. All diets were fed as total mixed ration (TMR). Pre- and post-parturition, cows were individually fed ad libitum. Orts for each cow were collected and weighed prior to each feeding. The ingredient and nutrient composition of the diets fed are reported in Appendix Table 1. All cows had free access to clean water during the entire experiment and were euthanized after 8 h of fasting at the end of the period.
Table 1.
Effect of rumen-protected glucose (RPG) on volatile fatty acid profile in the cecum of transition dairy cows1.
| Item | CON | RPG | SEM | P-value |
|---|---|---|---|---|
| TVFA, mmol/L | 68.02 | 59.78 | 3.629 | 0.174 |
| Acetate, mol/100 mol | 71.25 | 67.76 | 0.914 | 0.038 |
| Propinate, mol/100 mol | 15.83 | 18.08 | 0.508 | 0.021 |
| Butyrate, mol/100 mol | 6.11 | 6.63 | 0.265 | 0.728 |
| Isovalerate, mol/100 mol | 1.87 | 2.38 | 0.233 | 0.231 |
| Valerate, mol/100 mol | 2.92 | 2.95 | 0.289 | 0.190 |
| A/P, mol/mol | 4.51 | 3.77 | 0.157 | 0.939 |
VFA = total volatile fatty acid; A/P = acetate-to-propionate ratio.
CON: the control group which fed a basal diet add with coating fat; RPG: the RPG group which fed a basal diet add with RPG.
2.2. Sample collection and measure procedure
Total mixed ration samples were collected weekly and the dry matter (DM) content was determined by drying at 65 °C. Contents of DM, crude protein (CP), ash, ether extract was measured according to the method of AOAC (1990). The starch content was determined after pre-extraction with 80% ethanol, and the glucose released from the starch was measured via enzyme hydrolysis using α-amylase according to Karthner and Theurer (1981). The NDF and ADF contents were determined according to the Van Soest et al. (1991). Minerals were determined using inductively coupled plasma atomic emission spectroscopy according to the method described by Majewska et al. (2009).
The fresh cecal contents in the middle part of the cecum were sampled after the cows were euthanized. These contents were separated into 2 parts. One (approximately 2 g) was placed in a 2 mL sterilized centrifuge tube, frozen in liquid nitrogen immediately, and then stored at −80 °C for DNA extraction and subsequent microbial quantification. Another (approximately 5 g) was homogenized with an equal volume saline solution by vertexing continuously overnight. Then, the homogenate was centrifuged at 15,000 × g for 10 min at 4 °C, and the supernatants that were mixed with a one-tenth volume of 25% (wt/vol) metaphosphoric acid were stored at −20 °C for volatile fatty acid (VFA) analysis (Jiao et al., 2016). The cecal mucosa was carefully scraped from the middle cecum (approximately 10 cm) using a sterile glass slide, cut into several pieces approximately 1 cm2 in size and wrapped with sterilized tinfoil. Immediately, the cecal mucosa was snap-frozen using liquid nitrogen and then stored at −80 °C for follow-up molecular analysis of mucosal microbiota and gene expression testing.
2.3. Volatile fatty acid analyses
To analyze the concentration of VFA, frozen VFA samples (2 mL) were thawed and then centrifuged at 15,000 × g, at 4 °C, for 10 min in a temperature-controlled centrifuge. The supernatant (1 mL) was filtered using a 0.22-μm syringe filter and transferred into a 2.0-mL glass chromatograph vial. The solution for standard curves was prepared using appropriate fold dilutions of the mother solution. The VFA concentrations of cecal contents were measured on gas chromatograph (GC7890A, Agilent, USA). Fatty acids were separated with a DB-FFAP column (30 m length, 0.25 mm i.d, 0.25 μm film thickness). VFA was detected by a hydrogen flame ionization detector and the carrier gas in this study was nitrogen. The oven temperature of gas chromatograph was initially set at 60 °C for 2 min, and then increased with a gradient of 20 °C/min until it reached 220 °C. The detector temperature was set to 280 °C (Wang et al., 2014). The VFA was identified and quantified from chromatograph peak areas using calibration with external standards.
2.4. RNA extraction and cDNA synthesis
The total RNA of the collected mucosa samples was extracted by the guidelines of the Takara mini-BEST Universal RNA Extraction Kit (Takara, Kusatsu, Japan). First, lysis buffer was added to the liquid-nitrogen-ground samples, and the lysis solution was transferred to the gDNA Eraser Spin Column to remove impurities and gDNA. Then, the mixed-solution was transferred to the RNA Spin Column after adding an equal volume of 70% ethyl alcohol. Finally, the RNA was released from the washed Spin Filter membrane. The concentration was determined with a NanoDrop spectrophotometer (ND-1000; NanoDrop Technologies, Inc., Wilmington, DE, USA). The integrity was measured by 1% agarose gel electrophoresis (Jiao et al., 2018). Then, the remaining RNA was reverse-transcribed to synthesize cDNA according to the instructions of the Prime-Script 1st Strand cDNA Synthesis Kit (Takara, Japan). Briefly, the reverse–transcription reaction mixture containing RNase inhibitor was incubated for 60 min at 42 °C, followed by 95 °C for 5 min. The resulting cDNA was stored at −20 °C for subsequent analysis of gene expression.
2.5. Gene expression profile
Relative gene expression using real-time quantitative PCR was carried out on a Lightcycler 480 II Sequence Detection System (Roche, Basel, Switzerland) with a SYBR Premix Ex Taq II (Tli RNaseH Plus) kit (Takara, Kusatsu, Japan) according to the manufacturer's protocol (Zhang et al., 2019). All primers were synthesized by Sangon Biotech Co., Ltd (Shanghai, China). β-actin and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (Bustin et al., 2019; Frota et al., 2011; Modesto et al., 2013) were used as reference genes to normalize the expression levels of target genes. Details of the primers are listed in Appendix Table 2. The relative expression levels of targeted genes were calculated using the 2 –ΔΔCt method (Livak and Schmittgen, 2001).
Table 2.
Effect of rumen-protected glucose (RPG) on expression of genes involved in glucose transport and gluconeogenesis in the cecal mucosa of transition dairy cows1.
| Gene | CON | RPG | SEM | P-value |
|---|---|---|---|---|
| NHE1 | 1.40 | 1.85 | 0.40 | 0.730 |
| NHE2 | 1.08 | 1.04 | 0.20 | 1.000 |
| NHE3 | 1.01 | 1.56 | 0.15 | 0.076 |
| MCT1 | 1.16 | 1.26 | 0.20 | 0.413 |
| PAT1 | 1.61 | 0.52 | 0.36 | 1.000 |
| DRA | 1.74 | 1.29 | 0.43 | 0.191 |
| BDH1 | 1.22 | 0.42 | 0.21 | 0.111 |
| BDH2 | 1.94 | 0.56 | 0.52 | 0.389 |
| HMGCS2 | 1.60 | 0.53 | 0.39 | 0.556 |
| SREBP2 | 2.42 | 2.83 | 0.78 | 0.730 |
| FDPS | 1.02 | 1.10 | 0.08 | 0.413 |
NHE1 = sodium/hydrogen exchanger 1; NHE2 = sodium/hydrogen exchanger 2; NHE3 = sodium/hydrogen exchanger 3; MCT1 = monocarboxylate transporter 1; PAT1 = putative anion transporter 1; DRA = downregulated in adenoma; BDH = β-hydroxybutyrate dehydrogenases; HMGCS2 = 3-hydroxy-3-methylglutaryl coenzyme a synthase isoform 2; SRBBP2 = sterol regulatory element-binding protein 2; FDPS = squalene synthase 1.
CON: the control group which fed a basal diet add with coating fat; RPG: the RPG group which fed a basal diet add with RPG.
2.6. DNA extraction
The total DNA of the cecal digesta and mucosa extraction was extracted by the QIAamp DNA Stool Mini Kit (Qiagen GmbH, Germany), according to the instructions of this kit, with slight changes (Jiao et al., 2015a). After adding ASL buffer, the digesta was lysed at a higher temperature of 95 °C for 10 min compared to the original of 70 °C for 5 min to lyse both gram-positive and gram-negative microbial cells. The quantity of DNA was measured on the basis of absorbance at 260 nm and the A260/A280 ratio using a NanoDrop 2,000 (NanoDrop Technologies, Inc, Wilmington, DE, USA), and the quality of DNA was determined by gel electrophoresis. The extracted DNA was stored at −20 °C for subsequent bacterial quantification.
2.7. Microbiota analysis
Absolute quantification using real-time PCR for the copy number of total bacterial 16S rRNA gene was conducted on an ABI 7,900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with SYBR Premix Ex Taq II (Tli RNaseH Plus) Detection Kit (Takara, Kusatsu, Japan), according to the manufacturer's instructions, using universal primers (forward, 5′-CGGCAACGAGCGCAACCC-3′, and reverse, 5′-CCATTGTAGCACGTGTGTAGCC-3′) (Jiao et al., 2016; Denman and McSweeney, 2006). Standard curves were constructed using 10-fold serial dilutions of plasmid DNA containing bacterial 16S rRNA genes. The final absolute bacterial copy numbers per gram of ceca were calculated using the computational formula (MQ × C × VD)/(S × V) (Jiao et al., 2015b). To provide a better understanding for further statistical analysis, the mathematical conversion of the copy numbers to log 10 was performed.
The PCR amplification was conducted for the V3 to V4 region of the 16S rRNA gene using barcoded universal primers as detailed in our companion study (Zhang et al., 2019). The amplification products were purified using a QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany), and mixed at equal molar ratios. Afterward, the Illumina MiSeq PE250 platform was used to sequence the amplicons.
QIIME (Quantitative Insights Into Microbial Ecology) software was used for data quality control (Caporaso et al., 2010). Afterwards, the raw paired-end reads were overlapped into tags with FLASH software (Magoc and Salzberg, 2011). The UPARSE method was used to cluster clean reads into operational taxonomic units (OTU) of 97% similarity (Edgar et al., 2011). Taxonomic assignment was conducted by searching input sequences against the Greengenes database (release 13.8) (DeSantis et al., 2006). Alpha diversity and beta diversity were calculated using QIIME software. The distance matrix for principal coordinate analysis (PCoA) was calculated by the Bray–Curtis similarity index.
2.8. Statistical analysis
The data including the VFA profile, gene expression and bacterial copy numbers, were subjected to statistical analysis using SPSS version 23 with the independent-sample Student's t-test. The amplicon sequencing data were analyzed using R software with Wilcox's rank-sum test. Effects were declared significant at P < 0.05 and considered as tendencies when 0.05 < P < 0.10.
3. Results
3.1. Cecal volatile fatty acid profile
The total VFA concentration was similar between the 2 groups (P > 0.10, Table 1). The molar percentage of acetate was lower (P = 0.038) and the molar percentage of propionate was higher (P = 0.021) in the RPG group than in the CON group. Hence, the acetate-to-propionate ratio was lower (P = 0.016) in the RPG group than in the CON group. However, RPG supplementation did not alter (P > 0.10) the molar percentages of butyrate, isovalerate and valerate in the cecum of cows.
3.2. Expression of genes involved in ion transport and fatty acid metabolism of cecal mucosa
In terms of genes encoding ion transport, supplementation with RPG tended to upregulate the gene expression of Na+/H+ hydrogen exchanger 3 (NHE3, P = 0.076, Table 2), while the expression of NHE1 and NHE2 was similar (P > 0.10) between the RPG and CON groups. For genes encoding fatty acid metabolism, the expression of 3-hydroxy-3-methylglutaryl coenzyme a synthase isoform 2 (HMGCS2), sterol regulatory element-binding protein 2 (SREBP2), β-hydroxybutyrate dehydrogenases (BDH1, BDH2), and squalene synthase 1 (FDPS) displayed no differences (P > 0.10) between the RPG group and the CON group.
3.3. Expression of mucosal cytothesis genes
For genes involved in cytothesis (Table 3), supplemental RPG downregulated the expression of matrix metalloproteinases matrix metalloproteinase 1 (MMP1) (P = 0.016), MMP3 (P = 0.027), MMP9 (P = 0.016) and MMP13 (P = 0.019) compared with the CON group.
Table 3.
Effect of rumen-protected glucose (RPG) on expression of matrix metalloproteinase (MMP) genes in the cecal mucosa of transition dairy cows1.
| Gene | CON | RPG | SEM | P-value |
|---|---|---|---|---|
| MMP1 | 1.08 | 0.25 | 0.18 | 0.016 |
| MMP3 | 1.09 | 0.31 | 0.18 | 0.027 |
| MMP9 | 1.05 | 0.13 | 0.18 | 0.016 |
| MMP13 | 1.14 | 0.13 | 0.22 | 0.019 |
MMP1 = matrix metalloproteinase 1; MMP3 = matrix metalloproteinase 3; MMP9 = matrix metalloproteinase 9; MMP13 = matrix metalloproteinase 13.
CON: the control group which fed a basal diet add with coating fat; RPG: the RPG group which fed a basal diet add with RPG.
3.4. Expression of mucosal immunity genes
As illustrated in Table 4, supplemental RPG downregulated the mRNA expression of genes encoding the Toll-like receptor (TLR) pathways, e.g., TLR4 (P = 0.016), TLR6 (P = 0.027) and TLR7 (P = 0.019). Moreover, when compared to the CON group, the expression of the proinflammatory cytokines interleukin 17F (IL17F) (P = 0.019), IL17A (P = 0.016) and IL22 (P = 0.032), as well as immune interferon gamma (IFNG; P = 0.016), were downregulated by supplementation with RPG. Supplemental RPG upregulated the expression of the gene encoding tight junction protein, occludin (P = 0.032). Nonetheless, the expression of other TLR, proinflammatory interleukins, and tight junction proteins displayed no differences (P > 0.10) between the RPG and CON groups.
Table 4.
Effect of rumen-protected glucose (RPG) on expression of genes associated with TLR pathway and barrier function in the cecal mucosa of transition dairy cows1.
| Gene | CON | RPG | SEM | P-value |
|---|---|---|---|---|
| TLR2 | 1.19 | 1.35 | 0.18 | 0.623 |
| TLR4 | 1.05 | 0.39 | 0.14 | 0.016 |
| TLR6 | 1.10 | 0.44 | 0.17 | 0.027 |
| TLR7 | 1.03 | 0.20 | 0.16 | 0.019 |
| TOLLIP | 1.12 | 1.51 | 0.13 | 0.140 |
| IFNG | 1.05 | 0.30 | 0.16 | 0.016 |
| CD45 | 1.06 | 0.76 | 0.12 | 0.556 |
| IL6 | 1.13 | 0.22 | 0.20 | 0.032 |
| IL22 | 1.12 | 0.25 | 0.19 | 0.032 |
| IL17F | 1.02 | 0.34 | 0.14 | 0.019 |
| IL17A | 1.06 | 0.20 | 0.18 | 0.016 |
| Occludin | 1.03 | 2.56 | 0.39 | 0.032 |
| Claudin1 | 1.07 | 0.79 | 0.11 | 0.413 |
| Claudin4 | 1.11 | 1.54 | 0.21 | 0.286 |
| TPJ1 | 1.15 | 1.29 | 0.19 | 0.556 |
TLR = Toll-like receptor; TOLLIP = toll interacting protein; IFNG = immune interferon gamma; CD45 = protein tyrosine phosphatase receptor type C; IL = interleukin; TPJ1 = tight junction protein 1.
CON: the control group which fed a basal diet add with coating fat; RPG: the RPG group which fed a basal diet add with RPG.
3.5. Cecal microbial diversity and community
The total bacterial copy numbers are shown in Fig. 1, and there were no differences (P > 0.10) between the RPG and CON groups in either the cecal digesta or mucosa.
Fig. 1.
Copy numbers of bacterial 16S rRNA genes in the cecal digesta and the mucosa of dairy cows fed with rumen-protected glucose (RPG) diet or control (CON) diet. Values are shown as mean ± SEM, n = 5.
The alpha diversity analysis of the cecal microbiota revealed that there was no statistically significant effect of cecal digesta between the 2 groups (Fig. 2). In contrast, the observed and Chao1 diversity values were higher (P < 0.05) in the cecum of cows fed the RPG diet than in their companions fed the CON diet.
Fig. 2.
Alpha diversity metrics for microbiota in the cecal digesta and mucosa of transition dairy cows between the control (CON) and rumen-protected glucose (RPG) groups. MU.CON: the cecal mucosa from the control group; MU.RPG: the cecal mucosa from the RPG group; DI.CON: the digesta from the control group; DI.RPG: the digesta from the RPG group. Values are shown as mean ± SEM, n = 5.
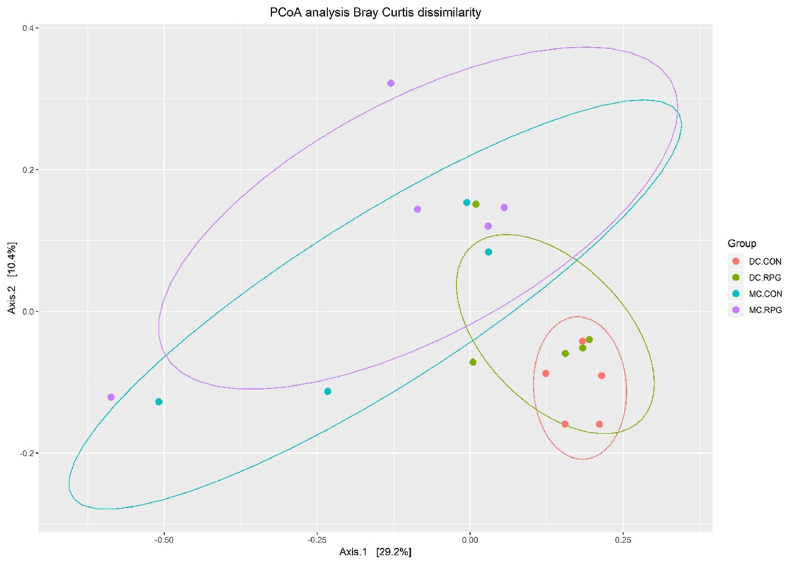
The beta diversity analysis (Bray–Curtis distance) of the composition of cecal microbiota showed no significant effect on the cecal microbiota community between the CON and RPG groups (Adonis, P > 0.10; beta dispersions, P > 0.10; Fig. 3), but there was distinct separation between the mucosa and the digesta (Adonis, P < 0.01; beta dispersions, P > 0.10).
Fig. 3.
Principal coordinate analysis (PCoA) of cecal digesta and mucosa bacterial community between CON and RPG groups.MU.CON: the cecal mucosa from the control group; MU.RPG: the cecal mucosa from the RPG group; DI.CON: the digesta from the control group; DI.RPG: the digesta from the RPG group. Values are shown as mean ± SEM, n = 5.
4. Discussion
When dietary starch is too much, it will escape microbial degradation in the rumen and enzymatic hydrolysis in the small intestine and will be subject to fermentation in the cecum and colon (Petri et al., 2019). In the cecum, the starch or glucose is fermented into acetate, propionate and butyrate by an enormous array of microorganisms (Rodriguez et al., 2017). The pattern of fermentation is determined by the structure of dietary carbohydrates, especially the starch and fiber ratio (Chatellard et al., 2016). In Holstein cows, it has been reported that the cecal total VFA concentration is usually greater, while the acetate-to-propionate ratio is lower when a high starch diet is offered compared to a high forage diet (Li et al., 2012; Plaizier et al., 2016). Similarly, the current study indicated a shift towards propionate fermentation in the cecum of dairy cows during RPG supplementation. As the lactose precursor, more propionate production by cecal microbiota during RPG supplementation might contribute to the drastic improvement in milk production (Li et al., 2019).
Gastrointestinal mucosal cellular function can be affected by the luminal environment, particularly the short-chain fatty acid profile (Penner et al., 2011). It is well characterized that the activities of transporters and exchangers, including NHE, putative anion transporter 1 (PAT1) and monocarboxylate transporters (MCT), as well as downregulated-in-adenoma (DRA), exert prominent roles in short chain fatty acids (SCFA) absorption (Aschenbach et al., 2011). In the gastrointestinal tract, the expression of genes involved in SCFA−/HCO3− and Na+/H+ exchangers, such as DRA, PAT1, NHE1, NHE2 and NHE3, are enhanced when goats are given a high-concentrate diet relative to a low-concentrate diet (Yan et al., 2014). Likewise, our data showed that the gene expression of NHE3 tended to be upregulated by RPG supplementation. NHE3 possesses the capacity to deliver hydrogen ions, which is dissociated from SCFA, thereby playing an indispensable role in maintaining the gut environment (Penner et al., 2011; Yan et al., 2014). Hence, the greater NHE3 expression by RPG supplementation might increase proton recycling to maintain intestinal ionic balance in transition dairy cows.
Matrix metalloproteinases play crucial roles in extracellular matrix degradation (Wang, 2011), apoptosis (Li et al., 2016), and inflammation (Guo et al., 2018). In dairy cows, the expression of MMP1, MMP3, MMP9 and MMP13 is significantly greater in cows with severe NEB than in cows with mild NEB (Wathes et al., 2011). Furthermore, Guo et al. (2018) demonstrated that quercetin supplementation decreases the expression of MMP2 and MMP9, thereby preventing laminitis, through inhibiting degradation of the extracellular matrix. The results presented in this study showed that RPG supplementation downregulated the expression of MMP1, MMP3, MMP9 and MMP13. Accordingly, it is inferred that RPG supplementation might relieve the degradation of cecal mucosal cells resulting from NEB in transition cows.
The participation of Toll-like signaling pathways, including Toll-like receptors and proinflammatory cytokines, in the pathophysiology of the immune system is well recognized. The present study showed that the gene expression of TLR such as TLR4, TLR6 and TLR7, together with proinflammatory cytokines, including IL17F, IL17A, IL22 and IFNG in the cecal mucosa was downregulated by RPG supplementation. These findings were consistent with our research on the ileum, in which RPG supplementation down-regulated the expression of TLR4 and IL17A in the ileal mucosa (Zhang et al., 2019). Moreover, previous research has also shown that the expression of TLR4 is greater during negative-energy balance states, indicating an immune imbalance (Moyes et al., 2010). Simultaneously, dietary supplementation with RPG elevated the gene expression of the tight junction protein, occludin. Tight junction proteins are regarded as doorways to the intestinal epithelial cells (Matter and Balda, 2003), as well as guardians that prevent permeations of harmful microorganisms or toxins from intruding into the gut epithelium (Jiao et al., 2018). Interestingly, our contemporary found that the plasma IL-8 concentration was lower in the RPG group (Li et al., 2019). Collectively, dietary supplementation with RPG probably inhibited the incidence of inflammation by restraining proinflammatory pathways and improving barrier function to enhance immunocompetence in the cecal mucosa.
Maintaining a healthy gut microbiota is critical to ruminant health and productivity (McKenney and Pamer, 2015). It is well established that dietary intervention can shift the structure and community of the gastrointestinal microbiota (Jiao et al., 2018). For instance, goats fed a high-concentrate diet exhibited a higher gene abundance of Clostridium and Turicibacter in the cecal digesta than low-concentrate diet-fed (Tao et al., 2017). In the current study, no difference was found in the structure and composition of the cecal microbiota between the RPG and CON groups, illustrating that RPG supplementation did not alter cecal microflora. This finding might be explained by the fact that the period and the supplemental amounts of RPG in the present trial may not be sufficient to significantly alter cecal microbiota composition. To clarify whether the RPG exerts its activity on gut microbiota, further studies with longer trial periods and higher RPG supplementation levels are recommended.
The limitation of the present study is that the number of animals used was slightly low, although n = 5 was statistically significant. Beyond that, considering the cost effect, the present study lacked a concentration gradient in the diet.
5. Conclusion
Supplementing transition dairy cows with a 25 g/kg RPG diet (DM) per day shifted fermentation towards propionate production in the cecal digesta. In addition, it also enhanced mucosal immune homeostasis in the cecal mucosa. Nonetheless, this quantity of RPG was insufficient to significantly alter cecal microbiota composition. These data will extend the basic understanding of RPG supplementation and utilization in transition dairy cows in terms of host microbe interplay in the cecum.
Author contributions
Conceptualization, Zhixiong He, Zhiliang Tan and Xuefeng Han; data curation, Jinzhen Jiao; formal analysis, Xiaoli Zhang, Xiaopeng Li and Jian Wu; supervision, Zhiliang Tan and Xuefeng Han; writing – original draft, Xiaoli Zhang; writing – review & editing, Jinzhen Jiao and Xuefeng Han.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the National Key Research and Development Program of China (grant no. 2016YFD0501206 and 2018YFD0501604), the Major Project for Science and Technology of Hunan Province (grants no. 2017NK1020), the CAS Pioneer Hundred Talents Program and Youth Innovation Team Project of ISA, CAS (2017QNCXTD_ZCS), and the CAS Science and Technology Service Network Initiative (KFJ-STS-ZDTP-056).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.08.002.
Appendix.
The following is the Supplementary data to this article:
References
- AOAC . 15th ed. Association of Official Analytical Chemists; Arlington, VA, USA: 1990. Official methods of analysis. [Google Scholar]
- Agrawal A., Khan M.J., Graugnard D.E., Vailati-Riboni M., Rodriguez-Zas S.L., Osorio J.S., Loor J.J. Prepartal energy intake alters blood polymorphonuclear leukocyte transcriptome during the peripartal period in Holstein cows. Bioinf Biol Insights. 2017;11 doi: 10.1177/1177932217704667. 1177932217704667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbach J.R., Penner G.B., Stumpff F., Gabel G. Ruminant nutrition symposium: role of fermentation acid absorption in the regulation of ruminal pH. J Anim Sci. 2011;89(4):1092–1107. doi: 10.2527/jas.2010-3301. [DOI] [PubMed] [Google Scholar]
- Batistel F., Arroyo J.M., Bellingeri A., Wang L., Saremi B., Parys C., Trevisi E., Cardoso F.C., Loor J.J. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci. 2017;100(9):7455–7467. doi: 10.3168/jds.2017-12689. [DOI] [PubMed] [Google Scholar]
- Batistel F., Arroyo J.M., Garces C.I.M., Trevisi E., Parys C., Ballou M.A., Cardoso F.C., Loor J.J. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J Dairy Sci. 2018;101(1):480–490. doi: 10.3168/jds.2017-13185. [DOI] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE Guidelines: minimum information for publication of quantitative Real-Time PCR experiments. Clin Chem. 2019;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Tumbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatellard L., Trably E., Carrere H. The type of carbohydrates specifically selects microbial community structures and fermentation patterns. Bioresour Technol. 2016;221:541–549. doi: 10.1016/j.biortech.2016.09.084. [DOI] [PubMed] [Google Scholar]
- Denman S.E., McSweeney C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol. 2006;58(3):572–582. doi: 10.1111/j.1574-6941.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- DeSantis T.Z., Hugenholtz P., Larsen N., Rojas M., Brodie E.L., Keller K., Huber T., Dalevi D., Hu P., Andersen G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2015;14:20. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27(16):2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frota I.M., Leitao C.C., Costa J.J., Brito I.R., van den Hurk R., Silva J.R. Stability of housekeeping genes and expression of locally produced growth factors and hormone receptors in goat preantral follicles. Zygote. 2011;19(1):71–83. doi: 10.1017/S0967199410000080. [DOI] [PubMed] [Google Scholar]
- Guo C., Li H., Sun D., Liu J., Mao S. Effects of abomasal supplementation of quercetin on performance, inflammatory cytokines, and matrix metalloproteinase genes expression in goats fed a high-grain diet. Livest Sci. 2018;209:20–24. [Google Scholar]
- Janovick N.A., Drackley J.K. Prepartum dietary management of energy intake affects postpartum intake and lactation performance by primiparous and multiparous Holstein cows. J Dairy Sci. 2010;93(7):3086–3102. doi: 10.3168/jds.2009-2656. [DOI] [PubMed] [Google Scholar]
- Jiao J., Lu Q., Forster R.J., Zhou C., Wang M., Kang J., Tan Z. Age and feeding system (supplemental feeding versus grazing) modulates colonic bacterial succession and host mucosal immune maturation in goats. J Anim Sci. 2016;94(6):2506–2518. doi: 10.2527/jas.2015-0081. [DOI] [PubMed] [Google Scholar]
- Jiao J.Z., Li X.P., Beauchemin K.A., Tan Z.L., Tang S.X., Zhou C.S. Rumen development process in goats as affected by supplemental feeding v. grazing: age-related anatomic development, functional achievement and microbial colonisation. Br J Nutr. 2015;113(6):888–900. doi: 10.1017/S0007114514004413. [DOI] [PubMed] [Google Scholar]
- Jiao J.Z., Z W., Guan L.L., Tan Z.L., Han X.F., Tang S.X., Zhou C.S. Postnatal bacterial succession and functional establishment of hindgut in supplemental feeding and grazing goats. J Anim Sci. 2015;93(7):3528–3538. doi: 10.2527/jas.2014-8706. [DOI] [PubMed] [Google Scholar]
- Jiao J.Z., Wu J., Wang M., Zhou C.S., Zhong R.Z., Tan Z.L. Rhubarb supplementation promotes intestinal mucosal innate immune homeostasis through modulating intestinal epithelial microbiota in goat kids. J Agric Food Chem. 2018;66(4):1047–1057. doi: 10.1021/acs.jafc.7b05297. [DOI] [PubMed] [Google Scholar]
- Karthner R.J., Theurer B. Comparison of hydrolysis methods used in feed, digesta, and fecal starch analysis. J Agric Food Chem. 1981;29:8–11. doi: 10.1021/jf00103a003. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Mikami D., Kimura H., Kamiyama K., Morikawa Y., Yokoi S., Kasuno K., Takahashi N., Taniguchi T., Iwano M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-α-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun. 2017;486(2):499–505. doi: 10.1016/j.bbrc.2017.03.071. [DOI] [PubMed] [Google Scholar]
- Li H., Zheng H.L., Li L.H., Shen X.G., Zang W.J., Sun Y.S. The effects of matrix metalloproteinase-9 on dairy goat mastitis and cell survival of goat mammary epithelial cells. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160989. e0160989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Khafipour E., Krause D.O., Kroeker A., Rodriguez-Lecompte J.C., Gozho G.N., Plaizier J.C. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci. 2012;95(1):294–303. doi: 10.3168/jds.2011-4447. [DOI] [PubMed] [Google Scholar]
- Li X.P., Tan Z.L., Jiao J.Z., Long D.L., Zhou C.S., Yi K.L., Liu C.H., Kang J.H., Wang M., Duan F.H., Tang S.X., He Z.X., Han X.F. Supplementation with fat-coated rumen-protected glucose during the transition period enhances milk production and influences blood biochemical parameters of liver function and inflammation in dairy cows. Anim Feed Sci Technol. 2019;252:92–102. [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Magoc T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27(21):2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska D., Jakubowska M., Ligocki M., Tarasewicz Z., Szczerbińska D., Karamucki T., Sales J. Physicochemical characteristics, proximate analysis and mineral composition of ostrich meat as influenced by muscle. Food Chem. 2009;117(2):207–211. [Google Scholar]
- Matter K., Balda M.S. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4(3):225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- McKenney P.T., Pamer E.G. From Hype to Hope: the gut microbiota in enteric infectious disease. Cell. 2015;163(6):1326–1332. doi: 10.1016/j.cell.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto P., Peletto S., Pisoni G., Cremonesi P., Castiglioni B., Colussi S., Caramelli M., Bronzo V., Moroni P., Acutis P.L. Evaluation of internal reference genes for quantitative expression analysis by real-time reverse transcription-PCR in somatic cells from goat milk. J Dairy Sci. 2013;96(12):7932–7944. doi: 10.3168/jds.2012-6383. [DOI] [PubMed] [Google Scholar]
- Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- Moyes K.M., Drackley J.K., Morin D.E., Loor J.J. Greater expression of TLR2, TLR4, and IL6 due to negative energy balance is associated with lower expression of HLA-DRA and HLA-A in bovine blood neutrophils after intramammary mastitis challenge with Streptococcus uberis. Funct Integr Genom. 2010;10(1):53–61. doi: 10.1007/s10142-009-0154-7. [DOI] [PubMed] [Google Scholar]
- Penner G.B., Steele M.A., Aschenbach J.R., McBride B.W. Ruminant nutrition symposium: molecular adaptation of ruminal epithelia to highly fermentable diets. J Anim Sci. 2011;89(4):1108–1119. doi: 10.2527/jas.2010-3378. [DOI] [PubMed] [Google Scholar]
- Petri R.M., Munnich M., Zebeli Q., Klevenhusen F. Graded replacement of corn grain with molassed sugar beet pulp modulates the fecal microbial community and hindgut fermentation profile in lactating dairy cows. J Dairy Sci. 2019;102(6):5019–5030. doi: 10.3168/jds.2018-15704. [DOI] [PubMed] [Google Scholar]
- Plaizier J.C., Li S., Tun H.M., Khafipour E. Nutritional models of experimentally-induced subacute ruminal acidosis (SARA) differ in their impact on rumen and hindgut bacterial communities in dairy cows. Front Microbiol. 2016;7:2128. doi: 10.3389/fmicb.2016.02128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigout S., Hurtaud C., Lemosquet S. Lactational effect of propionic acid and duodenal glucose in cows. J Dairy Sci. 2003;86:243–253. doi: 10.3168/jds.S0022-0302(03)73603-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Carro M.D., Valiente V., Formoso-Rafferty N., Rebollar P.G. Effects of dietary fish oil supplementation on performance, meat quality, and cecal fermentation of growing rabbits. J Anim Sci. 2017;95(8):3620–3630. doi: 10.2527/jas.2017.1690. [DOI] [PubMed] [Google Scholar]
- Tao S.Y., Tian P., Luo Y.W., Tian J., Hua C.F., Geng Y.L., Cong R.H., Ni Y.D., Zhao R.Q. Microbiome-metabolome responses to a high-grain diet associated with the hind-gut health of goats. Front Microbiol. 2017;8:1764. doi: 10.3389/fmicb.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wang M., Sun X.Z., Janssen P.H., Tang S.X., Tan Z.L. Responses of methane production and fermentation pathways to the increased dissolved hydrogen concentration generated by eight substrates in in vitro ruminal cultures. Anim Feed Sci Technol. 2014;194:1–11. [Google Scholar]
- Wang X.F. Decreased Expression of Matrix metalloproteinase-9 and increased expression of tissue inhibitors of matrix metalloproteinase-1 in paratuberculosis-infected cattle in the ELISA-negative subclinical stage. Anim Biotechnol. 2011;22(2):44–49. doi: 10.1080/10495398.2010.536096. [DOI] [PubMed] [Google Scholar]
- Wathes D.C., Cheng Z.R., Fenwick M.A., Fitzpatrick R., Patton J. Influence of energy balance on the somatotrophic axis and matrix metalloproteinase expression in the endometrium of the postpartum dairy cow. Reproduction. 2011;141(2):269–281. doi: 10.1530/REP-10-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Zhang B., Shen Z.M. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats. J Dairy Sci. 2014;97(9):5668–5675. doi: 10.3168/jds.2013-7807. [DOI] [PubMed] [Google Scholar]
- Zhang H.Y., Du M., Yang Q.Y., Zhu M.J. Butyrate suppresses murine mast cell proliferation and cytokine production through inhibiting histone deacetylase. JNB (J Nutr Biochem) 2016;27:299–306. doi: 10.1016/j.jnutbio.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Wu J., Han X.F., Tan Z.L., Jiao J.Z. Effects of rumen-protected glucose on ileal microbiota and genes involved in ileal epithelial metabolism and immune homeostasis in transition dairy cows. Anim Feed Sci Technol. 2019;254:114199. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.