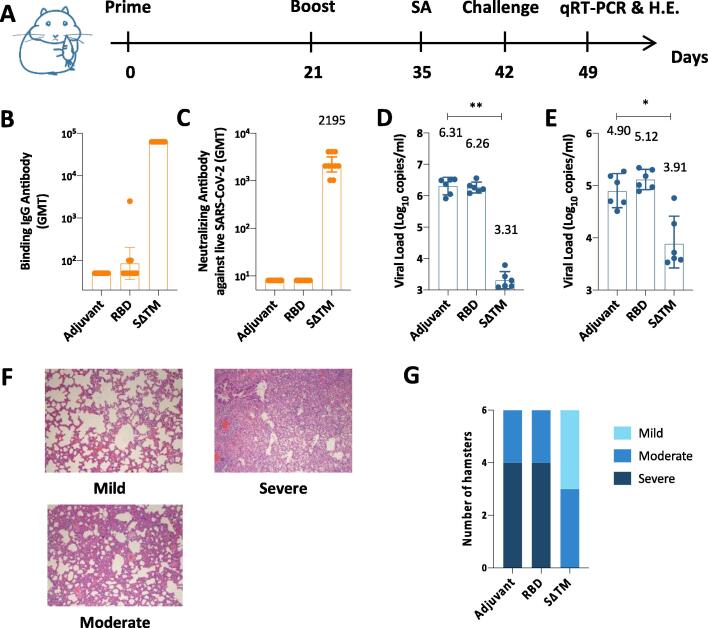
Fig. 5.
Immune responses and protective efficacy in vaccinated golden Syrian hamsters. (A) Experiment schedule. Hamsters from different groups (N = 10) were prime-boost immunized intramuscularly at Day 0 and Day 21. Adjuvant group vaccine contained 100 μg Alum and 100 μg CpG; RBD group contained 10 μg RBD, 100 μg Alum and 100 μg CpG, and SΔTM group contained 10 μg SΔTM, 100 μg Alum and 100 μg CpG. Blood was collected at Day 35 from each group to detect antibody responses. On Day 42, all hamsters were challenged intranasally with 105 TCID50 SARS-CoV-2. On Day 49, a subset of hamsters in each group (N = 6) was euthanized for detecting viral loads of lungs and nasal turbinates by qRT-PCR and evaluating lung histopathology by hematoxylin and eosin (H.E.) staining. (B) Binding antibody titers and (C) live virus neutralizing antibody titers of sera from each group of hamsters at Day 35. Viral loads of lungs (D) and nasal turbinates (E) determined by qRT-PCR. (F) Representative lung pathology scaled as mild, moderate or severe. Microscope images were taken at 100 × magnification. (G) Number of hamsters that displayed mild, moderate or severe lung pathology in each group. Each dot represents an individual hamster. Numbers on the top of each bar represent the value of geometric mean. Statistical analysis was performed using t-test with Welch’s correction. **P < 0.01; ****P < 0.0001.