Abstract
Background
During the ongoing COVID-19 pandemic, immunocompromised patients are at a higher risk of severe infection, since the immune system has an important role in defeating this disease. This study compares the severity of COVID-19 in patients taking methotrexate with the severity of their family members’ illness as patients with normal immune system function.
Methods
A total of 35 participants, including 14 patients taking methotrexate and 21 patients with normal immune function, entered this study, and the indicators of COVID-19 severity were compared between these two groups.
Results
The case group, who were on methotrexate therapy, had significantly less severe COVID-19 based on their symptoms, including fever (p = 0.000) and cough and dyspnea (p = 0.01) as well as in terms of COVID-19 severity indicators such as pulmonary involvement (p = 0.001), ferritin level (p = 0.001), white blood cell count (p = 0.008) and CRP level (p = 0.006), compared to the control group. There was a significant correlation between taking methotrexate and lower severity in COVID-19 disease.
Conclusion
The present findings demonstrated that methotrexate does not predispose patients to severe COVID-19; on the contrary, patients taking methotrexate may experience a milder disease, possibly due to their reduced severe inflammatory reactions as a result of inhibited TNFα, lowered IL6, and increased T regulatory cells. According to these findings, methotrexate appears to be a suitable treatment option for patients who need immunosuppressive medications during the COVID-19 pandemic.
Keywords: Methotrexate, COVID-19, Immunocompromised
1. Introduction
COVID-19 is a viral disease responsible for a major worldwide pandemic since 2019 [1]. By way of an acute pulmonary syndrome, COVID-19 leads to acute respiratory distress syndrome (ARDS), septic shock, and/or multiple organ failure in about 5% of the patients [2]. Direct person-to-person transmission through respiratory particles is the main route of transmission for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Host and virus-related factors such as transmission route, immune status, and a long and variable incubation period have helped the virus spread worldwide and cause many casualties [3]. SARS-Cov-2 binds to the ACE2 receptor, activates signaling pathways and promotes the production of inflammatory cytokines, such as initiating IL-6 release by viral proteins, which activate the transcription factor nuclear factor kappa B (NF-κB), the Janus kinase (JAK)/(STAT), and the (PI3K) pathways, potentially leading to pro-inflammatory cytokine release, interleukin 1β (IL-1β), interleukin 8 (IL-8) and interleukin17 (IL-17)[4]. Since adaptive immune system cells play a key role in fighting this disease [5], immunocompromised patients who acquire respiratory viral infections are at a higher risk of severe infections as well as increased bacterial and fungal infections compared to individuals with normal immune function [6]. The COVID-19 pandemic has affected the management of chronic diseases, such as Alopecia Areata (AA), due to the immune system dysregulation caused by their treatment regimens [7]. AA is an autoimmune disease in which the hair follicles are attacked by the immune system [8], and its severe forms, such as Alopecia Totalis and Universalis, may require treatment with immunosuppressive agents such as Methotrexate (MTX) [9]. MTX is an inhibitor of dihydrofolate reductase, i.e., the enzyme that reduces folate to tetrahydrofolate [10]. MTX suppresses inflammation and the immune response through various mechanisms, such as inhibition of purine and pyrimidine synthesis [11] and inhibition of transmethylation reactions [12], causing adenosine release, nitric oxide synthase uncoupling, regulation of the expression of long noncoding RNAs, inhibition of JAK–STAT signaling and inhibition of NF-κB signaling [13]. MTX is used to treat a wide range of autoimmune diseases, such as rheumatoid arthritis, juvenile idiopathic arthritis, psoriasis, inflammatory bowel disease and many other diseases [14]. This study aims to evaluate the severity of COVID-19 in people with AA on MTX therapy compared to patients with normal immune systems who were not taking any immunosuppressive medications.
2. Patients and Methods
This study was conducted as a retrospective observational cohort study. A total of 48 individuals, including 14 patients receiving MTX (15 mg weekly) for AA and 34 of their first-degree family members who all infected with COVID-19 between March 2020 and February 2021, were initially enrolled in the study. The first round of the statistical analysis revealed a significant difference between the two groups in terms of mean age; therefore, the inclusion and exclusion criteria were defined as follows.
Inclusion criteria: Being a COVID-19 patient with AA taking MTX or being a family member of the said patients, plus age under 45 years. Exclusion criteria: Age over 45 years or having underlying diseases predisposing the patient to severe COVID-19. After excluding those who did not meet the inclusion criteria, 35 participants remained and were divided into two groups: (A) Patients taking MTX were assigned to the case group (n = 14); (B) The family members of these patients were assigned to the control group (n = 21). The indicators of COVID-19 severity were extracted from literature [15], [16], and the two groups were accordingly compared for COVID-19 severity.
2.1. Ethical considerations
This study was reviewed by the ethics committee of Alborz University of Medical Sciences and approved with the code IR.ABZUMS.REC.1400.148. The participants were assured that their personal information would remain confidential.
2.2. Statistical analysis
Statistical analyses were performed in IBM statistics SPSS version 22. Data are expressed as mean ± SD for the continuous or discrete variables and as percentage for the categorical variables. The Chi-square test, Mann-Whitney’s U test, student’s t-test, Welch’s test, and Pearson/Spearman (nonparametric data) correlation tests were used to compare the data between the two groups.
3. Results
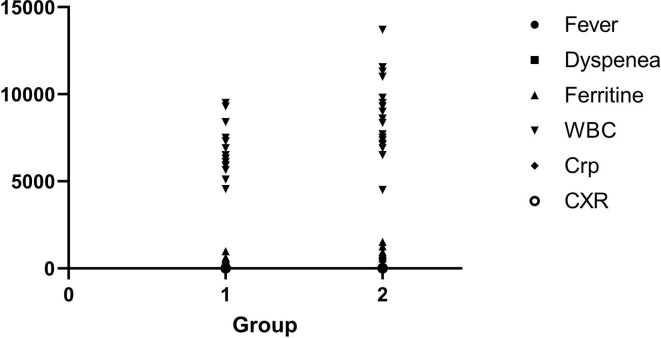
A total of 48 cases, including 14 patients on MTX therapy and 34 of their first-degree family members, were enrolled in this study, but after excluding the cases aged over 45 years, 35 cases remained for the comparisons (14 patients and 21 family members). There were no differences between the case and control groups in terms of age and gender. The mean age of the case group was 29.14 ± 8.637 years and the mean age of the control group was 27.67 ± 9.676 years (p = 0.648). Gender distribution in the groups was as follows: Four males (28.6%) and ten females (47.6%) in the case group, and ten males (71.4%) and 11 females (52.4%) in the control group (p = 0.267). The case group, which was on MTX therapy, had significantly less severe symptoms such as fever, cough, and dyspnea. The patients taking MTX also had significantly less severe COVID-19 compared to the control group, as determined by the following indicators: Pulmonary involvement (CXR abnormality), ferritin level, white blood cell count and C-Reactive Protein (CRP) level. Nevertheless, the two groups did not differ significantly in terms of lymphocyte count (Table 1 ). There were no significant differences between the two groups in terms of hospital stay, and none of the patients died of COVID-19 in any of the groups. Pearson/Spearman correlation test revealed a significant correlation between the use of low-dose MTX and a decrease in COVID-19 severity indicators, including fever (p ≤ 0.0001), dyspnea (p = 0.0082), ferritin level (p = 0.0013), WBC (p = 0.0079), CRP (p = 0.0064), and CXR (p = 0.0005) (Fig. 1 ).
Table 1.
Comparison of Alopecia Areata patients and their family members during their development of COVID-19.
| Case Group (Patients) | Control Group (Family) | P-Value | ||
|---|---|---|---|---|
| Age | 29.14 ± 8.637 | 27.67 ± 9.676 | 0.648 | |
| Gender |
Male | 4 (28.6%) | 10 (71.4%) | 0.267 |
| Female | 10 (47.6%) | 11 (52.4%) | ||
| Number of patients | 14 | 21 | ||
| Fever | 6 (42.8%) | 21(100%) | 0.000* | |
| Cough or dyspnea | 10 (71.4%) | 21(100%) | 0.01* | |
| Ferritin | 436.21 ± 190 | 730.62 ± 271.8 | 0.001* | |
| WBCa | 6778.57 ± 1482.42 | 8590.48 ± 2063.83 | 0.008* | |
| Lymphocyte | 1203.57 ± 199.985 | 1084.05 ± 186.062 | 0.08 | |
| CRPb | 73.64 ± 32.131 | 113.19 ± 43.438 | 0.006* | |
| CXR abnormality | 8 (57.1%) | 21(100%) | 0.001* | |
: White Blood Cells,
: C-Reactive Protein.
Fig. 1.
Pearson/Spearman correlation analysis of fever, dyspnea, ferritin level, WBC, CRP, and CXR (representative of pulmonary involvement) Group1: Patient who taking MTX, Group2: Control group. WBC (White Blood Cells), CRP (C-Reactive Protein), CXR (Chest X-ray).
3.1. Discussion
COVID-19 is a major concern for patients taking immunosuppressive medications [17]. Some studies have shown that immunocompromised patients are more vulnerable to comorbidities than the general population and suffer worse outcomes when admitted to the hospital for COVID-19 [18]. According to some studies [19] and given the viral nature of the disease [20], patients on MTX therapy were expected to experience more severe COVID-19 and worse outcomes than patients with intact immune systems. This study examined a sample of 35 patients with AA and their healthy family members who had developed COVID-19. The results showed that the symptoms of COVID-19 were significantly less severe in the patients with AA on MTX compared to their family members. As genetic diversity may affect disease severity [21], the control group was selected from the family members of patients receiving MTX to minimize the confounding effect of genetic diversity.
The patients in the control group, who did not take MTX, had a higher rate of fever, cough, dyspnea, pulmonary involvement, ferritin level, CRP level and white blood cell count than the patients with AA taking MTX. Other studies have also been conducted on a similar subject. A study in France on patients with inflammatory rheumatic and musculoskeletal diseases showed that corticosteroids made patients more susceptible to more severe disease; however, taking MTX, tumor necrosis factor alpha (TNF-α) inhibitors and IL-6 inhibitors were not associated with more severe disease [22]. A possible explanation might be the mechanisms of cytokine storm induction by the SARS-CoV-2 virus and the mechanisms of improvement of these effects by low-dose MTX.
The SARS-Cov2 virus activates the inflammatory cascade upon entry into the cells by inducing NF-κB, which stimulates the production of other inflammatory cytokines as well [4]. The virus also reduces the response of type-I interferons, which play an important role in antiviral activity [23]. MTX has reversal effects on these processes, e.g., it inhibits the activated T cells which play an important role in creating cytokine storms, as well as the expression of adhesion molecules [24], [25]. MTX can also reduce severe inflammatory reactions by inhibiting TNFα, reducing IL6, and increasing T regulatory cells [26]. TNF-α is produced by several cell types, including macrophages, mast cells, T cells, epithelial cells, and smooth muscle cells in the airways. Its synthesis is mainly stimulated by pathogen-associated molecular patterns and IL-1 via NF-κB activation and IL-17 [27]. TNF-α triggers bronchial hyper-responsiveness and inflammation of the airways and also promotes the production of other cytokines, such as IL-1β and IL-6 [28]. MTX reduces TNF-alpha synthesis in macrophages and T cells [29] and reduces their activity by inhibiting NF-κB [30].
IL6 is expressed in many cell types, such as T and B lymphocytes, monocytes/macrophages, dendritic cells, fibroblasts, and endothelial cells [31]. IL6 stimulates the growth and differentiation of B lymphocytes and increases the generation of platelets. It also activates the hepatocytes and induces the secretion of inflammation proteins such as CRP and fibrinogen [32]. MTX has been shown to reduce IL6 and could be one of the mechanisms involved in the reduced inflammatory response observed in patients [33]. IL-1β is one of the most important inflammatory pleiotropic cytokines that plays an important role in inflammatory diseases and seems to be involved in COVID-19 cytokine release syndrome [34]. Studies have shown that low-dose MTX can reduce IL1 β production [35].
IL-17 and TNF-α induce the expression of pro-coagulation factors, thereby stimulating thrombosis and inhibiting the endothelial anticoagulation pathways. IL-17 seems to increase the replication of some viruses and lead to viral persistence [4]. MTX has been reported to reduce IL-17 levels [36].
In the present study, a significant correlation was observed between MTX use and reduction in disease severity, which is consistent with the anti-inflammatory effects of MTX mentioned earlier. An in-vitro study also reported a significant dose-dependent inhibition of SARS-CoV-2 virus replication in Vero E6 cells by MTX at concentrations of 25, 2.5, or 0.25 µM [37].
In accordance with the present study, Smitten et al. (2008) also examined the association of nosocomial infections in patients with rheumatoid arthritis and a control group, and reported that patients taking oral corticosteroids had an increased risk of nosocomial infections, but taking MTX and hydroxychloroquine was associated with a reduced risk of nosocomial infections [38]. Ibrahim et al. (2019) conducted a systematic review and meta-analysis of randomized controlled trials to determine the risk of infection in adults with Inflammatory Rheumatic Disease (IRD) treated with MTX. Their results showed that MTX generally does not increase the risk of infection in patients with IRD, except in the case of rheumatoid arthritis. Furthermore, they reported no significant difference in the risk of general infections and serious infections in patients with IRD compared to the control group [39].
4. Conclusion
The present findings showed that taking low dose Methotrexate does not predispose patients to severe COVID-19; on the contrary, patients taking low dose MTX may experience a milder form of the disease. According to these results, MTX appears to be a suitable option for patients who need immunosuppressive medications during the COVID-19 pandemic, although further studies are required to determine the effectiveness of this medication against COVID-19 and to safely recommend it as a treatment option for this viral disease.
Study limitation
In this study, only patients with alopecia areata who received MTX were compared with the control group, and it is suggested that in future studies, patients from different groups of autoimmune diseases be compared with a control group. Patients receiving MTX in this study all received low doses of MTX, so this study is not generalizable to all patients taking MTX and more studies should be performed on patients receiving high doses of MTX.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Zakiye Ganjei: Conceptualization, Methodology, Project administration, Data curation. Hoorvash Faraji Dana: Supervision, Validation. Sepehr Ebrahimi-Dehkordi: Visualization, Writing – original draft. Fereshte Alidoust: Visualization, Writing – original draft. Kiumars Bahmani: Formal analysis, Investigation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.W.H. Organization, WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020, 2020.
- 2.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20(5):269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyerowitz E.A., Richterman A., Gandhi R.T., Sax P.E. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann. Intern. Med. 2020 doi: 10.7326/M20-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darif D., Hammi I., Kihel A., Saik I.E.I., Guessous F., Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: What goes wrong? Microb. Pathog. 2021;104799 doi: 10.1016/j.micpath.2021.104799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swain S.L., McKinstry K.K., Strutt T.M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 2012;12(2):136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuel O., Estabrook M. A.S.o.T.I.D.C.o. Practice, RNA respiratory viral infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019;33(9) doi: 10.1111/ctr.13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres T., Puig L. Managing cutaneous immune-mediated diseases during the COVID-19 pandemic. Am. J. Clin. Dermatol. 2020;21(3):307–311. doi: 10.1007/s40257-020-00514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. Hordinsky, M. Ericson, Autoimmunity: alopecia areata, Journal of Investigative Dermatology Symposium Proceedings, Elsevier, 2004, pp. 73–78. [DOI] [PubMed]
- 9.Asilian A., Fatemi F., Ganjei Z., Siadat A.H., Mohaghegh F., Siavash M. Oral Pulse Betamethasone, Methotrexate, and Combination Therapy to Treat Severe Alopecia Areata: A Randomized, Double-blind, Placebo-controlled, Clinical Trial. Iranian J. Pharm. Res. 2021;20(1):267–273. doi: 10.22037/ijpr.2020.113868.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyoun S.C., Običan S.G., Scialli A.R. Teratogen update: methotrexate, Birth Defects Research Part A: Clinical and Molecular. Teratology. 2012;94(4):187–207. doi: 10.1002/bdra.23003. [DOI] [PubMed] [Google Scholar]
- 11.Steffen J., Stolzmann W. Studies on in vitro lymphocyte proliferation in cultures synchronized by the inhibition of DNA synthesis: I. Variability of S plus G2 periods of first generation cells. Exp. Cell Res. 1969;56(2–3):453–460. doi: 10.1016/0014-4827(69)90039-1. [DOI] [PubMed] [Google Scholar]
- 12.Chan E.S., Cronstein B.N. Methotrexate—how does it really work? Nat. Rev. Rheumatol. 2010;6(3):175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 13.Cronstein B.N., Aune T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020;16(3):145–154. doi: 10.1038/s41584-020-0373-9. [DOI] [PubMed] [Google Scholar]
- 14.Bedoui Y., Guillot X., Sélambarom J., Guiraud P., Giry C., Jaffar-Bandjee M.C., Ralandison S., Gasque P. Methotrexate an old drug with new tricks. Int. J. Mol. Sci. 2019;20(20):5023. doi: 10.3390/ijms20205023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang K. 2020 International Conference on Public Health and Data Science (ICPHDS), IEEE. 2020. Risk factors and indicators for COVID-19 severity: Clinical severe cases and their implications to prevention and treatment; pp. 333–337. [Google Scholar]
- 16.Vargas-Vargas M., Cortés-Rojo C. Ferritin levels and COVID-19. Revista Panamericana de Salud Pública. 2020;44 doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L., Xu X., Ma K., Yang J., Guan H., Chen S., Chen Z., Chen G. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am. J. Transplant. 2020;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belsky J.A., Tullius B.P., Lamb M.G., Sayegh R., Stanek J.R., Auletta J.J. COVID-19 in immunocompromised patients: A systematic review of cancer, hematopoietic cell and solid organ transplant patients. J. Infect. 2021 doi: 10.1016/j.jinf.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vijenthira A., Gong I.Y., Fox T.A., Booth S., Cook G., Fattizzo B., Martín-Moro F., Razanamahery J., Riches J.C., Zwicker J. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients Blood. J. Am. Soc. Hematol. 2020;136(25):2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Englund J., Feuchtinger T., Ljungman P. Viral infections in immunocompromised patients. Biol. Blood Marrow Transplant. 2011;17(1):S2–S5. doi: 10.1016/j.bbmt.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F., Huang S., Gao R., Zhou Y., Lai C., Li Z., Xian W., Qian X., Li Z., Huang Y. Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discovery. 2020;6(1):1–16. doi: 10.1038/s41421-020-00231-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.F.R.S.S.S.C.I. consortium, contributors, Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients, Ann. Rheum. Dis. 80(4) (2021) 527–538. [DOI] [PMC free article] [PubMed]
- 23.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston A., Gudjonsson J.E., Sigmundsdottir H., Ludviksson B.R., Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin. Immunol. 2005;114(2):154–163. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Fajgenbaum D.C., June C.H. Cytokine storm. N. Engl. J. Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsufyani H.S., Rawas W.A., Alsufyani S.S., Alsaadi N.F., Felemban M.S., Awadain J.A., Albaqami M.M.A., Alasmari A.M., Halawani M.A.A., Alsaedi W.H. The effect of mehotrexate in the treatment of alopecia areata. Egyptian J. Hospital Med. 2017;67(2):599–604. [Google Scholar]
- 27.J.L. Muñoz-Carrillo, J.F. Contreras-Cordero, O. Gutiérrez-Coronado, P.T. Villalobos-Gutiérrez, L.G. Ramos-Gracia, V.E. Hernández-Reyes, Cytokine profiling plays a crucial role in activating immune system to clear infectious pathogens, Immune response activation and immunomodulation, IntechOpen2018.
- 28.Peiris J., Lai S., Poon L., Guan Y., Yam L., Lim W., Nicholls J., Yee W., Yan W., Cheung M. Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet. 2003;361(9366):1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker C., Barbulescu K., Hildner K., Zum büschenfelde K.H.M., Neurath M.F. Activation and Methotrexate-Mediated Suppression of the TNFα Promoter in T Cells and Macrophages. Ann. N. Y. Acad. Sci. 1998;859(1):311–314. doi: 10.1111/j.1749-6632.1998.tb11153.x. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar S., Aggarwal B.B. Methotrexate suppresses NF-κB activation through inhibition of IκBα phosphorylation and degradation. J. Immunol. 2001;167(5):2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 31.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18(12):773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspect. Biol. 2014;6(10) doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aggarwal A., Misra R. Methotrexate inhibits interleukin-6 production in patients with juvenile rheumatoid arthritis. Rheumatol. Int. 2003;23(3):134–137. doi: 10.1007/s00296-002-0267-y. [DOI] [PubMed] [Google Scholar]
- 34.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 35.Novaes G., Mello S., Laurindo I., Cossermelli W. Low dose methotrexate decreases intraarticular prostaglandin and interleukin 1 levels in antigen induced arthritis in rabbits. J. Rheumatol. 1996;23(12):2092–2097. [PubMed] [Google Scholar]
- 36.Li Y., Jiang L., Zhang S., Yin L., Ma L., He D., Shen J. Methotrexate attenuates the Th17/IL-17 levels in peripheral blood mononuclear cells from healthy individuals and RA patients. Rheumatol. Int. 2012;32(8):2415–2422. doi: 10.1007/s00296-011-1867-1. [DOI] [PubMed] [Google Scholar]
- 37.Caruso A., Caccuri F., Bugatti A., Zani A., Vanoni M., Bonfanti P., Cazzaniga M.E., Perno C.F., Messa C., Alberghina L. Methotrexate inhibits SARS-CoV-2 virus replication “in vitro”. J. Med. Virol. 2021;93(3):1780–1785. doi: 10.1002/jmv.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smitten A.L., Choi H.K., Hochberg M.C., Suissa S., Simon T.A., Testa M.A., Chan K.A. The risk of hospitalized infection in patients with rheumatoid arthritis. J. Rheumatol. 2008;35(3):387–393. [PubMed] [Google Scholar]
- 39.Ibrahim A., Ahmed M., Conway R., Carey J.J. Risk of infection with methotrexate therapy in inflammatory diseases: a systematic review and meta-analysis. J. Clin. Med. 2019;8(1):15. doi: 10.3390/jcm8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]