Abstract
Background and aim
Periodontitis involves a dynamic disease process, demanding the identification of biomarkers to diagnose the current state of disease activity. Therefore this study assessed the potential of “sTREM-1, IL-1β, and MMP-8” as a short panel of biomarkers of host biological process indicating the inflammatory burden in periodontium and thereby serving as a panel of diagnostic markers in periodontal disease.
Methods
Sixty eight patients were recruited and allotted into four groups comprising of subjects with clinically healthy gingiva and Stage III/IV Periodontitis with and without type 2 diabetes with HbA1c levels in the range of 6.5–7.9%. Periodontal parameters were measured and full mouth radiographic assessment was done. Whole saliva (unstimulated) samples were collected from all patients and estimation of the levels of markers was done employing ELISA.
Results
All the three biomarkers were noted to be the lowest in group I (sTREM-1: 75.63 ± 13.77; IL-1β: 15.67 ± 3.39; MMP-8: 85.83 ± 22.32) and highest in group IV (sTREM-1: 138.83 ± 14.89; IL-1β: 39.19 ± 7.20; MMP-8: 201.15 ± 50.32) with statistically significant difference. The difference observed between groups II and III for all the biomarkers assessed were statistically insignificant. The clinical parameters and HbA1c levels had positive correlation with the levels of biomarkers which was statistically significant.
Conclusion
This study unveils the potential of the short panel of biomarkers (“sTREM-1, IL-1β, and MMP-8”) to be used as diagnostic and possible prognostic markers for Periodontitis. It further corroborates the role of type 2 diabetes mellitus in amplifying the diverse processes that result in periodontal destruction.
Keywords: Periodontal disease, sTREM-1, Biomarkers, Diabetes, IL-1ß, MMP-8, Periodontitis-systemic interactions
1. Introduction
Periodontal disease is a chronic inflammatory disease, instigated by a dysbiotic microflora in the gingival sulcus which activates the innate immune response through an array of host cell-based receptors, that initiates the intra and inter-cellular signalling, thereby activating the host immuno-inflammatory response.1 TREM-1 is one of the innate immune surface receptors expressed on various immune cells such as, neutrophils, monocytes, macrophages, natural killer cells, immature dendritic cells, and non-immune cells like endothelial and epithelial cells of myeloid origin. Activation of the TREM-1/DAP12 signalling pathway promotes the synthesis of inflammatory cytokines like IL-1β, TNF-α, IL-6, IL-8.2 However, when it is activated in union with other PRRs, it brings about a significant intensification of the immune response.3
The “soluble triggering receptor expressed on myeloid cells (sTREM-1)” is a kind of secreted TREM-1 produced by the substitutive TREM-1 mRNA splice variant translation, which lacks the transmembrane domain or shedding of the membrane-bound TREM-1 from proteolytic cleavage by matrix metalloproteinases.4, 5, 6 The sTREM-1 so generated from the activated immune and non-immune cells is shed into various body fluids like blood, pleural fluid, broncho-alveolar lavage fluid, synovial fluid, urine and oral fluids such as saliva and GCF which warrants its use as a surrogate marker of TREM-1 activation in several systemic diseases etc. Being shed in oral fluids such as saliva and GCF, it has the potential to serve as a biomarker for the severity of local inflammation, and further serve as a metric to evaluate the systemic conditioning of the tissues towards inflammation.10
In the process of this immuno-inflammatory cascade, several inflammatory cytokines such as, IL-1β, an eloquent pro-inflammatory and bone destructive mediator are released which also initiate the expression of matrix metalloproteinases including MMP-8 from neutrophils, activated macrophages, fibroblasts and endothelial cells resulting in connective tissue breakdown of the periodontium expressed as attachment loss.11, 12, 13
Chronic inflammation is the underlying feature of pathogenesis in multiple systemic disorders, including Type 2 diabetes mellitus (T2DM) which was revealed in the early 1990's by Hotamisiligil et al.14 Various factors are known to influence the inter-relationship between T2DM and periodontal disease.15 Furthermore, diabetes is a well-established risk factor for periodontitis, while periodontitis is known to negatively influence glycemic control.16,17 As diabetes mellitus embodies dysregulation of inflammatory and immune mechanisms as in periodontal disease, the concentration of various validated markers to evaluate multiple host processes including immune dysregulation, bone loss, and connective tissue deterioration involved in periodontal destruction thus, assessing periodontal disease activity together with any associated risk burden.
Therefore, the current study assessed the salivary biomarker profiles of selected promising biomarkers – “sTREM-1, IL-1β, and MMP-8” that are inter-linked to each other, seeking to quantify and correlate the levels of these biomarkers in health and in Stage III/IV Periodontitis patients and to evaluate the role of Diabetes Mellitus, in the upregulation of these markers.
2. Materials and methods
The current study was devised as an analytical case-control study and performed in the Department of Periodontics, SRM Dental College, Ramapuram, Chennai from September 2019 to August 2020. It was approved by the ethical and scientific committees of the institutional review board (SRMDC/IRB/2018/MDS/No.507) and registered in the clinical trials registry (CTRI/2020/05/025032). Informed consent in verbal and written format was obtained from all participants.
A total of 68 patients were enrolled in this study and allotted into 4 groups with 17 subjects in each group. Groups I and II comprised of systemically healthy subjects with clinically healthy gingiva and Stage III/IV Periodontitis respectively. Groups III and IV involved T2DM patients with clinically healthy gingiva and Stage III/IV Periodontitis respectively.
The individuals in the age range of 25–65 years; Subjects with clinically healthy gingiva exhibiting normal sulcus depth and bone heights, no/minimal bleeding on probing; Subjects with Stage III/IV periodontitis exhibiting probing depth ≥ 6 mm, interdental clinical attachment loss at site of greatest loss ≥ 5 mm, radiographic bone loss extending to middle or apical third of root, tooth loss due to periodontitis, and more than 30% of teeth being involved18; Subjects with T2DM having HbA1c levels between 6.5 and 7.9%19 were recruited in the study. Pregnant and lactating women; Patients having other systemic inflammatory conditions; Patients who underwent periodontal management in the past 6 months or history of antibiotics usage in the last 3 months were excluded from the study.
3. Periodontal and laboratory examination
A full mouth periodontal examination was performed and the clinical parameters such as, plaque index (Silness and Loe 1964) (PI), probing pocket depth (PPD), clinical attachment level (CAL), and modified sulcular bleeding index (Mombelli et al., 1987) (mSBI) were evaluated prior to sample collection by a single examiner. Bone loss assessed radiographically using orthopantomogram aided in confirming the diagnosis of Periodontitis.
All subjects were tested for post-prandial glucose levels prior to sample collection. For values nearing the diabetic range and for individuals with diabetic history, HbA1c test measuring the blood levels of glycated haemoglobin was done and the results were interpreted according to the American Diabetes Association guidelines. Patients with HbA1c values in the range of 6.5–7.9% were included in this study.19
4. Saliva sample collection
After periodontal diagnosis, about 5 ml of unstimulated whole saliva was collected from every study subjects in a sterile uricup container by spitting method. The participants were required to let the saliva pool in the floor of the mouth and spit every 60 s. The collected samples were then centrifuged for 10 min at 2700 rpm. Following centrifugation, the supernatants were collected and aliquoted into three 0.5 ml Eppendorf tubes for each sample and labelled appropriately. The supernatants were then stockpiled at −80 °C in the deep freezer until ELISA procedures were performed.20
5. Estimation of “sTREM-1, IL-1β, and MMP-8” levels
The salivary levels of “sTREM-1, IL-1β, and MMP-8” were quantified using commercially available ELISA kits procured from Abbkine Scientific Co., Ltd., and Ray Biotech Inc, USA respectively. The assays were executed just as the manufacturer's recommendations.
6. Statistical analysis
One-way ANOVA was employed for comparing mean values among the groups and Tukey's HSD analysis was used for multiple pairwise comparisons. Karl Pearson correlation was employed to know the linear relationship between the variables. To analyse the data, SPSS (IBM SPSS Statistics for Windows, Version 26.0, Released 2019) was used. The level of significance was considered at 5% and power of the study was 80%.
7. Results
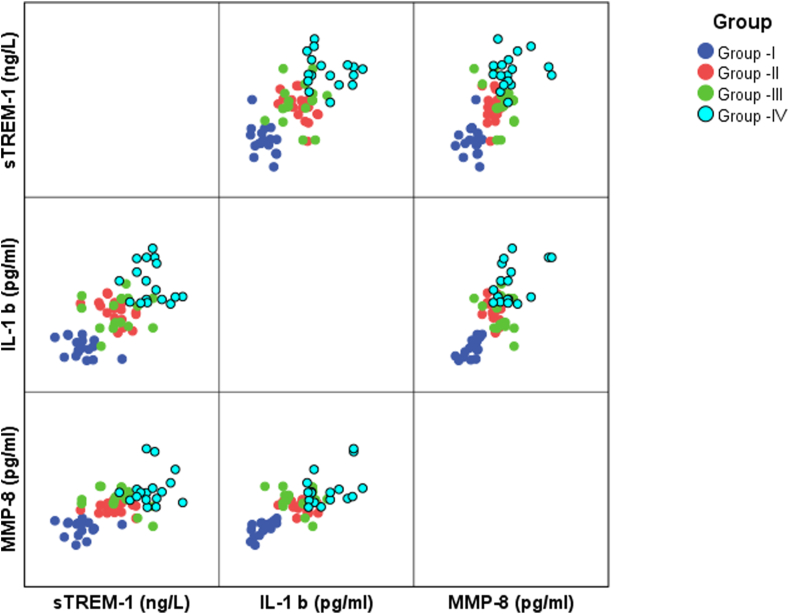
The mean age of the participants among the study population was 47.69 ± 8.07. The four groups had no significant differences based on age and gender. All the saliva samples showed detectable range of “sTREM-1, IL-1β, and MMP-8” and the values are expressed in mean and standard deviation. The measurements of clinical parameters, HbA1c, and the mean values of the biomarkers concentration assessed in the study using ELISA are given in Table 1. All the three biomarkers were noted to be lowest in the group I (sTREM-1: 75.63 ± 13.77 ng/l; IL-1β: 15.67 ± 3.39 pg/ml; MMP-8: 85.83 ± 22.32 pg/ml) and highest in the group IV (sTREM-1: 138.83 ± 14.89 ng/l; IL-1β: 39.19 ± 7.20 pg/ml; MMP-8: 201.15 ± 50.32 pg/ml). No significant difference was noted for any of the biomarkers assessed (“sTREM-1, IL-1β, and MMP-8”) between groups II and III. All other pairwise comparisons in biomarkers expression were statistically significant (p < 0.001) (Table 2). The clinical parameters PI, mSBI, PPD, CAL, HbA1c were analysed in Table 3 using Pearson correlation which shows statistically significant positive correlation with “sTREM-1, IL-1β, and MMP-8” (p < 0.001) and the no. of teeth present shows negative correlation with “sTREM-1, IL-1β, and MMP-8”. A scatter plot analysis (Fig. 1) was done to correlate the biomarkers which reveals statistically significant positive correlation within the three biomarkers.
Table 1.
Measurement of clinical parameters and expression of biomarkers in all four study groups.
| Variable | Group I (n = 17) | Group II (n = 17) | Group III (n = 17) | Group IV (n = 17) |
|---|---|---|---|---|
| Plaque Index (PI) | 0.33 ± 0.09 | 1.85 ± 0.31 | 0.31 ± 0.07 | 2.33 ± 0.18 |
| Bleeding Index (mSBI) | 0.00 ± 0.00 | 1.67 ± 0.39 | 0.00 ± 0.00 | 2.20 ± 0.25 |
| PPD (mm) | 1.83 ± 0.23 | 5.00 ± 0.59 | 2.10 ± 0.25 | 6.03 ± 0.44 |
| CAL (mm) | 0.00 ± 0.00 | 5.88 ± 0.71 | 0.00 ± 0.00 | 6.68 ± 0.72 |
| Number of teeth present | 30.76 ± 1.09 | 28.94 ± 2.58 | 29.53 ± 1.73 | 25.12 ± 5.17 |
| HbA1c (%) | NA | NA | 6.82 ± 0.23 | 7.29 ± 0.45 |
| sTREM-1 (ng/L) | 75.63 ± 13.77 | 106.14 ± 13.02 | 109.16 ± 18.41 | 138.83 ± 14.89 |
| IL-1β (pg/ml) | 15.67 ± 3.39 | 28.84 ± 4.28 | 28.26 ± 6.07 | 39.19 ± 7.20 |
| MMP-8 (pg/ml) | 85.83 ± 22.32 | 147.16 ± 15.25 | 170.95 ± 32.12 | 201.15 ± 50.32 |
Table 2.
Tukey HSD Post Hoc pairwise analysis between groups for clinical parameters and expression of biomarkers.
| Variable | Group | Mean Difference | p-value | |
|---|---|---|---|---|
| sTREM-1 (ng/L) | Group –I | Group –II | −30.509294 | <0.001** |
| Group –III | −33.530765 | <0.001** | ||
| Group –IV | −63.206294 | <0.001** | ||
| Group –II | Group –III | −3.021471 | 0.938 | |
| Group –IV | −32.697000 | <0.001** | ||
| Group –III | Group –IV | −29.675529 | <0.001** | |
| IL-1β (pg/ml) | Group –I | Group –II | −13.171294 | <0.001** |
| Group –III | −12.584353 | <0.001** | ||
| Group –IV | −23.516118 | <0.001** | ||
| Group –II | Group –III | 0.586941 | 0.989 | |
| Group –IV | −10.344824 | <0.001** | ||
| Group –III | Group –IV | −10.931765 | <0.001** | |
| MMP-8 (pg/ml) | Group –I | Group –II | −61.330529 | <0.001** |
| Group –III | −85.117294 | <0.001** | ||
| Group –IV | −115.319000 | <0.001** | ||
| Group –II | Group –III | −23.786765 | 0.159 | |
| Group –IV | −53.988471 | <0.001** | ||
| Group –III | Group –IV | −30.201706 | 0.044* | |
*p < 0.05 – statistically significant.
**p < 0.001 – highly significant.
Table 3.
Overall correlation of clinical parameters with biomarkers using Pearson correlation.
| Clinical Parameters | sTREM-1 (ng/L) | IL-1β (pg/ml) | MMP-8 (pg/ml) | |
|---|---|---|---|---|
| HbA1c (%) | r- value | 0.412 | 0.514 | 0.409 |
| P-value | 0.015* | 0.002* | 0.016* | |
| N | 34 | 34 | 34 | |
| Plaque Index (PI) | r- value | 0.634 | 0.655 | 0.459 |
| P-value | <0.001** | <0.001** | <0.001** | |
| N | 68 | 68 | 68 | |
| Bleeding Index (mSBI) | r- value | 0.597 | 0.670 | 0.482 |
| P-value | <0.001** | <0.001** | <0.001** | |
| N | 68 | 68 | 68 | |
| PPD (mm) | r- value | 0.661 | 0.688 | 0.516 |
| P-value | <0.001** | <0.001** | <0.001** | |
| N | 68 | 68 | 68 | |
| CAL (mm) | r- value | 0.597 | 0.636 | 0.476 |
| P-value | <0.001** | <0.001** | <0.001** | |
| N | 68 | 68 | 68 | |
| Number of teeth present | r- value | −0.381 | −0.432 | −0.480 |
| P-value | <0.001** | <0.001** | <0.001** | |
| N | 68 | 68 | 68 | |
*p < 0.05 – statistically significant.
**p < 0.001 – highly significant.
Fig. 1.
Scatter plot representing correlation between biomarkers in all groups.
8. Discussion
Periodontitis being a common inflammatory condition of the supporting structures of the teeth, is epitomized by dysregulated immune response and exacerbated production of pro-inflammatory molecules that result in the breakdown of the extracellular matrix and alveolar bone destruction. While the associations between periodontal inflammatory disease and various systemic disorders have been validated through longitudinal studies, the bond with diabetes mellitus is deep-rooted in medical evidence. Multiple controlled trials and systematic reviews have established diabetes mellitus to be the strongest risk factor for inflammatory periodontal disease. The presence of untreated periodontal disease in turn, is known to negatively influence the hyperglycaemic state. It would therefore be sensible to identify corresponding biomarkers of the various channels of periodontal disease process that estimate the extent of inflammatory activity to aid in the detection of periodontitis along with the associated systemic inflammatory burden, and to evolve appropriate treatment strategies based on the insight of the current state of disease activity. Saliva is an oral fluid exhibiting various molecules of local and systemic inflammation and immune dysregulation, thereby becoming a ready reckoner of choice to assess the inflammatory burden.21 The present study therefore, sought to assess the potential of three salivary markers of the biologic processes that occur in periodontitis, to evaluate their efficacy in monitoring the disease progression associated with periodontitis patients, with and without T2DM.
In our study, all the three evaluated biomarkers (“sTREM-1, IL-1β and MMP-8”) were found to be elevated in the periodontitis groups compared to the healthy groups. In the Bostanci et al study,22 they had observed a 3.3–5.6 fold raised salivary sTREM-1 levels in advanced periodontitis cases and similarly, Belibasakis et al in 201423 and Surabhigigras et al in 201724 reported increased sTREM-1 levels in disease sites and all forms of periodontitis. The results of our study also supports these findings. Thus, the contribution of sTREM-1 in periodontal disease progression is justified and their salivary levels denote the host's ability in modulating and settling the microbial challenge and the concomitant raise in the levels of multiple pro-inflammatory molecules.25 At the same time, “IL-1β and MMP-8” are well-established markers of periodontal disease and their elevated levels in inflammatory diseases including periodontitis and diabetes is supported by enormous studies in the literature.26, 27, 28 On comparison with the clinical parameters, “sTREM-1, IL-1β and MMP-8” levels had significant (p < 0.001) positive correlation with PI, mSBI, PPD, and CAL measurements in our study. Chen et al in 201629 reported an indirect association between higher GCF sTREM-1 concentrations and bleeding index. Further, increased expression of sTREM-1 was found to be correlated with unresolved deep pockets after periodontal therapy in the Dubar et al study in 2020.25 A strong association of periodontal parameters with “IL-1β and MMP-8” levels were suggested by Gaphor et al in 201427 and Zhang et in 201826 respectively. The results of our study reinforces these findings which corroborates sTREM-1 activity in propagating inflammatory response to this multifactorial disease and it could be pinned to the aspect of increased influx of over-expressive inflammatory cells delivering a broad range of inflammation-promoting mediators and the disparity in the regulation of MMPs and their inhibitors which might have contributed to the disease severity as well as increased incidence of periodontitis in diabetics in our study.
The results of the current study revealed the presence of highest inflammatory burden in group IV (T2DM with Stage III/IV Periodontitis). It affirms the synergistic effect of both the hyper-inflammatory conditions. Additionally, the HbA1c values had a significant favourable correlation (P < 0.05) with all the inflammatory markers confirming the raising inflammatory burden accompanied by weak glycaemic control thereby posing a risk of increased periodontal disease severity. It is also important to understand that the diabetic state modifies the individual to become more profoundly pro-inflammatory, and this is independent of a microbiome driven periodontal disease, further highlighting the risk burden that is carried by patients with diabetes mellitus. The enhanced sTREM-1 expression among the diabetic sub-population in this study matches with the in-vitro and in-vivo findings of higher sTREM-1 levels associated with insulin resistance, obesity, and diabetic nephropathy.30,31
Further, no statistically significant difference in the expression of “sTREM-1, IL-1β, and MMP-8” between the groups II and III was observed. Therefore, it must be noted with caution that, although a diabetic patient presents with a clinically disease-free periodontium, a shift towards advanced periodontitis can occur in no time due to the rapidity of disease progression as a result of the increased inflammatory burden. Hence, patients with T2DM need to be maintained under regular supportive periodontal care. This finding can also be viewed from the other perspective that, Stage III/IV Periodontitis gives rise to an inflammatory burden that is comparable to that of a common systemic disease, Diabetes mellitus thus advocating the literature finding of Periodontitis being the sixth complication of T2DM.
In assessing the mean concentrations of the evaluated markers “sTREM-1, IL-1β, and MMP-8”, this study highlights the potential for sTREM-1 to be a specific marker for periodontitis. The sensitivity of sTREM-1 in periodontitis is comparable to the effects of sTREM-1 on various systemic diseases as indicated in multiple studies.7, 8, 9, 10
Our study also illustrates the positive correlation between the evaluated biomarkers. “sTREM-1, IL-1β, and MMP-8” are positively correlated with each other achieving statistical significance (p < 0.001) and this substantiates the strong inflammatory profile of this short panel of markers besides validating the underlying inter-connection between these 3 molecules. Although the nature of the study doesn't propose a direct correlation, it enables us to understand the concomitant association between this short panel of markers which is in compliance with the findings of other studies showing enhanced IL-1β synthesis following TREM-1 activation,32 activation of MMP-8 by inflammatory molecules including IL-1β, and the involvement of MMP-8 in proteolytic cleavage to produce sTREM-1.33 Similar favourable correlation between salivary “sTREM-1/PGLYRP-1 and IL-1β, MMP-8” was observed in a study by Karita M Nylund et al in 2018.34 The positive correlational statistics between sTREM-1 with IL-1β and sTREM-1 with MMP-8 were also reported by Bostanci 201132 and Texeira 2019.35
To our best knowledge, this is the first study to propose “sTREM-1, IL-1β and MMP-8” as a short panel of markers in evaluating the inflammatory burden of Periodontitis and also to compare sTREM-1 levels between periodontitis and a T2DM cohort. Almost all studies in the literature investigating periodontitis and T2DM, have missed out the evaluation of a heathy diabetic control group, and recruited patients with a wide range of glycaemic control. The present study has addressed these shortfalls by including a group of diabetic patients with clinically healthy gingiva and all the subjects in both the T2DM study groups were under good control which would help to decipher the sole influence of systemic state of insulin resistance on periodontitis. This study being in cross-sectional nature did not assess the history of diabetes and diabetic medication which could be a possible limitation.
9. Conclusion
This study unveils the potential of the short panel of multi-process biomarkers consisting of “sTREM-1, IL-1β, and MMP-8” to be used as diagnostic markers that will indicate the inflammatory burden in periodontium. It also emphasises the colossal influence of type 2 diabetes mellitus on the upregulation of inflammatory reactions and thereby periodontal disease progression highlighting the importance of regular supportive periodontal care for diabetic patients. It further strengthens the role of the less investigated yet potential sTREM-1, in the propagation of periodontal inflammation. Future long-term studies are required to validate these findings and transform the use of this multi-process panel of markers into implementation in clinical forum.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Source(s) of support
Nil.
Presentation at a meeting
No.
Ethics approval
SRMDC/IRB/2018/MDS/No.507.
Patient consent statement
Verbal and written consent was obtained from all study participants.
Registration number
CTRI/2020/05/025032 [Clinical Trials Registry India].
Declaration of competing interest
None.
Acknowledgements
Nil.
References
- 1.Hasturk H., Kantarci A. Activation and resolution of periodontal inflammation and its systemic impact. Periodontol. 2000 2015;69(1):255–273. doi: 10.1111/prd.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tessarz A.S., Cerwenka A. The TREM-1/DAP12 pathway. Immunol Lett. 2008;116(2):111–116. doi: 10.1016/j.imlet.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Arts R.J., Joosten L.A.B., van der Meer JWM & Netea. MG TREM-1: intracellular signaling pathways and interaction with pattern recognition receptors. J Leukoc Biol. 2013;93:209–215. doi: 10.1189/jlb.0312145. [DOI] [PubMed] [Google Scholar]
- 4.Baruah S., Keck K., Vrenios M. Identification of a novel splice variant isoform of TREM-1 in human neutrophil granules. J Immunol. 2015;195(12):5725–5731. doi: 10.4049/jimmunol.1402713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gómez-Piña V., Soares-Schanoski A., Rodríguez-Rojas A. Metalloproteinases shed TREM-1 ectodomain from lipopolysaccharide-stimulated human monocytes. J Immunol. 2007;179(6):4065–4073. doi: 10.4049/jimmunol.179.6.4065. [DOI] [PubMed] [Google Scholar]
- 6.Klesney-Tait J., Turnbull I.R., Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7(12):1266–1273. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 7.Chang W., Peng F., Meng S.S., Xu J.Y., Yang Y. Diagnostic value of serum soluble triggering expressed receptor on myeloid cells 1 (sTREM-1) in suspected sepsis: a meta-analysis. BMC Immunol. 2020;21(1):2. doi: 10.1186/s12865-020-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorlier C., Gottenberg J.E., Laurans L., Simon T., Ait-Oufella H., Sellam J. Serum level of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) is a biomarker of synovitis in rheumatoid arthritis. Int J Rheum Dis. 2019;22(9):1616–1618. doi: 10.1111/1756-185X.13656. [DOI] [PubMed] [Google Scholar]
- 9.Ye W., Hu Y., Zhang R., Ying K. Diagnostic value of the soluble triggering receptor expressed on myeloid cells‐1 in lower respiratory tract infections: a meta‐analysis. Respirology. 2014;19(4):501–507. doi: 10.1111/resp.12270. [DOI] [PubMed] [Google Scholar]
- 10.Lemarié J., Gibot S. Soluble triggering receptor expressed on myeloid cells-1: diagnosis or prognosis? Crit Care Clin. 2020;36(1):41–54. doi: 10.1016/j.ccc.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura I., Jimi E. Regulation of osteoclast differentiation and function by interleukin‐1. Vitam Horm. 2006;74:357–370. doi: 10.1016/S0083-6729(06)74015-8. [DOI] [PubMed] [Google Scholar]
- 12.Murayama R., Kobayashi M., Takeshita A., Yasui T., Yamamoto M. MAPKs, activator protein‐1 and nuclear factor‐κB mediate production of interleukin‐1β‐stimulated cytokines, prostaglandin E2 and MMP‐1 in human periodontal ligament cells. J Periodontal Res. 2011;46(5):568–575. doi: 10.1111/j.1600-0765.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- 13.Birkedal‐Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–484. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 14.Hotamisligil G.S., Peraldi P., Budavari A., Ellis R., White M.F., Spiegelman B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-α-and obesity-induced insulin resistance. Science. 1996;271(5249):665–670. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 15.Hanes P.J., Krishna R. Characteristics of inflammation common to both diabetes and periodontitis: are predictive diagnosis and targeted preventive measures possible? EPMA J. 2010;1(1):101–116. doi: 10.1007/s13167-010-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang P.C., Lim L.P. Interrelationships of periodontitis and diabetes: a review of the current literature. J Dent Sci. 2012;7(3):272–282. doi: 10.1016/j.jds.2012.02.002. [DOI] [Google Scholar]
- 17.Preshaw P.M., Alba A.L., Herrera D. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55(1):21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonetti M.S., Greenwell H., Kornman K.S. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 2018;89:S159–S172. doi: 10.1002/JPER.18-0006. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association Standards of medical care in diabetes—2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14. doi: 10.2337/cd17-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaza‐Guzmán D.M., Medina‐Piedrahíta V.M., Gutiérrez‐Henao C., Tobón‐Arroyave S.I. Salivary levels of NLRP3 inflammasome‐related proteins as potential biomarkers of periodontal clinical status. J Periodontol. 2017;88(12):1329–1338. doi: 10.1902/jop.2017.170244. [DOI] [PubMed] [Google Scholar]
- 21.Kc S., Wang X.Z., Gallagher J.E. Diagnostic sensitivity and specificity of host‐derived salivary biomarkers in periodontal disease amongst adults: systematic review. J Clin Periodontol. 2020;47(3):289–308. doi: 10.1111/jcpe.13218. [DOI] [PubMed] [Google Scholar]
- 22.Bostanci N., Öztürk V.Ö., Emingil G., Belibasakis G.N. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in periodontitis. J Dent Res. 2013;92(2):161–165. doi: 10.1177/0022034512470691. [DOI] [PubMed] [Google Scholar]
- 23.Belibasakis G.N., Öztürk V.Ö., Emingil G., Bostanci N. Soluble triggering receptor expressed on myeloid cells 1 (sTREM‐1) in gingival crevicular fluid: association with clinical and microbiologic parameters. J Periodontol. 2014;85(1):204–210. doi: 10.1902/jop.2013.130144. [DOI] [PubMed] [Google Scholar]
- 24.Gigras S., Patil S.R., Veena H.R., Dani S. Gingival crevicular fluid level of soluble triggering receptor expressed on myeloid cells-1(strem-1) in periodontal health and disease: a case-control study. Int J Dent Res. 2017;5(2):83–88. doi: 10.14419/ijdr.v5i2.7689. [DOI] [Google Scholar]
- 25.Dubar M., Frippiat J.P., Remen T. Comparison of sTREM‐1 and associated periodontal and bacterial factors before/after periodontal therapy, and impact of psychosocial factors. J Clin Periodontol. 2020;47(9):1064–1078. doi: 10.1111/jcpe.13339. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Li X., Yan H., Huang L. Salivary matrix metalloproteinase (MMP)-8 as a biomarker for periodontitis: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2018;97(3) doi: 10.1097/MD.0000000000009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaphor S.M., Ali S.H., Abdullah M.J. Evaluation of salivary interleukin-1beta (IL-1β) level in relation to the periodontal status in smoker and non-smoker individuals. J Interdiscipl Med Dent Sci. 2014;2 doi: 10.4172/2376-032X.1000120. 2376-032X. [DOI] [Google Scholar]
- 28.Yi X., Zhang L., Lu W. The effect of NLRP inflammasome on the regulation of AGE s‐induced inflammatory response in human periodontal ligament cells. J Periodontal Res. 2019;54(6):681–689. doi: 10.1111/jre.12677. [DOI] [PubMed] [Google Scholar]
- 29.Chen S.S., Wang K., Zhao J., Wu W.C., Wu Y.F., Zhao L. Increased expression of triggering receptor expressed on myeloid cells 1 and 2 in inflamed human gingiva. J Periodontal Res. 2017;52(3):512–521. doi: 10.1111/jre.12417. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian S., Pallati P.K., Rai V., Sharma P., Agrawal D.K., Nandipati K.C. Increased expression of triggering receptor expressed on myeloid cells‐1 in the population with obesity and insulin resistance. Obesity. 2017;25(3):527–538. doi: 10.1002/oby.21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X., Zhao Y., Zhu X. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT‐1‐TREM‐1 pathway in diabetic nephropathy. J Cell Physiol. 2019;234(5):6917–6926. doi: 10.1002/jcp.27450. [DOI] [PubMed] [Google Scholar]
- 32.Bostanci N., Thurnheer T., Belibasakis G.N. Involvement of the TREM-1/DAP12 pathway in the innate immune responses to Porphyromonas gingivalis. Mol Immunol. 2011;49(1-2):387–394. doi: 10.1016/j.molimm.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Bleharski J.R., Kiessler V., Buonsanti C. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol. 2003;170(7):3812–3818. doi: 10.4049/jimmunol.170.7.3812. [DOI] [PubMed] [Google Scholar]
- 34.Nylund K.M., Ruokonen H., Sorsa T. Association of the salivary triggering receptor expressed on myeloid cells/its ligand peptidoglycan recognition protein 1 axis with oral inflammation in kidney disease. J Periodontol. 2018;89(1):117–129. doi: 10.1902/jop.2017.170218. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira M.K., Lira-Junior R., Lourenço E.J. The modulation of the TREM-1/PGLYRP1/MMP-8 axis in peri-implant diseases. Clin Oral Invest. 2020;24(5):1837–1844. doi: 10.1007/s00784-019-03047-z. [DOI] [PubMed] [Google Scholar]