Abstract
The objective of this experiment was to evaluate the effect of supplementing rumen-protected Lys based on a Lys-deficient diet on liver metabolism in growing Holstein heifers. The experiment was conducted for 3 months with 36 Holstein heifers (initial body weight: 200 ± 9.0 kg; 7-month-old). Heifers were randomly assigned to 2 diets based on corn, soybean meal, alfalfa hay, and wheat bran: control, Lys-deficient diet (LD; 0.66% Lys in diet), and Lys-adequate diet (LA; 1.00% Lys in diet). The results showed no difference in growth performance between the 2 groups (P > 0.05). However, there was a clear trend of increasing feed conversion rate with Lys supplementation (0.05 < P < 0.01). The serum urea nitrogen concentration was significantly decreased, and the aspartate aminotransferase-to-alanine aminotransferase ratio was significantly decreased by Lys supplementation (P < 0.05). Moreover, growing heifers fed a Lys-adequate diet had lower levels of urine nitrogen excretion and higher levels of the biological value of nitrogen (P < 0.05). Metabolomic analysis revealed that 5 types of phosphatidylcholine and 3 types of ceramide were significantly increased and enriched in sphingolipid metabolism and glycerophospholipid metabolism (P < 0.05). His, Leu, and Asp levels were significantly decreased in the liver following Lys supplementation (P < 0.05). In conclusion, Lys supplementation may promote the synthesis of body tissue proteins, as evidenced by significantly decreased amino acids in the liver and urine N excretion, it also improves hepatic lipid metabolism by providing lipoprotein precursors.
Keywords: Growing Holstein heifer, Lysine, Liver, Untargeted metabolomics
1. Introduction
Lys has been identified as an amino acid (AA) that can limit milk and milk protein synthesis and growth in growing heifers (Li et al., 2019; Noftsger and St-Pierre, 2003). Therefore, feeding a Lys-deficient diet leads to decreased nitrogen (N) efficiency, causing increased N excretion (Wang et al., 2012). Accordingly, supplementation with rumen-protected AA, specifically Lys, is an effective way to optimize dietary AA patterns and achieve optimal body weight (BW) and withers heifer height. Thus, the management of growing dairy heifers has a considerable effect on future milk yield, fertility, and longevity (Handcock et al., 2019).
Optimal Lys supplementation is characterized by improved efficiency of N metabolism (Li et al., 2019; Tan et al., 2021; Wang et al., 2012). However, recent studies have revealed that Lys is also an important bioactive molecule that plays key roles in signaling pathways and metabolic regulation, including energy, glucose, and lipid metabolism (Hu and Guo, 2020; Kong et al., 2020; Wu, 2009). For example, a study indicated that dietary Lys restriction could enhance milk fat accumulation in dairy cows (Křížová et al., 2010), and prepartum supply of intestinally available Lys in Holstein cows could improve milk fat and energy-corrected milk yields (Fehlberg et al., 2020). However, we speculate that Lys metabolism and functions in dairy cows are inferred from pre-ruminants, and adult ruminants are distinguished from pre-ruminants by rumen microbial fermentation (Malmuthuge and Guan, 2017). Unfortunately, very few studies have investigated the functions of Lys in ruminants. In our previous study, we found that changes in the livers of 3–6-month-old calves with dietary Lys deficiency were related to lipid metabolism and the sectional urea cycle (Kong et al., 2020).
Furthermore, the addition of Met plus Lys at higher amounts to cow rations during transition periods was shown to protect the cows against liver lipid accumulation (Lin et al., 2014; Grummer, 1993). However, the objectives of post-weaning calves and transition cows are to adapt to rumen volume expansion and the onset of lactation, respectively (Roche et al., 2017), and that of growing heifers is to obtain the appropriate target BW and muscle development (Kertz et al., 2017). Thus, we hypothesized that the effects of Lys supplementation on liver metabolism differ among these stages.
After Lys is absorbed by the small intestine, compared with the other organs, the liver plays the most important role in Lys metabolism (Lapierre et al., 2009; Pink et al., 2011; Wan et al., 2015), by converting glucogenic AA and propionic acid to glucose (Larsen and Kristensen, 2013; Van der Drift et al., 2012) and non-esterified fatty acids (NEFA) to ketone bodies (Grum et al., 2002). However, the liver has many vital functions that are difficult to measure and quantify. Recently, the increased ease of use and efficiency of mass spectrometry (MS)-based metabolomics has facilitated our understanding of the mechanisms underlying the variations in metabolite concentrations by injecting samples for chromatographic separation before MS analysis (Patti et al., 2012). Thus, we chose an integrative approach to explore the effects of Lys supplementation on liver function in growing heifers.
The present experiment was designed to determine whether Lys affects liver metabolism in growing Holstein heifers fed diets based on corn, soybean meal, alfalfa hay, and wheat bran when Met and Thr are not limited. Additionally, the effects of Lys supply on growth performance, N flow, and serum biochemistry were investigated. We expected to observe significant liver metabolism responses to Lys supplementation in these heifers.
2. Materials and methods
2.1. Ethics statement for animal experiments
The experimental protocol was approved by the Ethics Committee of the Chinese Academy of Agricultural Sciences (CAAS, Beijing, China). The experiment was performed under the animal welfare practices and procedures in the Guidelines for Experimental Animals of the Ministry of Science and Technology (permit number AEC-CAAS-2017-01).
2.2. Animals, diets, and experimental design
Thirty-six growing Holstein heifers (age = 22 ± 0.5-weeks old; BW = 200 ± 9.0 kg; mean ± standard deviation) from the Third Ranch Branch, Good Earth Group Co., Ltd. (Heze City, Shandong, China) were randomly allocated to 2 treatments based on BW and age, and fed with 2 total mixed rations (TMR): (1) Lys-deficient TMR (LD) containing 0.66% Lys on a dry matter (DM) basis (n = 18), providing 70% of Lys requirements of the heifers, and (2) Lys-adequate TMR (LA) containing 1.00% Lys on DM basis (n = 18), providing 100% of Lys requirements of the heifers. In the LD and LA groups, the Met requirement was calculated according to a BW of 250 kg, average daily gain (ADG) of 1.1 kg, and the formula proposed by Zinn and Shen (1998), in which the Met requirement = 1.956 + 0.0292 × ADG × [268 – (29.4 × 0.0557 × BW0.75 × ADG1.097)/ADG] + 0.112 × BW0.75. The Thr requirement was calculated according to a Thr:Met ratio of 72:33 (Wang et al., 2012) to meet the requirements of Met and Thr. In the LA group, the Lys requirement was calculated according to a Lys:Met ratio of 100:33 (Abe et al., 1998). The Lys:Met ratio was 100:22 in the LD group. The heifers were fed a ration based on NRC (2001) recommendations for 1.1 kg/d ADG and 250 kg BW, estimated to contain 10.46 MJ/kg metabolizable energy and 15.1% crude protein (CP) on a DM basis. After a 3-week adaptation period, an experimental feeding period of 90 d (September to November) commenced. Rumen-protected Lys, Met, and Thr products were used to meet the AA requirements based on the AA levels in the basal diet. The basal diet nutrient levels and compositions are shown in Table 1. The AA requirement and composition of diets as well as metabolizable AA were calculated and are shown in Table 2.
Table 1.
Ingredients and composition of basal total mixed ration (%, dry matter basis).
| Ingredients | Content | Nutrient levels2 | Level |
|---|---|---|---|
| Corn | 45.67 | Metabolizable energy, MJ/kg | 10.13 |
| Soybean meal | 11.97 | Crude protein | 14.95 |
| Wheat bran | 15.00 | Rumen degradable protein3 | 9.85 |
| Alfalfa hay | 25.00 | Rumen undegradable protein3 | 5.10 |
| Limestone | 1.06 | Ether extract | 3.04 |
| Salt | 0.30 | Ash | 7.58 |
| Premix1 | 1.00 | Neutral detergent fiber | 29.22 |
| Total | 100.00 | Acid detergent fiber | 13.99 |
| Calcium | 1.12 | ||
| Phosphorus | 0.60 |
The premix provided the following minerals and vitamins for total mixed ration: Cu, 12.5 mg/kg; Fe, 90 mg/kg; Zn, 90 mg/kg; Mn, 30 mg/kg; I, 1.0 mg/kg; Se, 0.3 mg/kg; Co, 0.5 mg/kg; vitamin A, 15,000 IU/kg; vitamin D3, 5,000 IU/kg; vitamin E, 50 mg/kg.
Nutrient levels were measured, except for metabolizable energy, rumen degradable protein, and rumen undegradable protein. Metabolizable energy was measured and calculated using digestibility and metabolism trials. The energy of CH4 was calculated using the equation suggested by Eggleston et al. (2006).
Values estimated based on NRC (2001). The rumen undegradable protein content (% crude protein) of corn, soybean meal, wheat bran, and alfalfa hay was 32.8%, 24.3%, 14.6%, and 31.6%, respectively.
Table 2.
Dietary amino acid composition and metabolizable amino acid supply in heifers fed Lys-deficient and Lys-adequate diets.
| Item | Diet1 |
|
|---|---|---|
| LD | LA | |
| Lys, % of DM | ||
| Required | 1.00 | 1.00 |
| Supply from basal diet | 0.51 | 0.51 |
| Supply from RPLys2 | 0.16 | 0.49 |
| Balance | −0.33 | 0 |
| Met, % of DM | ||
| Required | 0.33 | 0.33 |
| Supply from basal diet | 0.07 | 0.07 |
| Supply from RPMet3 | 0.26 | 0.26 |
| Balance | 0 | 0 |
| Thr, % of DM | ||
| Required | 0.72 | 0.72 |
| Supply from basal diet | 0.49 | 0.49 |
| Supply from RPThr4 | 0.23 | 0.23 |
| Balance | 0 | 0 |
| Metabolizable Lys, % of DM | 0.97 | 1.24 |
| Supply from microbial protein5 | 0.68 | 0.68 |
| Supply from rumen undegradable protein6 | 0.17 | 0.17 |
| Supply from RPLys2 | 0.12 | 0.39 |
| Metabolizable Met, % of DM | 0.34 | 0.34 |
| Supply from microbial protein5 | 0.19 | 0.19 |
| Supply from rumen undegradable protein6 | 0.02 | 0.02 |
| Supply from RPMet3 | 0.13 | 0.13 |
| Metabolizable Thr, % of DM | 0.71 | 0.71 |
| Supply from microbial protein5 | 0.45 | 0.45 |
| Supply from rumen undegradable protein6 | 0.17 | 0.17 |
| Supply from RPThr4 | 0.09 | 0.09 |
| Metabolizable Lys:Metabolizable Met:Metabolizable Thr ratio | 100:35:73 | 100:27:57 |
DM = dry matter, RPLys = rumen-protected Lys, RPMet = rumen-protected Met, RPThr = rumen-protected Thr.
The growing heifers of the LD group were fed a Lys-deficient diet providing 66% of the Lys requirements of the heifers. The growing heifers of the LA group were fed a Lys-adequate diet that provided 100% of the Lys requirements of the heifers. The treatment was achieved by using rumen-protected Lys based on Lys levels in the basal diet.
Rumen-protected Lys (Yahe Nutrition High Tech Co., Ltd, Beijing, China) contained 36% Lys and 64% hydrogenated fat. The passage rate from the rumen to the small intestine was 80%.
Rumen-protected Met (Bluestar Adisseo, Antony, France) contained 44% Met and some silicon dioxide. The passage rate from the rumen to the small intestine was 50%.
Rumen-protected Thr (King Technology Co., Ltd, Hangzhou, China) contained 40% Thr. The passage rate from the rumen to the small intestine is 90%.
Rumen degradable protein was used with 85% efficiency for microbial protein (NRC 2001). The Lys, Met, and Thr content in microbial proteins was 8.10%, 2.29%, and 5.34%, respectively (Sok et al., 2017).
The amino acid composition of crude protein in the diet was used as the amino acid composition of rumen undegradable protein.
The growing heifers were housed in well-ventilated individual pens (2.6 m long × 2.2 m wide × 2.2 m high) bedded with fermented feces, which were replaced once a month to maintain comfort and hygiene. The growing heifers were offered the TMR twice daily at 08:00 and 17:00 and had ad libitum access to water. The environmental conditions were continuously recorded, the mean air temperature was 11.87 ± 7.54 °C.
2.3. Growth performance
The quantities of feed offered and refused were recorded daily for each growing heifer by electronic scale (YPHX-3Ex, ANFEI Environmental Technology Co., Ltd, Guangzhou, China). Feed samples were collected monthly and used to determine the chemical compositions, including DM, ash, CP, ether extract, neutral detergent fiber (NDF), acid detergent fiber (ADF), calcium, and phosphorus (AOAC, 2006). The DM content in samples were analyzed by drying the samples in an AirForce oven at 105 °C for 2 h. The N content was determined by the Kjeldahl method. The contents of NDF and ADF were quantified using Van Soest et al. (1991) method. The ether extract was measured using the weight loss of DM upon extraction with diethyl ether in a Soxhlet extraction apparatus for 8 h. The calcium content was determined using an atomic absorption spectrophotometer (M9W-700, Perkin–Elmer Corp., Norwalk, CT, USA). The phosphorus content was analyzed using the molybdovanadate colorimetric method using a spectrophotometer (UV-600, Mapada Instruments Co., Ltd., Shanghai, China). Lys, Met, and Thr concentrations in the diet were analyzed by a Hitachi L-8900 automatic AA analyzer. The growing heifers were weighed at the start and end of the experiment. The ADG, feed conversion ratio (FCR), and dry matter intake (DMI) were calculated.
2.4. Blood sampling and analysis
At the end of the experiment (90 d), blood samples were collected from 5 heifers which were close to the average BW through coccygeal venipuncture before morning feeding in each treatment. Then, the serum was separated by centrifugation (L420-A centrifuge, Cence Group, Hunan, China) at 3,000 × g at 4 °C for 10 min and then stored at −20 °C until analysis. The serum metabolites and enzyme activities were determined by a biochemical auto-analyzer (Hitachi automatic biochemical analyzer 7600, Tokyo, Japan) using commercially available kits according to the manufacturer's instructions: NEFA, serum urea nitrogen (SUN), total cholesterol (TC), triglyceride (TG), glucose, enzyme activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
2.5. Diet apparent digestibility and N balance
A digestibility trial was conducted by selecting 4 heifers from each group on the last week of this experiment (83 to 90 d) with a 3-d adaptation period and a 4-d sampling period. Total feces and urine were recorded at the end of each 24-h period daily at 07:00. The feces were mixed, and 100 g subsamples were sampled as a fixed proportion of total volume to produce a representative sample with 10 mL 10% dilute hydrochloric acid and stored at −20 °C for analysis. In addition, 100 mL urine samples were collected using the same procedure as in the feces collection. Fecal and urine samples were analyzed for DM, CP, and ash concentration, as described above for feed samples (Li et al., 2019).
2.6. Liver sampling and analysis
Liver samples from 5 same heifers used for sampling serum were obtained at the end of the experiment, as described in a previous study (Kong et al., 2020). Briefly, liver samples were collected through an incision (1 cm) on the right side of the growing heifer between the 10th and 11th intercostal space on a line from the mid-humerus to the tuber coxae. Multiple liver biopsies (approximately 15 mg each sample) were taken from the incision site using stainless steel, frozen immediately after collection in liquid N, and stored at −80 °C. After sampling, postoperative heifers were injected with 6 mL of 5% flunixin meglumine (Hisun Pharmaceutical Co., Ltd, Zhejiang, China) to prevent inflammation.
Tissue pieces were pulverized in the presence of liquid N in a ceramic mortar and then freeze-dried. Fifty milligrams of freeze-dried liver power were weighed for each sample (placed in ice water until the next step). Metabolite extraction was carried out using 1.5 mL of methanol/water solution (methanol:water = 4:1, vol:vol) in the presence of a 5 mm diameter stainless steel bead using a homogenizer (Spex Sample PREP, Stanmore, UK) operating at 20 Hz for 2 min and stood for 60 min at −20 °C (Yin et al., 2015). The mixture was then centrifuged at 30,000 × g for 3 min at 4 °C. A quality control (QC) sample was prepared by pooling an equal amount of extract from the entire set of 10 samples. The extracts were dried in a vacuum concentrator and then reconstituted using 250 μL of methanol-water solution (80:20, vol/vol). The mix was finally centrifuged (30,000 × g for 5 min at 4 °C), and the supernatants were finally transferred into glass vials for analysis. Additionally, 3 blank samples were injected before starting the QC for the baseline stabilization of the Liquid Chromatogram (LC) -MS system.
The chromatographic separation of the liver extracts by reversed-phase liquid chromatography (RPLC) was achieved using a water UPLC HSS T3 (100 mm × 2.1 mm, 1.8 μm particle size; Waters Corporation, MA, USA) operated at 45 °C. Mobile phase A was acetonitrile-water (60:40, vol/vol), and mobile phase B was isopropanol-acetonitrile (90:10, vol/vol). Both A and B contained 0.1% formic acid and 10 mmol/L ammonium acetate. The gradient conditions for RPLC are shown in Appendix Table 1. The flow rate was 300 μL/min, and the injection volume was 1 μL.
Conversely, the hydrophilic interaction liquid chromatography (HILIC) separation for the liver extracts was performed using the Waters UPLC BEH Amide (100 mm × 2.1 mm; 1.7 μm particle size, Waters Corporation, MA, USA) operated at 40 °C. Mobile phase A was acetonitrile, and mobile phase B was water. Both A and B contained 0.1% formic acid and 10 mmol/L ammonium acetate. The gradient conditions for HILIC are shown in Appendix Table 2. The flow rate was 300 μL/min, and the injection volume was 1 μL.
A Thermo Scientific Q Exactive hybrid quadrupole Orbitrap mass spectrometer equipped with a HESI-II probe was employed. The positive and negative HESI-II spray voltages were 3.7 kV and 3.5 kV, respectively, and the heated capillary temperature was 320 °C. Both the sheath gas and the auxiliary gas were N. The collision gas was also N with 1.5 mTorr pressure. The parameters of the full mass scan were as follows: a resolution of 70,000, an auto gain control target under 1 × 106, a maximum isolation time of 50 ms, and a mass-to-charge ratio (m/z) range of 50 to 1,500. The LC-MS system was controlled using Xcalibur 2.2 SP1.48 software (Thermo Fisher Scientific), and data were collected and processed using the same software.
2.7. Data analysis
LC-MS data was processed using Progenesis QI data analysis software (Nonlinear Dynamics, Newcastle, UK) for imputing raw data, peaks alignment, and normalization to produce peak intensities for the retention time and m/z data pair. Before performing the multivariate analysis, additional data preprocessing procedures were required. The maximum allowed CV% value measured for each frame in the QC sample injected before, during, and after the acquisition sequence was set to 30%. After filtering the unneeded variables, the data were normalized against the sum of the peak areas. Orthogonal partial least squares discriminant analysis (OPLS-DA) was used to visually discriminate between samples in the LD group and LA group. Mass ions with variable important in projection (VIP) value greater than 1 were considered discriminant key characteristic metabolites. The t-test was performed, and a P-value less than 0.05 was considered significant. The Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used to analyze and visualize the affected pathway.
The growth performance, serum parameters, apparent digestibility, and N balance data were analyzed by one-way ANOVA using SAS (version 9.1, SAS Institute, Inc., Cary, NC, USA). The statistical differences among the means of the treatments were compared using Duncan's Multiple Range Test. Treatment differences with P < 0.05 were considered statistically significant.
3. Results
3.1. Growth performance
Table 3 shows the experimental data on growth performance. No significant differences were found between the 2 groups (P > 0.05). However, there was a clear trend of increasing FCR with Lys supplementation (0.05 < P < 1).
Table 3.
Effects of Lys supplementation on growth performance in growing Holstein heifers.
| Item | Treatments1 |
SEM | P-value | |
|---|---|---|---|---|
| LD | LA | |||
| Initial BW, kg | 227.31 | 229.20 | 1.661 | 0.58 |
| Final BW, kg | 326.23 | 333.90 | 2.586 | 0.15 |
| ADG, kg/d | 1.10 | 1.16 | 0.024 | 0.19 |
| DMI, kg/d | 7.15 | 7.05 | 0.046 | 0.33 |
| FCR, gain/feed | 0.15 | 0.17 | 0.003 | 0.09 |
BW = body weight; ADG = average daily gain; DMI = dry matter intake; FCR = feed conversion rate.
LD group: growing heifers fed a Lys-deficient diet; LA group: growing heifers fed a Lys-adequate diet. (n = 18).
3.2. Serum biochemical parameters
The serum biochemical parameters are shown in Table 4. No significant differences were found in the serum TC, TG, glucose, and NEFA levels between the 2 groups (P > 0.05). The concentration of SUN was significantly lower in the LA group than in the LD group at 9 months of age (P < 0.05). The enzyme activities of AST and AST/ALT were significantly lower in the LA group than those in the LD group at 9 months of age (P < 0.05).
Table 4.
Effects of Lys supplementation on serum biochemical parameters in growing Holstein heifers.
| Item | Months of age | Treatments1 |
SEM | P-value | |
|---|---|---|---|---|---|
| LD | LA | ||||
| TC, mmol/L | 7 | 1.90 | 1.91 | 0.089 | 0.98 |
| 9 | 2.79 | 2.62 | 0.165 | 0.63 | |
| TG, mmol/L | 7 | 0.17 | 0.15 | 0.011 | 0.50 |
| 9 | 0.18 | 0.19 | 0.010 | 0.59 | |
| SUN, mmol/L | 7 | 3.61 | 3.34 | 0.115 | 0.27 |
| 9 | 5.03 | 4.15 | 0.219 | 0.03 | |
| Glucose, mmol/L | 7 | 4.75 | 4.62 | 0.064 | 0.32 |
| 9 | 5.12 | 5.26 | 0.134 | 0.63 | |
| NEFA, μmon/L | 7 | 346.45 | 350.39 | 3.588 | 0.61 |
| 9 | 353.07 | 340.20 | 4.142 | 0.13 | |
| AST, U/L | 7 | 53.69 | 53.67 | 0.740 | 0.99 |
| 9 | 51.21 | 43.07 | 1.793 | 0.01 | |
| ALT, U/L | 7 | 14.06 | 16.07 | 0.623 | 0.11 |
| 9 | 17.06 | 16.67 | 0.321 | 0.57 | |
| AST/ALT | 7 | 3.83 | 3.40 | 0.141 | 0.14 |
| 9 | 3.00 | 2.59 | 0.103 | 0.03 | |
TC = total cholesterol; TG = triglyceride; SUN = serum urea nitrogen; NEFA = non-esterified fatty acid; ALT = enzyme activity of alanine aminotransferase; AST = enzyme activity of aspartate aminotransferase.
LD group: growing heifers fed a Lys-deficient diet; LA group: growing heifers fed a Lys-adequate diet. (n = 5).
3.3. Diet apparent digestibility and N metabolism
No significant differences were observed in the apparent DM and organic matter digestibility (P > 0.05; Table 5). For N balance, there were no differences in N intake and fecal N (P > 0.05). Urine N was 12.10% lower in the LA group than in the LD group (P < 0.05), and N retention rate tended to be higher in the LA group (0.05 < P < 1). The biological value of N was also higher in the LA group (P < 0.05).
Table 5.
Effects of Lys supplementation on the apparent digestibility of nutrient and nitrogen metabolism in growing Holstein heifers.
| Item | Treatment1 |
SEM | P-value | |
|---|---|---|---|---|
| LD | LA | |||
| Apparent digestibility of nutrient | ||||
| Dry matter, % | 71.71 | 70.94 | 0.474 | 0.46 |
| Organic matter, % | 72.47 | 70.88 | 0.656 | 0.25 |
| Nitrogen metabolism2 | ||||
| Intake N, g/d | 225.65 | 224.90 | 0.315 | 0.26 |
| Fecal N, g/d | 67.33 | 69.51 | 0.885 | 0.24 |
| Urine N, g/d | 94.66 | 83.21 | 2.682 | 0.02 |
| Total N excretion, g/d | 161.98 | 152.72 | 2.601 | 0.07 |
| Absorbed N, g/d | 158.33 | 155.39 | 1.044 | 0.18 |
| Retained N, g/d | 63.67 | 72.18 | 2.555 | 0.09 |
| N digestibility, % | 70.16 | 69.09 | 0.410 | 0.21 |
| N retention rate, % | 28.21 | 32.10 | 1.140 | 0.09 |
| Biological value of N, % | 40.17 | 46.46 | 1.650 | 0.04 |
N = nitrogen.
LD group: growing heifers fed a Lys-deficient diet; LA group: growing heifers fed a Lys-adequate diet. (n = 4).
Total N excretion = fecal N + urine N; Absorbed N = intake N – fecal N; Retained N = intake N – fecal N – urine N; N digestibility = absorbed N/intake N; N retention rate = retained N/intake N; Biological value of N = retained N/absorbed N.
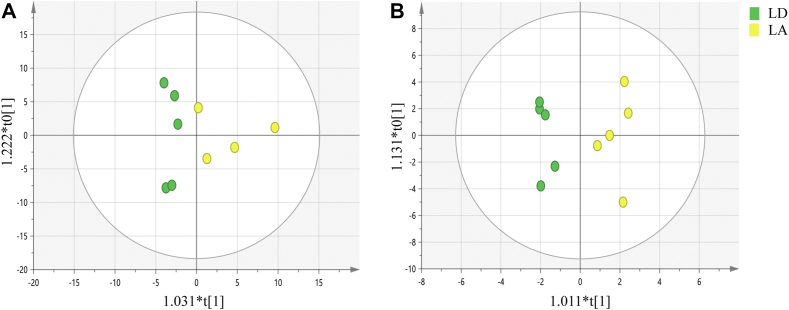
3.4. RPLC-MS analysis
Score plots from the OPLS-DA showed an obvious separation between the LD and LA groups in both positive (Fig. 1a) and negative modes (Fig. 1b). The VIP of the OPLS-DA models were applied to filter the significant metabolites, in which the ions far from the origin were likely to be the significant metabolites in the S-plots (Appendix Fig. 1). A VIP value > 1 and P-value < 0.05 were considered statistically significant. Ten metabolites differentially expressed between the LD and LA groups were selected, as summarized in Table 6, with 8 metabolites acquired from the positive mode and 2 from the negative mode (P < 0.05). It is interesting to note that 5 types of phosphatidylcholine (PC), PC (16:0/20:4(8Z, 11Z, 14Z, 17Z)), PC (16:0/18:2(9Z, 12Z)), PC (16:0/20:3(8Z, 11Z, 14Z)), PC (18:0/20:4(8Z, 11Z, 14Z, 17Z)), and PC (18:0/20:3(5Z, 8Z, 11Z)), and 3 types of ceramide (Cer), namely Cer (d18:1/24:1(15Z)), Cer (d18:1/22:0), and Cer (d18:1/23:0), were increased by Lys supplementation (P < 0.05). In addition, fatty acids, including arachidic acid and 11Z-eicosenoic acid were decreased (P < 0.05).
Fig. 1.
Orthogonal partial least squares discriminant analysis score plots for the liver samples obtained from the RPLC-MS analysis in the positive mode (A) and negative mode (B). LD group, growing heifers fed a Lys-deficient diet; LA group, growing heifers fed a Lys-adequate diet. The X-axis indicated score of the principal component. The Y-axis indicated orthogonal T score. RPLC-MS = reversed-phase liquid chromatography-mass spectrometry.
Table 6.
Summary of the metabolites that changed significantly in the livers of growing heifers fed a Lys-adequate diet through RPLC-MS analysis.
| Metabolites1 | ID2 | Formula | Mode3 | VIP | P-value | FC4 | Direction5 |
|---|---|---|---|---|---|---|---|
| Arachidic acid | HMDB02212 | C20H40O2 | – | 1.47 | 0.027 | 0.63 | ↓ |
| 11Z-Eicosenoic acid | HMDB02231 | C20H38O2 | – | 1.44 | 0.043 | 0.74 | ↓ |
| PC (16:0/20:4(8Z, 11Z, 14Z, 17Z)) | HMDB08462 | C44H80NO8P | + | 1.39 | 0.048 | 1.54 | ↑ |
| PC (16:0/18:2(9Z, 12Z)) | HMDB08133 | C42H80NO8P | + | 1.17 | 0.049 | 1.28 | ↑ |
| PC (16:0/20:3(8Z, 11Z, 14Z)) | HMDB07981 | C44H82NO8P | + | 1.16 | 0.015 | 1.41 | ↑ |
| PC (18:0/20:4(8Z, 11Z, 14Z, 17Z)) | HMDB08049 | C46H84NO8P | + | 1.23 | 0.049 | 1.47 | ↑ |
| PC (18:0/20:3(5Z, 8Z, 11Z)) | HMDB08046 | C46H86NO8P | + | 1.39 | 0.012 | 1.59 | ↑ |
| Cer (d18:1/24:1(15Z)) | HMDB04953 | C42H81NO3 | + | 1.11 | 0.025 | 1.61 | ↑ |
| Cer (d18:1/22:0) | HMDB04952 | C40H79NO3 | + | 1.10 | 0.021 | 1.59 | ↑ |
| Cer (d18:1/23:0) | HMDB00950 | C41H81NO3 | + | 1.02 | 0.047 | 1.47 | ↑ |
RPLC-MS = reversed-phase liquid chromatography-mass spectrometry; VIP = variable important in projection; FC = fold change; PC = phosphatidylcholine; Cer = ceramide.
The different metabolites were filtered using significance estimate of P < 0.05 and VIP > 1.0 (n = 5). LD group: growing heifers fed a Lys-deficient diet; LA group: growing heifers fed a Lys-adequate diet.
Compound ID of metabolites in human metabolome database.
‘-’ indicates negative mode and ‘+’ indicates the positive mode.
Fold change indicates the relative amounts of LA group compared with LD group.
‘↑’ indicates the metabolite in LA group was increased when compared with LD group; ‘↓’ indicates the metabolite in LA group was decreased when compared with LD group.
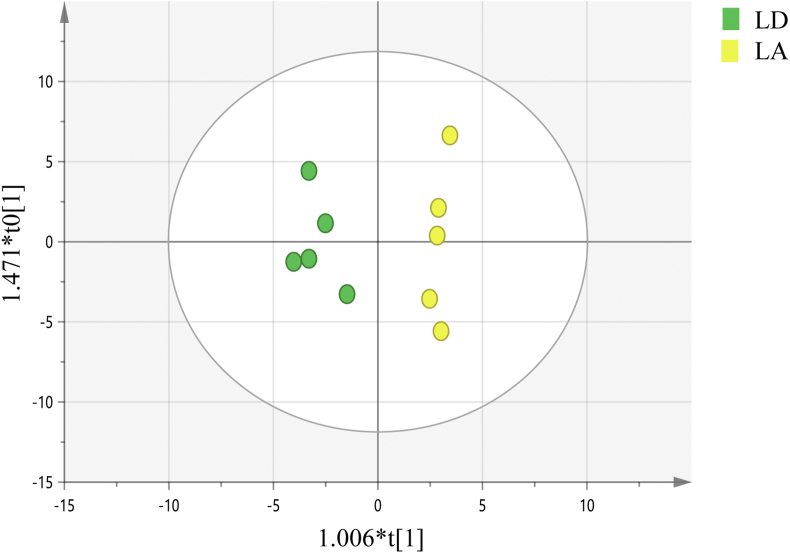
3.5. HILIC-MS analysis
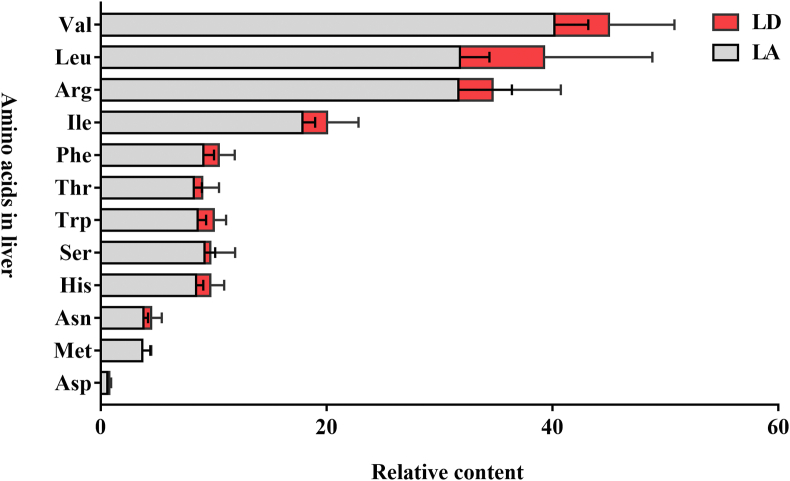
The OPLS-DA obtained from HILIC-MS analysis is shown in Fig. 2. Similarly, the differences between the LA and LD groups are highlighted in this figure. Significant metabolites were identified by comparing the P-value and VIP. The S-plot of the OPLS-DA is shown in Appendix Fig. 2. Table 7 summarizes the significant metabolites obtained by HILIC-MS analysis. His, Leu, and Asp levels were decreased by Lys supplementation (P < 0.05). Although other AA were not filled, the relative contents of these AA were lower in the LA group than that in the LD group (Fig. 3).
Fig. 2.
OPLS-DA scores plots for the liver samples obtained from the HILIC-MS analysis. LD group, growing heifers fed a Lys-deficient diet; LA group, growing heifers fed a Lys-adequate diet. The X-axis indicated score of the principal component. The Y-axis indicated orthogonal T score. OPLS-DA = orthogonal partial least squares discriminant analysis; HILIC-MS = hydrophilic interaction liquid chromatography-mass spectrometry.
Table 7.
Summary of the metabolites that changed significantly in the livers of growing heifers fed a Lys-adequate diet through HILIC-MS analysis.
| Metabolites1 | ID2 | Formula | VIP | P-value | FC3 | Direction4 |
|---|---|---|---|---|---|---|
| L-Histidine | HMDB00177 | C6H9N3O2 | 1.41 | 0.044 | 0.87 | ↓ |
| L-Leucine | HMDB00687 | C6H13NO2 | 1.39 | 0.047 | 0.81 | ↓ |
| L-Aspartic acid | HMDB00191 | C4H7NO4 | 1.39 | 0.018 | 0.78 | ↓ |
HILIC-MS = hydrophilic interaction liquid chromatography-mass spectrometry; VIP = variable important in projection; FC = fold change.
The different metabolites were filtered using significance estimate of P < 0.05 and VIP > 1.0 (n = 5). LD group: growing heifers fed a Lys-deficient diet; LA group: growing heifers fed a Lys-adequate diet.
Compound ID of metabolites in human metabolome database.
Fold change indicates the relative amounts of LA group compared with LD group.
‘↓’ indicates the metabolite in LA group was decreased when compared with LD group.
Fig. 3.
The relative content of AA detected in the livers of growing heifers fed a Lys-deficient diet or a Lys-adequate diet. The X-axis indicates the different amino acids in liver. The Y-axis indicates the relative content of amino acids generated from metabolomics. The values are the mean ± SEM (n = 5); LD group, growing heifers fed a Lys-deficient diet; LA group, growing heifers fed a Lys-adequate diet. AA = amino acid.
3.6. KEGG pathways
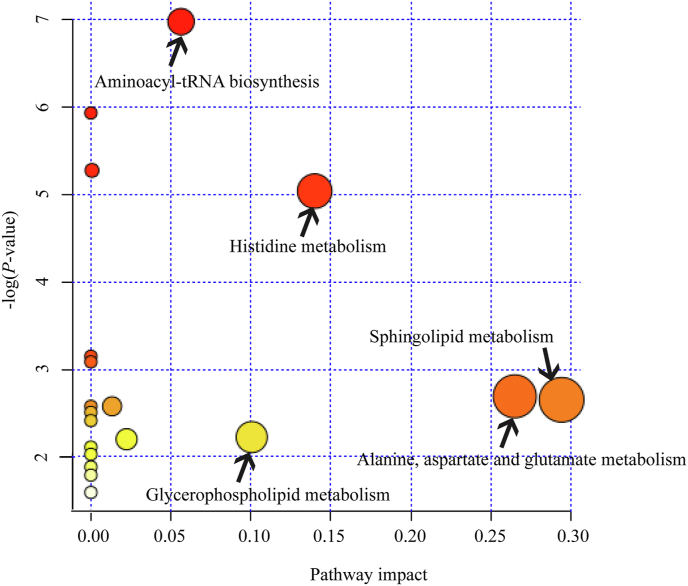
Nineteen pathways were identified when 13 significantly different metabolites were imported into the KEGG database. After enrichment and pathway topology analysis of the identified pathways, only 8 pathways showed an impact value at the comprehensive level (Table 8): sphingolipid metabolism; Ala, Asp, and Glu metabolism; His metabolism; glycerophospholipid metabolism, aminoacyl-tRNA biosynthesis, Val, Leu, and Ile degradation, Val, Leu, and Ile biosynthesis; N metabolism. A comprehensive analysis of the -lop (P-value) and impact value showed that the pathways that differed the most were aminoacyl-tRNA biosynthesis; His metabolism; sphingolipid metabolism; Ala, Asp, and Glu metabolism; and glycerophospholipid metabolism (Fig. 4).
Table 8.
Enrichment of the KEGG pathway using significantly changed metabolites from the combined HILIC-MS and RPLC-MS analyses.
| KEGG Pathway name | -log (P-value)1 | Impact value2 |
|---|---|---|
| Sphingolipid metabolism | 2.410 | 0.294 |
| Alanine, aspartate and glutamate metabolism | 2.449 | 0.265 |
| Histidine metabolism | 4.525 | 0.140 |
| Glycerophospholipid metabolism | 1.988 | 0.101 |
| Aminoacyl-tRNA biosynthesis | 6.150 | 0.056 |
| Valine, leucine and isoleucine degradation | 1.965 | 0.022 |
| Valine, leucine and isoleucine biosynthesis | 2.336 | 0.013 |
| Nitrogen metabolism | 4.759 | <0.001 |
KEGG = Kyoto Encyclopedia of Genes and Genomes; HILIC-MS = hydrophilic interaction liquid chromatography-mass spectrometry; RPLC-MS = reversed-phase liquid chromatography-mass spectrometry.
The KEGG pathway P-values were calculated by comparing the proportion of metabolites in this pathway.
The impact value is calculated by adding up the importance measures of each of the matched metabolites and then dividing by the sum of the important measures of all metabolites in each pathway.
Fig. 4.
The metabolome view map of the significant metabolic pathways characterized in the liver of the growing heifers of the LD and LA group. This figure shows the pathways that are significantly changed based on enrichment and related indicators. The X-axis represents the pathway impact, and the Y-axis represents pathway enrichment. Larger sizes and darker colors indicate greater pathway enrichment and higher pathway impact values, respectively. LD group, growing heifers fed a Lys-deficient diet; LA group, growing heifers fed a Lys-adequate diet.
4. Discussion
Lys has frequently been identified as a limiting AA (Li et al., 2019; Wang et al., 2012). It regulates key metabolic pathways necessary for maintenance, growth, reproduction, and immunity (Wu, 2009). The present study was conducted to evaluate the effects of Lys supplementation on liver metabolism in growing Holstein heifers through metabolomics. First, we found that FCR was improved by Lys supplementation, but the improvement was too marginal to differ from results obtained in calves (Abe et al., 1997; Kong et al., 2020). This might be owing to the full rumen development and ruminal microbial protein synthesis in 7 to 9-month-old heifers (Abe et al., 1997). Furthermore, it is possible that the theoretical Lys addition in this experiment was relatively higher than the actual requirement of growing heifers because of the absence of accurate data regarding Lys requirement. Further research is needed to determine the Lys requirement of growing heifers, and our results indicate that changes in the Lys profile may affect the endogenous metabolism of growing heifers.
Dietary digestion was not altered by Lys supplementation. The digestive efficiency of the diet always depends on rumen fermentation and subsequent absorption in the small intestine. Gln, Glu, and Asp rather than Lys are important energy sources in the small intestine and are responsible for intestinal ATP-dependent metabolic processes (Cabrera et al., 2013). Cabrera et al. (2013) found that supplementation with Gln and Glu improved the intestinal health and FCR of piglets. Keulen et al. (2020) also reported comparable results in calves. Lys has been traditionally considered not to be utilized by the intestinal mucosa, which is consistent with our observation on nutrient digestibility.
Apart from the small intestine, the liver is the largest organ that responds to Lys metabolism. Studies have shown that the amount of dietary Lys is negatively correlated with SUN concentration, which is an end metabolite of the urea cycle in the liver through AA deamination or a product of rumen fermentation (Wang et al., 2012; Xue et al., 2011). Owing to Lys supplementation in the LA group, lower body protein degradation and relatively infrequent protein turnover might contribute to decreased AA concentration in the liver, including Asp, His, and Leu, and the disruption of relative AA pathways (Fig. 5). The data from the N balance experiment also proved that the balance between protein degradation and synthesis and the urea cycle in the liver was more optimal in the presence of Lys supplementation; more N was retained and less urea N discharged, thereby leading to increased FCR. Rius et al. (2012) fed milk replacers containing high protein to calves and reported that a greater intake of nutrients increased the net intake of AA to support rapid body growth. Wray-Cahen et al. (1997) infused AA into the mesenteric vein of dry cows and showed that the liver removed Thr, Leu, Val, and Lys to a greater extent. The results from these studies, combined with our results, indicate that the metabolic efficiency of the liver depends on the metabolizable AA pattern and Lys supplementation and that Lys supplementation in the present study could enhance the N retention rate of other AA in the liver and increase the efficiency of N deposition.
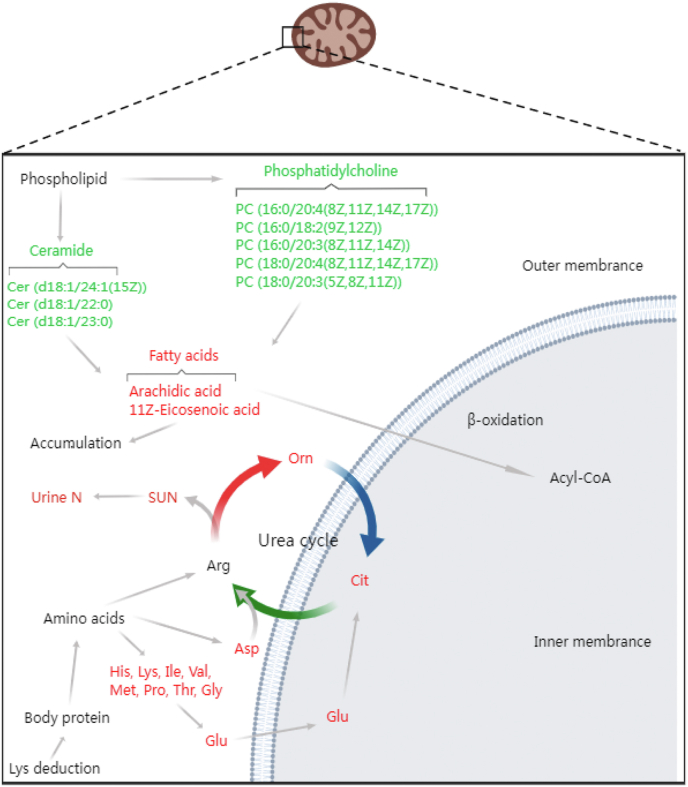
Fig. 5.
Schematic diagram of the metabolic pathway. Red represents the decreased metabolites. Green represents the increased metabolites. The metabolites changed in the LA group (growing heifers fed a Lys-adequate diet) compared with the LD group (growing heifers fed a Lys-deficient diet) from the metabolomics used to draw this figure. SUN = serum urea nitrogen; N = nitrogen.
Whitehouse et al. (2017) reported that plasma-free Lys was increased linearly to increase the amount of metabolizable Lys, and the plasma-free Lys dose-response method was effective in determining the relative bioavailability of rumen-protected Lys. Interestingly, Lys supplementation decreased the Lys level in the liver, suggesting that hepatic Lys concentration depended on many factors. This index, rather than the plasma Lys concentration, made it difficult to determine the availability of rumen-protected Lys, as the Lys concentration in the liver depended not only on portal vein supplementation but also on hepatic artery supplementation. Additionally, we found that glycogenic AA, such as Ala and Gln, were not affected by Lys supplementation. This may explain the same glucose concentration in the serum.
Using metabolomics, we found that many phospholipids and sphingolipids, including 5 types of PC and 3 types of Cer, were increased after Lys supplementation. In the past 2 decades, these have been referred to as “bioactive lipids” because of their pivotal role in immune regulation, inflammation, and maintenance of tissue homeostasis (Chiurchiù et al., 2018; Furse and de Kroon, 2015). Among them, PC is a phospholipid attached to choline particles. It is a storage lipid for arachidic acid residues (Kramer and Deykin, 1983), which is consistent with our results (Fig. 5). Moreover, PC is a principal component of very-low-density lipoprotein (VLDL) (McFadden, 2020), a key means of triacylglycerol export from the liver. This implies that an increased PC content might alleviate the accumulation of triacylglycerols in the liver, partially explaining the decreased fatty acid levels in the liver. PC are regarded as a potential set of predictive biomarkers of hepatic lipidosis (Imhasly et al., 2014) and feed efficiency (Artegoitia et al., 2017). Suppressed levels of unsaturated PC have been observed in Holstein cows with moderate fatty liver disease (Sina et al., 2018). These results suggest that lipid metabolism in the liver is affected by Lys supplementation.
A previous study showed that rabbits fed a diet supplemented with Lys and Met had increased hepatic PC levels and choline phosphotransferase activity, the last enzyme in the PC biosynthetic pathway (Giroux et al., 1999). Alternatively, DeLong et al. (1999) reported that the hepatic synthesis of PC relied on the cytidine diphosphate-choline pathway and Met transmethylation cycles. Hence, Lys supplementation may consume Met and improve choline phosphotransferase activity in the liver. However, the growing heifers in the 2 groups had the same Met intake, and the Met content in the liver was the same between the 2 groups. Thus, an optimal balance and interaction exist among the AA in the diet, especially between Lys and Met (Tsiplakou et al., 2020; Wang et al., 2010). Although our interpretation was limited by the lack of data on enzyme activity and Met metabolism in the liver, the aforementioned factors likely played a role in the response. Overall, these findings suggest that the role of Lys in the PC biosynthetic pathway could become a concern, and an investigation into the relationship between Lys and Met in the liver should be conducted.
Cer are component lipids that comprise sphingolipids. Similar to PC, Cer are found in high concentrations in the cell membrane and VLDL (Wiesner et al., 2009). Thus, sphingolipid and phospholipid metabolism are intertwined. Cer are recognized as biomarkers for metabolic disease because of their crucial role in liver homeostasis to protect the liver from fatty liver disease (Luukkonen et al., 2016; Pewzner-Jung et al., 2010). Therefore, circulating Cer concentrations were increased in early lactation cows, which experienced adipose tissue free fatty acid mobilization and liver steatosis. Similar observations were made in dairy cows supplemented with Cer through an abomasal fistula (Myers et al., 2019) and those subjected to intravenous triacylglycerol infusion (Rico et al., 2018). These recent studies have demonstrated that Cer in lipoproteins enhance lipid transport. Considering our previous work on evaluating the effects of Lys reduction on liver metabolism in calves (Kong et al., 2020), we were not surprised to detect metabolites involved in lipid metabolism in growing heifers. The amount of Cer in the liver depends on the de novo synthetic pathway and sphingomyelin hydrolysis (McFadden and Rico, 2019). Although the regulatory mechanism remains to be defined, we conclude that Lys is pertinent to lipid metabolism by Cer.
Many studies have investigated serum-sensitive biomarkers of fatty liver in dairy cows. For example, serum proteins, such as fibroblast growth factor-21, hemoglobin, and angiopoietin-like protein 4 can be used as fatty liver biomarkers in lactating dairy cows (Shen et al., 2018; Wang et al., 2018). Gerspach et al. (2016) found that AST, rather than NEFA, TC, or TG, is a biochemical parameter that distinguishes cows with fatty liver. Studies have also indicated that the AST to ALT ratio is positively correlated with disease severity in several chronic liver diseases (Mansoor et al., 2015; Sheth et al., 1998). Owing to liver diseases, high concentrations of AST in hepatic cells are released as a result of apoptosis and enter the circulating plasma. In our study, we found no effects of Lys supplementation on serum NEFA, TC, and TG levels in growing heifers. Significantly different results were obtained for the AST concentration and AST to ALT ratio. Both values were within the normal range. Thus, we suspected that Lys supplementation could alleviate hepatic cell apoptosis.
5. Conclusion
In this study, Lys supplementation and meeting the Lys requirement of growing Holstein heifers led to less N emission from AA deamination in the urea cycle and regulated lipid metabolism by increasing apolipoprotein precursors, including PC and Cer, and further increased FCR and the biological value of N. The role of Lys in hepatic metabolism is further emphasized by involving not only N metabolism but also lipid metabolism. This study partially revealed the function of Lys in the liver and explained the mechanism of changes in apparent performance. Further studies should be conducted to investigate the pathway mediated by Lys with hepatic cell culture. Additionally, we speculated that Lys might be important for the transition dairy cows in relieving lipid accumulation and alleviate negative energy balance.
Author contributions
Fanlin Kong carried out this study and then did the sampling and laboratory works. Yuan Li finished statistical work and critically reviewed the manuscript. Yanliang Bi also did the sampling and drafted the manuscript. Yan Tu and Qiyu Diao designed and approved the study plan. All authors read and approved the final manuscript.
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgment
The author would like to thank Yijia Zhang (College of veterinary medicine, China agricultural university) for her help in producing figures. This work was supported, in part, by the Key Research and Development Program of Hebei Province (19226621D), Earmarked Fund for Beijing Dairy Industry Innovation Consortium of Agriculture Research System, Chinese Academy of Agricultural Science and Technology Innovation Project (CAAS-XTCX-2016011-01), Fundamental Research Funds for Central Non-profit Scientific Institution (Y2019CG08), Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2017-FRI-04), and Science and Technology Open Cooperation Project of Henan Province (182106000035)-Study on the Pattern of Diet Amino Acid for Different Physiological Stages of Heifers.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2021.10.001.
Contributor Information
Yanliang Bi, Email: vetbi2008@163.com.
Yan Tu, Email: tuyan@caas.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abe M., Iriki T., Funaba M. Lysine deficiency in postweaned calves fed corn and corn gluten meal diets. J Anim Sci. 1997;75:1974–1982. doi: 10.2527/1997.7571974x. [DOI] [PubMed] [Google Scholar]
- Abe M., Iriki T., Funaba M., Onda S. Limiting amino acids for a corn and soybean meal diet in weaned calves less than three months of age. J Anim Sci. 1998;76:628–636. doi: 10.2527/1998.762628x. [DOI] [PubMed] [Google Scholar]
- AOAC . 18th ed. AOAC INTERNATIONAL; 2006. Official methods of analysis. [Google Scholar]
- Artegoitia V.M., Foote A.P., Lewis R.M., Freetly H.C. Liver metabolomics analysis associated with feed efficiency on steers. J Anim Sci. 2017;95:258–259. doi: 10.1093/jas/skab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera R.A., Usry J.L., Arrellano C., Nogueira E.T., Kutschenko M., Moeser A.J., Odle J. Effects of creep feeding and supplemental glutamine or glutamine plus glutamate (aminogut) on pre- and post-weaning growth performance and intestinal health of piglets. J Anim Sci Biotechnol. 2013;4:29. doi: 10.1186/2049-1891-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiurchiù V., Leuti A., Maccarrone M. Bioactive lipids and chronic inflammation: managing the fire within. Front Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong C.J., Shen Y.J., Thomas M.J., Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem. 1999;274:29683–29688. doi: 10.1074/jbc.274.42.29683. [DOI] [PubMed] [Google Scholar]
- Eggleston H.S., Buendia L., Kyoko M., Ngara T., Tanabe K. IPCC guidelines for national greenhouse gas inventories. Institute for Global Environmental Strategies; Japan: 2006. [Google Scholar]
- Fehlberg L.K., Guadagnin A.R., Thomas B.L., Sugimoto Y., Cardoso P. Feeding rumen-protected lysine prepartum increases energy-corrected milk and milk component yields in Holstein cows during early lactation. J Dairy Sci. 2020;103:11386–11400. doi: 10.3168/jds.2020-18542. [DOI] [PubMed] [Google Scholar]
- Furse S., de Kroon A.I. Phosphatidylcholine's functions beyond that of a membrane brick. Mol Membr Biol. 2015;32:117–119. doi: 10.3109/09687688.2015.1066894. [DOI] [PubMed] [Google Scholar]
- Gerspach C., Ruetten M., Riond B. Investigation of coagulation and serum biochemistry profiles in dairy cattle with different degrees of fatty liver. Schweiz Arch Tierheilkd. 2016;158:811–818. doi: 10.17236/sat00096. [DOI] [PubMed] [Google Scholar]
- Giroux I., Kurowska E.M., Freeman D.J., Carroll K.K. Addition of arginine but not glycine to lysine plus methionine-enriched diets modulates serum cholesterol and liver phospholipids in rabbits. J Nutr. 1999;129:1807–1813. doi: 10.1093/jn/129.10.1807. [DOI] [PubMed] [Google Scholar]
- Grum D.E., Drackley J.K., Clark J.H. Fatty acid metabolism in liver of dairy cows fed supplemental fat and nicotinic acid during an entire lactation. J Dairy Sci. 2002;85:3026–3034. doi: 10.3168/jds.S0022-0302(02)74388-9. [DOI] [PubMed] [Google Scholar]
- Grummer R.R. Etiology of lipid-related metabolic disorders in periparturient dairy cows. J Dairy Sci. 1993;76:3882–3896. doi: 10.3168/jds.S0022-0302(93)77729-2. [DOI] [PubMed] [Google Scholar]
- Handcock R.C., Lopez-Villalobos N., McNaughton L.R., Back P.J., Edwards G.R., Hickson R.E. Positive relationships between body weight of dairy heifers and their first-lactation and accumulated three-parity lactation production. J Dairy Sci. 2019;102:4577–4589. doi: 10.3168/jds.2018-15229. [DOI] [PubMed] [Google Scholar]
- Hu X.M., Guo F.F. Amino acid sensing in metabolic homeostasis and health. Endocr Rev. 2020;42:56–76. doi: 10.1210/endrev/bnaa026. [DOI] [PubMed] [Google Scholar]
- Imhasly S., Naegeli H., Baumann S., Von Bergen M., Luch A., Jungnickel H., Potratz S., Gerspach C. Metabolomic biomarkers correlating with hepatic lipidosis in dairy cows. BMC Vet Res. 2014;10:122. doi: 10.1186/1746-6148-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertz A.F., Hill T.M., Quigley J.D., Heinrichs A.J., Linn J.G., Drackley J.K. A 100-year review: calf nutrition and management. J Dairy Sci. 2017;100:10151–10172. doi: 10.3168/jds.2017-13062. [DOI] [PubMed] [Google Scholar]
- Keulen P.V., Khan A., Dijkstra J., Knol F., McCoard S.A. Effect of arginine or glutamine supplementation and milk feeding allowance on small intestine development in calves. J Dairy Sci. 2020;103:4754–4764. doi: 10.3168/jds.2019-17529. [DOI] [PubMed] [Google Scholar]
- Kong F., Bi Y., Wang B., Cui K., Li Y., Fu T., Diao Q., Tu Y. Integrating RNA-sequencing and untargeted LC-MS metabolomics to evaluate the effect of lysine deficiency on hepatic functions in Holstein calves. Amino Acids. 2020;52:781–792. doi: 10.1007/s00726-020-02852-1. [DOI] [PubMed] [Google Scholar]
- Kramer R.M., Deykin D. Arachidonoyl transacylase in human platelets. Coenzyme A-independent transfer of arachidonate from phosphatidylcholine to lysoplasmenylethanolamine. J Biol Chem. 1983;258:13806–13811. [PubMed] [Google Scholar]
- Křížová L., Třináctý J., Svobodová J., Richter M., Černý V., Jarošová A. Effect of supplemental rumen-protected lysine, methionine or both added to diet of lactating dairy cows on milk fatty acids profile. Acta Univ Agric Silvic Mendelianae Brunensis. 2010;58:87–94. [Google Scholar]
- Lapierre H., Doepel L., Milne E., Lobley G.E. Responses in mammary and splanchnic metabolism to altered lysine supply in dairy cows. Animal. 2009;3:360–371. doi: 10.1017/S1751731108003571. [DOI] [PubMed] [Google Scholar]
- Larsen M., Kristensen N.B. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal. 2013;7:1640–1650. doi: 10.1017/S1751731113001171. [DOI] [PubMed] [Google Scholar]
- Li Y., Bi Y., Diao Q., Piao M., Wang B., Kong F., Hu F., Tang M., Sun Y., Tu Y. The limiting sequence and appropriate amino acid ratio of lysine, methionine, and threonine for seven- to nine-month-old Holstein heifers fed corn-soybean m-based diet. Animals. 2019;9:750. doi: 10.3390/ani9100750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H.Y., Chen C.C., Chen Y.J., Lin Y.Y., Mersmann H.J., Ding S.T. Enhanced amelioration of high-fat diet-induced fatty liver by docosahexaenoic acid and lysine supplementations. BioMed Res Int. 2014;2014:310981. doi: 10.1155/2014/310981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen P.K., Zhou Y., Sädevirta S., Leivonen M., Arola J., Orešič M., Hyötyläinen T., Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Malmuthuge N., Guan L.L. Understanding host-microbial interactions in rumen: searching the best opportunity for microbiota manipulation. J Anim Sci Biotechnol. 2017;8:8. doi: 10.1186/s40104-016-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoor S., Collyer E., Alkhouri N. A comprehensive review of noninvasive liver fibrosis tests in pediatric nonalcoholic fatty liver disease. Curr Gastroenterol Rep. 2015;17:23. doi: 10.1007/s11894-015-0447-z. [DOI] [PubMed] [Google Scholar]
- McFadden J.W. Review: lipid biology in the periparturient dairy cow: contemporary perspectives. Animal. 2020;14:s165–s175. doi: 10.1017/S1751731119003185. [DOI] [PubMed] [Google Scholar]
- McFadden J.W., Rico J.E. Invited review: sphingolipid biology in the dairy cow: the emerging role of ceramide. J Dairy Sci. 2019;102:7619–7639. doi: 10.3168/jds.2018-16095. [DOI] [PubMed] [Google Scholar]
- Myers W.A., Rico J.E., Davis A.N., Fontoura A.B.P., Dineen M.J., Tate B.N., McFadden J.W. Effects of abomasal infusions of fatty acids and one-carbon donors on hepatic ceramide and phosphatidylcholine in lactating Holstein dairy cows. J Dairy Sci. 2019;102:7087–7101. doi: 10.3168/jds.2018-16200. [DOI] [PubMed] [Google Scholar]
- Noftsger S., St-Pierre N.R. Supplementation of methionine and selection of highly digestible rumen undegradable protein to improve nitrogen efficiency for milk production. J Dairy Sci. 2003;86:958–969. doi: 10.3168/jds.S0022-0302(03)73679-0. [DOI] [PubMed] [Google Scholar]
- NRC . National Academic Press; Washington: 2001. Nutrient requirement of dairy cattle. [Google Scholar]
- Patti G.J., Yanes O., Siuzdak G. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. doi: 10.1038/nrm3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner-Jung Y., Park H., Laviad E.L., Silva L.C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., Prieto M., Merrill A.H., Jr., Futerman A.H. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. J Biol Chem. 2010;285:10902–10910. doi: 10.1074/jbc.M109.077594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink D.B., Gatrell S.K., Elango R., Turchinsky J., Kiess A.S., Blemings K.P., Dixon W.T., Ball R.O. Lysine α-ketoglutarate reductase, but not saccharopine dehydrogenase, is subject to substrate inhibition in pig liver. Nutr Res. 2011;31:544–554. doi: 10.1016/j.nutres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Rico J.E., Giesy S.L., Haughey N.J., Boisclair Y.R., McFadden J.W. Intravenous triacylglycerol infusion promotes ceramide accumulation and hepatic steatosis in dairy cows. J Nutr. 2018;148:1529–1535. doi: 10.1093/jn/nxy155. [DOI] [PubMed] [Google Scholar]
- Rius A.G., Weeks H.A., Cyriac J., Akers R.M., Bequette B.J., Haniqan M.D. Protein and energy intakes affected amino acid concentrations in plasma, muscle, and liver, and cell signaling in the liver of growing dairy calves. J Dairy Sci. 2012;95:1983–1991. doi: 10.3168/jds.2011-4688. [DOI] [PubMed] [Google Scholar]
- Roche J.R., Burke C.R., Crookenden M.A., Heiser A., Loor J.L., Meier S., Mitchell M.D., Phyn C.V.C., Turner S.A. Fertility and the transition dairy cow. Reprod Fertil Dev. 2017;30:85–100. doi: 10.1071/RD17412. [DOI] [PubMed] [Google Scholar]
- Shen Y., Chen L., Yang W., Wang Z. Exploration of serum sensitive biomarkers of fatty liver in dairy cows. Sci Rep. 2018;8:13574. doi: 10.1038/s41598-018-31845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth S.G., Flamm S.L., Gordon F.D., Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- Sina S.S., Yu Z., William A.M., Ester G., Joseph W.M. A lipidomic analysis of bovine liver during metabolic disease. J Dairy Sci. 2018;101:4. [Google Scholar]
- Sok M., Ouellet D.R., Firkins J.L., Pellerin D., Lapierre H. Amino acid composition of rumen bacteria and protozoa in cattle. J Dairy Sci. 2017;100:5241–5249. doi: 10.3168/jds.2016-12447. [DOI] [PubMed] [Google Scholar]
- Tan P., Liu H., Zhao J., Gu X., Wei X., Zhang X., Ma N., Johnston L.J., Bai Y., Zhang W., Nie C., Ma X. Amino acids metabolism by rumen microorganisms: nutrition and ecology strategies to reduce nitrogen emissions from the inside to the outside. Sci Total Environ. 2021;800:149596. doi: 10.1016/j.scitotenv.2021.149596. [DOI] [PubMed] [Google Scholar]
- Tsiplakou E., Mavrommatis A., Skliros D., Righi F., Flemetakis E. The impact of rumen-protected amino acids on the expression of key- genes involved in the innate immunity of dairy sheep. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Drift S.G.A., Houweling M., Schonewille J.T., Tielens A.G.M., Jorritsma R. Protein and fat mobilization and associations with serum β-hydroxybutyrate concentrations in dairy cows. J Dairy Sci. 2012;95:4911–4920. doi: 10.3168/jds.2011-4771. [DOI] [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Wan P.J., Yuan S.Y., Tang Y.H., Li K.L., Yang L., Fu Q., Li G.Q. Pathways of amino acid degradation in nilaparvata lugens (stål) with special reference to lysine-ketoglutarate reductase/saccharopine dehydrogenase (LKR/SDH) PLoS One. 2015;10 doi: 10.1371/journal.pone.0127789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu H.Y., Wang Y.M., Yang Z.Q., Liu J.X., Wu Y.M., Yan T., Ye H.W. Effects of dietary supplementation of methionine and lysine on milk production and nitrogen utilization in dairy cows. J Dairy Sci. 2010;93:3661–3670. doi: 10.3168/jds.2009-2750. [DOI] [PubMed] [Google Scholar]
- Wang J., Diao Q., Tu Y., Zhang N., Xu X. The limiting sequence and proper ratio of lysine, methionine and threonine for calves fed milk replacers containing soy protein. Asian-Australas J Anim Sci. 2012;25:224–233. doi: 10.5713/ajas.2011.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhu X., She G., Kong Y., Guo Y., Wang Z., Liu G., Zhao B. Serum hepatokines in dairy cows: periparturient variation and changes in energy-related metabolic disorders. BMC Vet Res. 2018;14:236. doi: 10.1186/s12917-018-1560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse N.L., Schwab C.G., Brito A.F. The plasma free amino acid dose-response technique: a proposed methodology for determining lysine relative bioavailability of rumen-protected lysine supplements. J Dairy Sci. 2017;100:9585–9601. doi: 10.3168/jds.2017-12695. [DOI] [PubMed] [Google Scholar]
- Wiesner P., Leidl K., Boettcher A., Schmitz G., Liebisch G. Lipid profiling of FPLC-separated lipoprotein fractions by electrospray ionization tandem mass spectrometry. J Lipid Res. 2009;50:574–585. doi: 10.1194/jlr.D800028-JLR200. [DOI] [PubMed] [Google Scholar]
- Wray-Cahen D., Metcalf J.A., Backwell F.R., Bequette B.J., Brown D.S., Sutton J.D., Lobley G.E. Hepatic response to increased exogenous supply of plasma amino acids by infusion into the mesenteric vein of Holstein-Friesian cows in late gestation. Br J Nutr. 1997;78:913–930. doi: 10.1079/bjn19970209. [DOI] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Xue F., Zhou Z., Ren L., Meng Q. Influence of rumen-protected lysine supplementation on growth performance and plasma amino acid concentrations in growing cattle offered the maize stalk silage/maize grain-based diet. Anim Feed Sci Technol. 2011;169:61–67. [Google Scholar]
- Yin P., Zhou L., Zhao X., Xu G. Sample collection and preparation of biofluids and extracts for liquid chromatography-mass spectrometr. Methods Mol Biol. 2015;1277:51–59. doi: 10.1007/978-1-4939-2377-9_5. [DOI] [PubMed] [Google Scholar]
- Zinn R.A., Shen Y. An evaluation of ruminally degradable intake protein and metabolizable amino acid requirements of feedlot calves. J Anim Sci. 1998;76:1280. doi: 10.2527/1998.7651280x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.