Abstract
While looking for a solution to treat COVID-19, the massive off-label use of several drugs in COVID-19 has generated concerns in the early phase of the pandemic because of possible arrhythmogenic effects in relation to QTc interval prolongation. Indeed, some of these drugs have been historically associated with QT prolongation and Torsade de Point, a potentially lethal ventricular arrhythmia, and their first-time use on a very large scale has raised several concerns in the scientific community. This work aims to summarize the underlying arrhythmogenic mechanisms related to the use of potentially QT-prolonging drugs used during the pandemic to treat COVID-19.
Keywords: QT interval, COVID-19, Ventricular arrhythmias, Torsade de point, Hydroxychloroquine, Antivirals
Key points
-
•
COVID-19 patients might experience an increased arrhythmic risk due to QT prolongation, as for their clinical status or for the massive off-label use of potentially QT-prolonging drugs.
-
•
In such patients, a complete baseline QT assessment at a 12-lead ECG should be performed, as well as with Tisdale score calculation and ECG monitoring during drug administration.
-
•
Among the most important clinical factors predisposing to QT prolongation and ventricular arrhythmias, genetic predisposition, older age, female gender, electrolyte disorders, pharmacologic interactions, and bradycardia represent the most relevant features.
-
•
Chloroquine and hydroxychloroquine are associated with QT prolongation especially when used in combination with macrolides, such as azithromycin, or fluoroquinolones.
-
•
A scarce body of evidence exists on antivirals and immunomodulators, with lopinavir/ritonavir appearing to be the most frequently associated with QT prolongation.
Introduction
Apart from a well-known respiratory involvement,1 , 2 several reports have described the presence of a significant myocardial injury in coronavirus disease (COVID-19), often sustained by macrothrombosis and microthrombosis, as well as a direct cardiac damage.3, 4, 5, 6, 7, 8, 9, 10 Indeed, as highlighted in different studies, acute coronary syndromes and cardiac arrhythmias have been reported as potential complications in hospitalized patients, often impairing COVID-19 patients' prognosis.11, 12, 13, 14, 15, 16, 17, 18 Besides a disease-related cardiac involvement, the massive off-label use of several drugs,19 , 20 including immunosuppressive agents (eg, anakinra or tocilizumab), different antivirals (eg, oseltamivir, remdesivir, or the lopinavir/ritonavir combination), and antimalarial drugs such as chloroquine (CQ) and hydroxychloroquine (HCQ) with or without azithromycin (AM), has generated concerns in the early phase of the pandemic because of their possible arrhythmogenic effects in relation to QT interval prolongation. Indeed, some of these drugs have never been used on a large scale and little is known about their possible arrhythmogenic effects in elderly, critically ill patients, often showing multiple comorbidities, being treated with multiple drugs. Most of these drugs may prolong the QT interval both with direct (channel blocking activity) or indirect effects (eg, liver and/or kidney toxicity, cytochrome interactions, electrolyte imbalance), potentially increasing the arrhythmic (eg, Torsade de Pointes [TdP]) and nonarrhythmic mortality. The aim of this work is to summarize the underlying arrhythmogenic mechanisms related to the use of potentially QT-prolonging drugs used during the COVID-19 pandemic.
The QT interval
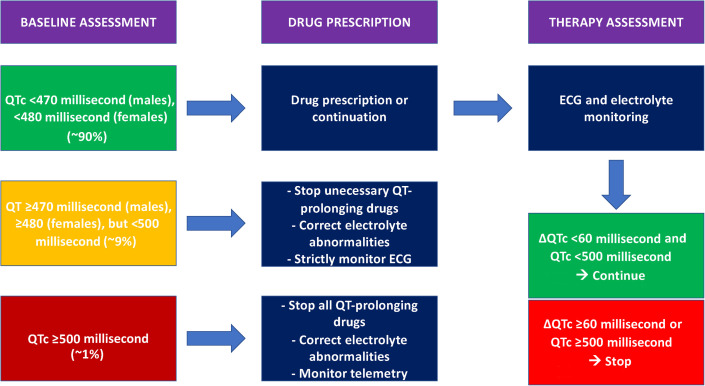
The QT interval is the interval from the beginning of ventricular depolarization to the completion of the repolarization of the entire ventricular mass. Ideally, the QT interval should be measured at a paper rate of 25 mm/s from the beginning of the QRS until the return to baseline of the T wave. The QT interval should be calculated in a total of 6 leads, with 3 leads taken from peripheral leads (avoiding DIII and aVR because of frequent low voltages and inverted polarity, respectively) and 3 precordial leads (preferably V2, V4, and V6). The QT value should be derived from the median of the 6 individual leads. Although QT calculation is a well-known and standardized methodology, a correct and consistent measurement of the QT interval is always difficult to obtain in clinical practice. Deciding if QT interval is normal or prolonged is often challenging, as reported by Viskin and colleagues, underlining that most physicians, including many cardiologists, cannot accurately calculate a QT and cannot correctly identify a long QT.21 Moreover, as the heart rate is the major determinant of the QT interval, a corrected QT interval value (QTc) should always be preferred in clinical practice. Several formulae may commonly be adopted to calculate QTc, with the Bazett formula being the most frequently used. Nevertheless, Vandenberk and colleagues22 showed that the Bazett formula may overestimate the number of patients at potential risk of dangerous QTc prolongation when compared with Fridericia and Framingham formulae. To sum up different clinical data, the Framingham formula still seems to be the best choice to predict drug-induced QTc prolongation.23 Different cut-offs have been traditionally proposed to define when a QTc is prolonged, with the most significantly reliable being 450 msec, with values of greater than 500 msec being considered definitely abnormal and potentially arrhythmogenic24 , 25; thus, drug-induced changes in QTc of greater than 50 msec are often used as safety endpoints when evaluating drug effects and may justify a treatment interruption. A proposed algorithm to identify patients at risk of developing ventricular arrhythmias (VAs) when treating with one or more QT-prolonging drugs during the COVID-19 pandemic is reported in Fig. 1 .
Fig. 1.
Proposed algorithm to identify patients at risk of developing ventricular arrhythmias when treating with one or more QT-prolonging drugs.
(Adapted from Giudicessi JR, Noseworthy PA, Friedman PA, Ackerman MJ. Urgent Guidance for Navigating and Circumventing the QTc-Prolonging and Torsadogenic Potential of Possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19). Mayo Clin Proc. 2020;95(6):1213-1221. https://doi.org/10.1016/j.mayocp.2020.03.024; with permission)
Mechanisms of QT prolongation
QT prolongation is associated with an increase in both arrhythmic and nonarrhythmic mortality, and it is often used as a metric of drug safety.26 Indeed, QT prolongation is related to a mix of modifiable and unmodifiable risk factors that may determine why at same drugs dosages, drug-induced long-QT may happen only in some cases. Drug-induced effects are one of the most frequent reasons for QT prolongation: an updated list of the medications associated with QT prolongation and risk of TdP is reported on https://www.crediblemeds.org. It should be noted that, besides the direct impact of some medications on the QT interval, drug-to-drug and drug-to-cytochrome interactions should always be considered, especially in COVID-19, when assessing the risk of QT prolongation in the COVID-19 clinical setting.
Nonmodifiable Risk Factors
Genetic background, as well as older age and female gender, are the most important unmodifiable risk factors. Long QT syndrome (LQTS) represents a heterogeneous family of inherited primary arrhythmia syndromes characterized by QT interval prolongation and T-wave abnormalities on the ECG. Patients affected by LQTS have been identified all over the world and in all ethnic groups; among Caucasians, the prevalence of LQTS has been 1:2000 apparently healthy newborns.27 Risks of VAs related to LQTS are mainly due to adrenergic activation, and the annual rate of sudden cardiac death (SCD) in patients with untreated LQTS is estimated to be between 0.33% and 0.9%.28 , 29 Mutations in 13 genes have been traditionally associated with LQTS—among those, mutations in potassium-channel genes KCNQ1 (LQT1 locus) and KCNH2 (LQT2 locus) and the sodium-channel gene SCN5A (LQT3 locus) are the most common causes of the LQTS and account for approximately 75% of cases.30 Once diagnosis is made, risk stratification is mandatory to tailor lifestyle changes and to deliver the adequate therapy, such as implantable cardioverter-defibrillator (ICD) in high-risk patients, with the modern subcutaneous ICD potentially being the most appropriate therapeutic option.31, 32, 33, 34, 35 All LQTS patients, regardless of the SCD risk, should avoid QT-prolonging drugs, promptly correct electrolyte abnormalities (hypokalemia, hypomagnesemia, and hypocalcemia) that may occur during diarrhea, vomiting, or metabolic conditions and avoid genotype-specific triggers for arrhythmias (strenuous swimming, especially in LQTS1, and exposure to loud noises in LQTS2 patients).36 There are more than 260 medicines on the “drugs to avoid” list for patients with LQTS, that are generally grouped as follows:
-
•
Known risk: drugs that should never or very rarely use because of clear danger—if administered, LQTS patients should be treated by cardiologists with expertise in arrhythmias management.
-
•
Possible risk: drugs that have been found to increase QT interval and may be dangerous in some LQTS patients—if necessary, those drugs may be prescribed by specialists.
-
•
Conditional risk: drugs that may increase risk in LQTS patients only in certain conditions (eg, overdose, prolonged treatments, use in combination with other drugs that may change their clearance)—most of those drugs can be prescribed safely.
-
•
Special risk: drugs that have a theoretic risk of causing arrhythmias in LQTS patients because of their adrenergic effect—most of those drugs can be prescribed to carefully selected LQTS patients37 .
Given the pandemic nature of COVID-19, even a rare congenital genetic predisposition, may result in tremendous consequences if undetected, in terms of drug-induced TdP and SCD. The most widely used QT-prolonging drugs that should be avoided in patients with LQTS and their associated risk have been summarized in Table 1 .
Table 1.
Most widely used potentially QT-prolonging drugs that should be avoided in patients with long QT and their associated risk.66
| Risk | AAD | AB/AFA/AM | AP/AD | Anesthetic | Other |
|---|---|---|---|---|---|
| Known | Amiodarone Dronedarone Disopyramide Dofetilide Flecainide Ibutilide Procainamide Quinidine Sotalol |
Azithromycin Ciprofloxacin Clarithromycin Erythromycin Fluconazole Gatifloxacin Levofloxacin Moxifloxacin Roxithromycin Chloroquine Hydroxychloroquine |
Chlorpromazine Citalopram |
Propofol Sevoflurane |
Cocaine Methadone Domperidone Levosulpiride Ondansetron Other antineoplastic drugs |
| Possible | Norfloxacin Ofloxacin |
Lithium Venlafaxine Aripiprazole Clozapine |
Tramadol | Alfuzosin Nicardipine Oxytocin Other antineoplastic drugs |
|
| Conditional | Ivabradine Propafenone Ranolazine |
Piperacillin/tazobactam Amphotericin B Ketoconazole Metronidazole Voriconazole |
Amisulpride Amitriptyline Fluoxetine Olanzapine Paroxetine Quetiapine Risperidone Sertraline Trazodone |
Hydrochlorothiazide Torasemide Amantadine Indapamide Furosemide Loperamide Metolazone Metoclopramide Omeprazole Lansoprazole Pantoprazole Esomeprazole |
|
| Special | Drugs used for the treatment of asthma, –ADHD, or nasal congestion | ||||
Abbreviations: AAD, antiarrhythmic drugs; AB, antibiotics; AD, antidepressants; ADHD, attention-deficit hyperactivity disorder; AFA, antifungal agents; AM, antimalarials; AP, antipsychotics.
Modifiable Risk Factors
Electrolyte abnormalities are the most common modifiable risk factors associated with QT prolongation. Among those, hypokalemia has a particular arrhythmogenic effect, not only prolonging the QT interval but also being a major risk factor for drug-induced LQTS as it increases the tendency of Kv11.1 channels to remain inactivated and decreases repolarizing currents. Hypocalcemia and hypomagnesemia as well may show a QT-prolonging effect. Kidney and liver failure have both been associated with the risk of QT prolongation because of their role in metabolite/toxin clearance; finally, bradycardia is a relevant additional risk factor.
Patients accessing intensive care such as COVID-19 severe infections should therefore be strictly monitored because of their potential exposure to these risk factors. Lastly, to estimate the risk of drug-induced QT prolongation, all the patients treated with a potential QT-prolonging drug should be evaluated with a Tisdale score at baseline (Table 2 ).38 Indeed, the Tisdale risk classes are, respectively, associated with 15%, 37%, and 73% risk of QT prolongation, and can be extremely useful for a quick but reliable baseline risk assessment.
Table 2.
Tisdale score to identify hospitalized patients at risk for QT interval prolongation could lead to interventions to reduce the risk of TdP38
| Age ≥ 68 y Female Sex Loop Diuretic Treatment |
1 point |
| Serum K+ ≤ 3.5mEq/L Acute myocardial infarction Admission QTc ≥ 450 msec |
2 points |
| 1-QTc-prolonging drugs 2+ QTc-prolong drugs Sepsis Heart failure hospitalization |
3 points |
TISDALE SCORE:
Low risk (≤6 points).
Intermediate risk (7–10 points).
High risk (≥11 points).
QT-prolonging drugs and arrhythmogenic risk in COVID-19
While waiting for the massive vaccination campaign to be completed to reach the herd immunity, several drugs proposed as potential treatments are still used worldwide to treat COVID-19. However, most of these drugs are not specific and targeted against SARS-CoV-2, so that using pre-existing drugs has represented a fast and very useful strategy with known safety, characteristics, and dosage used during the early and even late phase of the pandemic.19 If some of these drugs have been investigated for their efficacy and safety in treating COVID-19, some others are still undergoing clinical trials to test their profile. One of the main concerns regarding the use of some of these repurposed drugs is the potential impact on the QT interval and their arrhythmogenic effects, which is particularly noteworthy because of the common coprescription of several drugs that may show combined effects on the QT interval, as well as several clinical characteristics that may eventually lead to arrhythmic manifestations.39 The knowledge on these potential adverse events is mostly derived from the historical data collected according to the European Union Drug Regulatory Authorities (EUDRA) vigilance by the European Medical Agency (EMA). If data on chloroquine (CQ) and hydroxychloroquine (HCQ) are more robust, data on other less commonly used drugs are weaker.
Antimalarial Agents
CQ and HCQ are antimalarial drugs that inhibit lysosomes functions increasing pH and thereby blocking endosome-mediated entry. These drugs can also interfere with cell replication, viral protein glycosylation, virus assembly, and release. CQ use is restricted because of potential overdose, acute poisoning, and death, whereas HCQ (a derivative of CQ) has been demonstrated to be far less toxic than CQ.19 In the early phase of the pandemic, these antimalarial drugs have been suggested to be effective in treating COVID-19,40 and they have been thereby extensively used both in mild and in severe COVID-19. Randomized trials and metanalysis have, however, shown that HCQ was not effective as it was initially supposed. In the randomized, controlled, open-label RECOVERY trial,41 comparing a range of possible treatments with usual care in patients hospitalized with COVID-19, patients receiving HCQ did not have a lower incidence of death at 28 days than those who received usual care. Also, the TOGETHER trial42 showed that an early treatment with HCQ did not have any significant benefit in decreasing COVID-19–associated hospitalization or other secondary clinical outcomes. These results were confirmed by Ghazy and colleagues43 in a metanalysis, showing that neither CQ nor HCQ were able to decrease mortality, improve virological cure, reduce the risk for noninvasive ventilation and shorten the conversion to negative polymerase chain reaction, prevent radiological progression, and affect clinical worsening of the disease. Considering this evidence, showing a complete lack of efficacy and an increase in adverse events, most American and European medical associations and drugs associations do not recommend the use of HCQ in hospitalized COVID-19 patients and in the early stages of the disease.
Nevertheless, several trials have been performed to test the cardiac safety of CQ/HCQ in the early phase of the pandemic, and although their results may now appear outdated in the light of this recent discovery of CQ/HCQ inefficacy in COVID-19, all these analyses gave the scientific community the possibility to test these drugs during a mass-use on critically ill patients. The importance of these reports is undoubtedly related to the idea that CQ and HCQ are known to be associated with a risk of QT prolongation, so that they are classified as drugs associated with TdP on the Credible Meds Web site. Hence, between 0.5% and 2% of all the side effects of these drugs reported to the European Medicines Agency (EMA) are major arrhythmic events with non-negligible rates of cardiac arrest. Moreover, is noteworthy to underline that HCQ, besides malaria, is currently used to treat discoid or systemic lupus erythematosus, rheumatoid arthritis (RA), and systemic sclerosis.
Indeed, research on this topic is beneficial to better understand its arrhythmic safety also for these patients. Specifically, Gasperetti and colleagues extensively evaluated the arrhythmic safety of HCQ in different clinical settings.44 In this study, enrolled patients were followed in 3 different clinical settings, defined as home management, medical ward, or intensive care unit (ICU) management, depending on the COVID-19 severity, and were all tested through serial ECG monitoring. The authors concluded that HCQ administration, alone or in combination with other potentially QTc-prolonging drugs, although potentially causing only modest QTc prolongation, did not result in significant arrhythmic events, representing a safe option for patients with COVID-19 infection. Indeed, no TdP were noticed in the entire cohort, and the described ventricular fibrillation (VF) events occurred in the ICU cohort, with acute myocardial infarction as the underlying cause. These results were confirmed by 3 different studies, enrolling patients with COVID-19 treated with HCQ. First, Mazzanti and colleagues45 did not document any life-threatening arrhythmic event, with only a modest effect on QTc prolongation, that was attributed to the short duration of HCQ treatment in COVID-19, as HCQ reaches the steady state after 180 days of HCQ therapy.46 Therefore, caution should be adopted when extending these safety results to patients treated for several years for other indications, that could experience a more severe QTc prolongation and related arrhythmic effects. The authors recommend to always perform a baseline ECG before starting HCQ, followed by a subsequent recording “on therapy” for patients with a normal baseline QTc, with an advisable daily monitoring for patients with baseline QTc greater than 480 msec. Furthermore, Bernardini and colleagues47 and Chorin and colleagues48 evaluated the safety of the HCQ plus AM combination regimen that might surely have higher proarrhythmic effects than HCQ alone. In both cohorts, a significant increase of QT interval was noted, especially in the elderly, with 8% and 23% patients treated with HCQ + AM showing a QTc greater than 500 msec, respectively. Besides this difference, if in the first cohort no arrhythmic fatalities occurred, in the second one QT prolongation has led to 1 life-threatening arrhythmia (0.4%) in the form of TdP. These dissimilarities might be due to concurrent modifiable risk factors, such as electrolyte imbalance, comorbidities, or COVID-19 severity, that could have contributed to QT prolongation. Indeed, the safety of HCQ large-scale use in acutely ill patients with multiple comorbidities, possibly receiving several QT-prolonging drugs and potentially at risk of electrolyte disbalance, still needs to be properly tested. Even if data point toward a general arrhythmic safety of HCQ, especially when used alone or in the short-term period, a baseline ECG and a periodic QTc interval monitoring should be advisable when this drug regimen is given.
Antiviral Drugs
Lopinavir/ritonavir
Lopinavir and ritonavir (LPV/RTN) are antiretroviral protease inhibitors that are used in combination to treat human immunodeficiency virus. RTN increases the half-life of LPV by inhibiting the half-life of cytochrome P450 half-life, and thereby acting as a pharmacokinetic enhancer; LPV acts against viral 3-chymotrypsin-like protease (3CLpro). This combination has shown promising in vitro results against SARS-CoV and MERS-CoV, but clinical randomized trials did show no benefit with LPV/RTN combination beyond standard of care in hospitalized adult patients with severe COVID-19.49 Nevertheless, some researchers, interpreting the findings of this clinical trial, suggested the earlier usage of LPV/RTN in the course of the disease may be overall beneficial in some cases.50 Therefore, the evaluation of the arrhythmogenic effects is of pivotal importance. Indeed, this combination has an intrinsic risk of ventricular tachycardia (0.03%), VF (0.03%), and TdP (0.09%) reported in the literature, according to the EUDRA vigilance from EMA. During the pandemic, Haghjoo and colleagues51 investigated the potential QT-prolonging role of LPV/RTN, showing a significant increase in QTc during drug therapy (along with CQ, HCQ, atazanavir/ritonavir, oseltamivir, favipiravir, and remdesivir alone in combination with AM). Nevertheless, in this cohort, TdP occurred overall rarely (n = 9; 0.385), with 4 patients treated with HCQ + AM, whereas 5 patients were treated with LPN/RTV + AM. Interestingly, in this analysis, although critical QT prolongation was associated with a higher risk of TdP, only treatment with LPN/RTV, simultaneous administration of amiodarone (known to prolong QT interval52) or furosemide and hypokalemia could predict the occurrence of TdP in this cohort; instead, HCQ use was only modestly associated with TdP (0.3% of patients). Other cases of QT prolongation with LPN/RTV treatment have been described during the pandemic,53 so that careful QTc duration evaluation and monitoring should be performed at baseline and during this drug therapy to identify patients at high risk of arrhythmias.
Remdesivir
Remdesivir is an adenosine analog that inserts itself into viral RNA chains, blocking viral replication. Although nothing has been reported in the FDA and EMA databases regarding links with QT prolongation, some case reports and scarce data have suggested that also this drug may prolong QT, as well as induce sinus bradycardia, as reported by Gupta and colleagues. 54 It should be noted that in this case, patients were also on AM while receiving remdesivir, which is well-known to prolong the QT interval. Remdesivir monotherapy has indeed shown to prolong QTc in the Haghjoo and colleagues51 analysis, although keeping a low-risk profile in terms of QT prolongation and TdP induction. Moreover, no major cardiac arrhythmia events were described in the largest trial assessing remdesivir efficacy and safety profile. Nevertheless, cardiac safety of remdesivir remains largely uncertain and these effects were described as reversible upon stopping remdesivir therapy, caution should be taken with this antiviral agent.
Favipiravir
Favipiravir is a guanine analog that selectively inhibits viral RNA-dependent RNA polymerase and it was approved for influenza and Ebola virus infection. Çap and colleagues55 specifically evaluated any change in the QTc interval in patients who were hospitalized due to COVID-19, receiving favipiravir treatment. No significant QTc prolongation was noted with monotherapy, when compared to HCQ or HCQ + favipiravir. On the other side, Haghjoo and colleagues51 observed a mild QTc prolongation in most cases, without TdP events, even if they concluded that favipiravir monotherapy was safer than other COVID-19 mediations in terms of QTc prolongation.
Oseltamivir
Oseltamivir is an antiviral drug that inhibits neuraminidase, expressed on the viral surface, which plays an essential role in viral entry to host cells, viral release from infected cells, and subsequent viral spread. Although its role in COVID-19 is very limited, it is noteworthy to mention that Haghjoo and colleagues51 reported that this drug may significantly prolong QTc when used in combination with HCQ, as also suggested by Çelik and colleagues.56 No TdP were noted in these patients, as in previous preclinical models that tested oseltamivir therapy alone, as this drug is capable to inhibit both inward and outward currents.57 However, caution should be taken when prescribing oseltamivir plus other COVID-19 medications potentially prolonging QTc during the influenza season.
Antibacterial Drugs
Azithromycin
AM, a macrolide antibacterial agent, has an established role against a broad spectrum of gram-positive and gram-negative agents, as well as act as an immunomodulator. During the COVID-19 pandemic, it has been used in combination with HCQ because of promising in vitro findings, even further clinical trials have demonstrated that a routine use of AM for reducing time to recovery or risk of hospitalization for people with suspected COVID-19 in the community was not justified.58 AM is a well-known QT-prolonging drug, that should be avoided in all LQTS cases, even when used as a stand-alone therapy. The arrhythmogenic potential of AM has been discussed in the previous sections when assessing the combination of this drug with HCQ. Indeed, in the PRINCIPLE trial,58 no difference was found regarding a prolonged QTc interval between the AM group and the standard care group, but a QTc interval prolongation was most common in the HCQ + AM group.
Immunomodulators
Tocilizumab
Tocilizumab (TCZ) is an anti–interleukin (IL)-6 receptor antibody that potently inhibits inflammatory activation and is used to treat RA, systemic juvenile idiopathic arthritis, and chimeric antigen receptor–cell-induced cytokine release syndrome. In a clinical trial conducted on patients with RA, Lazzerini and colleagues59 showed that TCZ treatment was associated with a rapid and significant reduction to mean values less than 440 msec in patients who had prolonged QTc interval at baseline. This effect seems to be driven by TCZ action against systemic inflammation, thus providing further evidence of the close correlation between the degree of systemic inflammation and QTc duration in RA patients.60 In this light, the administration of anti–IL-6 targeted therapies (TCZ, sarilumab) to patients with COVID-19, particularly those severely ill, has been supposed not only to promote the recovery from multiorgan dysfunction but also mitigate the associated high arrhythmic risk, in the early phase of the pandemic. However, randomized trials have shown that the use of TCZ did not result in significantly better clinical status or lower mortality than placebo at 28 days, being not effective for preventing intubation or death in moderately ill hospitalized patients with COVID-19.61 , 62 Specific data on the supposed antiarrhythmic effects, associated with the anti-inflammatory effects, were not specifically reported.
Sarilumab
Sarilumab (SAR) is a humanized monoclonal antibody, inhibiting the IL-6 receptor; it is approved for the treatment of adults with moderately to severely active RA. The rate of cardiovascular arrest reported in the EMA registry is relatively high (3.2%). Nevertheless, no specific data concerning QT prolongation and/or VAs in patients treated with SAR have been reported, and even a protective role (similar to TCZ) has been otherwise suggested, because of its immunomodulating effect, potentially decreasing the extent of myocardial injury frequently observed in COVID-19.63 However, data in COVID-19 are scarce, and specific investigations on its effect on QT interval are lacking.
IL-1 inhibitors (anakinra and canakinumab)
Anakinra (ANA) and canakinumab (CAN) are the only 2 IL-1 inhibitors approved in Europe. Owing to the massive COVID-19 inflammatory reaction, it has been suggested that intravenous ANA and CAN could be used against the cytokine storm that seems to be associated with some extent of the lung damage in COVID-19. A metanalysis has shown that the administration of ANA in COVID-19 patients could be associated with reductions in both mortality and need for mechanical ventilation.64 As for TCZ and SAR, specific data on the proarrhythmic or antiarrhythmic effect are lacking.
Summary
Severe systemic inflammation and the off-label use of some drugs in COVID-19 may significantly prolong the QTc interval, potentially leading to a non-negligible risk of VAs. Among these drugs, CQ and HCQ have shown the higher risk of QTc prolongation and TdP, that is, however, overall low, even in association with other QT-prolonging drugs, such as AM.
In line with other authors,65 also this panel believes that the ultimate aim of QTc surveillance during the COVID-19 pandemic should not result in an exclusion from potentially beneficial treatments or experimental clinic trials, but instead to identify patients at risk, in order to counterbalance and mitigate all potentially drug-induced arrhythmogenic side-effects.
Clinics care points
-
•
Arrhythmic risk assessment is of pivotal importance when administering drug therapy in COVID-19.
-
•
Several drugs may prolong QT interval in COVID-19, and particular attention should be paid to specific drugs combinations (e.g. chloroquine and hydroxychloroquine + macrolides).
References
- 1.Gattinoni L., Coppola S., Cressoni M., et al. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busana M., Gasperetti A., Giosa L., et al. Prevalence and outcome of silent hypoxemia in COVID-19. Minerva Anestesiol. 2021;87(3):325–333. doi: 10.23736/S0375-9393.21.15245-9. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiavone M., Gasperetti A., Mancone M., et al. Oral anticoagulation and clinical outcomes in COVID-19: an Italian multicenter experience. Int J Cardiol. 2021;323:276–280. doi: 10.1016/j.ijcard.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi M., Thachil J., Iba T., et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiavone M., Gasperetti A., Mancone M., et al. Redefining the prognostic value of high-sensitivity troponin in COVID-19 patients: the importance of concomitant coronary artery disease. J Clin Med. 2020;9(10):3263. doi: 10.3390/jcm9103263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Rocca D.G., Magnocavallo M., Lavalle C., et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J Thromb Thrombolysis. 2021;51(3):571–576. doi: 10.1007/s11239-020-02330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S., Qin M., Shen B., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J.F., Huang F.Y., Xiong T.Y., et al. Acute myocardial injury is common in patients with COVID-19 and impairs their prognosis. Heart. 2020;106(15):1154–1159. doi: 10.1136/heartjnl-2020-317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiavone M., Gobbi C., Biondi-Zoccai G., et al. Acute coronary syndromes and Covid-19: exploring the uncertainties. J Clin Med. 2020;9(6):1683. doi: 10.3390/jcm9061683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Guan B., Su T., et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitacchione G., Schiavone M., Gasperetti A., et al. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur Hear J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antwi-Amoabeng D., Beutler B.D., Singh S., et al. Association between electrocardiographic features and mortality in COVID-19 patients. Ann Noninvasive Electrocardiol. 2021 doi: 10.1111/anec.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero J., Alviz I., Parides M., et al. T-wave inversion as a manifestation of COVID-19 infection: a case series. J Interv Card Electrophysiol. 2020;59(3):485–493. doi: 10.1007/s10840-020-00896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarighi P., Eftekhari S., Chizari M., et al. A review of potential suggested drugs for coronavirus disease (COVID-19) treatment. Eur J Pharmacol. 2021;895:173890. doi: 10.1016/j.ejphar.2021.173890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitacchione G., Schiavone M., Curnis A., et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: data from tertiary referral hospitals during COVID-19 pandemic in Italy. J Clin Lipidol. 2021;15(1):68–78. doi: 10.1016/j.jacl.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viskin S., Rosovski U., Sands A.J., et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2(6):569–574. doi: 10.1016/j.hrthm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Vandenberk B., Vandael E., Robyns T., et al. Which QT correction formulae to use for QT monitoring? J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Batchvarov V.N., Ghuran A., Smetana P., et al. QT-RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol Heart Circ Physiol. 2002;282(6) doi: 10.1152/ajpheart.00860.2001. [DOI] [PubMed] [Google Scholar]
- 24.Postema P.G., De Jong J.S.S.G., Van der Bilt I.A.C., et al. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5(7):1015–1018. doi: 10.1016/j.hrthm.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Postema P.G., Arthur A.M.W. The measurement of the QT interval. Curr Cardiol Rev. 2014;10(3):287–294. doi: 10.2174/1573403X10666140514103612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simpson T.F., Salazar J.W., Vittinghoff E., et al. Association of QT-prolonging medications with risk of autopsy-defined causes of sudden death. JAMA Intern Med. 2020;180(5):698–706. doi: 10.1001/jamainternmed.2020.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz P.J., Stramba-Badiale M., Crotti L., et al. Prevalence of the congenital long-qt syndrome. Circulation. 2009;120(18):1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss A.J., Schwartz P.J., Crampton R.S., et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84(3):1136–1144. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 29.Priori S.G., Schwartz P.J., Napolitano C., et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348(19):1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 30.Nakano Y., Shimizu W. Genetics of long-QT syndrome. J Hum Genet. 2016;61(1):51–55. doi: 10.1038/jhg.2015.74. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz P.J., Spazzolini C., Priori S.G., et al. Who are the long-QT syndrome patients who receive an implantable cardioverter-defibrillator and what happens to them?: data from the European Long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) registry. Circulation. 2010;122(13):1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 32.Forleo G.B., Gasperetti A., Breitenstein A., et al. Subcutaneous implantable cardioverter defibrillator and defibrillation testing: a propensity-matched pilot study. Heart Rhythm. 2021 doi: 10.1016/j.hrthm.2021.06.1201. [DOI] [PubMed] [Google Scholar]
- 33.Gasperetti A., Schiavone M., Ziacchi M., et al. Long term complications in patients implanted with subcutaneous implantable defibrillators Real-world data from the Extended ELISIR experience. Heart Rhythm. 2021 doi: 10.1016/j.hrthm.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Lambiase P.D., Eckardt L., Theuns D.A., et al. Evaluation of subcutaneous implantable cardioverter-defibrillator performance in patients with ion channelopathies from the EFFORTLESS cohort and comparison with a meta-analysis of transvenous ICD outcomes. Heart Rhythm O2. 2020;1(5):326–335. doi: 10.1016/j.hroo.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gasperetti A., Schiavone M., Ziacchi M., et al. Long-term complications in patients implanted with subcutaneous implantable cardioverter-defibrillators: real-world data from the extended ELISIR experience. Heart Rhythm. 2021 doi: 10.1016/j.hrthm.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz P.J., Priori S.G., Spazzolini C., et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 37.Moss A.J., Zareba W., Hall W.J., et al. Effectiveness and limitations of β-blocker therapy in congenital long- QT syndrome. Circulation. 2000;101(6):616–623. doi: 10.1161/01.cir.101.6.616. [DOI] [PubMed] [Google Scholar]
- 38.Tisdale J.E., Jaynes H.A., Kingery J.R., et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6(4):479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasperetti A., Schiavone M., Tondo C., et al. QT interval monitoring and drugs management during COVID-19 pandemic. Curr Clin Pharmacol. 2020;15 doi: 10.2174/1574884715666201224155042. [DOI] [PubMed] [Google Scholar]
- 40.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 41.The RECOVERY Collaborative Group Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis G., Moreira Silva E.A.D.S., Medeiros Silva D.C., et al. Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial. JAMA Netw Open. 2021;4(4):e216468. doi: 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghazy R.M., Almaghraby A., Shaaban R., et al. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci Rep. 2020;10(1):1–18. doi: 10.1038/s41598-020-77748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasperetti A., Biffi M., Duru F., et al. Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace. 2020;22(12):1855–1863. doi: 10.1093/europace/euaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzanti A., Briani M., Kukavica D., et al. Association of hydroxychloroquine with QTc interval in patients with COVID-19. Circulation. 2020;142(5):513–515. doi: 10.1161/CIRCULATIONAHA.120.048476. [DOI] [PubMed] [Google Scholar]
- 46.Thémans P., Belkhir L., Dauby N., et al. Population pharmacokinetics of hydroxychloroquine in COVID-19 patients: implications for dose optimization. Eur J Drug Metab Pharmacokinet. 2020;45(6):703–713. doi: 10.1007/s13318-020-00648-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernardini A., Ciconte G., Negro G., et al. Assessing QT interval in COVID-19 patients:safety of hydroxychloroquine-azithromycin combination regimen. Int J Cardiol. 2021;324:242–248. doi: 10.1016/j.ijcard.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chorin E., Wadhwani L., Magnani S., et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17(9):1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao B., Wang Y., Wen D., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owa A.B., Owa O.T. Lopinavir/ritonavir use in Covid-19 infection: is it completely non-beneficial? J Microbiol Immunol Infect. 2020;53(5):674–675. doi: 10.1016/j.jmii.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haghjoo M., Golipra R., Kheirkhah J., et al. Effect of COVID-19 medications on corrected QT interval and induction of torsade de pointes: results of a multicenter national survey. Int J Clin Pract. 2021;75(7):e14182. doi: 10.1111/ijcp.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohanty S., Di Biase L., Mohanty P., et al. Effect of periprocedural amiodarone on procedure outcome in patients with longstanding persistent atrial fibrillation undergoing extended pulmonary vein antrum isolation: results from a randomized study (SPECULATE) Heart Rhythm. 2015;12(3):477–483. doi: 10.1016/j.hrthm.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Zhu S., Wang J., Wang Y., et al. QTc prolongation during antiviral therapy in two COVID-19 patients. J Clin Pharm Ther. 2020;45(5):1190–1193. doi: 10.1111/jcpt.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta A.K., Parker B.M., Priyadarshi V., et al. Cardiac adverse events with remdesivir in COVID-19 infection. Cureus. 2020 doi: 10.7759/cureus.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Çap M., Bilge Ö., Işık F., et al. The effect of favipiravir on QTc interval in patients hospitalized with coronavirus disease 2019. J Electrocardiol. 2020;63:115–119. doi: 10.1016/j.jelectrocard.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Çelik H.G., Keske Ş., Şener Ü., et al. Why we should be more careful using hydroxychloroquine in influenza season during COVID-19 pandemic? Int J Infect Dis. 2021;102:389–391. doi: 10.1016/j.ijid.2020.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura Y., Sasaki R., Cao X., et al. Intravenous anti-influenza drug oseltamivir will not induce torsade de pointes: evidences from proarrhythmia model and action-potential assay. J Pharmacol Sci. 2016;131(1):72–75. doi: 10.1016/j.jphs.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Butler C.C., Dorward J., Yu L.M., et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397(10279):1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lazzerini P.E., Acampa M., Capecchi P.L., et al. Antiarrhythmic potential of anticytokine therapy in rheumatoid arthritis: tocilizumab reduces corrected qt interval by controlling systemic inflammation. Arthritis Care Res. 2015;67(3):332–339. doi: 10.1002/acr.22455. [DOI] [PubMed] [Google Scholar]
- 60.Panoulas V.F., Toms T.E., Douglas K.M.J., et al. Prolonged QTc interval predicts all-cause mortality in patients with rheumatoid arthritis: an association driven by high inflammatory burden. Rheumatol (Oxford) 2014;53(1):131–137. doi: 10.1093/rheumatology/ket338. [DOI] [PubMed] [Google Scholar]
- 61.Stone J.H., Frigault M.J., Serling-Boyd N.J., et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384(16):1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazzerini P.E., Laghi-Pasini F., Acampa M., et al. IL-6 (interleukin 6) blockade and heart rate corrected QT interval prolongation in COVID-19. Circ Arrhythm Electrophysiol. 2020;13(9):e008791. doi: 10.1161/CIRCEP.120.008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasin L., Cavalli G., Navalesi P., et al. Anakinra for patients with COVID-19: a meta-analysis of non-randomized cohort studies. Eur J Intern Med. 2021;86:34–40. doi: 10.1016/j.ejim.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giudicessi J.R., Noseworthy P.A., Friedman P.A., et al. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible Pharmacotherapies for Coronavirus Disease 19 (COVID-19) Mayo Clin Proc. 2020;95(6):1213–1221. doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drugs to be avoided by congenital long QT patients. www.crediblemeds.org Available at: Accessed July 18, 2021.