Abstract
Numerous systemic manifestations, including cardiac involvement in the form of myocardial infarction, myocarditis, and electrocardiographic changes, have been associated with COVID-19..In this review, the authors describe the electrocardiographic features that have been reported to date in patients affected by this disease and their possible underlying mechanisms.
Keywords: COVID 19, ECG, Electrocardiography
Key points
-
•
Electrocardiographic (ECG) changes seen in association with COVID-19 include QRS axis changes, conduction abnormalities, and ST segment and T-wave changes.
-
•
QTc interval changes, commonly described at the beginning of the pandemic, are now less frequently seen due to increased awareness for the need of QTc monitoring and avoidance of QT prolonging drugs, resulting in a reduction of possibly fatal arrhythmic events.
-
•
Atrial and ventricular arrhythmias are more common in critically ill patients with COVID-19, with atrial fibrillation being the most commonly reported rhythm disturbance.
-
•
The mechanisms for the development of ECG changes and arrhythmias are multifactorial.
Introduction
Since its declaration as a pandemic more than a year ago, the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has affected more than 180 million people worldwide and sadly claimed more than 4 million lives, including more than 33 million cases and 600,000 deaths in the United States alone. SARS-CoV-2’s effects continue to unravel, as it affects nations worldwide, health care systems and economies adjust to battle it, and the daily livelihoods of billions of people and patients are touched. In addition, patients, a lot of whom suffer from the aftermaths and morbidity inflicted by the disease, are chronically influenced by SARS-CoV-2, and this has led to escalation of efforts by physicians and researchers worldwide for the development of treatments and vaccinations to limit its extension and decrease morbidity and mortality.1 Subsequently, the abundance of data regarding disease processes, disease associations, different manifestations, and pathophysiology of the COVID-19 has improved our understanding of the disease and how to approach it. Varying mortality rates have been described, ranging from 1 per 100,000 to 100 per 100,000,2 and several factors have been associated with higher morbidity and mortality, including previously known comorbidities, advanced age, obesity, or male gender.3 , 4
Different organ systems are involved as a result of SARS-CoV-2 infection including pulmonary involvement with hypoxemic respiratory (which is commonly responsible for deterioration and mortality), renal involvement,5 , 6 multisystem inflammatory syndromes,7 and a wide array of cardiac manifestations. Numerous studies have described the existence of cardiac involvement in patients infected with SARS-CoV-2, with increased mortality.8, 9, 10 In addition, cardiac involvement has been demonstrated on cardiac imaging such as magnetic resonance imaging (MRI) in patients who recovered from COVID-19,11 and by evidence of right ventricular (RV) dysfunction, left ventricular (LV) systolic dysfunction, and LV diastolic dysfunction on transthoracic echocardiography.12 In addition, cardiac manifestations have been described in electrocardiographic (ECG) features that include cardiac arrhythmias, ST segment changes, and QT prolongation.
Methods
In this review, the authors aim to summarize ECG characteristics in patients with COVID-19, the pathophysiology behind them, and their implications. They performed a database search of PubMed, Embase, and Cochrane Central Register of Clinical Trials for articles using the keywords “COVID” OR “coronavirus” OR “SARS-CoV-2” OR “SARS” AND “electrocardiogram” OR “EKG” OR “ECG” OR “arrhythmia.” The search yielded 1433 results. Prospective and retrospective studies, systematic reviews, meta-analyses, case reports and series, narrative reviews, letters, and clinical guidelines were reviewed. The authors reviewed relevant articles for data to comprehensively discuss and describe the ECG features encountered thus far in COVID-19.
Discussion
Axis, QRS, and Atrioventricular Conduction
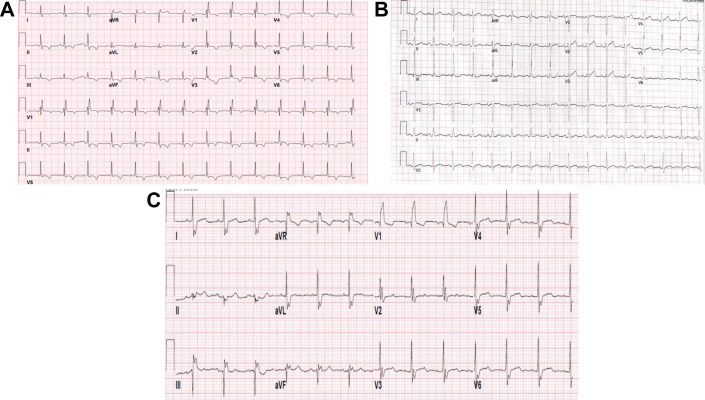
Changes to cardiac axis, in addition to QRS complex morphology and atrioventricular (AV) conduction, have been reported with SARS-CoV-2 (Fig. 1 ). In order to assess ECG changes in patients admitted with COVID-19, McCullough and colleagues analyzed ECGs of 756 patients in a New York City hospital.13 On review, 19.3% had an abnormal QRS axis: 13.8% in the form of left axis deviation and 5.5% in the form of right or right superior axis deviation. In other studies, ECG changes in the form of right axis deviation have been reported in patients with evidence of RV strain, such as in patients with extensive pulmonary disease and pneumonia, or when pulmonary embolism occurs as a complication of COVID-19 with significant clot burden.14 Conduction abnormalities were also noted in 11.8% of the cohort, with left bundle branch block in 1.5% of the patients, right bundle branch block in 7.8%, and nonspecific intraventricular block in 2.5%. In addition, 29% of patients had nonspecific repolarization abnormalities. AV block was observed in 2.6%, with the majority (2.5%) being first-degree AV block. Atrial premature contractions occurred in 7.7% and ventricular premature contractions in 3.4%. Atrial premature contractions (OR 2.31, 95% CI 1.27-4.21, P = .006), repolarization abnormalities (OR 2.31, 95% CI 1.27-4.21, P = .006), and right bundle branch block/intraventricular block (OR 2.61, 95% CI 1.32-5.18, P = .002) were predictors of increased mortality.13
Fig. 1.
Axis and QRS in patients with COVID-19. (A) Incomplete right bundle branch block. Diffuse T wave inversion involving septal, anterior, inferior and lateral leads is also observed; (B) right axis deviation; (C) left axis deviation. Right bundle branch block is also present.
ST Segment and T-Wave Changes
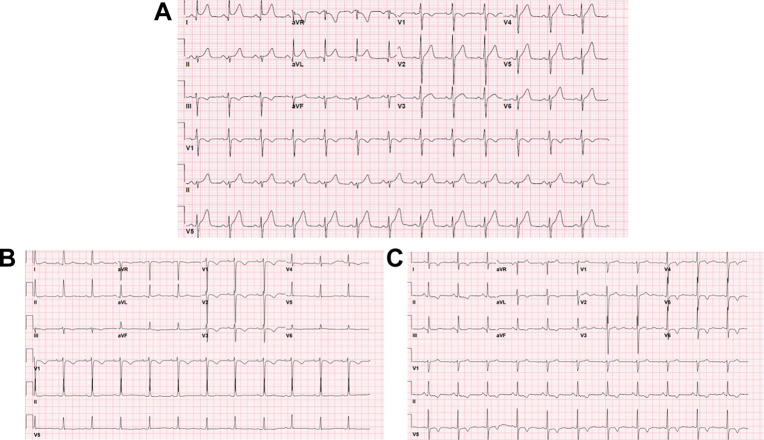
ST segment deviations, whether elevations or depressions, are amongst the ECG changes present in patients infected with SARS-CoV-2. (Fig. 2 A) A case series of patients admitted with COVID-19 reviewed 18 patients who either presented with ST segment elevations or developed ST elevations during hospitalizations. After stratification based on echocardiographic features and wall motion, peak troponin levels, and patient symptoms, 50% of these patients underwent coronary angiography, of which 67% were found to have obstructive coronary disease. Forty-four percent of the patients described in the cohort were diagnosed with myocardial infarction, whereas 56% were thought to have noncoronary-related myocardial injury.15 In addition to myocardial infarction, ST segment changes have been attributed to myocardial injury in the form of myocarditis or microthrombi.16, 17, 18 For instance, in patients with COVID-19 myocarditis, nearly 50% of patients had ST-segment elevation on ECG. Echocardiographic features observed in these patients include cardiomegaly or increased wall thickness, decreased ejection fraction, or global hypokinesis in nearly two-thirds of patients,19 as well as late gadolinium enhancement on MRI in all patients. In addition, surrogates of myocardial injury such as troponin I and T are frequently elevated.
Fig. 2.
ST and T-wave changes in COVID-19. (A) ST segment elevation; (B) T-wave inversions in anterior leads; (C) T-wave inversions in anterolateral leads.
Furthermore, in a recent case series, the authors described the finding of new T-wave inversion (TWI) in patients with SARS-CoV-2 and its association with increased mortality, need for intubation and mechanical ventilation, particularly in patients with concomitant elevation of cardiac troponins (Fig. 2B, C).20 In this study of 3225 patients admitted with COVID-19, 6% of patients had either new TWI or pseudonormalization, with 23% of these patients having concomitant troponin elevation. TWI were observed in the lateral leads (71%), anterior leads (64%), inferior leads (57%), and septal leads (26%). In addition, roughly one-quarter of the patients who had a transthoracic echocardiogram were found to have regional wall motion abnormalities, and mortality was 35%, 52%, and 80% if TWI were diffuse, accompanied by elevated troponin levels, or both, respectively. In addition, other studies have corroborated ST and T-wave changes and their relation to cardiac injury and mortality in COVID-19.13 , 21 An analysis by Barman and colleagues of ECG findings in 219 patients admitted with COVID-19 based on clinical severity (95 severe and 124 nonsevere infection) revealed more frequent ST depressions (28% vs 14%), ST-T changes (36% vs 21%), and TWI (29% vs 16%) in patients with severe infection compared with nonsevere infection.22
Although the exact mechanisms of myocardial injury contributing to ST-segment and T-wave morphology changes are unknown, it is hypothesized that this occurs as a result of myocardial microthrombi formation (involving primarily capillaries, arterioles and small muscular arteries),23 direct damage to the cardiomyocytes, systemic inflammatory response syndromes, cytokine-mediated responses by helper T cells, and interferon-mediated immune response. In addition, hypoxia as a result of COVID-19–associated respiratory failure and coronary plaque destabilization may be other contributors.16 These theories are supported by evidence of myocyte necrosis and interstitial edema with predominant lymphocytic infiltration in an animal model of coronavirus infection, as well as endomyocardial biopsy in a human subject revealing interstitial and endocardial inflammation, and viral particle presence in interstitial cells with loss of cytoplasmic membrane integrity.24 , 25 COVID-19 severity and the presence of cardiac injury defined as elevated high-sensitivity troponin I were found to be independent predictors of ECG changes in the form of ST segments and T-wave changes (odds ratio [OR] 1.87 and 3.32, respectively) in an analysis of patients with COVID-19.22 In another study, the investigators conferred that the number of abnormal T waves was an indicator of myocardial injury (OR 2.36, 95% confidence interval [CI], 1.38–4.04], P = .002) and that the presence of T-wave changes in itself was a predictor of mortality (hazard ratio, 3.57 [1.40, 9.11], P = .008).21 Moreover, ST segment/T-wave changes suggestive of right heart strain such as ST depressions or T-wave inversions in leads V1 to V3 or the inferior leads, or presence of the S1Q3T3 pattern, may correlate with disease outcomes and mortality. However, in a study of 15 patients with pulmonary embolism and COVID-19, only 1 patient (7%) had the pathognomonic pattern of S1Q3T3.26
QT Interval Changes
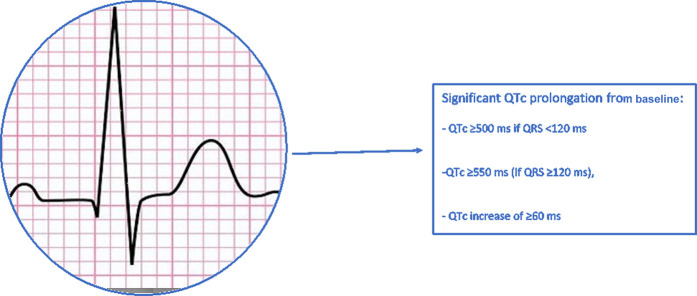
Previous studies with other coronaviruses have demonstrated QT prolongation caused by the infection itself.25 QT interval prolongation has been particularly described extensively in patients with COVID-19, due to the potential risk for arrhythmias or torsades de pointes (TdP).27 For instance, in an international multicenter registry that enrolled 110 patients, 14% of patients developed QTc prolongation after 7 days of hospitalization (mean QTc increase 66 ± 20 msec, +16%, P < .001), with a 3.6% incidence of life-threatening arrhythmias.28 Independent predictors of QTc prolongation included older age, higher basal heart rate, and treatment with dual antiviral therapy. In earlier stages of the pandemic, concerns regarding QTc-prolonging drugs that were believed to be beneficial for treatment of COVID-19, such as azithromycin, chloroquine, hydroxychloroquine (HCQ), and lopinavir/ritonavir, triggered calls for monitoring and caution while using these drugs.29 In one study that included 90 patients with SARS-CoV-2 infection,30 for example, patients receiving concomitant azithromycin and HCQ were noted to have a greater median (interquartile range) change in QT interval compared with hydroxychloroquine alone (23 [10–40] vs 5.5 [−15.5 to 34.25] msec; respectively; P = .03). In addition, seven patients (19%) who received hydroxychloroquine alone developed prolonged QTc of greater than 500 msec and 3 patients (3%) had a QTc increase of 60 msec or more. Of those who received concomitant azithromycin, 11 of 53 (21%) had prolonged QTc of 500 msec or more and 7 of 53 (13%) had a change in QTc of 60 msec or more. Moreover, HCQ was discontinued in one patient due to TdP.30 Another study of 98 patients assessed critical QTc prolongation, defined as maximum QTc greater than or equal to 500 ms (if QRS <120 ms) or QTc greater than or equal to 550 ms (if QRS ≥ 120 ms), and QTc increase of greater than or equal to 60 ms in patients receiving HCQ, azithromycin, or a combination of both. In this cohort, 12% of patients met the criteria for critical QTc prolongation, with a higher incidence in patients taking combination therapy.31 Additional studies described similar results, with higher mortality rates in patients with either QTc greater than 500 ms or an increase of 60 ms32 (Fig. 3 ). Consequently, this led to health care systems using policies for baseline and follow-up ECG monitoring, remote and telemonitoring of QTc, and the FDA granting emergency authorization for the use of the KardiaMobile 6L (AliveCor, Inc) device for this purpose. However, more recent studies suggest that the QTc prolongation seen with SARS-CoV-2 infection and some of the medications used in earlier treatment protocols does not necessarily translate into higher mortality or incidence of cardiac arrhythmias;32 this may be due to the QTc prolongation not meeting the threshold for arrhythmogenesis or better monitoring leading to cessation of drugs when certain thresholds are met (QTc ≥ 500 ms or increase in QTc interval ≥ 60 ms).33 In addition, with emerging evidence of lack of benefit of HCQ and azithromycin, administration of these medications, particularly in combination, has been greatly limited.
Fig. 3.
QTc changes that warrant frequent QTc monitoring in SARS-CoV-2 infection.
Arrhythmias in COVID-19
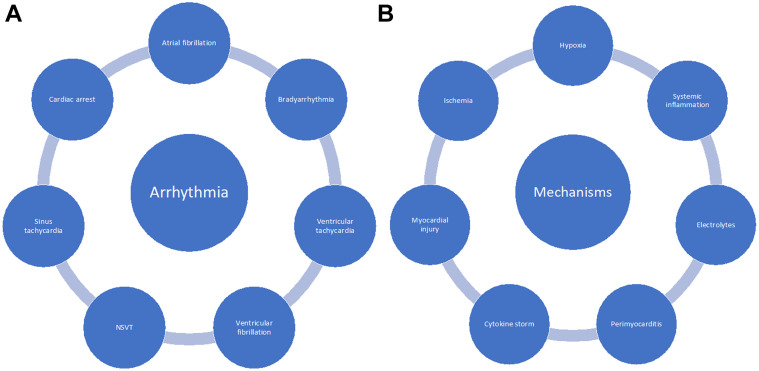
Although sinus rhythm and sinus tachycardia remain the most common rhythms encountered in patients with COVID-19, cardiac arrhythmias, as other cardiac manifestations of COVID-19, have been of interest, given their relevance and possible effects on morbidity and mortality inflicted by the disease (Fig. 4 A). Earlier data emerging from China33 at the onset of the pandemic reported incidences of up to 17% of patients admitted with SARS-COV-2 infection having arrhythmias, higher rates of up to 44% in patients requiring intensive care unit (ICU) care, and around 7% of admitted patients having ventricular tachycardia or ventricular fibrillation. Subsequent data from 700 patients admitted in a US hospital, 11% of which required ICU admission, reported 25 incident atrial fibrillation (AF) events, 10 nonsustained ventricular tachycardias (NSVTs), 9 clinically significant bradyarrhythmias, and 9 cardiac arrests—all the arrests occurred in ICU-admitted patients.34 Furthermore, ICU admission was associated with arrhythmias such as incident AF and NSVT (OR 4.68 [95% confidence interval [CI] 1.66–13.18] and 8.92 [95% CI 1.73–46.06]; respectively), and in-hospital mortality only increased with cardiac arrests. Similarly, a meta-analysis of studies that included 5815 patients with COVID-19 reported an arrhythmia incidence of 9.3%,35 and a Heart Rhythm Society survey of electrophysiologists worldwide reported that atrial fibrillation was the most commonly encountered arrhythmia (21%) by professionals treating patients with COVID-19.36
Fig. 4.
(A) Arrhythmias in COVID-19; (B) mechanism of arrhythmias. NSVT, nonsustained ventricular tachycardia.
Multiple factors likely contribute to arrhythmias in patients affected by COVID-19. Acute myocardial injury occurring in some patients, can in itself trigger cardiac arrhythmias. In a study of 1284 patients with severe COVID-19, 170 patients had evidence of myocardial injury as evidenced by elevated cardiac troponin I, of which 26% had arrhythmias.37 Conversely, in a meta-analysis that assessed the role of cardiac injury as a predictor of mortality and severe disease, incidence of new arrhythmias was found to be associated with higher risk of severe disease and the need for ICU admission (risk ratio 13, 95% CI 7.00–24.47, P < .001).38 Moreover, hypoxia and electrolyte abnormalities may contribute to bradyarrhythmias, atrial fibrillation, and ventricular arrhythmias; and systemic inflammation, viral perimyocarditis, and proarrhythmogenicity induced by certain medications may predispose to atrial and ventricular arrhythmias (Fig. 4B). In addition, bradyarrhythmias, interestingly, have been suggested to be a possible sign associated with developing cytokine storm.39
Summary
ECG features in patients with COVID-19 range from changes in morphologies of QRS complexes, ST segments, and T waves, to changes in cardiac axis, QTc interval, and cardiac arrhythmias, which may be atrial or ventricular in origin. Often, these findings are due to severe systemic illness inflicted by hypoxic injury, electrolyte abnormalities, endothelial or myocardial injury, microthrombi and plaque rupture, and cytokine storm. Knowledge of these ECG features, paired with patients’ clinical status, cardiac imaging findings, and cardiac biomarkers, can assist clinicians in accurately assessing and tailoring care through an understanding of the underlying disease processes.
Clinics care points
-
•
Clinicians should be cognizant of some of the reported ECG changes, such as abnormal QRS axis in nearly 20% of patients, conduction abnormalities in approximately 20%, AV block in about 2.6%, and premature beats in nearly 10% of patients.
-
•
ST segment and T-wave changes in patients with COVID-19 can be due to myocardial infarction or myocardial injury secondary to myocarditis, inflammatory responses, or microthrombi and should therefore be interpreted in the correct clinical context, as they can be associated with illness severity and mortality.
-
•
Baseline and follow-up ECG for QTc monitoring is suggested in hospitalized patients. Clinically significant QTc prolongation can be defined as intraventricular QTc of greater than or equal to 500 ms with a normal QRS interval, greater than or equal to 550 ms if QRS greater than or equal to 120 ms, or QTc increase of greater than or equal to 60 ms from baseline.
-
•
Although sinus rhythm is the most common, nearly 9.3% of patients admitted with COVID-19 are reported to have arrhythmias, with atrial fibrillation being the most incident. Arrhythmias can be a sign of myocardial injury and increased disease severity, and the treatment focus should be on the underlying infection and any potential triggers.
Acknowledgments
None
Disclosure
Dr L. Di Biase is a consultant for Stereotaxis, Biosense Webster, Boston Scientific, and Abbott Medical and has received speaker honoraria/travel support from Medtronic, Atricure, Bristol Meyers Squibb, Pfizer, and Biotronik. Dr A. Natale is a consultant for Biosense Webster, Stereotaxis, and Abbott and has received speaker honoraria/travel support from Medtronic, Atricure, Biotronik, and Janssen. Remaining authors report no conflict of interest.
Footnotes
Funding: none.
References
- 1.Ferrari R., Maggioni A.P., Tavazzi L., et al. The battle against COVID-19: mortality in Italy. Eur Heart J. 2020;41(22):2050–2052. doi: 10.1093/eurheartj/ehaa326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilinski A., Emanuel E.J. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. 2020;324(20):2100–2102. doi: 10.1001/jama.2020.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guzik T.J., Mohiddin S.A., Dimarco A., et al. COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666–1687. doi: 10.1093/cvr/cvaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitencourt L., Pedrosa A.L., Soares de Brito S.B.C., et al. COVID-19 and renal diseases: an update. Curr Drug Targets. 2021;22(1):52–67. doi: 10.2174/1389450121999201013151300. [DOI] [PubMed] [Google Scholar]
- 6.Santoriello D., Khairallah P., Bomback A.S., et al. Postmortem Kidney Pathology Findings in Patients with COVID-19. J Am Soc Nephrol. 2020 Sep;31(9):2158–2167. doi: 10.1681/ASN.2020050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris S.B., Schwartz N.G., Patel P., et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection - United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonow R.O., O'Gara P.T., Yancy C.W. Cardiology and COVID-19. JAMA. 2020;324(12):1131–1132. doi: 10.1001/jama.2020.15088. [DOI] [PubMed] [Google Scholar]
- 9.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoso A., Pranata R., Wibowo A., et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2020;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szekely Y., Lichter Y., Taieb P., et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough S.A., Goyal P., Krishnan U., et al. Electrocardiographic findings in Coronavirus Disease-19: insights on mortality and underlying myocardial processes. J Card Fail. 2020;26(7):626–632. doi: 10.1016/j.cardfail.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias P., Poterucha T.J., Jain S.S., et al. The prognostic value of electrocardiogram at presentation to Emergency Department in patients with COVID-19. Mayo Clin Proc. 2020;95(10):2099–2109. doi: 10.1016/j.mayocp.2020.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangalore S., Sharma A., Slotwiner A., et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382(25):2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babapoor-Farrokhran S., Gill D., Walker J., et al. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253:117723. doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castiello T., Georgiopoulos G., Finocchiaro G., et al. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Fail Rev. 2021 Mar 24:1–11. doi: 10.1007/s10741-021-10087-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guagliumi G., Sonzogni A., Pescetelli I., et al. Microthrombi and ST-segment-elevation myocardial infarction in COVID-19. Circulation. 2020;142(8):804–809. doi: 10.1161/CIRCULATIONAHA.120.049294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kariyanna P.T., Sutarjono B., Grewal E., et al. A systematic review of COVID-19 and myocarditis. Am J Med Case Rep. 2020;8(9):299–305. [Google Scholar]
- 20.Romero J., Alviz I., Parides M., et al. T-wave inversion as a manifestation of COVID-19 infection: a case series. J Interv Card Electrophysiol. 2020;59(3):485–493. doi: 10.1007/s10840-020-00896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L., Feng Y., Tang J., et al. Surface electrocardiographic characteristics in coronavirus disease 2019: repolarization abnormalities associated with cardiac involvement. ESC Heart Fail. 2020;7(6):4408–4415. doi: 10.1002/ehf2.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barman H.A., Atici A., Alici G., et al. The effect of the severity COVID-19 infection on electrocardiography. Am J Emerg Med. 2020;46:317–322. doi: 10.1016/j.ajem.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation. 2021 Mar 9;143(10):1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 24.Tavazzi J., Pellegrini C., Maurelli M., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020 May;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander L.K., Keene B.W., Yount B.L., et al. ECG changes after rabbit coronavirus infection. J Electrocardiol. 1999;32(1):21–32. doi: 10.1016/S0022-0736(99)90018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kho J., Ioannou A., Van den Abbeele K., et al. Pulmonary embolism in COVID-19: clinical characteristics and cardiac implications. Am J Emerg Med. 2020;38(10):2142–2146. doi: 10.1016/j.ajem.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankelson L., Karam G., Becker M.L., et al. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020;17(9):1472–1479. doi: 10.1016/j.hrthm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoro F., Monitillo F., Raimondo P., et al. QTc interval prolongation and life-threatening arrhythmias during hospitalization in patients with COVID-19. Results from a multi-center prospective registry [published online ahead of print, 2020 Oct 24]. Clin Infect Dis. 2020;ciaa1578 doi: 10.1093/cid/ciaa1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giudicessi J.R., Noseworthy P.A., Friedman P.A., et al. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for Coronavirus Disease 19 (COVID-19) Mayo Clin Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercuro N.J., Yen C.F., Shim D.J., et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramireddy A., Chugh H., Reinier K., et al. Experience with hydroxychloroquine and azithromycin in the coronavirus disease 2019 pandemic: implications for QT interval monitoring. J Am Heart Assoc. 2020;9(12):e017144. doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsia B.C., Greige N., Quiroz J.A., et al. QT prolongation in a diverse, urban population of COVID-19 patients treated with hydroxychloroquine, chloroquine, or azithromycin. J Interv Card Electrophysiol. 2020;59(2):337–345. doi: 10.1007/s10840-020-00822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang D., Saleh M., Gabriels J., et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J Am Coll Cardiol. 2020;75(23):2992–2993. doi: 10.1016/j.jacc.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunutsor S.K., Laukkanen J.A. Cardiovascular complications in COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):e139–e141. doi: 10.1016/j.jinf.2020.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopinathannair R., Merchant F.M., Lakkireddy D.R., et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59(2):329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Si D., Du B., Ni L., et al. Death, discharge and arrhythmias among patients with COVID-19 and cardiac injury. CMAJ. 2020;192(28):E791–E798. doi: 10.1503/cmaj.200879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X., Pan X., Li Y., et al. Cardiac injury associated with severe disease or ICU admission and death in hospitalized patients with COVID-19: a meta-analysis and systematic review. Crit Care. 2020 Jul 28;24(1):468. doi: 10.1186/s13054-020-03183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolis A.S., Manolis A.A., Manolis T.A., et al. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30(8):451–460. doi: 10.1016/j.tcm.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]