Abstract
Adversity exposure is a risk factor for psychopathology, which most frequently onsets during adolescence, and prior research has demonstrated that alterations in cortico-limbic connectivity may account in part for this association. In a sample of youth from the Adolescent Brain Cognitive Development (ABCD) Study (N = 4006), we tested a longitudinal structural equation model to examine the indirect effect of adversity exposure (negative life events) on later psychopathology via changes in cortico-limbic resting-state functional connectivity (rsFC). We also examined the potential protective effects of parental acceptance. Generally, cortico-limbic connectivity became more strongly negative between baseline and year 2 follow-up, suggesting that stronger negative correlations within these cortico-limbic networks may reflect a more mature phenotype. Exposure to a greater number of negative life events was associated with stronger negative cortico-limbic rsFC which, in turn, was associated with lower internalizing (but not externalizing) symptoms. The indirect effect of negative life events on internalizing symptoms via cortico-limbic rsFC was significant. Parental acceptance did not moderate the association between negative life events and rsFC. Our findings highlight how stressful childhood experiences may accelerate neurobiological maturation in specific cortico-limbic connections, potentially reflecting an adaptive process that protects against internalizing problems in the context of adversity.
Keywords: Adversity, Cortico-limbic, Psychopathology, Resting-state fMRI
Highlights
-
•
Childhood adversity shapes cortico-limbic connectivity and mental health.
-
•
In the ABCD Study, cortico-limbic functional connectivity changed over time.
-
•
These changes explain the association between adversity and internalizing symptoms.
-
•
Adversity exposure may accelerate corticolimbic development.
1. Introduction
Exposure to adversity during childhood and adolescence is highly prevalent, with more than half of individuals experiencing at least one adverse event prior to age 18 (McLaughlin, 2016, Merrick et al., 2018). Experiences such as maltreatment, parental separation, family financial stress, and parental mental illness can disrupt developmental processes and have consequences across multiple domains of development, shaping brain structure and function (Tomalski and Johnson, 2010) as well as mental health outcomes (Felitti et al., 1998, Kessler et al., 2010). Indeed, adversity during childhood accounts for approximately 30% of all mental illness (Green et al., 2010). Accumulating evidence suggests that the association between adversity and mental health outcomes may be explained by alterations in the developing brain (McLaughlin et al., 2016, McLaughlin et al., 2020, Rakesh et al., 2021, VanTieghem and Tottenham, 2018). However, many youth demonstrate trajectories associated with resilience, and there is a critical need for the identification of precise protective factors and adaptations that mitigate the effects of adversity. Adolescence is a particularly important developmental period during which to examine these associations due to the frequent onset of psychopathology (Kessler et al., 2005, Lee et al., 2014) along with ongoing maturation in stress-sensitive regions of the brain (Casey et al., 2016, Gee and Casey, 2015, Tottenham and Sheridan, 2010).
The impact of adversity on mental health outcomes has been well-documented (e.g., Green et al., 2010; McLaughlin et al., 2010); however, it remains unclear what neurobiological mechanisms may underlie this association. Accumulating empirical evidence suggests that aberrant functional connectivity may be a key mechanism, given that the development of neural circuitry can be altered under conditions of stress (Gee et al., 2013a, Herzberg and Gunnar, 2020, Sripada et al., 2014). In particular, connections between the prefrontal cortex and subcortical, limbic regions are highly sensitive to environmental inputs, in part due to dense innervation with glucocorticoid receptors and the developmental timing of circuit maturation (Lupien et al., 2009). Both human and animal studies have demonstrated that chronic stress is associated with functional alterations in amygdala-prefrontal-hippocampal circuitry (Eiland et al., 2012, Weems et al., 2019). For example, greater severity of early life stress has been linked to more negative amygdala-dorsolateral prefrontal cortex (dlPFC) resting-state functional connectivity (rsFC) (Herringa et al., 2013, Kaiser et al., 2018). These connections between top-down executive networks and subcortical, affective systems underlie cognitive and emotional processing and support successful regulation of behavior and affective states. For example, the cingulo-opercular (CO) network, which includes the dorsal anterior cortex (dACC), insula, and anterior prefrontal cortex, supports cognitive control (Dosenbach et al., 2007), and projections between this network and subcortical regions such as the hippocampus and amygdala comprise key emotion circuits that may underlie psychiatric disorders (Sylvester et al., 2020). Thus, development of CO-hippocampus and CO-amygdala rsFC may be a potential mechanism linking youth adversity and levels of psychopathology.
Cortico-limbic circuits demonstrate age-related changes (Gabard-Durnam et al., 2014, Gee et al., 2013b, Silvers et al., 2017, Wu et al., 2016), with acute development and reorganization during adolescence (Casey et al., 2019, Stevens, 2016). Theoretical and empirical work across species suggests that exposure to adversity may accelerate these patterns of maturation (Callaghan and Tottenham, 2016, Gee et al., 2013a, Herzberg et al., 2021, Miller et al., 2020, Silvers et al., 2016, Thijssen et al., 2017). For example, stressful family environments have been linked to accelerated pubertal maturation, which in turn was associated with altered CO-amygdala rsFC, among youth in the Adolescent Brain Cognitive Development Study℠ (ABCD Study®; Thijssen et al., 2020a, Thijssen et al., 2020b). In this way, accelerated maturation may reflect adaptation to stressful environments and promote short-term resilience to mental health problems. At the same time, other work has suggested that adversity exposure is associated with delays or weakening connectivity in certain circuits, and in turn, increased risk for psychopathology (e.g., Rakesh et al., 2021). Much of this work has relied on cross-sectional designs (e.g., Gee et al., 2013a; Herzberg et al., 2021; Silvers et al., 2016; Thijssen et al., 2020a, Thijssen et al., 2020b). Additional research, especially with longitudinal neuroimaging, is necessary to disentangle the directions of these associations across development. Similarly, the literature is relatively mixed regarding the valence of changes in rsFC across development. Both strengthening and weakening of functional connectivity have been observed (Fair et al., 2010, Stevens, 2016) depending on the specific circuit under consideration, imaging modality, and the age of participants. For this reason, it can be difficult to identify deviations from expected patterns of maturation (e.g., acceleration versus delay) in a given circuit. Further research with repeated measures of rsFC is necessary to identify typical directionality of changes in connectivity, as well as deviations from typical trajectories.
Functional alterations in neural circuitry may have downstream consequences for youth psychosocial adjustment. Indeed, the same circuits that are highly sensitive to stress are also implicated in a host of psychopathology outcomes in youth. For example, aberrant patterns of cortico-limbic rsFC may underlie both internalizing (Rakesh et al., 2021, VanTieghem and Tottenham, 2018) and externalizing (Rubia, 2011, Silveira et al., 2021, Thijssen et al., 2020a, Thijssen et al., 2020b) symptomatology. More specifically, connectivity between the CO network and subcortical regions has been linked to psychopathology among youth in the ABCD Study. In cross-sectional analyses, Lees et al. (2021) found that hyperconnectivity between the CO network and the putamen was associated with heightened internalizing symptomatology among 9–10 year-olds. However, research has not yet examined CO connectivity with the stress-sensitive limbic system, as well as how developmental changes over time in functional connections may contribute to psychopathology outcomes.
Not all youth who are exposed to adversity demonstrate alterations in neurobiology. Indeed, many children and adolescents demonstrate resilient trajectories, and there is a critical need for the identification of precise protective factors that mitigate the effects of adversity on neurodevelopment, which may ultimately disrupt pathways to psychopathology. In particular, features of the home and family environment may confer resilience to stressors. Prior research points to the benefits of parental support and caregiving behaviors in the development of higher-order cognitive processes and emotion regulation (Deater-Deckard, 2014, Morris et al., 2017). Parenting that is characterized by high sensitivity, warmth, and emotional support may offset the potentially negative consequences of adversity exposure (Whittle et al., 2017). For example, initial evidence indicates that supportive parenting can buffer against the effects of adolescent poverty on rsFC in networks involved in emotion regulation and executive control (Brody et al., 2019).
Taken together, prior research demonstrates that adversity is associated with individual differences in cortico-limbic circuitry that may underlie internalizing and externalizing symptomatology. However, only a few studies have integrated measures of adversity, functional connectivity, and psychopathology in order to formally investigate potential mediating effects (Barch et al., 2018, Callaghan et al., 2017, Rakesh et al., 2021, Silveira et al., 2021). As one example, Rakesh et al. (2021) found that longitudinal increases in between-network connectivity (e.g., between the default mode network, frontoparietal network, dorsal attention network, and salience network) mediated the association between maltreatment history and adolescent depressive symptoms. This type of prospective, longitudinal design and repeated measures of resting-state functional connectivity is rare, but critical in order to appropriately delineate the neurobiological and clinical sequelae of adversity. The ABCD Study (Casey et al., 2018) is uniquely positioned to elucidate these developmental processes and address limitations of prior research (Karcher and Barch, 2021). Specifically, the longitudinal design of this multi-site study, repeated neuroimaging measures, and unprecedented sample size offer novel opportunities to advance our understanding of risk and resilience in neurodevelopment and mental health outcomes. To this end, we utilized data from the ABCD Study at baseline, year 1 follow-up, and year 2 follow-up in order to test whether adversity was associated with later psychopathology via changes in cortico-limbic connectivity. Consistent with the stress acceleration hypothesis, we hypothesized that greater stress exposure would be associated with more mature patterns of rsFC between the CO network and the amygdala and hippocampus, which in turn would be associated with lower internalizing and externalizing symptomatology. Furthermore, we expected that parental acceptance would moderate the association between adversity and cortico-limbic rsFC such that the effect of adversity would be weaker for adolescents with greater parental acceptance.
2. Method
2.1. Participants
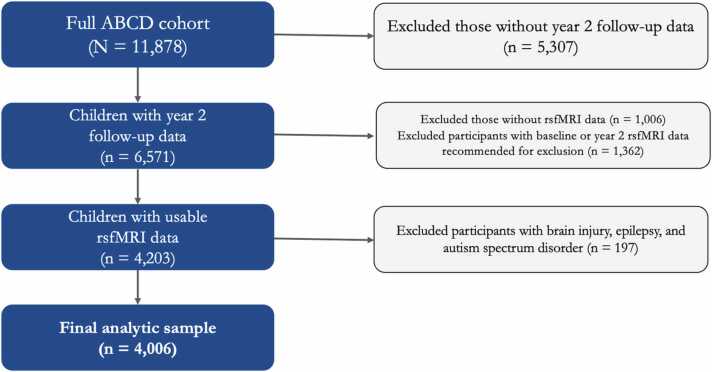
Data were drawn from the Adolescent Brain Cognitive Development (ABCD) Study, an ongoing longitudinal study of 11,878 children across 21 study sites in the United States (Casey et al., 2018). We used data from the 3.0 release (DOI:10.15154/1519007) which includes baseline data, year 1 data, and approximately half of the anticipated year 2 data (the year 2 follow-up data collection is actively underway as of this writing, and thus was not available for analysis). Participants were excluded if i) their year 2 follow-up data were not yet released, ii) they did not have resting-state functional magnetic resonance imaging (rsfMRI) data, iii) their rsfMRI data were recommended for exclusion by the ABCD analytic core (detailed in ABCD 3.0 release notes), or iv) they had a history of brain injury, epilepsy, or autism spectrum disorder. These criteria are detailed in Fig. 1 and resulted in a final sample of N = 4006. Participants in the final sample (51% male) were 9–10 years old at baseline, 10–11 years old at year 1 follow-up, and 11–12 years old at year 2 follow-up. Median household income fell between $75,000 - $99,000. Participants identified as White (58%), Black (11%), Hispanic (20%), Asian (2%), or another race (10%). There were small but significant differences between participants who were included versus excluded, such that those who were included were more likely to be White (χ2 = 89.07, p < .001), female (χ2 = 4.54, p = .03), have higher income (t10287.85 = −6.38, p < .001), have higher parent education (t10854.20 = −5.79, p < .001), and report fewer negative life events (t10462.60 = 4.14, p < .001). All participants provided informed consent or assent (see Clark et al., 2018 for ethics and oversight in the ABCD Study). The participant IDs included in these analyses, as well as further details on the measures used, can be found in this project’s NDA study (DOI 10.15154/1522544).
Fig. 1.
Inclusion and exclusion criteria for the present study’s analytic sample.
2.2. Measures
2.2.1. Negative life events
At the year 1 follow-up, children completed the Life Events Scale (Grant et al., 2004, Hoffman et al., 2019, Tiet et al., 1998), a 26-item measure that describes a variety of experiences. Children are asked to report whether or not they have ever experienced a given event, and if so, to report if the event was “mostly good” or “mostly bad” for them. Example items include, “Someone in family died”, “Was a victim of crime/violence/assault”, and “Family member had a mental or emotional problem”. We used a sum score of the total number of lifetime events that children endorsed as bad (negative), with higher scores reflecting greater exposure to negative life events.
2.2.2. Parent acceptance
At the year 1 follow-up, children also completed the Children’s Report of Parental Behavior Inventory (CRPBI; Schaefer, 1965). The parental acceptance subscale includes 5 items that are rated on a 3-point Likert-type scale ranging from “0 = Not like him/her” to “3 = A lot like him/her”. Example items include, “My parent is able to make me feel better when I am upset”, “My parent is easy to talk to”, and “My parent believes in showing his/her love for me”. Children completed the CRPBI for the parent/caregiver that participated in the study with them as well as their secondary parent/caregiver (if applicable). The CRPBI demonstrated good reliability in the current sample for both primary caregiver (α = 0.69) and secondary caregiver (α = 0.75) reports. Scores were averaged between primary and secondary caregivers in order to create an overall parental acceptance variable, with higher scores reflecting greater parental acceptance.
2.2.3. Psychopathology
Parents reported on their child’s psychopathology symptoms using the Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001). Symptoms were rated on a 3-point Likert-type scale ranging from “0 = Never” to “2 = Often”. We used T-scores from the internalizing and externalizing symptom subscales to evaluate child psychopathology at year 2 follow-up.
2.2.4. Resting-state cortico-limbic functional connectivity
In the present study, we used rsfMRI data from baseline and the year 2 follow-up. We used indices of functional connectivity from the ABCD Study’s tabulated data, which were pre-processed and analyzed by the consortium’s data analytic core (Hagler et al., 2019) using a seed-based correlational approach (Van Dijk et al., 2010). During pre-processing, T1-weighted images were corrected for gradient non-linearity distortions (Jovicich et al., 2006). Head motion was corrected by registering each frame to the first using AFNI’s 3dvolreg (Cox, 1996) and B0 distortions were corrected using the reversing gradient method (Holland et al., 2010). Mutual information was used to register T2-weighted images to T1-weighted structural images. Initial frames were discarded to ensure equilibration of the T1 signal. Frames with displacement > 0.30 mm were excluded from the regression (Power et al., 2014).
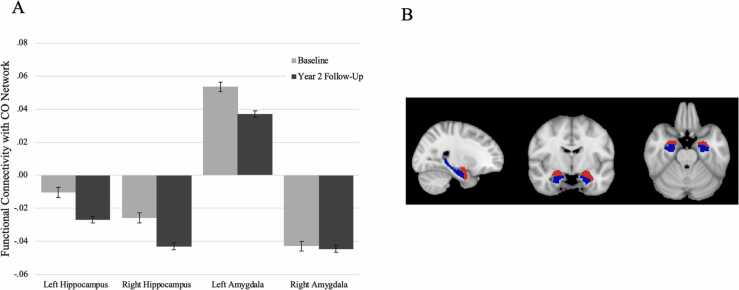
Pre-processed time courses were sampled onto the cortical surface for each participant. Average cortical time courses were calculated using the Gordon functional parcellation based on resting-state functional connectivity patterns (Gordon et al., 2016) and subcortical time-courses were also calculated (Fischl et al., 2002). Average correlations between the CO network and subcortical gray matter ROIs were calculated by averaging the correlations between each ROI within the CO network and the given subcortical ROI (e.g., left amygdala). Correlation values were Fisher Z-transformed to provide summary measures of connectivity strength (Van Dijk et al., 2010). Given prior work demonstrating that regions in the CO network, amygdala, and hippocampus are both stress-sensitive (Huang et al., 2021, Lupien et al., 2009) and changing across development (Dosenbach et al., 2010, Gee et al., 2013b, Marek et al., 2015), we specifically focused our analyses on CO network connectivity with the hippocampus and amygdala (see Fig. 2b for subcortical segmentations).
Fig. 2.
A) Means and standard errors of resting-state functional connectivity at baseline and year 2 follow-up. CO = Cingulo-Opercular. B) Bilateral amygdala (red) and hippocampus (blue) segmentations.
3. Analytic plan
First, in order to identify patterns of change in cortico-limbic connectivity, we used paired samples t-tests to evaluate mean differences in connectivity between baseline and year 2 follow-up. Primary hypotheses were tested using Structural Equation Modeling (SEM) in Mplus Version 8.3 (Muthén and Muthén, 1998). Longitudinal mediation models were estimated with MODEL INDIRECT to test the indirect effect of negative life events on psychopathology via cortico-limbic connectivity. We also tested the interaction between negative life events and parental acceptance (mean-centered) on cortico-limbic connectivity. Four total models were estimated in order to test the mediating effect of CO network connectivity with the left and right amygdala and left and right hippocampus. All models controlled for the effects of sex and age.1 We also included baseline cortico-limbic connectivity as a predictor in the model, regressing connectivity (at year 2 follow-up) on itself at a prior occasion (baseline). In doing so, the outcome is residualized such that any variability accounted for by baseline connectivity is regressed out, leaving only the variance that is unexplained by baseline and can thus be understood as variability likely due to developmental change (Castro-Schilo and Grimm, 2018). To account for the complex sampling structure in the ABCD Study, we specified stratification by study site and clustering of siblings within families (using TYPE = COMPLEX) in all models. RMSEA values of less than .05 were considered a close fit while values less than .08 were considered a reasonable fit (Browne and Cudeck, 1993). CFI values of greater than .90 were considered an acceptable fit while values greater than .95 were considered an excellent fit (Bentler, 1990). Full information maximum likelihood (FIML) estimation was used to handle missing data on study variables (after applying exclusion criteria; see Table 1 for Ns for each variable). FIML uses maximum likelihood estimation based on all available data, and is superior to alternative missing data methods such as listwise deletion or imputation (Enders and Bandalos, 2001).
Table 1.
Correlation coefficients and descriptive statistics for study variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | M (SD) | Min-Max | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Negative life events | 2.30 (2.22) | 0.00–20.00 | 3942 | |||||||||||
| 2. Parental acceptance | -0.14** | 2.79 (0.25) | 1.20–3.00 | 3938 | ||||||||||
| 3. CO-left amygdala connectivity (baseline) | -0.07** | .05** | 0.05 (0.14) | -0.91-0.97 | 4006 | |||||||||
| 4. CO-right amygdala connectivity (baseline) | -0.06** | .03 | .54** | -0.04 (0.18) | -1.52–1.15 | 4006 | ||||||||
| 5. CO-left hippocampus connectivity (baseline) | .00 | -0.03 | .01 | -0.01 | -0.01 (0.15) | -0.93–1.20 | 4006 | |||||||
| 6. CO-right hippocampus connectivity (baseline) | -0.05** | .04* | .43** | .32** | .01 | -0.02 (0.13) | -0.83-0.77 | 4002 | ||||||
| 7. CO-left amygdala connectivity (follow-up) | -.09** | .01 | .22** | .13** | .02 | .20** | 0.04 (0.13) | -0.67-0.69 | 4006 | |||||
| 8. CO-right amygdala connectivity (follow-up) | -.07** | .02 | .14** | .13** | .01 | .14** | .63** | -0.04 (0.16) | -1.28-0.89 | 4006 | ||||
| 9. CO-left hippocampus connectivity (follow-up) | -.001 | -0.02 | .06** | .02 | .09** | .03 | .01 | .05** | -0.03 (0.13) | -1.07-0.74 | 4006 | |||
| 10. CO-right hippocampus connectivity (follow-up) | -.07** | .02 | .16** | .11** | .04* | .22** | .56** | .44** | .07** | -0.04 (0.13) | -0.89-0.85 | 3996 | ||
| 11. Internalizing symptomatology | .09** | -.09** | .03 | .03 | .01 | .02 | .03* | .05** | .01 | .05** | 47.48 (10.35) | 33.00–84.00 | 4005 | |
| 12. Externalizing symptomatology | .17** | -.16** | -.02 | -0.03 | .02 | -0.03 | -0.03 | -0.01 | -0.01 | -0.02 | .56** | 44.18 (9.50) | 33.00–82.00 | 4005 |
Note. CO = Cingulo-Opercular network. N = number of participants with data for each variable. All participants in the sub-sample (N = 4006) were included in final analyses and missing data were handled with Full Information Maximum Likelihood (FIML).
p < .05.
p < .01.
4. Results
Descriptive statistics and correlations for all study variables are presented in Table 1. All variables were normally distributed with levels of skewness less than 3 and kurtosis less than 10 (Kline, 2011).
4.1. Changes in cortico-limbic connectivity
First, we examined changes in cortico-limbic rsFC between the baseline scan and the year 2 follow-up. Results indicated that cortico-limbic rsFC became more strongly negative between baseline and year 2 follow-up for CO-left hippocampus (t4005 = 6.67, p < .001) and CO-right hippocampus (t3991 = 7.10, p < .001) connectivity. CO-right amygdala rsFC was also negative in valence, but did not change significantly over time (t4005 = 0.28, p = .78). CO-left amygdala rsFC was positively valenced and became weaker between time points (t4005 = 6.31, p < .001). These results are illustrated in Fig. 2a.
4.2. Cingulo-opercular network—Amygdala connectivity
4.2.1. CO-left amygdala connectivity
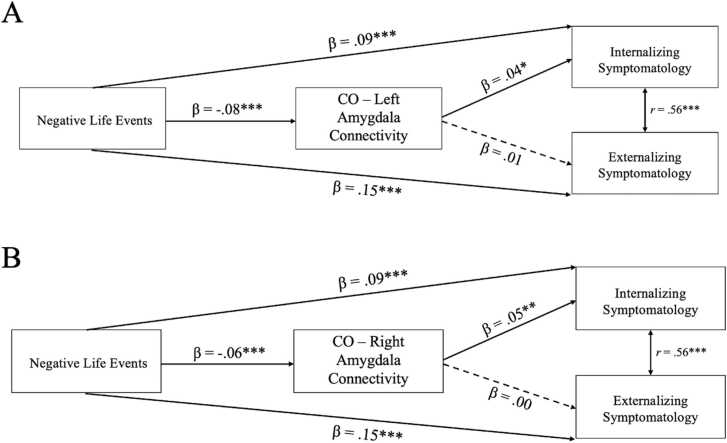
The path model with CO-left amygdala connectivity as a mediator (Fig. 3a) demonstrated acceptable model fit (χ2 = 26.34, df = 12, p = .01, RMSEA =0.02, CFI =0.99). All model parameters, with effect sizes, are presented in Table 2. More negative life events predicted greater internalizing symptomatology and externalizing symptomatology. Negative life events were also associated with changes in CO-left amygdala rsFC. Specifically, more negative life events were associated with weaker positive (i.e., more mature) functional connectivity, which in turn was associated with lower internalizing but not externalizing symptomatology. The indirect effect of negative life events on later psychopathology via CO-left amygdala rsFC was significant (β = −0.003, SE = 0.001, p = .03). There was not a significant main effect of parental acceptance on changes in CO-left amygdala rsFC, or a significant interaction between parental acceptance and negative life events. However, parental acceptance was associated with both internalizing and externalizing symptomatology, such that higher parental acceptance at year 1 was associated with lower symptomatology at year 2.
Fig. 3.
Standardized estimates for the associations between negative life events, cingulo-opercular (CO) network-amygdala connectivity, and psychopathology. A) Model with CO-left amygdala connectivity, B) Model with CO-right amygdala connectivity. All models controlled for the effects of age, sex, and baseline CO-amygdala connectivity, and included main and interaction effects of parental acceptance on connectivity (not pictured for clarity). Non-significant paths illustrated by dashed lines. * p < .05, ** p < .01, *** p < .001.
Table 2.
Mediation Model Parameter Estimates.
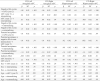 |
Note. CO = Cingulo-Opercular network; rsFC = resting-state functional connectivity. Indirect effect = the effect of negative life events on internalizing symptomatology via cortico-limbic connectivity.
4.2.2. CO-right amygdala connectivity
The path model with CO-right amygdala connectivity as a mediator (Fig. 3b) demonstrated acceptable model fit (χ2 = 14.84, df = 12, p = .02, RMSEA =0.02, CFI = 1.00). All model parameters, with effect sizes, are presented in Table 2. Negative life events were associated with changes in CO-right amygdala rsFC, such that more negative life events were associated with stronger negative (i.e., more mature) functional connectivity, which in turn was associated with lower internalizing, but not externalizing, symptomatology. The indirect effect of negative life events on later psychopathology via CO-right amygdala rsFC was significant (β = −0.003, SE = 0.001, p = .02). There was not a significant main effect of parental acceptance on changes in CO-right amygdala rsFC or a significant interaction between parental acceptance and negative life events.
4.3. Cingulo-opercular network—Hippocampus connectivity
4.3.1. CO-left hippocampus connectivity
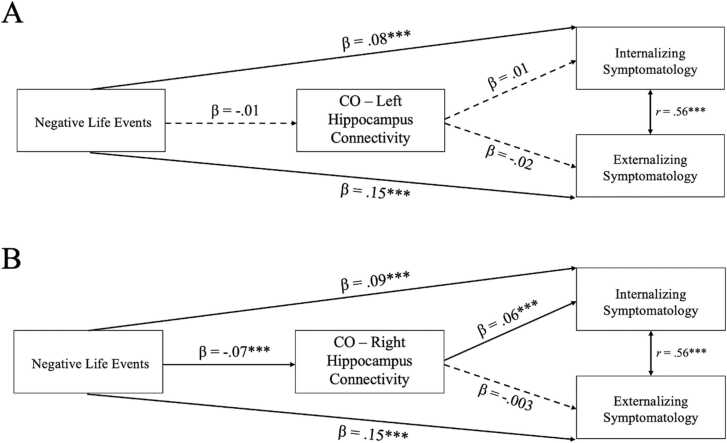
The path model with CO-left hippocampus rsFC as a mediator (Fig. 4a) demonstrated acceptable model fit (χ2 = 10.66, df = 12, p = .10, RMSEA =0.01, CFI = 1.00). All model parameters, with effect sizes, are presented in Table 2. Negative life events were not associated with differences in CO-left hippocampus rsFC and CO-left hippocampus rsFC was not associated with internalizing or externalizing symptomatology. There was not a significant main effect of parental acceptance on changes in CO-left hippocampus rsFC or a significant interaction between parental acceptance and negative life events.
Fig. 4.
Standardized estimates for the associations between negative life events, cingulo-opercular (CO) network-hippocampus connectivity, and psychopathology. A) Model with CO-left hippocampus connectivity, B) Model with CO-right hippocampus connectivity. All models control for the effects of age, sex, and baseline CO-hippocampus connectivity, and included main and interaction effects of parental acceptance on connectivity (not pictured for clarity). Non-significant paths illustrated by dashed lines. *p<.05, **p<.01, ***p<.001.
4.3.2. CO-right hippocampus connectivity
The path model with CO-right hippocampus rsFC as a mediator (Fig. 4b) demonstrated acceptable model fit (χ2 = 18.79, df = 12, p = .001, RMSEA =0.02, CFI =0.99). All model parameters, with effect sizes, are presented in Table 2. Negative life events were associated with differences in CO-right hippocampus rsFC. Specifically, more negative life events were associated with stronger negative (i.e., more mature) functional connectivity, which in turn was associated with lower internalizing, but not externalizing, symptomatology. The indirect effect of negative life events on later psychopathology via CO-right hippocampus rsFC was significant (β = −0.004, SE = 0.001, p = .01). There was not a significant main effect of parental acceptance on changes in CO-right hippocampus rsFC or a significant interaction between parental acceptance and negative life events.
4.4. Sensitivity analysis
In order to evaluate whether outliers contributed to bias in model estimates, we conducted a sensitivity analysis excluding participants with values greater than 3.29 SD from the mean (Tabachnick and Fidell, 2001) on any model variables. After applying this exclusion, the sample included 3348 participants. The results were highly consistent with results that included all participants, with only very small changes in parameter estimates across all models.
5. Discussion
The purpose of the present study was to test the indirect effect of adversity on later psychopathology via changes in cortico-limbic resting-state functional connectivity, as well as to explore the protective role of parental acceptance. Leveraging data from the ABCD Study, we found that more negative life events were significantly associated with longitudinal changes in cortico-limbic functional connectivity. In turn, changes in cortico-limbic rsFC were associated with lower internalizing, but not externalizing, symptoms. The indirect effect of negative life events on internalizing symptoms via cortico-limbic rsFC was significant, suggesting that adaptations in cortico-limbic connectivity may explain lower symptomatology among youth with greater stress exposure. We did not find evidence for protective effects of parental acceptance in these specific associations.
Results indicated that higher levels of adversity were associated with more mature patterns of functional connectivity between the CO network and left and right amygdala and right hippocampus. It is possible that these findings reflect stress acceleration effects (rather than developmental delays) and adaptation to environments laden with stressful experiences. That is, under conditions of stress, more rapid development may benefit children and adolescents by prematurely activating neural circuitry that supports affective processing and regulation (Callaghan and Tottenham, 2016). Thus, adolescents in this sample who had experienced more negative life events may have adapted to more stressful contexts via accelerated maturation in CO-amygdala-hippocampal connections. Consistent with this finding, a recent investigation with ABCD data found that accelerated pubertal maturation mediated the association between stressful family environments and CO network-amygdala rsFC (Thijssen et al., 2020a, Thijssen et al., 2020b). The current longitudinal findings also extend prior cross-sectional evidence of stress acceleration in cortico-limbic circuitry (Gee et al., 2013a, Herzberg et al., 2021, Miller et al., 2020, Silvers et al., 2016, Thijssen et al., 2017).
Our findings further indicated that more mature functional connectivity was associated with lower levels of internalizing symptomatology, corroborating the idea that these adaptations may be beneficial for psychosocial adjustment. That is, within the context of increased risk for psychopathology that accompanies early adversity, acceleration in cortico-limbic circuitry was associated with lower internalizing symptoms. One possibility is that stress-induced acceleration may be beneficial in the short term in order to promote the emotion regulation skills necessary to navigate stressful environments and mitigate psychopathology symptoms. However, we expect that in the long-term, adolescents with greater stress exposure will ultimately demonstrate higher internalizing symptomatology. The trade-off of early adaptation could be a shortened period of developmental plasticity, which may increase vulnerability to stress-related psychopathology later in development (Callaghan and Tottenham, 2016). As future releases of ABCD follow-up data become available, it will be possible to formally test this hypothesis. Finally, the indirect effects we observed were specific to internalizing, rather than externalizing, symptomatology. Though prior work in this area has overwhelmingly focused on disorders such as depression and anxiety, it is also important to consider specificity of neurobiological mechanisms that lead to different types of psychopathology. It is possible that distinct networks (e.g., fronto-striatal, inferior frontal gyrus) more strongly relate to externalizing symptomatology (Barch et al., 2018, Rubia, 2011), and this is an important line of inquiry for future work.
More broadly, our results are generally consistent with work suggesting that alterations in neural circuitry related to emotion regulation may underlie the association between adversity exposure and youth mental health (McLaughlin et al., 2019, VanTieghem and Tottenham, 2018). In a recent longitudinal study, Rakesh et al. (2021) found that greater maltreatment severity was associated with widespread changes in functional connectivity across several major functional networks between age 16 and age 19. In turn, changes in connectivity were associated with higher depressive symptoms. Further evidence suggests that ventral-striatal-mPFC connectivity significantly mediates the association between early institutionalization and adolescent social problems (Fareri et al., 2017). Our findings add to a growing literature illustrating the mediating effect of resting-state networks in the association between adversity and adolescent adjustment. Specifically, we found both a direct effect of adversity on psychopathology, as well as an indirect effect via changes in cortico-limbic connectivity, suggesting that connectivity partially mediated this association. This aligns with prior work showing that a more mature pattern of amygdala-mPFC connectivity partially mitigated the effect of parental deprivation on anxiety (Gee et al., 2013a). Additional research linking early adversity with mental health via alterations in functional connectivity highlights important variability in these associations. For example, whereas increasing (i.e., more strongly negative) cortico-limbic connectivity was associated with lower internalizing symptoms following negative life events in the current study, Rakesh and colleagues (2021) found that widespread increases in connectivity were associated with higher depressive symptoms following maltreatment. These findings may reflect effects that are specific to a given type of stressor, neural circuit, and developmental stage.
By accounting for baseline connectivity in our model we were able to observe developmental changes in cortico-limbic circuitry. Results indicated that between baseline and year 2 follow-up, connectivity between the CO network and the hippocampus (left and right hemispheres) became more strongly negative. Though no studies to our knowledge have examined longitudinal changes in these particular connections, this finding is broadly consistent with studies that show age-related increases (i.e., directional strengthening) of functional connectivity in certain circuits (Fair et al., 2010, Rakesh et al., 2021). At the same time, conclusions regarding developmental changes in resting-state connectivity are quite mixed (Stevens, 2016), perhaps due to the fact that the directionality and degree of development is region-specific (van Duijvenvoorde et al., 2019). Indeed, our results show that mean connectivity between the CO network and left amygdala across the sample was positive and decreased (became weaker) between baseline and year 2 follow-up (as opposed to stronger negative connectivity with the hippocampus and right amygdala). It is difficult to evaluate how these patterns compare to prior work given that small samples and heterogeneity in analytic decisions and participant ages limit comparison across studies, and so these findings should be interpreted with care. Prior work illustrates differential processing in the left and right amygdalae (e.g., Baker and Kim, 2004; Polli et al., 2009) which may account in part for this finding. As additional data are released from the ABCD Study, we will be better able to disentangle typical developmental trajectories and patterns of cortico-limbic connectivity.
In the present study, the effect of adversity on cortico-limbic rsFC did not vary by levels of parental acceptance. We had hypothesized that greater parental acceptance would buffer against the effect of adversity based on prior work that has demonstrated the protective role of warm and supportive caregiving on brain development (Brody et al., 2019, Whittle et al., 2017, Yap et al., 2008). However, we did not observe main or interactive effects of parental acceptance. Another recent ABCD study similarly did not find a buffering effect of parental acceptance against ecological stress. Specifically, the interaction between ecological stress and parental acceptance was not significantly associated with amygdala reactivity during the emotional EN-back task (Demidenko et al., 2021). It is possible that this specific index of caregiving (i.e., five items from the CRPBI) may not be capturing the more nuanced ways in which caregivers can protect against adversity, particularly with regard to the specific cortico-limbic connections considered in our analyses.
We acknowledge that the participants included in these analyses experienced relatively low numbers of negative life events and reported relatively high parental acceptance. This was expected given the fact that the ABCD Study includes a community sample of youth who were not selected based on any criteria such as stress or trauma exposure. Relatedly, participants included in these analyses tended to have lower exposure to negative life events and higher socioeconomic status relative to participants who were excluded, which may have limited generalizability. However, we expect that the effects of adversity on cortico-limbic connectivity may be even more pronounced in a sample of youth with greater exposure to negative life events. Furthermore, youth with higher exposure to adversity may benefit more from protective factors such as high parental acceptance (relative to youth with lower exposure), consistent with protective-enhancing models of resilience (Luthar et al., 2000). This may serve as one explanation for the non-significant interaction effects between negative life events and parental acceptance in this sample, though future work with adversity-exposed youth will be important to formally test these hypotheses.
Although our results demonstrate novel developmental findings related to adversity, neural circuitry, and psychopathology, they should be interpreted in the context of several limitations. First, our analyses focused on specific, a priori functional connections selected based on prior evidence of stress acceleration in cortico-limbic circuitry (e.g., Callaghan and Tottenham, 2016; Gee et al., 2013a; Herzberg et al., 2021; Miller et al., 2020; Silvers et al., 2016; Thijssen et al., 2017). The effects of adversity are likely to also have more distributed effects that differ in other circuits (e.g., Herzberg et al., 2021; Rakesh et al., 2021). Second, the effects of adversity may vary depending on timing of exposure (Gee and Casey, 2015), but we were only able to evaluate the effects of overall lifetime exposure. The Life Events Scale does not differentiate by age of exposure, thus precluding any conclusions regarding timing effects. Future work with more detailed assessments will be better suited to evaluate how stress exposure may interact with sensitive periods of development. Relatedly, using an overall index of negative life events may have obscured important heterogeneity in adversity experiences; indeed, different types of environmental exposures have been linked to distinct neurobiological sequelae (Cohodes et al., 2020, Hong et al., 2021, McLaughlin and Sheridan, 2016), and consideration of these heterogeneous effects and broader social contexts is a critical direction for future work (Simmons and Conley et al., 2021). Finally, consistent with the increased statistical power to observe small effects in large samples such as the ABCD Study, the effect sizes in this study are small relative to previously published work. Many of these previously reported effects, especially in neuroimaging studies, are from small samples that may contribute to inflated effect sizes or are underpowered to detect small effects (Dick et al., 2021). The effects found in the present analyses may in reality be very small. Though we note that even small effects may still hold clinical or practical significance (Rosenthal et al., 2000), future work will be important to inform the extent to which the current findings are clinically meaningful (Anvari et al., 2021).
The ABCD Study offers unprecedented opportunities to elucidate the effects of environmental exposures on neurodevelopmental processes across adolescence. By taking advantage of the longitudinal neuroimaging data in the ABCD Study, we have highlighted novel neurodevelopmental mechanisms that may underlie the association between adversity and internalizing symptomatology. Delineating how adversity contributes to alterations in cortico-limbic circuitry, as well as evaluating potential protective factors that may or may not buffer against these effects, is critical to understanding the mechanisms that link early adversity with psychopathology and to identifying potential intervention targets for youth.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by funding from the Susan Nolen-Hoeksema Postdoctoral Fellowship for AEB, the National Science Foundation Graduate Research Fellowship Program award (NSF DGE-1752134) for LMS, and the National Institutes of Health (NIH) Director’s Early Independence Award (DP5OD021370), National Institute on Drug Abuse (U01DA041174), Brain & Behavior Research Foundation (NARSAD Young Investigator Award), Jacobs Foundation Early Career Research Fellowship, and The Society for Clinical Child and Adolescent Psychology (Division 53 of the American Psychological Association) Richard "Dick" Abidin Early Career Award and Grant for DGG. Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development® (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and Additional Federal Partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortiummembers/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from NDA Release 3.0 (doi:10.15154/1519007).
Footnotes
We explored models that also controlled for the effect of total household income on connectivity and psychopathology; results were highly consistent, and all significant effects held even when accounting for income. These model estimates are included in the Supplementary Material.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2021.101022.
Appendix A. Supplementary material
Supplementary material
.
Data Availability
All data analyzed in this study are accessible through an official request to the NIH Data Archive (https://nda.nih.gov/abcd). An NDA study detailing the list of participants, variables, and related information for this study may also be accessed at the following doi:10.15154/1522544.
References
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, and Families; Burlington, VT: 2001. Manual for the ASEBA Adult Forms & Profiles. [Google Scholar]
- Anvari F., Kievit R., Lakens D., Pennington C.R., Przybylski A.K., Tiokhin L., Orben A. Evaluating the practical relevance and significance of observed effect sizes in psychological research. PsyArXiv. 2021 doi: 10.31234/osf.io/g3vtr. [DOI] [Google Scholar]
- Baker K.B., Kim J.J. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav. Neurosci. 2004;118:15–23. doi: 10.1037/0735-7044.118.1.15. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Belden A.C., Tillman R., Whalen D., Luby J.L. Early childhood adverse experiences, inferior frontal gyrus connectivity, and the trajectory of externalizing psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2018;57:183–190. doi: 10.1016/j.jaac.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Brody G.H., Yu T., Nusslock R., Barton A.W., Miller G.E., Chen E., Holmes C., McCormick M., Sweet L.H. The protective effects of supportive parenting on the relationship between adolescent poverty and resting-state functional brain connectivity during adulthood. Psychol. Sci. 2019;30:1040–1049. doi: 10.1177/0956797619847989. 0956797619847989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M.W., Cudeck R. In: Testing Structural Equation Models. Bollen K.A., Long J.S., editors. Sage; Beverly Hills: 1993. Alternative ways of assessing model fit; pp. 136–162. [Google Scholar]
- Callaghan B.L., Dandash O., Simmons J.G., Schwartz O., Byrne M.L., Sheeber L., Whittle S. Amygdala resting connectivity mediates association between maternal aggression and adolescent major depression: a 7-year longitudinal study J. Am. Acad. Child Adolesc. Psychiatry. 2017;56:983–991. doi: 10.1016/j.jaac.2017.09.415. .e3. [DOI] [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The stress acceleration hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Chaarani B., Mejia M.H., Hagler D.J., J.r, Daniela Cornejo M., Sicat C.S., Harms M.P., Dosenbach N., Rosenberg M., Earl E., Bartsch H., Watts R., Polimeni J.R., Kuperman J.M., Fair D.A., Dale A.M., ABCD Imaging Acquisition W. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B., Galván A., Somerville L.H. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Heller A.S., Gee D.G., Cohen A.O. Development of the emotional brain. Neurosci. Lett. 2019;693:29–34. doi: 10.1016/j.neulet.2017.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Schilo L., Grimm K.J. Using residualized change versus difference scores for longitudinal research. J. Soc. Pers. Relatsh. 2018;35:32–58. doi: 10.1177/0265407517718387. [DOI] [Google Scholar]
- Clark D.B., Fisher C.B., Bookheimer S., Brown S.A., Evans J.H., Hopfer C., Yurgelun-Todd D. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: the ABCD experience. Dev. Cogn. Neurosci. 2018;32:143–154. doi: 10.1016/j.dcn.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohodes E.M., Kitt E.R., Baskin-Sommers A., Gee D.G. Influences of early-life stress on frontolimbic circuitry: harnessing a dimensional approach to elucidate the effects of heterogeneity in stress exposure. Dev. Psychobiol. 2020;63:153–172. doi: 10.1002/dev.21969. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Family matters: intergenerational and interpersonal processes of executive function and attentive behavior. Curr. Dir. Psychol. Sci. 2014;23:230–236. doi: 10.1177/0963721414531597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidenko M.I., Ip K.I., Kelly D.P., Constante K., Goetschius L.G., Keating D.P. Ecological stress, amygdala reactivity, and internalizing symptoms in preadolescence: is parenting a buffer? Cortex. 2021;140:128–144. doi: 10.1016/j.cortex.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A.S., Lopez D.A., Watts A.L., Heeringa S., Reuter C., Bartsch H., Fan C.C., Kennedy D.N., Palmer C., Marshall A., Haist F., Hawes S., Nichols T.E., Barch D.M., Jernigan T.L., Garavan H., Grant S., Pariyadath V., Hoffman E., Neale M., Stuart E.A., Paulus M.P., Sher K.J., Thompson W.K. Meaningful associations in the adolescent brain cognitive development study. NeuroImage. 2021;239 doi: 10.1016/j.neuroimage.2021.118262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A.T., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Nardos B., Cohen A.L., Fair D.A., Power J.D., Church J.A., Schlaggar B.L. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C.K. van, Westhoff B., Vos F. de, Wierenga L.M., Crone E.A. A three-wave longitudinal study of subcortical–cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum. Brain Mapp. 2019;40:3769–3783. doi: 10.1002/hbm.24630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L., Ramroop J., Hill M.N., Manley J., McEwen B.S. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders C., Bandalos D. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equ. Model.: Multidiscip. J. 2001;8:430–457. doi: 10.1207/S15328007SEM0803_5. [DOI] [Google Scholar]
- Fair D.A., Bathula D., Mills K.L., Dias T.G., Blythe M.S., Zhang D., Snyder A.Z., Raichle M.E., Stevens A.A., Nigg J.T., Nagel B.J. Maturing thalamocortical functional connectivity across development. Front. Syst. Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Gabard-Durnam L., Goff B., Flannery J., Gee D.G., Lumian D.S., Tottenham N. Altered ventral striatal-medial prefrontal cortex resting-state connectivity mediates adolescent social problems after early institutional care. Dev. Psychopathol. 2017;29:1865–1876. doi: 10.1017/S0954579417001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti V.J., Anda R.F., Nordenberg D., Williamson D.F., Spitz A.M., Edwards V., Marks J.S. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Casey B.J. The impact of developmental timing for stress and recovery. Neurobiol. Stress. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U.S.A. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M., Tottenham N. A developmental shift from positive to negative connectivity in human amygdala – prefrontal circuitry. J. Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Adeyemo B., Huckins J.F., Kelley W.M., Petersen S.E. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex. 2016;26:288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant K.E., Compas B.E., Thurm A.E., McMahon S.D., Gipson P.Y. Stressors and child and adolescent psychopathology: measurement issues and prospective effects. J. Clin. Child Adolesc. Psychol. 2004;33:412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr, Hatton S., Cornejo M.D., Makowski C., Fair D.A., Dick A.S., Sutherland M.T., Casey B.J., Barch D.M., Harms M.P., Watts R., Bjork J.M., Garavan H.P., Hilmer L., Pung C.J., Sicat C.S., Kuperman J., Bartsch H., Xue F., Heitzeg M.M., Laird A.R., Trinh T.T., Gonzalez R., Tapert S.F., Riedel M.C., Squeglia L.M., Hyde L.W., Rosenberg M.D., Earl E.A., Howlett K.D., Baker F.C., Soules M., Diaz J., de Leon O.R., Thompson W.K., Neale M.C., Herting M., Sowell E.R., Alvarez R.P., Hawes S.W., Sanchez M., Bodurka J., Breslin F.J., Morris A.S., Paulus M.P., Simmons W.K., Polimeni J.R., van der Kouwe A., Nencka A.S., Gray K.M., Pierpaoli C., Matochik J.A., Noronha A., Aklin W.M., Conway K., Glantz M., Hoffman E., Little R., Lopez M., Pariyadath V., Weiss S.R., Wolff-Hughes D.L., DelCarmen-Wiggins R., Feldstein Ewing S.W., Miranda-Dominguez O., Nagel B.J., Perrone A.J., Sturgeon D.T., Goldstone A., Pfefferbaum A., Pohl K.M., Prouty D., Uban K., Bookheimer S.Y., Dapretto M., Galvan A., Bagot K., Giedd J., Infante M.A., Jacobus J., Patrick K., Shilling P.D., Desikan R., Li Y., Sugrue L., Banich M.T., Friedman N., Hewitt J.K., Hopfer C., Sakai J., Tanabe J., Cottler L.B., Nixon S.J., Chang L., Cloak C., Ernst T., Reeves G., Kennedy D.N., Heeringa S., Peltier S., Schulenberg J., Sripada C., Zucker R.A., Iacono W.G., Luciana M., Calabro F.J., Clark D.B., Lewis D.A., Luna B., Schirda C., Brima T., Foxe J.J., Freedman E.G., Mruzek D.W., Mason M.J., Huber R., McGlade E., Prescot A., Renshaw P.F., Yurgelun-Todd D.A., Allgaier N.A., Dumas J.A., Ivanova M., Potter A., Florsheim P., Larson C., Lisdahl K., Charness M.E., Fuemmeler B., Hettema J.M., Maes H.H., Steinberg J., Anokhin A.P., Glaser P., Heath A.C., Madden P.A., Baskin-Sommers A., Constable R.T., Grant S.J., Dowling G.J., Brown S.A., Jernigan T.L., Dale A.M. Image processing and analysis methods for the adolescent brain cognitive development study. NeuroImage. 2019;202 doi: 10.1016/j.neuroimage.2019.116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa R.J., Birn R.M., Ruttle P.L., Burghy C.A., Stodola D.E., Davidson R.J., Essex M.J. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc. Natl. Acad. Scie. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M.P., Gunnar M.R. Early life stress and brain function: activity and connectivity associated with processing emotion and reward. NeuroImage. 2020;209 doi: 10.1016/j.neuroimage.2019.116493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M.P., McKenzie K.J., Hodel A.S., Hunt R.H., Mueller B.A., Gunnar M.R., Thomas K.M. Accelerated maturation in functional connectivity following early life stress: circuit specific or broadly distributed? Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E.A., Clark D.B., Orendain N., Hudziak J., Squeglia L.M., Dowling G.J. Stress exposures, neurodevelopment and health measures in the ABCD study. Neurobiol. Stress. 2019;10 doi: 10.1016/j.ynstr.2019.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D., Kuperman J.M., Dale A.M. Efficient correction of inhomogeneous static magnetic field-induced distortion in echo planar imaging. Neuroimage. 2010;50:175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.-J., Sisk L.M., Caballero C., Mekhanik A., Roy A.K., Milham M.P., Gee D.G. Decomposing complex links between the childhood environment and brain structure in school-aged youth. Dev. Cogn. Neurosci. 2021;48 doi: 10.1016/j.dcn.2021.100919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D., Liu Z., Cao H., Yang J., Wu Z., Long Y. Childhood trauma is linked to decreased temporal stability of functional brain networks in young adults. J. Affect. Disord. 2021;290:23–30. doi: 10.1016/j.jad.2021.04.061. [DOI] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R., Kennedy D., Schmitt F., Brown G., Macfall J., Fischl B., Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kaiser R.H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., Pizzagalli D.A. Childhood stress, grown-up brain networks: corticolimbic correlates of threat-related early life stress and adult stress response. Psychol. Med. 2018;48:1157–1166. doi: 10.1017/S0033291717002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher N.R., Barch D.M. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. 2021;46:131–142. doi: 10.1038/s41386-020-0736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Williams D.R. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R.B. Principles and Practice of Structural Equation Modeling. third ed. Guildford Press; New York, NY: 2011. [Google Scholar]
- Lees B., Squeglia L.M., McTeague L.M., Forbes M.K., Krueger R.F., Sunderland M., Mewton L. Altered neurocognitive functional connectivity and activation patterns underlie psychopathology in preadolescence. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2021;6:387–398. doi: 10.1016/j.bpsc.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F.S., Heimer H., Giedd J.N., Lein E.S., Šestan N., Weinberger D.R., Casey B.J. Adolescent mental health—Opportunity and obligation. Science. 2014;346:547–549. doi: 10.1126/science.1260497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luthar S.S., Cicchetti D., Becker B. The construct of resilience: a critical evaluation and guidelines for future work. Child Dev. 2000;71:543–562. doi: 10.1111/1467-8624.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek S., Hwang K., Foran W., Hallquist M.N., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A. Future directions in childhood adversity and youth psychopathology. J. Clin. Child Adolesc. Psychol. 2016;45:361–382. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Colich N.L., Rodman A.M., Weissman D.G. Mechanisms linking childhood trauma exposure and psychopathology: a transdiagnostic model of risk and resilience. BMC Med. 2020;18:96. doi: 10.1186/s12916-020-01561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Kubzansky L.D., Dunn E.C., Waldinger R., Vaillant G., Koenen K.C. Childhood social environment, emotional reactivity to stress, and mood and anxiety disorders across the life course. Depress. Anxiety. 2010;27:1087–1094. doi: 10.1002/da.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr. Direct. Psychol. Sci. 2016;25:239–245. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Gold A.L., Duys A., Lambert H.K., Peverill M., Pine D.S. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. 2016;41:1956–1964. doi: 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Weissman D., Bitrán D. Childhood adversity and neural development: a systematic review. Annu. Rev. Dev. Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M.T., Ford D.C., Ports K.A., Guinn A.S. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr. 2018;172:1038–1044. doi: 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.G., Ho T.C., Humphreys K.L., King L.S., Foland-Ross L.C., Colich N.L., Ordaz S.J., Lin J., Gotlib I.H. Early life stress, frontoamygdala connectivity, and biological aging in adolescence: a longitudinal investigation. Cereb. Cortex. 2020;30:4269–4280. doi: 10.1093/cercor/bhaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A.S., Criss M.M., Silk J.S., Houltberg B.J. The impact of parenting on emotion regulation during childhood and adolescence. Child Dev. Perspect. 2017;11:233–238. doi: 10.1111/cdep.12238. [DOI] [Google Scholar]
- Muthén, L.K., Muthén, B.O. (1998–2019). Mplus user’s guide (8th ed.). Los Angeles: United States.
- Polli F.E., Wright C.I., Milad M.R., Dickerson B.C., Vangel M., Barton J.J., Rauch S.L., Manoach D.S. Hemispheric differences in amygdala contributions to response monitoring. Neuroreport. 2009;20:398–402. doi: 10.1097/WNR.0b013e328324edb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakesh D., Kelly C., Vijayakumar N., Zalesky A., Allen N.B., Whittle S. Unraveling the consequences of childhood maltreatment: deviations from typical functional neurodevelopment mediate the relationship between maltreatment history and depressive symptoms. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging. 2021;6:329–342. doi: 10.1016/j.bpsc.2020.09.016. [DOI] [PubMed] [Google Scholar]
- Rosenthal R., Rosnow R.L., Rubin D.B. Cambridge University Press; 2000. Contrasts and Effect Sizes in Behavioral Research: A Correlational Approach. [Google Scholar]
- Rubia K. “Cool” inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus “hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2011;69:e69–e87. doi: 10.1016/j.biopsych.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Schaefer E.S. Children’s reports of parental behavior: an inventory. Child Dev. 1965;36:413–424. doi: 10.2307/1126465. [DOI] [PubMed] [Google Scholar]
- Silveira S., Boney S., Tapert S.F., Mishra J. Developing functional network connectivity of the dorsal anterior cingulate cortex mediates externalizing psychopathology in adolescents with child neglect. Dev. Cogn. Neurosci. 2021;49 doi: 10.1016/j.dcn.2021.100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Insel C., Powers A., Franz P., Helion C., Martin R.E., Ochsner K.N. vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb. Cortex. 2017;27:3502–3514. doi: 10.1093/cercor/bhw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Lumian D.S., Gabard-Durnam L., Gee D.G., Goff B., Fareri D.S., Tottenham N. Previous institutionalization is followed by broader amygdala–hippocampal–pfc network connectivity during aversive learning in human development. J. Neurosci. 2016;36:6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C., Conley M.I., Gee D.G., Baskin-Sommers A., Barch D.M., Hoffman E.A., Casey B.J. Responsible use of open-access developmental data: the adolescent brain cognitive development (ABCD) study. Psychol. Sci. 2021:1–5. doi: 10.1177/09567976211003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., Swain J.E., Evans G.W., Welsh R.C., Liberzon I. Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology. 2014;39:2244–2251. doi: 10.1038/npp.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C. The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation. Neurosci. Biobehav. Rev. 2016;70:13–32. doi: 10.1016/j.neubiorev.2016.07.027. [DOI] [PubMed] [Google Scholar]
- Sylvester C.M., Yu Q., Srivastava A.B., Marek S., Zheng A., Alexopoulos D., Dosenbach N.U.F. Individual-specific functional connectivity of the amygdala: a substrate for precision psychiatry. Proc. Natl. Acad. Sci. U.S.A. 2020;117:3808–3818. doi: 10.1073/pnas.1910842117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnick B.G., Fidell L.S. Vol. 5. Allyn & Bacon; Nedham Heights, MA: 2001. (Using Multivariate Statistics). [Google Scholar]
- Thijssen S., Collins P.F., Luciana M. Pubertal development mediates the association between family environment and brain structure and function in childhood. Dev. Psychopathol. 2020;32:687–702. doi: 10.1017/S0954579419000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Collins P.F., Weiss H., Luciana M. The longitudinal association between externalizing behavior and frontoamygdalar resting-state functional connectivity in late adolescence and young adulthood. J. Child Psychol. Psychiatry. 2020 doi: 10.1111/jcpp.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., Ijzendoorn M.H.V. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Tiet Q.Q., Bird H.R., Davies M., Hoven C., Cohen P., Jensen P.S., Goodman S. Adverse life events and resilience. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37:1191–1200. doi: 10.1097/00004583-199811000-00020. [DOI] [PubMed] [Google Scholar]
- Tomalski P., Johnson M.H. The effects of early adversity on the adult and developing brain. Curr. Opin. Psychiatry. 2010;23:233–238. doi: 10.1097/YCO.0b013e3283387a8c. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3 doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem M.R., Tottenham N. Neurobiological programming of early life stress: functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci. 2018;38:117–136. doi: 10.1007/7854_2016_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems C.F., Russell J.D., Neill E.L., McCurdy B.H. Annual research review: pediatric posttraumatic stress disorder from a neurodevelopmental network perspective. J. Child Psychol. Psychiatry. 2019;60:395–408. doi: 10.1111/jcpp.12996. [DOI] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Simmons J.G., Dennison M., Schwartz O., Pantelis C., Sheeber L., Byrne M.L., Allen N.B. Role of positive parenting in the association between neighborhood social disadvantage and brain development across adolescence. JAMA Psychiatry. 2017;74:824–832. doi: 10.1001/jamapsychiatry.2017.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D., Phan K.L. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood. Hum. Brain Mapp. 2016;37:1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap M.B.H., Whittle S., Yücel M., Sheeber L., Pantelis C., Simmons J.G., Allen N.B. Interaction of parenting experiences and brain structure in the prediction of depressive symptoms in adolescents. Arch. Gen. Psychiatry. 2008;65:1377. doi: 10.1001/archpsyc.65.12.1377. 1377–1377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
All data analyzed in this study are accessible through an official request to the NIH Data Archive (https://nda.nih.gov/abcd). An NDA study detailing the list of participants, variables, and related information for this study may also be accessed at the following doi:10.15154/1522544.