Abstract
We describe a case of Trousseau's syndrome in a patient with lung carcinoma. A 69-year-old man presented with pleural effusion. Further evaluation revealed EGFR mutation-positive non-small cell carcinoma in the upper lobe with extensive lymph node, bone, and brain metastases. Administration of osimertinib, an EGFR tyrosine kinase inhibitor, resulted in partial tumor response, but caused osimertinib-induced pneumonitis 10 weeks later. Prednisolone restrained lung injury progression and was gradually tapered. However, he presented with impaired consciousness and right hemiplegia. Magnetic resonance imaging revealed a left middle cerebral artery M1 segment occlusion. D-dimer level was elevated to 19.5 μg/mL. In the absence of atherosclerotic or cardiogenic thrombi, these findings led to the diagnosis of Trousseau syndrome. Endovascular therapy, but not tissue plasminogen activator, improved his condition with no recurrences. These treatment strategies are crucial to restore function in patients with potentially disabling cerebral infarction due to Trousseau syndrome.
Keywords: Cerebral infarction, Endovascular therapy, Lung cancer, Trousseau syndrome, Osimertinib
Highlights
-
•
Systemic anticoagulation for Trousseau syndrome generally has a poor prognosis.
-
•
There is no established treatment for cancer-associated cerebral infarction.
-
•
We performed endovascular therapy for cerebral infraction due to Trousseau syndrome.
-
•
Endovascular therapy was effective for recanalization of the occluded artery.
-
•
An NIHSS score of 0 was achieved with no recurrence.
1. Introduction
Trousseau syndrome is a malignancy-associated thromboembolic disorder. The pathophysiologic mechanism of thrombus formation in Trousseau syndrome remains unknown. Trousseau syndrome is a common cause of cerebral infarction in patients with advanced non-small cell lung cancer, and it can affect their quality of life. The prognosis for such patients is reportedly extremely poor [1]. In such cases, systemic anticoagulation for thrombus removal, which is generally performed for Trousseau syndrome, has a limited effect. Recently, a few patients have been reported to have undergone endovascular therapy with subsequent functional recovery. Here, we present the endovascular treatment of cerebral infarction due to Trousseau syndrome in a patient with non-small cell lung cancer (NSCLC).
2. Case presentation
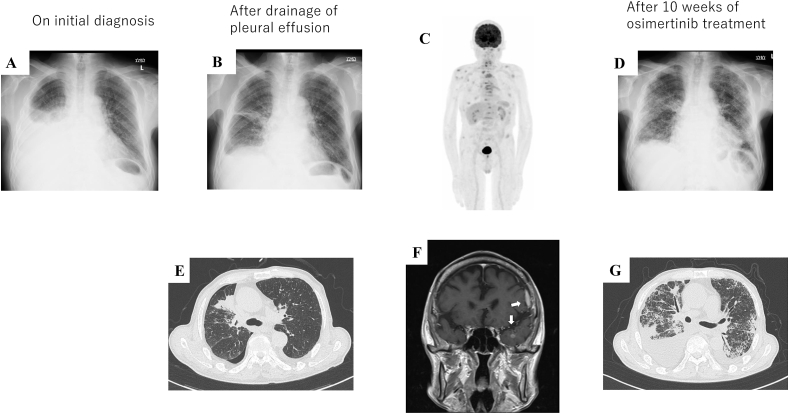
A 69-year-old man with a 49-pack-year history of smoking was admitted to our hospital due to progressive dyspnea; his past medical history was unremarkable, and exposure to asbestos status was unknown. Plain chest radiography revealed massive right pleural effusion (Fig. 1A), which was drained to improve his dyspnea. Cytologic analysis of the effusion was consistent with adenocarcinoma, and immunohistochemistry confirmed the diagnosis of adenocarcinoma with an exon 19 deletion of the epidermal growth factor receptor (EGFR) gene. Chest radiography and computed tomography (CT) revealed an irregularly shaped lung mass in the right upper lobe with pleural effusion and air space limitation due to poor lung expansion (Fig. 1B and E). An 18F-labeled fluorodeoxyglucose (18F-FDG)-positron emission tomography (PET) scan revealed an abnormal accumulation of FDG in the right upper lobe mass, mediastinal lymph nodes, and multiple bones (Fig. 1C). Magnetic resonance imaging (MRI) showed a small contrast-enhancing lesion and a thickened endocranium in the left cranial vault, indicating meningeal dissemination (Fig. 1F). These metastatic findings suggest a stage IV (cT1cN2M1c) NSCLC. The patient's Eastern Cooperative Oncology Group (ECOG) performance status was grade 1. Osimertinib (80 mg/day), EGFR tyrosine kinase inhibitors (TKIs), was administered as first-line chemotherapy, and he was discharged without any complications.
Fig. 1.
Chest radiographic images taken at the following time points: (A) initial diagnosis, (B) after drainage, and (D) after 10 weeks of osimertinib treatment. High-resolution computed tomography taken at the following time points: (E) after drainage and (G) after 10 weeks of osimertinib treatment. (C) 18F-labeled fluorodeoxyglucose (FDG) positron emission tomography before osimertinib treatment revealed an abnormal accumulation of FDG in the right upper lobe mass, mediastinal lymph nodes, and multiple bones. (F) Brain magnetic resonance imaging before osimertinib administration shows a small contrast-enhancing lesion and an endocranium thickening in the left cranial vault (white arrows).
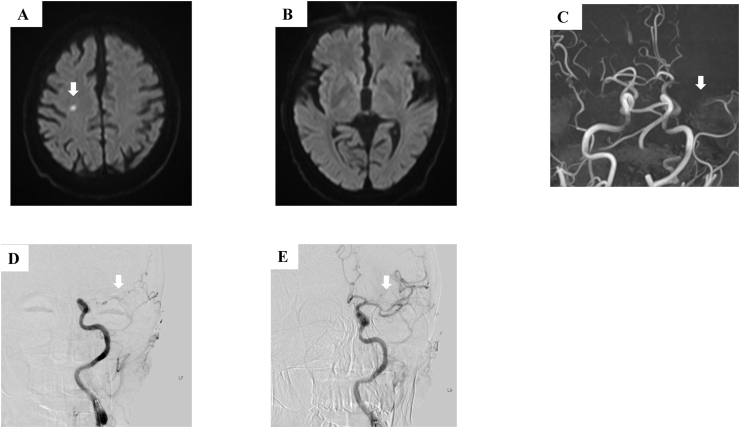
Ten weeks after osimertinib treatment, the patient presented with fever and dyspnea. Although chest radiography and high-resolution CT revealed a partial response of the primary tumor, bilateral infiltrates were detected (Fig. 1D and G). We suspected interstitial lung disease due to osimertinib. The patient was treated with 1000 mg methylprednisolone for three days, and subsequently with 40 mg prednisone until the lung infiltrates showed improvement. During tapering of prednisolone 13 weeks after osimertinib treatment, the patient suddenly experienced impaired consciousness and right hemiplegia. Diffusion-weighted magnetic resonance imaging revealed small subacute infarcts in the right hemisphere (Fig. 2A). However, these radiologic findings were not consistent with his symptom laterality (Fig. 2B). Further evaluation with magnetic resonance angiography revealed a left middle cerebral artery (MCA) M1 segment occlusion (Fig. 1C). The patient's plasma D-dimer level was elevated (19.5 μg/mL). Electrocardiography showed no atrial fibrillation. Echocardiography revealed no atrial thrombi. He was diagnosed with cerebral infarction due to Trousseau syndrome with a National Institutes of Health Stroke Scale (NIHSS) score of 7. As there were no endovascular specialists at our hospital to prescribe appropriate treatment, we initially decided to administer tissue plasminogen activator (t-PA) therapy. The patient did not respond to intravenous t-PA administration 1.25 hours after symptom onset; consequently, he was transferred to a tertiary hospital for endovascular thrombectomy. Endovascular therapy was administered 3 hours and 40 minutes after symptom onset, following which, the complete recanalization of the left MCA was achieved (Fig. 2D and E) and the NIHSS score improved to 1. Heparin was initiated following thrombectomy. After 10 days, an NIHSS score of 0 was achieved with no recurrence. The patient and his family did not wish to continue receiving osimertinib or cytotoxic chemotherapy for fear of an exacerbation of drug-induced pneumonitis. Therefore, no further chemotherapy was administered. Unfortunately, the patient's disease progressed and he died four months after cerebral infarction.
Fig. 2.
Diffusion-weighted magnetic resonance imaging revealed (A) subacute small infarcts in the right hemisphere (white arrows) with (B) no evidence of infarction in left hemisphere. (C) Magnetic resonance angiography revealed a left middle cerebral artery M1 segment occlusion (white arrows). (D and E) Endovascular therapy completely recanalized the left middle cerebral artery (white arrows).
3. Discussion
Cancer is a recognized major risk factor for cerebral infarction. Trousseau syndrome is a common complication of advanced cancers that may reduce the quality of life or cause death. The median survival was 4.5 months from the diagnosis of stroke and the mortality within 30 days was 25% [2]. A therapeutic approach for patients with cancer-associated cerebral infarction has not been established. Reports have shown that anticoagulant therapy alone was not effective for Trousseau syndrome [3]. Tumor cells produce prothrombotic agents, such as tissue factor and mucin [4]; thus, Trousseau syndrome treatment must target the underlying tumor [4].
EGFR-TKIs are a standard first-line therapy, so we selected Osimertinib [5]. CT revealed a partial tumor response according to the RECIST criteria. However, the osimertinib caused a lung injury, which prompted termination of the osimertinib therapy. Moreover, intravascular fibrin deposition is frequently found [6]. KL-6 was elevated in cases with mucin secretion. Mucins are large glycosylated ligands for selectins, which may induce coagulation [4]. Tumor size reduction due to osimertinib might lower the risk of cerebral infarction; however, termination of osimertinib due to secondary lung injury may induce cerebral infarction. The pronounced reduction in tumor burden achieved using osimertinib might have contributed to the lowered risk of cerebral infarction [5]. In our patient, the cerebral infarction due to Trousseau syndrome occurred during the time of osimertinib-induced lung injury.
In our case, endovascular therapy was effective for recanalization of the occluded artery. Only seven other cases of endovascular therapy for Trousseau syndrome have been reported (Table 1). Most of these patients have stage Ⅳ advanced cancers (e.g., lung cancer, pancreatic cancer, and ovarian cancer) [[7], [8], [9], [10], [11]].
Table 1.
Characteristics of cases of treatment of endovascular therapy for malignant tumor with Trousseau Syndrome.
| Age | Sex | Malignancy (Histology) | Stage | Location of Occlusion | References | |
|---|---|---|---|---|---|---|
| Case 1 | 55 | F | Ovarian cancer (N/A) | Ⅳ | Left MCA | [7] |
| Case 2 | 71 | F | Lung cancer (Adenocarcinoma) | N/A | Left MCA (M1) × 2 Right MCA (M2) |
[8] |
| Case 3 | 57 | M | Lung cancer (N/A) | Ⅳ | Left MCA (M1) | [9] |
| Case 4 | 60 | M | Lung cancer (N/A) | N/A | Right MCA (M1) | [9] |
| Case 5 | 75 | F | Ovarian cancer (Adenocarcinoma) | Ⅳ | Left MCA (M1) | [10] |
| Case 6 | 67 | M | Lung cancer (N/A) | Ⅳ | Basilar artery | [11] |
| Case 7 | 84 | F | Pancreatic body cancer (N/A) | Ⅳ | Left MCA | [11] |
MCA, middle cerebral artery.
Herein, we reported the treatment of Trousseau syndrome using these modalities in a patient with stage Ⅳ advanced lung cancer. Endovascular therapy for Trousseau syndrome may improve the symptoms and quality of life in terminal patients with advanced cancer, provided that informed consent from the patient and family is obtained.
For cerebral infarction, endovascular therapy has evolved and become the standard therapy for patients with acute occlusion of the internal carotid artery or proximal M1 segment in the MCA [12]. However, endovascular therapy is one among the limited therapeutic options available to treat patients with cerebral infarction. Currently, intravenous t-PA therapy prior to endovascular therapy is also considered standard therapy within the accepted time window (4.5 hours from the last known normal), as seen in this case [13]. As endovascular therapy for Trousseau syndrome cases is not established, further research is needed to identify effective therapy for cerebral infarction caused by Trousseau syndrome.
4. Conclusion
-
•
Treatment of Trousseau syndrome mainly consists of heparin administration and treatment of primary cancer.
-
•
The effectiveness of endovascular therapy should be studied in the future.
-
•
Endovascular therapy is a viable treatment option for Trousseau syndrome.
Disclosure statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Authors contribution statement
Y.K. wrote the manuscript. All authors contributed to editing the manuscript and approved the final version of the manuscript.
Funding statement
No funding was received for this study.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
- 1.Ikushima S., Ono R., Fukuda K., Sakayori M., Awano N., Kondo K. Trousseau's syndrome: cancer-associated thrombosis, Japanese. J. Clin. Oncol. 2016;46:204–208. doi: 10.1093/jjco/hyv165. [DOI] [PubMed] [Google Scholar]
- 2.Cestari D.M., Weine D.M., Panageas K.S., Segal A.Z., DeAngelis L.M. Stroke in patients with cancer: incidence and etiology. Neurology. 2004;62:2025–2030. doi: 10.1212/01.wnl.0000129912.56486.2b. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida K., Kimura T., Aburakawa Y., Suzuki Y., Kuroda K., Yahara O. Recurrent ischemic stroke in a patient with the Trousseau syndrome treated with dabigatran. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014;23:1724–1726. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2007;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nonagase Y., Takeda M., Tanaka K., Hayashi H., Iwasa T., Nakagawa K. Treatment of EGFR mutation-positive non-small cell lung cancer complicated by Trousseau syndrome with gefitinib followed by osimertinib: a case report. Oncotarget. 2018;9:29532–29535. doi: 10.18632/oncotarget.25687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am. J. Respir. Cell Mol. Biol. 2000;22:401–404. doi: 10.1165/ajrcmb.22.4.f184. [DOI] [PubMed] [Google Scholar]
- 7.Sakuta K., Mukai T., Fujii A., Makita K., Yaguchi H. Endovascular therapy for concurrent cardio-cerebral infarction in a patient with Trousseau syndrome. Front. Neurol. 2019;10:965. doi: 10.3389/fneur.2019.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicilioni K., Cristiano B., Jacobson J.P., Hoss D., Lund M., Cheung S., Dye J. Multiple thrombectomies in the same patient within one month: case report of a patient with Trousseau syndrome and acute ischemic stroke. Brain Sci. 2020;10 doi: 10.3390/brainsci10090590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balci S., Arsava E.M., Topcuoglu M.A., Arat A. Floating aortic thrombus: a rare cause of acute ischemic stroke necessitating modification of access route for thrombectomy. J. Stroke Cerebrovasc. Dis. : Off. J. Natl. Stroke Assoc. 2019;28:104291. doi: 10.1016/j.jstrokecerebrovasdis.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Kuroda N., Hiramatsu H., Mori M., Tanaka T. Mechanical thrombectomy for Trousseau syndrome in a terminally ill cancer patient. J. Pain Symptom Manag. 2019;57:688–694. doi: 10.1016/j.jpainsymman.2018.12.327. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto N., Fukuda H., Handa A., Kawasaki T., Kurosaki Y., Chin M., Yamagata S. Histological examination of Trousseau syndrome-related thrombus retrieved through acute endovascular thrombectomy: report of 2 cases. J. Stroke Cerebrovasc. Dis. : off. J. Natl. Stroke Assoc. 2016;25:e227–e230. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Cho Y.H., Choi J.H. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a literature review. J. Cerebrovasc. Endovasc. Neurosurg. 2021;23:193–200. doi: 10.7461/jcen.2021.E2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang A., Beheshtian E., Llinas E.J., Idowu O.R., Marsh E.B. Intravenous tissue plasminogen activator in combination with mechanical thrombectomy: clot migration, intracranial bleeding, and the impact of "Drip and Ship" on effectiveness and outcomes. Front. Neurol. 2020;11:585929. doi: 10.3389/fneur.2020.585929. [DOI] [PMC free article] [PubMed] [Google Scholar]