Abstract
During the coronavirus disease 2019 (COVID-19) worldwide pandemic, patients with cardiac implantable electronic device (CIED) refused scheduled follow-up visits because of the risk of infection. In this scenario, different telemedicine strategies have been implemented to ensure continuity of care to CIED patients. Patients can be monitored through dedicated applications, telephone calls, or virtual visits providing easy access to valuable information, such as arrhythmic events, acute decompensation manifestations, and device-related issues, without the need for in-person visits. This review provides a comprehensive description of the many possible applications of telemedicine for CIED patients during the COVID-19 period.
Keywords: COVID-19, Remote monitoring, Telemedicine, CIED, Telehealth, Pacemaker, Implantable cardiac defibrillator
Key points
-
•
The aim of remote monitoring is to optimize the clinical management of CIED patients, improve quality of life, and reduce hospitalization and emergency department access.
-
•
A well-established organizational model should include an adequately structured team, a valid integration with the primary health care centers, and an appropriate response to clinical alerts.
-
•
The development and refinement of telemedicine during the pandemic period suggest that remote monitoring should be recommended for all CIED patients.
Introduction
On December 31, 2019, a cluster of pneumonia cases of unknown origin was reported in the city of Wuhan; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was then discovered as the causative agent of the respiratory disease named coronavirus disease 2019 (COVID-19).1 The epidemic spread rapidly through China and subsequently to the rest of the world, leading the World Health Organization to declare the pandemic state on March 11, 2020.
COVID-19 has caused a global impact on public health services that led to the reorganization of hospital settings, including in-office visits for patients with cardiac implantable electronic device (CIED). Remote monitoring (RM) of CIED patients represents an appropriate strategy to minimize any potential risk of virus exposure for patients and health care providers, without compromising the quality of care.2, 3, 4, 5 RM offers access to the same information as an in-office visit and may contribute to the early detection of atrial and ventricular arrhythmias,6 , 7 prevent heart failure (HF) decompensation, and manage device-related issues.8, 9, 10, 11, 12 RM has also confirmed its usefulness in decreasing the hospitalization rate and improving clinical outcomes.13 Moreover, since the onset of the COVID-19 pandemic, physicians have suspended nonurgent scheduled visits and made a rapid transition to virtual visits (VV).14, 15, 16 Thanks to the technological improvement, patients utilizing wearable sensors for the measurement of hemodynamic parameters (blood pressure and saturation, heart rate) and adopting virtual health platforms may be monitored directly from home without any risk of infection.
In this review, we provide an overview of the many possible applications of RM, its limitations and challenges in patients with CIED during the COVID-19 pandemic.
The role of RM in CIED management during COVID-19
RM of CIED patients has become increasingly popular in clinical practice, especially during the COVID-19 pandemic.17 Indeed, in the new guidelines of the European Society of Cardiology (ESC) on cardiac pacing, RM is recommended to reduce the number of in-office follow-up in patients with pacemaker (PMK) who have difficulties to attend in-person visits. RM may also be useful in case of a device component that has been recalled or is on advisory, to enable early detection of actionable events in patients at high risk.18 RM provides the same information as an in-person visit ensuring an early identification of cardiac arrhythmias, such as ventricular tachycardias or atrial fibrillation, device therapy, and device-related issues like lead malfunction and early battery discharge.19, 20, 21, 22 Additional benefits were also demonstrated among HF patients in terms of preventing unfavorable cardiovascular events and reducing hospital readmissions.12 , 23
Although guidelines recommended the use of RM for the follow-up of patients with CIED, the coverage of RM was limited because of the organizational problems of health care systems and reimbursement issues.18 , 20 The COVID-19 pandemic forced all health care providers to minimize interpersonal contacts to limit the spread of the virus, which led to a total reshaping of outpatient cardiology management and accelerated the deployment and widespread use of RM.24 Indeed, the consensus document of the Heart Rhythm Society (HRS) and ESC for the management of cardiovascular disease during the COVID-19 pandemic recommended that RM should replace in-office visits for device interrogation and, whenever possible, postpone the scheduled in-person visit.3 , 4 , 14 , 24 , 25
A questionnaire-based survey by the European Heart Rhythm Association (EHRA) to assess the influence of the COVID-19 pandemic on RM in CIEDs demonstrated a strong implementation of RM in patients with PMKs and implantable loop recorders (ILRs; PMK 24.2 vs 39.9%; P = .002; ILR 61.5 vs 73.5%; P = .028). A nonsignificant increasing trend was registered for RM of cardiac resynchronization therapy-pacemaker (CRT-P) devices (44.5 vs 55%; P = .063), implantable cardioverter defibrillators (ICDs; 65.2 vs 69.6%; P = .408) and CRT-defibrillators (CRT-D; 65.2 vs 68.8%; P = .513).26
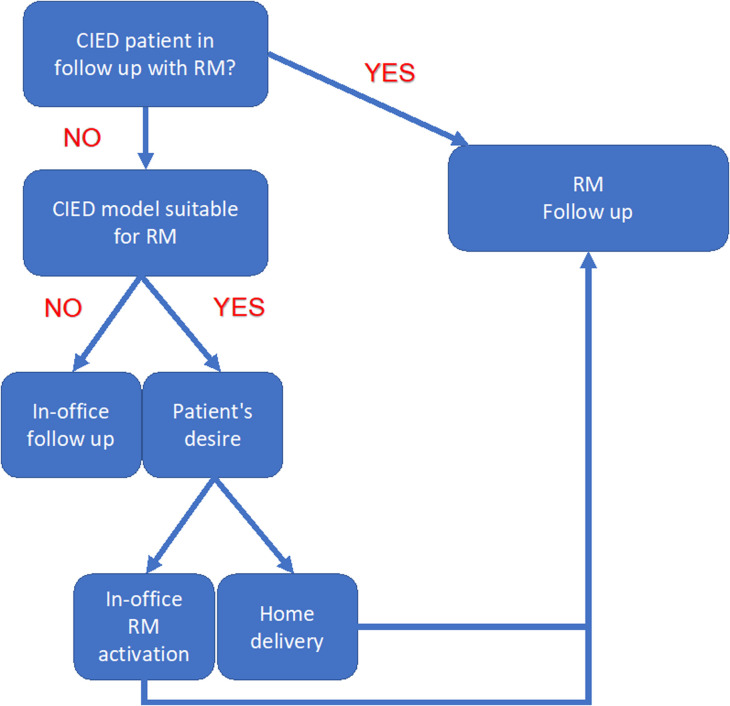
Home delivery of the transmitter for RM should be preferred over in-office delivery, as it limits the exposure of patients to the hospital environment. To date, home delivery of transmitter is feasible for Boston Scientific and Abbott PM and ICDs, as well as for the latest Medtronic CIEDs with BlueSync.24 As demonstrated in a recent multicenter study, the communicator LATITUDE was home delivered to 1324 patients from 49 different Italian centers and successful activation through telephone training was achieved in 92% of cases.27 Moreover, De Larochellière and colleagues confirmed that switching from a follow-up model with in-person visits to an RM model did not impair the management of ICD patients, and significantly reduced the number of in-person visits.28 In Fig. 1 , we summarized a protocol for setting up and managing RM during the COVID-19 pandemic. When the device is suitable for RM, home delivery of the transmitter is an appropriate strategy for minimizing any potential risk of virus exposure for patients and health care providers.
Fig. 1.
Remote monitoring setup protocol. CIED, cardiac implantable electronic device; RM, remote monitoring.
Overall, the available evidence confirmed that RM is an easy-to-use and effective tool for the management of CIED patients even during the pandemic and demonstrated that the home delivery and activation of communicators without an in-patient visit is a potential opportunity to further extend RM in the future.
Supplementation of teleconsultation in CIED patients
The ESC and HRS consensus documents stated that in-person visits should be replaced by telemedicine consultations in order to prevent the spread of the virus among cardiovascular patients.3 , 24 Indeed, the worldwide survey by Han and colleagues about the use of eHealth technologies during the COVID-19 pandemic showed a significant increase in the use of teleconsultations in the management of cardiological patients (5.9% vs 58.6%; P < .001) for all types of consultations compared with the prepandemic period.25
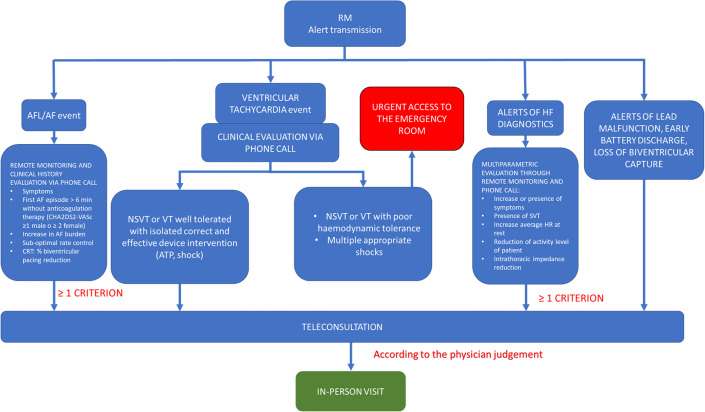
Supplementing teleconsultation in CIED patients could be a key tool in the management of these patients during the COVID-19 pandemic, especially for those affected by HF. ICD and CRT have the capability of monitoring HF by measuring thoracic impedance (Optivol, Medtronic; CorVue, Abbott)29 or by integration of several indices (HeartLogic, Boston Scientific).30 Nevertheless, guidance statements issued by experts in electrophysiology and HF recommend that every effort should be made to convert in-office visits to telehealth and VV. Specifically, the VVs used for decades to reach remote communities,31 but less commonly used in advanced health care systems, have now emerged as the cornerstone of ambulatory care in all subspecialties.32 The potential benefits of VV for HF patients are providing access to care and medical advice, which would be otherwise difficult to obtain and reducing in-person exposure to SARS-CoV-2. Cardiac rhythm professionals are advantaged by having wireless technology available to transmit monitored information to keep them connected.33 Moreover, VVs have the advantage of detecting and alerting caregivers about relevant parameter changes, allowing earlier hospitalization of the patient, even in a presymptomatic phase.34 A flowchart for VV is summarized in Fig. 2 ; VV was recommended in patients with atrial arrhythmias, alert for HF decompensation and nonsustained ventricular tachycardia.
Fig. 2.
Remote monitoring management protocol. AF, atrial fibrillation; AFL, atrial flutter; HF, heart failure; HR, heart rate; NSVT, non-sustained ventricular tachycardia; RM, remote monitoring; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
Overall, new technologies and digital platforms to aid in remote care should be developed and further research on the role of telehealth, continuous data collecting, advanced automotive features, and RM is needed to guide best practices.
Patient acceptability and satisfaction of RM during COVID-19
As in any health care interaction, patient involvement plays an important role, and in the case of RM, active participation is fundamental. Patients must adhere to transmission timetables and keep in contact with the physician to guarantee a successful health care system based on RM. Therefore, from a positive reciprocal interaction between patient and caregiver usually derives a high acceptability and satisfaction. From the point of view of the patient, especially during the COVID-19 period, RM should be ease of use,35, 36, 37 even when manual transmission of the data is requested, and guarantee a positive relationship with their health care provider at enrollment and during all the monitored period.36 , 38, 39, 40 The Home Monitoring Acceptance and Satisfaction Questionnaire is administered to evaluate the acceptability and satisfaction of RM (HoMASQ) and showed that ICD patients had a higher level of acceptance and satisfaction than patients with PMK.35 , 39 Moreover, RM was demonstrated to be easy to use and well accepted even for older people and patients with a low level of scholarity.41 Otherwise, the most frequent causes of noncompliance seem to be:
-
•
age-related: age under 40 years was associated with lower compliance.
-
•
Health care systems–related: high volume clinics were associated with better compliance.
-
•
Device-related: wireless devices are characterized by a better compliance compared with those requiring use of a wand.42
The institution of an RM patient agreement supported by the HRS enhances the compliance because patients can freely share information and experiences.17
During the COVID-19 pandemic, also the delivery of the communicator for RM could reduce the risk of contagion and influence patient’s acceptability. Piro and colleagues demonstrated that home delivery of the communicator and intensive transtelephonic support for its activation resulted in an easy understanding of the device activation process, as well as high satisfaction with the use of the transmitter.2 In addition, despite the ongoing pandemic and national lockdown, patients referred a sense of security and expressed interest in continuing with RM; also, in-office modem delivery and activation was associated with a higher prevalence of anxiety symptoms due to COVID-19 pandemic, compared with home modem delivery.2 , 27
In conclusion, several studies showed a high level of patient satisfaction and compliance, making it possible to extend this form of management to a growing volume of patients, especially in times of pandemics.
RM for CIED patients with other comorbidities
CIED patients are usually affected by multiple comorbidities: neurologic syndromes, chronic kidney disease, chronic obstructive pulmonary disease, diabetes mellitus, and other endocrinological disorders. New technologies and the adaptation of existing telemedicine tools represent an alternative option for an integrated monitoring.3 , 32 For example, diabetes patients need recurrent medical consultations to optimize drug therapy and blood sugar levels, and telemedicine can be a valuable alternative, especially during a pandemic when contacts need to be limited.43 To confirm the effectiveness of RM for diabetes management, a recent meta-analysis demonstrated a reduction in glycated hemoglobin in the RM group compared with controls.44 Moreover, continuous glucose monitoring is effective in the management of high-risk patients with type 1 diabetes mellitus without any diabetic ketoacidosis.45
Telemedicine also spread into the field of neurology and a telestroke unit was established to allow remote assessment of patients with suspected stroke to minimize unnecessary in-person visits.46 The latest evidence demonstrated that in-hospital management of end-stage renal disease patients increased the risk of infection up to 4 times compared with telemedicine-based home management and was more expensive. Similarly, RM appeared effective in the rehabilitation and management of chronic obstructive pulmonary disease patients, leading to a reduction in hospitalizations and emergency department visits.47
All this evidence shows how the pandemic escalated the adoption of telemedicine and all aspects of digital health, and this new reality is now likely to define medicine in the future not only in cardiology but also in other branches of medicine.
Economic aspects
In addition to primary analyses focusing on cardiovascular outcomes (hospitalizations, cardiovascular death, overall death), another important aspect to consider for the adoption of digital health solutions is their impact on health care expenditure.48 , 49 Owing to the outbreak of the SARS-CoV-2, a prompt reorganization of health care services was necessary with a related new economic-financial business plan.
The TARIFF study demonstrated that the overall mean annual cost per patient for in-office follow-up was significantly higher than an RM-based one (−53.87% in the RM group). The main reason for cost reduction is due to the cost of cardiovascular hospitalizations (€ 886.67 ± €1979.13 vs €432.34 ± €2488.10; P = .0030).50 The same findings were reported in the EVOLVO study, a multicenter clinical trial aiming at measuring the benefits of RM for HF patients with ICDs. The results of this study showed that RM was cost-effective with an average saving of €888.10 per patient.48 Notably, cost-effectiveness between countries varied considerably depending on whether there was specific reimbursement for RM services. In fact, there was heterogeneity among countries, with RM generating less profits for providers in the absence of specific reimbursements and similar or increased profits in cases such reimbursements existed.51 Indeed, according to a recent European survey, the absence of reimbursement in many countries is generally considered the major barrier to the implementation of RM in standard practice.20
RM was cost-effective for health care systems because of lower follow-up costs and hospitalization reductions; the future challenge will be a more uniform deployment of appropriate reimbursement systems.
Summary
The COVID-19 pandemic imposed challenges to the traditional rules of access and delivery of health care worldwide.52 It accelerated the adoption of telemedicine and digital health, confirming a new era in the management of CIED patients. Patient outcomes could be improved with device-based intensive monitoring compared with traditional in-clinic follow-up at regular intervals.53 The pandemic experience promoted the search for alternative solutions for an effective patient follow-up, such as validation of digital technologies, data management strategies, implementation of predictive analytics, cybersecurity, development of limited forms of remote CIED programming, and reimbursement.19 , 51 , 54
Clinics care points
-
•
Remote Monitoring should be proposed in all CIED patients.
-
•
Remote Monitoring is safe and effective also during COVID pandemic.
-
•
Virtual Visit might be used in patients with multiple cardiac comorbidities.
Acknowledgments
Disclosures
The authors have nothing to disclose.
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piro A., Magnocavallo M., Della Rocca D.G., et al. Management of cardiac implantable electronic device follow-up in COVID-19 pandemic: lessons learned during Italian lockdown. J Cardiovasc Electrophysiol. 2020;31:2814–2823. doi: 10.1111/jce.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varma N., Marrouche N.F., Aguinaga L., et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. EP Europace. 2021;23:313. doi: 10.1093/europace/euaa187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohanty S., Lakkireddy D., Trivedi C., et al. Creating a safe workplace by universal testing of SARS-CoV-2 infection in asymptomatic patients and healthcare workers in the electrophysiology units: a multi-center experience. J Interv Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Della Rocca D.G., Magnocavallo M., Lavalle C., et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Della Rocca D.G., Santini L., Forleo G.B., et al. Novel perspectives on arrhythmia-induced cardiomyopathy: pathophysiology, clinical manifestations and an update on invasive management strategies. Cardiol Rev. 2015;23:135–141. doi: 10.1097/CRD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q., Xu J., Gianni C., et al. Simple electrocardiographic criteria for rapid identification of wide qrs complex tachycardia: the new limb lead algorithm. Heart Rhythm. 2020;17:431–438. doi: 10.1016/j.hrthm.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Forleo G.B., Panattoni G., Schirripa V., et al. Device monitoring of heart failure in cardiac resynchronization therapy device recipients: a single-center experience with a novel multivector impedance monitoring system. J Cardiovasc Med. 2013;14:726–732. doi: 10.2459/JCM.0b013e3283650587. [DOI] [PubMed] [Google Scholar]
- 9.Ong M.K., Romano P.S., Edgington S., et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition–heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. 2016;176:310. doi: 10.1001/jamainternmed.2015.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García-Fernández F.J., Osca Asensi J., Romero R., et al. Safety and efficiency of a common and simplified protocol for pacemaker and defibrillator surveillance based on remote monitoring only: a long-term randomized trial (RM-ALONE) Eur Heart J. 2019;40:1837–1846. doi: 10.1093/eurheartj/ehz067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pignalberi C., Mariani M.V., Castro A., et al. Sporadic high pacing and Shock impedance on remote monitoring in Hybrid implantable cardioverter-Defibrillator systems: clinical impact and management. Heart Rhythm. 2021;18:1292–1300. doi: 10.1016/j.hrthm.2021.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Boehmer J.P., Hariharan R., Devecchi F.G., et al. A multisensor algorithm predicts heart failure events in patients with implanted devices. JACC: Heart Fail. 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G., Taborsky M., Glikson M., et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a Randomised Controlled trial. Lancet. 2014;384:583–590. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 14.AIAC Ricerca Network Investigators. Boriani G., Palmisano P., Guerra F., et al. Impact of COVID-19 pandemic on the clinical activities related to arrhythmias and electrophysiology in Italy: results of a survey promoted by AIAC (Italian association of arrhythmology and cardiac pacing) Intern Emerg Med. 2020;15:1445–1456. doi: 10.1007/s11739-020-02487-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollander J.E., Carr B.G. Virtually perfect? Telemedicine for covid-19. N Engl J Med. 2020;382:1679–1681. doi: 10.1056/NEJMp2003539. [DOI] [PubMed] [Google Scholar]
- 16.Lakkireddy D.R., Chung M.K., Gopinathannair R., et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the heart rhythm Society COVID-19 Task Force; electrophysiology section of the American College of cardiology; and the Electrocardiography and arrhythmias Committee of the Council on clinical cardiology, American heart association. Heart Rhythm. 2020;17:e233–e241. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slotwiner D., Varma N., Akar J.G., et al. HRS expert consensus statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm. 2015;12:e69–e100. doi: 10.1016/j.hrthm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Glikson M., Nielsen J.C., Kronborg M.B., et al. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021;2021 doi: 10.1093/eurheartj/ehab699. ehab364. [DOI] [PubMed] [Google Scholar]
- 19.Saxon L.A., Varma N., Epstein L.M., et al. Factors influencing the decision to proceed to firmware upgrades to implanted pacemakers for cybersecurity risk mitigation. Circulation. 2018;138:1274–1276. doi: 10.1161/CIRCULATIONAHA.118.034781. [DOI] [PubMed] [Google Scholar]
- 20.Mairesse G.H., Braunschweig F., Klersy K., et al. Implementation and reimbursement of remote monitoring for cardiac implantable electronic devices in Europe: a survey from the health economics Committee of the European heart rhythm association. EP Europace. 2015;17:814–818. doi: 10.1093/europace/euu390. [DOI] [PubMed] [Google Scholar]
- 21.Della Rocca Domenico G., Albanese M., Placidi F., et al. Feasibility of automated detection of sleep apnea using implantable pacemakers and defibrillators: a comparison with simultaneous polysomnography recording. J Interv Card Electrophysiol. 2019;56:327–333. doi: 10.1007/s10840-019-00631-x. [DOI] [PubMed] [Google Scholar]
- 22.Forleo G.B., Tesauro M., Panattoni G., et al. Impact of continuous intracardiac st-segment monitoring on mid-term outcomes of ICD-Implanted patients with coronary artery disease. Early results of a prospective comparison with conventional ICD outcomes. Heart. 2012;98:402–407. doi: 10.1136/heartjnl-2011-300801. [DOI] [PubMed] [Google Scholar]
- 23.Dang S., Dimmick S., Kelkar G. Evaluating the evidence Base for the Use of home telehealth remote monitoring in Elderly with heart failure. Telemed e-Health. 2009;15:783–796. doi: 10.1089/tmj.2009.0028. [DOI] [PubMed] [Google Scholar]
- 24.The European Society for Cardiology. ESC Guidance for the Diagnosis and Management of CV Disease during the COVID-19 Pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology Availlable at:
- 25.Han J.K., Al-Khatib S.M., Albert C.M. Changes in the digital health landscape in cardiac electrophysiology: a pre-and peri-pandemic COVID-19 era survey. Cardiovasc Digital Health J. 2021;2:55–62. doi: 10.1016/j.cvdhj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simovic S., Providencia R., Barra S., et al. The use of remote monitoring of cardiac implantable devices during the COVID-19 pandemic: an EHRA physician survey. EP Europace. 2021 doi: 10.1093/europace/euab215. euab215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnocavallo M., Bernardini A., Mariani M.V., et al. Home delivery of the communicator for remote monitoring of cardiac implantable devices: a multicenter experience during the Covid-19 lockdown. Pacing Clin Electrophysiol. 2021;44:995–1003. doi: 10.1111/pace.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Larochellière H., Champagne J., Sarrazin J.-F., et al. Findings of remote monitoring of implantable cardioverter defibrillators during the COVID-19 pandemic. Pacing Clin Electrophysiol. 2020;43:1366–1372. doi: 10.1111/pace.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham W.T., Compton S., Haas G., et al. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the fluid accumulation status trial (FAST) Congest Heart Fail. 2011;17:51–55. doi: 10.1111/j.1751-7133.2011.00220.x. [DOI] [PubMed] [Google Scholar]
- 30.Boehmer J.P., Hariharan R., Devecchi F.G., et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017;5:216–225. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Bagchi S. Telemedicine in Rural India. Plos Med. 2006;3:e82. doi: 10.1371/journal.pmed.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayed S. COVID-19 and diabetes; possible role of polymorphism and rise of telemedicine. Prim Care Diabetes. 2021;15:4–9. doi: 10.1016/j.pcd.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinberg J.S., Varma N., Cygankiewicz I., et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and External cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55–e96. doi: 10.1016/j.hrthm.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 34.Varma N., Epstein A.E., Irimpen A., et al. TRUST investigators efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the lumos-t safely reduces routine office device follow-up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 35.Morichelli L., Porfili A., Quarta L., et al. Implantable cardioverter defibrillator remote monitoring is well Accepted and easy to use during long-Term follow-up. J Interv Card Electrophysiol. 2014;41:203–209. doi: 10.1007/s10840-014-9935-6. [DOI] [PubMed] [Google Scholar]
- 36.Ricci R.P., Morichelli L., Quarta L., et al. Long-term patient Acceptance of and satisfaction with implanted device remote monitoring. Europace. 2010;12:674–679. doi: 10.1093/europace/euq046. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld M.H., Compton S.J., Mead R.H., et al. Remote monitoring of implantable cardioverter defibrillators: a prospective analysis. Pacing Clin Electrophysiol. 2004;27:757–763. doi: 10.1111/j.1540-8159.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 38.Marzegalli M., Lunati M., Landolina M., et al. Remote monitoring of CRT-ICD: the multicenter Italian CareLink evaluation--ease of use, acceptance, and organizational implications. Pacing Clin Electrophysiol. 2008;31:1259–1264. doi: 10.1111/j.1540-8159.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 39.Petersen H.H., Larsen M.C.J., Nielsen O.W., et al. Patient satisfaction and suggestions for improvement of remote ICD monitoring. J Interv Card Electrophysiol. 2012;34:317–324. doi: 10.1007/s10840-012-9675-4. [DOI] [PubMed] [Google Scholar]
- 40.Hindricks G., Elsner C., Piorkowski C., et al. Quarterly vs. Yearly clinical follow-up of remotely monitored recipients of prophylactic implantable cardioverter-defibrillators: results of the REFORM trial. Eur Heart J. 2014;35:98–105. doi: 10.1093/eurheartj/eht207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morichelli L., Ricci R., Sassi A., Quarta L., Porfili A., al CadedduNet. ICD remote monitoring is well Accepted and easy to use even for elderly. Eur Heart J. 2011;32(Suppl. 1):32. [Google Scholar]
- 42.Rosenfeld L.E., Patel A.S., Ajmani V.B., et al. Compliance with remote monitoring of ICDS/CRTDS in a real-world population. Pacing Clin Electrophysiol. 2014;37:820–827. doi: 10.1111/pace.12358. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh A., Gupta R., Misra A. Telemedicine for diabetes care in India during COVID19 pandemic and national lockdown period: guidelines for physicians. Diabetes Metab Syndr Clin Res Rev. 2020;14:273–276. doi: 10.1016/j.dsx.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhai Y.-K., Zhu W.-J., Cai Y.-L., et al. Clinical- and cost-effectiveness of telemedicine in type 2 diabetes mellitus: a systematic review and meta-analysis. Medicine (Baltimore) 2014;93:e312. doi: 10.1097/MD.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters A.L., Garg S.K. The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technology Ther. 2020;22:449–453. doi: 10.1089/dia.2020.0187. [DOI] [PubMed] [Google Scholar]
- 46.Majersik J.J., Reddy V.K. Acute neurology during the COVID-19 pandemic: supporting the front line. Neurology. 2020;94:1055–1057. doi: 10.1212/WNL.0000000000009564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velayati F., Ayatollahi H., Hemmat M. A systematic review of the effectiveness of telerehabilitation interventions for therapeutic purposes in the elderly. Methods Inf Med. 2020;59:104–109. doi: 10.1055/s-0040-1713398. [DOI] [PubMed] [Google Scholar]
- 48.Zanaboni P., Landolina M., Marzegalli M., et al. Cost-utility analysis of the EVOLVO study on remote monitoring for heart failure patients with implantable defibrillators: randomized controlled trial. J Med Internet Res. 2013;15:e106. doi: 10.2196/jmir.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guédon-Moreau L., Lacroix D., Sadoul N., et al. ECOST trial Investigators costs of remote monitoring vs. Ambulatory follow-Ups of implanted cardioverter defibrillators in the Randomized ECOST study. Europace. 2014;16:1181–1188. doi: 10.1093/europace/euu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricci R.P., Vicentini A., D’Onofrio A., et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: results of the health economics evaluation registry for remote follow-up (TARIFF) study. Heart Rhythm. 2017;14:50–57. doi: 10.1016/j.hrthm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 51.Heidbuchel H., Hindricks G., Broadhurst P., et al. EuroEco (European health economic trial on home monitoring in ICD patients): a provider perspective in five european countries on costs and net financial impact of follow-up with or without remote monitoring. Eur Heart J. 2015;36:158–169. doi: 10.1093/eurheartj/ehu339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Udwadia Z.F., Raju R.S. How to protect the protectors: 10 lessons to learn for doctors fighting the COVID-19 Coronavirus. Med J Armed Forces India. 2020;76:128–131. doi: 10.1016/j.mjafi.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hindricks G., Varma N., Kacet S., et al. Daily remote monitoring of implantable cardioverter-defibrillators: Insights from the Pooled patient-level data from three Randomized Controlled trials (IN-TIME, ECOST, TRUST) Eur Heart J. 2017;38:1749–1755. doi: 10.1093/eurheartj/ehx015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slotwiner D.J., Abraham R.L., Al-Khatib S.M., et al. HRS white paper on interoperability of data from cardiac implantable electronic devices (CIEDs) Heart Rhythm. 2019;16:e107–e127. doi: 10.1016/j.hrthm.2019.05.002. [DOI] [PubMed] [Google Scholar]