Highlights
-
•
First report demonstrating successful use of temporary-immersion bioreactors (TIBs) for the somatic embryogenesis process in the commercially important conifer hybrid larch Larix × eurolepis Henry
-
•
Two-fold increases in both multiplication (fresh weight of pro-embryogenic masses; PEMs) and yield of cotyledonary embryos in the TIBs compared to solid medium in plates
-
•
First report on germination of conifer somatic embryos in TIBs
-
•
Higher number of roots per plant, longer roots and taller plants in TIBs compared to germination on solid medium
Keywords: Germination, Horizontal TIB, Larix × eurolepis, Scale up, Somatic embryogenesis, Vertical TIB
Abstract
Somatic embryogenesis (SE) has high potential for large-scale clonal propagation of conifers. Different types of bioreactor cultures have been tested for the conifer SE process where the temporary immersion bioreactors (TIBs) have proved to be useful across the different developmental steps of the SE process. In the present study the use of TIBs was tested for hybrid larch (Larix × eurolepis Henry). The results showed two-fold increases in both fresh weight (FW) of pro-embryogenic masses (PEMs) and yield of cotyledonary embryos in the TIBs compared to solid medium in plates. For the germination phase, the highest number of roots per plant, the root length and height of plants were also obtained in the TIBs. The results show that the TIB system can be successfully used to support scale up of plant production in all steps of the SE process from proliferation to germination of hybrid larch (Larix × eurolepis Henry).
1. Introduction
The need for wood products is increasing globally at the same time as land available for forestry is decreasing. Tree plantations and reforestation efforts therefore receive attention on the global scale. To accommodate the demand for wood worldwide, forest tree breeding must increase productivity from managed forests whilst preserving natural forests through ecologically sustainable forestry practices. Application of plant biotechnology methods to forest tree improvement programs can greatly enhance the outcome and reduce the time to implementation of the breeding efforts. For commercially valuable conifer tree species, propagation by somatic embryogenesis (SE) in particular can have major benefits when compared with traditional propagation methods as a method for testing in breeding programs and mass-propagation of high-value woody plants [1], [2], [3]. Norway spruce (Picea abies) and larch (Larix decidua) are important commercial conifer species in Europe, both also used as model species for embryogenesis research. Extensive breeding program exists for both species [4,5] and SE is currently being optimized for implementation for forestry applications, in the breeding programs and for plant production [6], [7], [8]. A specific field of application for SE lays in the propagation of new hybrids with high potential for forestry but low propagation capacity, like the larch variety REVE-VERT first described and established at French National Institute for Agricultural Research (INRA) [9]. However, The practical use of this species for reforestation has been hindered because of low quality of seeds and poor germination [10].The successful use of SE for propagation of several SE lines of this hybrid was demonstrated earlier [10].
The SE process for clonal propagation proceeds through several developmental steps. In conifers, the SE process in essence corresponds to cloning of seed embryos starting the proliferating culture with a zygotic (seed) embryo. The zygotic embryo gives rise to early-stage somatic embryos (pro-embryogenic masses; PEMs) that continues to multiply until moved to a maturation medium. The cotyledonary embryo formed on the maturation medium can then germinate and form a plant that can grow ex vitro [11], [12], [13]. Despite the benefits for forestry applications, the standard methods for the SE process still in use in most laboratories, is labour intensive and with limited scalability [6,14]. To achieve cost-effective mass-propagation of SE plants, it is necessary to scale up the whole SE process from multiplication, maturation and germination. Bioreactors based on liquid culture medium offer many advantages both for improved growth of plant cell cultures in general due to the better accessibility of nutrients in the culture medium and by easier handling for the operator. Bioreactor systems are already proven to be unequalled units for large-scale production of plant cells, tissue and organ cultures [1,15,16]. For plant production, temporary immersion bioreactors (TIBs) have been shown to provide a fast, low-cost, efficient propagation systems that can produce high quality plants of valuable plants like Coffea arabica, Hevea brasiliensis, Kalopanax septemlobus, Bactris gasipaes, which survive transfer to ex vitro conditions [17], [18], [19], [20].
Bioreactors also offers many areas of application for propagation of forest trees [1]. In particular, bioreactors offers advantages for cost effective scale up production of SE plants by the opportunities for automation based on the liquid medium [21]. Different types of bioreactor systems have been explored for SE cultures of conifer species including Picea mariana and Picea glauca engelmannii [22,23], Picea glauca [24], Picea sitchensis [25], Picea abies [26], and Abies nordmanniana [27].
The following different bioreactor systems were tested for growth and maturation of P. sitchensis somatic embryos: stirred tank bioreactor, air-lift bioreactor, bubble bioreactor and hanging stirrer bar bioreactor. The results implied that the configuration, design, and operating conditions of the bioreactor systems affected the SE process. The highest number of cotyledonary embryos were produced from cultures in bubble bioreactor while no somatic embryo maturation occurred with the hanging stirrer bar bioreactor [25]. In Pinus kesiya, the embryogenic biomass increased significantly in the self-designed bubble bioreactor when compared to shake flask culture [28]. The TIB system developed for use together with the SE fluidics system [29] was demonstrated to stimulate shoot multiplication rates in Betula pendula, Betula pubescens, Eucalyptus grandis x urophylla, and to increase the rate of proliferation of PEMs and yield of cotyledonary embryo of A. nordmanniana [30] and P. abies when compared to cultures on solid medium [30]. Furthermore, disposable wave-bioreactors were tested for multiplication of A. nordmanniana somatic embryos however with limited success [27]. Full liquid immersion during suspension culture can be applied for proliferation of conifer somatic embryo cultures whereas the maturation process has only been demonstrated in suspension culture in one cell lines of P. abies [31].
The present study demonstrates enhanced proliferation rates and cotyledonary embryo yields from hybrid larch (Larix × eurolepis Henry) in a TIB system compared to cultures on solid medium in plates. Furthermore, development of improved quality plantlets in both horizontal and vertical germination TIB systems are shown.
2. Materials and methods
2.1. Plant materials
Two half-sib lines Q3 and Q10 of hybrid larch Larix × eurolepis Henry were established in 2009 from immature seeds using standard methods at INRA Orléans, France [10]. The PEM cultures were maintained on basal MSG medium [32] supplemented with 1.46 g•L − 1 (10 mM) l-glutamine, 9 µM 2,4-dichlorophenoxyacetic acid (2,4-D), 2.3 µM 6-benzyladenine (BA) and 60 mM sucrose. The pH was adjusted to 5.8 before autoclaved at 121 °C for 20 min.
2.2. PEM proliferation
Two culture systems were used in the present study: solid medium in Petri plates and liquid medium in TIBs. For solid cultures in Petri plates (150 × 20 mm, Sarstedt AG & Co. KG, Germany), the media were solidified with 4 g•L − 1 gelrite (Duchefa, Netherlands), and small clumps of PEMs with a total inoculum density of 3 ± 0.25 g was placed on the solid medium in each Petri plate. For bioreactor cultures, temporary immersion bioreactors developed specifically for conifer SE and for use with the SE Fluidics system (Bioautomaton System Inc, Atlanta, GA, USA) were utilized as described before [33]. To start a TIB, 3 ± 0.25 g PEM culture was suspended in a 50 mL falcon tube with 20 mL of basal MSG liquid medium and plated onto the filter paper on the TIB screen holder. The cultures were immersed in liquid medium twice per day and subcultured every two weeks by exchanging the medium bottle. All cultures were kept at 23 ± 1 °C in darkness. Three bioreactors were started for each of the 3 time points when fresh weight (FW) was to be measured. At each time point, the three bioreactors were terminated after the FW was recorded.
2.3. Somatic embryo maturation
Two-week old PEM culture was placed directly on solid maturation medium in clumps (each approximately 100 mg FW); or approximately 200 mg PEM culture was dispersed on filter paper. The maturation medium was MSG medium [32] supplemented with 1 µM indolebutyric acid (IBA), 60 µM abscisic acid (ABA), 0.2 M sucrose, 10 mM l-glutamine, and solidified with 8 g•L − 1 gelrite (Duchefa, Netherlands) [10]. PEM cultures were also plated onto the filter paper (Whatman, No. 113, UK Ltd.) placed on the TIB screen by suspending approximately 3 g PEM culture in a 50 mL falcon tube with 20 mL of liquid maturation medium. After 8 weeks of culture, the number of cotyledonary embryos in each bioreactor were counted. The maturation yield was given as number of cotyledonary embryos per g starting FW of PEM culture measured at the start of maturation. Different initial inoculum densities of PEM culture (0.5, 1.0, 3.0 and 5.0 g per TIB) of cell line Q3 were also tested to determine the optimal inoculum density for maturation of hybrid larch in the TIB system. After eight weeks of culture without subculture, the number of cotyledonary embryos per TIB was determined. The FW of the whole culture was also noted (Fig. S3). The TIB cultures were immersed in liquid medium once per day for 15–20 s. All cultures were maintained in darkness at 23 ± 1 °C.
2.4. Germination of somatic embryos
Cotyledonary embryos with elongated hypocotyls and distinctly visible separated cotyledons were selected and transferred to germination medium. Four culture systems including two solid culture systems (Petri plates 92 × 16 mm, and 150 × 20 mm, Sarstedt AG & Co. KG, Germany), and two TIB systems (horizontal and vertical) were utilized for comparing the germination frequency of somatic embryos in hybrid larch. In addition to the horizontal TIB which is described above, TIBs for vertical germination (Bioautomaton Systems Inc., Atlanta, GA, USA) were also utilized for the germination experiment. The vertical TIBs have the same shell as the horizontal TIB but are equipped with 30 vertical tubes per bioreactor for germination of cotyledonary embryos. Each tube holds a 10 mm diameter disc filter paper (Whatman, No. 113, UK Ltd.) with the cotyledonary embryo enclosed for germination stimulated by temporary immersion of the filter papers through the regular function of the TIB [34]. In this study, 30 cotyledonary embryos per Petri plate irrespective of size, or per TIB, was cultured with MSG medium [32] containing 10 mM l-glutamine and 60 mM sucrose [10]. The embryos were immersed in liquid medium once per day in both horizontal and vertical TIB systems. Each Petri plate (92 × 16 mm) contained 20 mL medium and the 150 × 20 mm Petri plate contained 100 mL medium. The number of somatic embryos forming a root; and the number of roots per plantlet, root length (mm), and shoot height (mm) were measured after 10 weeks of germination without subculture.
The germination experiments were conducted using LED light (VENSO EcoSolution AB, model PFL-600–1-RBC-30, Sweden) with 16-h photoperiod (setting with light intensity 90–100 µmol•m − 2•s − 1 at 23 ± 1 °C). After 10 weeks of germination, plantlets which shoot- and root formation were transferred into HIKO-V13 (BCC AB, Landskrona, Sweden) trays placed into seed starter trays (Nelson garden, Tingsryd, Sweden) containing a 3:1 soil-perlite mixture (Kekkilä Professional, Vantaa, Finland) with the lids initially to keep the relative humidity around 90 - 95%. The seed starter trays were placed in a culture room with the LED light, FL300 Sunlight from Senmatic, Sonderso, Denmark at 21 ± 1 °C and fertilized once per week with 10 ml•L − 1 WH Bouyant Rika S (Weibulls Horto, Hammenhög, Sweden). The percentage of survival was recorded after three months in ex vitro conditions.
2.5. Double staining of PEM with acetocarmine and evan's blue
An aliquot of two weeks old PEM culture (20 mg) was transferred to a microscope slide. The double staining protocol using Evan's blue and acetocarmine was conducted according to Gupta et al., 2005 [35]. The PEMs were visualized under a light microscope (Axioplan Imaging, Zeiss, Germany) and photographed with an attached camera (Axiocam HRc, Zeiss, Germany).
2.6. Statistical analysis
All experiments were carried out with three technical replicates. The results were calculated as means ± standard error. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range test, using the SPSS statistic 16.0 software (SPSS Inc., Chicago, IL, USA). Different letter indicated significant differences at P < 0.05.
3. Results
3.1. Proliferation of embryogenic cultures in TIB
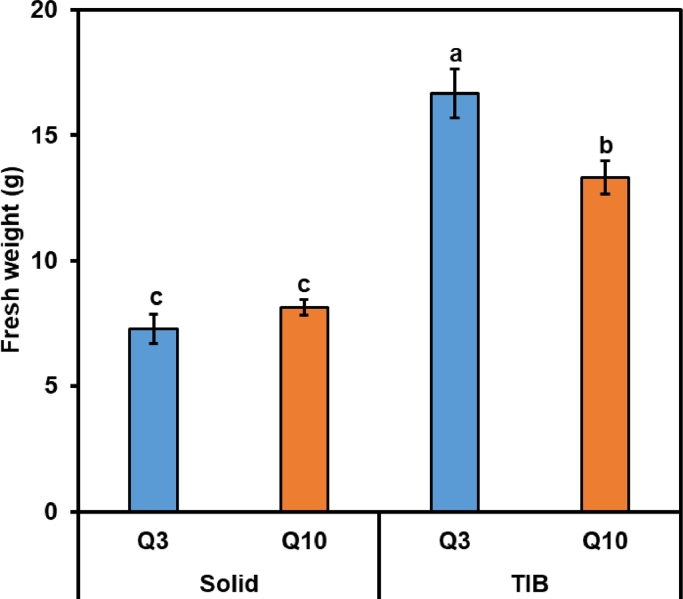
PEM proliferation rate was evaluated by comparison of FW increase in TIBs to standard proliferation rate on solid medium in Petri plates. Significant differences were observed between TIB and solid medium for both hybrid larch lines tested. After two weeks, the PEM culture FW increased more than twice in both cell lines Q3 and Q10 cultured on solid medium and five to six times (Q10 and Q3, respectively) when cultured in TIBs (Fig. 1). Cell lines Q3 and Q10 showed similar FW increase on solid medium whereas in TIBs, Q3 show significantly higher proliferation rate compared to Q10.
Fig. 1.
Fresh weight increase from starting fresh weight of 3 g PEMs per TIB during proliferation of hybrid larch (Larix × eurolepis Henry) embryogenic cultures in Petri plates and in the TIBs. Data represent mean ± SE from three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan's multiple range test (n = 3).
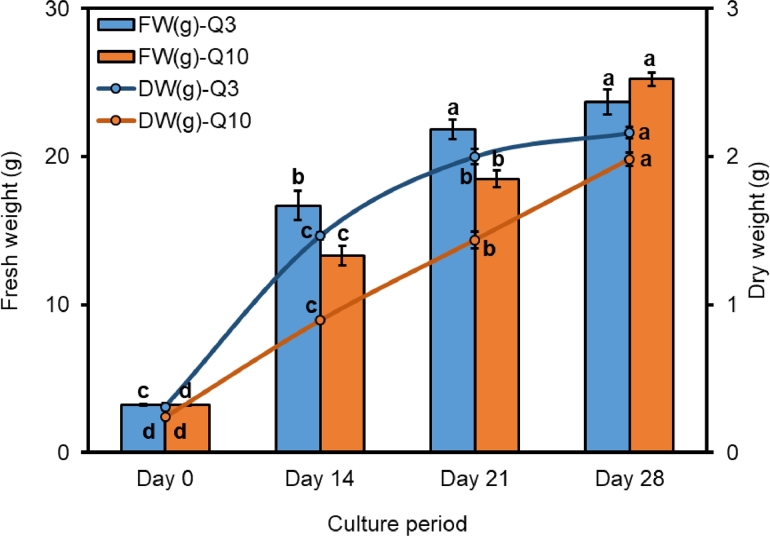
The FW increases of cell lines Q3 and Q10 in TIBs were linear without an observable lag phase over a period of four weeks with subculture to fresh medium of the same composition every two weeks and without removing any tissue from the TIBs during the culture period. The FW accumulation of Q3 line reached a maximum after three weeks then followed by a stationary phase during the fourth week of culture (Fig. 2). Cell line Q10 line grew slower than Q3 in the TIBs and the maximum FW was obtained after four weeks of culture (Fig. 2). After four weeks in culture, the PEM cultures had overgrown the surface of the filter paper in the TIB (Fig. 5A, B). There was a significant difference in FW increase between the cell lines after one, two and three weeks (Fig. 2). After four weeks in culture, the FW accumulation was however not significantly different between the cell lines. Similar results were shown for dry biomass (Fig. 2).
Fig. 2.
Accumulated fresh weight increase of cell lines Q3 and Q10 of hybrid larch (Larix × eurolepis Henry) embryogenic cultures in the TIBs. Data represent mean ± SE from three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan's multiple range test (n = 3).
Fig. 5.
Temporary immersion bioreactosr (TIBs) for somatic embryogenesis of hybrid larch (Larix × eurolepis Henry). A and B. Proliferation of PEMs. C. Double-staining of a PEM showing blue suspensor cells and red meristematic cells in the embryonic region. D, E. Maturation of somatic embryos. F. close-up of cotyledonary embryo. G, H, I, K. Different systems for germination of somatic embryos: 92 × 16 mm deep Petri plate (G), 150 × 20 mm Petri plate (H), horizontal TIB where the cotyledonary embryos are placed on the horizontal surface of the bottom of the TIB (I) and vertical TIB in which the cotyledonary embryos are placed in vertical position inside tubes (K). J and L. Larch plantlets in horizontal TIB and vertical TIB, respectively. M. SE plants grown in the greenhouse 15 months after transplanting from in vitro culture. EC, embryogenic cells stained with acetocarmine; SC, suspensor cells stained with Evan's Blue. Scale bar: 1 cm.
Typical PEM structures composed of an embryonic region and suspensor cells were present in the proliferating embryogenic cultures both in TIBs and cultures grown on solid medium. Early stage embryos (PEMs) visualized by the double-staining method show the meristematic regions of the pro-embryos within the PEM culture in red and suspensor cells in blue (Fig. 5C).
3.2. Somatic embryo maturation in TIBs
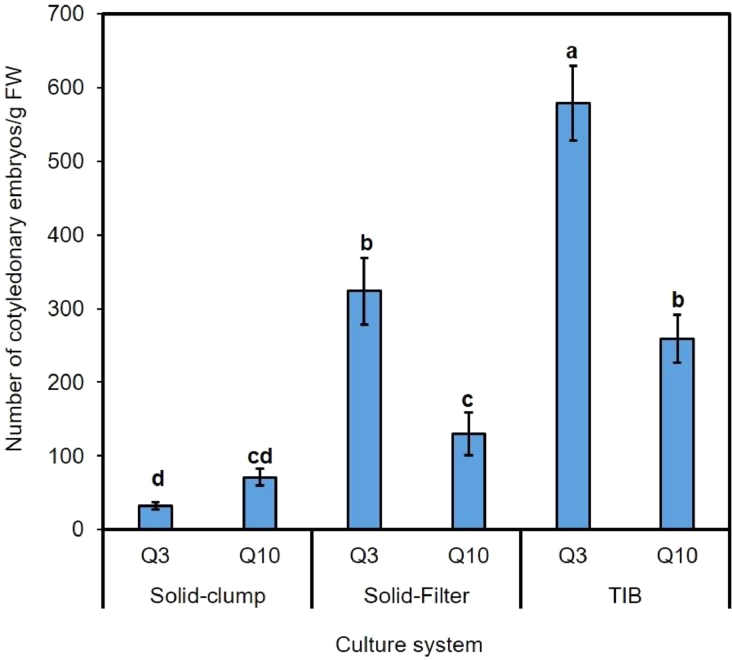
For comparison of maturation yields, the two culture systems included: small clumps of PEMs directly on solid medium, and suspended PEMs plated on filter paper on solid medium in plates; or suspended PEMs plated on filter paper in TIBs (Fig. 5d-F). After eight weeks on maturation medium, the highest yield of somatic embryos (579.33 ± 50.61 per g PEMs) was obtained in cell line Q3 cultured in the TIB system (Fig. 3). On solid medium, the yield of somatic embryos in cell line Q3 was significantly greater when cultured on filter paper (323.7 ± 45.29 per g PEMs) than when cultured as clumps (31.67 ± 4.85 per g PEMs) (Fig. 3). The highest yield of cotyledonary embryos in cell line Q10 (258.77 ± 32.3 per g PEMs) was also produced from culture in the TIB system while significantly lower yields were determined on solid medium for both filter paper and clumps culture systems (129.95 ± 28.95 and 70.93 ± 11.56 per g PEMs, respectively; Fig. 3). Overall, the embryo formation frequency in the TIB system was 1.8–2 times higher compared to the cultures plated on filter paper and 3.7–18.7 times higher when compared to clumps cultured on solid medium (Fig. 3).
Fig. 3.
Yield of cotyledonary embryos of hybrid larch (Larix × eurolepis Henry) after eight weeks of maturation per g FW of embryogenic culture in three different culture systems: clumps of embryogenic culture on solid maturation medium, suspended embryogenic culture plated onto filter paper placed on solid maturation medium, and TIBs. Data represent mean ± SE from three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan's multiple range test (n = 3).
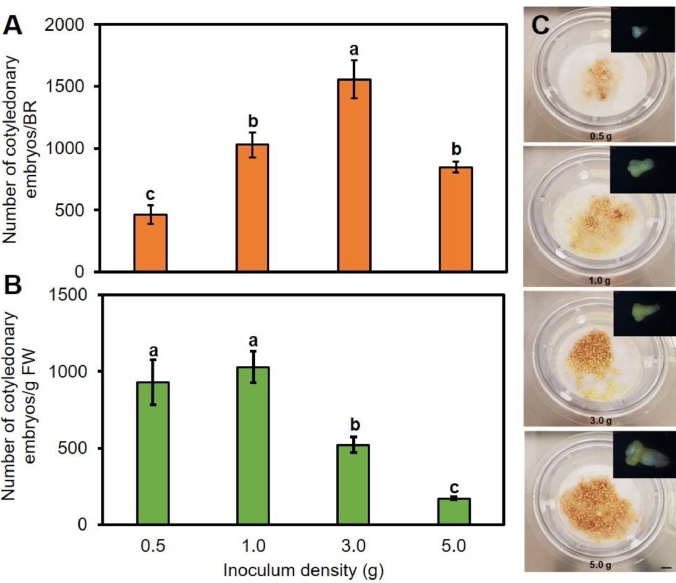
Due to the higher yield of cotyledonary embryos in cell line Q3, further maturation experiments were conducted only with the Q3 line. To determine the optimal initial inoculation density of embryogenic culture for maximum maturation frequency, embryogenic cultures of cell line Q3 were inoculated at densities varying from 0.5 to 5.0 g FW per TIB. The number of cotyledonary embryos increased linearly and the highest number of cotyledonary embryos per TIB (1558 ± 154.26 embryos per TIB) was reached with 3 g of PEM culture inoculum (Fig. 4A). However, the yield of cotyledonary embryos was highest at 1 g of PEM culture inoculum and significantly lower at higher inoculum densities of 3 g and 5 g (Fig. 4B). Interestingly, larger size cotyledonary embryos were formed at the two higher inoculum densities (Fig. 4C).
Fig. 4.
Effect of initial inoculum density on yield of cotyledonary embryos of hybrid larch (Larix × eurolepis Henry) in TIBs of cell line Q3 after eight weeks of maturation. A. Number of cotyledonary embryos per TIB. B. Yield of cotyledonary embryos. C. Overview of somatic embryo formation from different inoculum densities in the TIBs. Data represent mean ± SE from three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan's multiple range test (n = 3). Scale bar: 1 cm.
3.3. Germination of somatic embryos in TIBs and plant regeneration
For most conifer species, the germination of cotyledonary embryo is conducted by placing the cotyledonary embryo horizontally on solid germination medium in Petri plates. In this study, the effect of four different germination systems were investigated. The systems were constituted by conventional culture on solid medium in 92 × 16 mm deep Petri plates and 150 × 20 mm Petri plates, and horizontal and vertical TIB systems (Fig. 5G-L). The highest germination rates when considering either shoot- and root formation respectively for both cell lines were obtained on solid culture media in Petri plates whereas the longest roots and the tallest shoots were recorded in the horizontal TIBs (Table 1). The highest number of shoot formation (up to 63.33%) was recorded in cell line Q3 from embryos placed on 92 × 16 mm Petri plates (Table 1). The highest number of embryos that formed roots was observed in both cultures in 92 × 16 mm Petri plates and horizontal TIBs. However, the highest number of roots/plantlet (1.67 ± 0.33 roots/plantlet), the length of root (37.33 ± 4.33 mm) and the height of shoot (37.33 ± 1.45 mm) appeared on the embryos cultured in horizontal TIBs (Table 1, Fig. 5I, J). With Q10 line, 84.43% of embryos formed root on 92 × 16 mm Petri plates with solid culture, following to embryos culture on vertical TIBs (70%) (Table 1). The highest number of somatic embryos formed root was recorded in both 92 × 16 mm and 150 × 20 mm Petri plates with solid culture (12.33 ± 1.20 and 13.00 ± 1.00, respectively) (Table 1). The embryos cultured on 92 × 16 mm Petri plates showed the highest number of root/plantlet while the longest roots were observed in embryos placed on horizontal TIBs (Table 1). After 10 weeks of germination, the plantlets were planted in seed starter trays. Three months after planting, more than 65% of the rooted plantlets had successfully acclimatized and showed vigorous growth 15 months of planting (Fig. 5M).
Table 1.
Effect of different culture systems for germination of hybrid larch (Larix × eurolepis Henry) somatic embryos. Data represent mean ± SE from three replicates. Different letters indicate significant differences at P < 0.05 according to Duncan's multiple range test (n = 3).
| Line | System | No. forming shoot (%) | No. forming root (%) | No of root/plantlet | Root length (mm) | Shoot height (mm) |
|---|---|---|---|---|---|---|
| Q3 | 92 × 16 mm petri plate | 19.00 ± 3.21b (63.3) | 12.00 ± 1.00a (40.0) | 1.00 ± 0.00b | 11.33 ± 1.20c | 14.33 ± 1.45cde |
| 150 × 20 mm petri plate | 10.67 ± 1.20c (35.6) | 5.00 ± 1.53bc (16.6) | 1.00 ± 0.00b | 8.00 ± 0.58c | 12.67 ± 1.45de | |
| Vertical TIB | 7.67 ± 0.88c (25.6) | 3.33 ± 1.33c (11.1) | 1.00 ± 0.00b | 5.00 ± 1.15c | 19.67 ± 1.76b | |
| Horizontal TIB | 13.00 ± 1.00c (43.3) | 12.33 ± 1.45a (41.1) | 1.67 ± 0.33a | 37.33 ± 4.33a | 37.33 ± 1.45a | |
| Q10 | 92 × 16 mm petri plate | 25.33 ± 1.20a (84.4) | 12.33 ± 1.20a (41.1) | 1.67 ± 0.33a | 22.33 ± 1.76b | 16.00 ± 0.58bcd |
| 150 × 20 mm petri plate | 19.00 ± 0.58b (63.3) | 13.00 ± 1.00a (43.3) | 1.00 ± 0.00b | 26.00 ± 3.79b | 14.00 ± 0.58cde | |
| Vertical TIB | 21.00 ± 1.53ab (70.00) | 5.00 ± 1.00bc (16.67) | 1.00 ± 0.00b | 7.00 ± 1.15c | 11.00 ± 1.15e | |
| Horizontal TIB | 11.33 ± 2.96c (37.78) | 9.67 ± 2.67ab (32.22) | 1.00 ± 0.00b | 37.33 ± 4l33a | 17.33 ± 1.45bc |
4. Discussion
The availability of effective scale up methods for SE plant production in conifers is central to the success of SE applications in forest tree breeding programs and for plant production [1,36]. Automation of the most labour-intensive steps of the conifer SE process has been shown to work particularly well for propagation methods based on liquid culture medium [34].
Liquid culture medium in suspension culture in general works well for proliferation of PEMs although alternative containers like WAVE -bag systems appear less effective in keeping the PEM structures intact [27]. This is also reflected in the fact that there is only a single report on maturation in full liquid immersion demonstrating that PEMs of a conifer (P. abies) has limited capacity to mature under full liquid immersion in a suspension culture [31]. Arguably the shear stress on the PEMs in liquid suspension cultures acts negatively on formation of the more polarized PEMs that can respond to the maturation treatment [31,37]. The TIB systems offers both the advantages of a liquid culture medium and a solid support allowing polarized PEMs to develop.
Different types of bioreactors have been tested for conifer SE cultures both for proliferation of A. nordmanniana, P. sitchensis, Pinus kesiya, P. mariana and P. glauca-engelmannii [25,27,28,38] and maturation of A. nordmanniana, P. sitchensis, P. glauca, P. abies, Pinus kesiya, P. mariana and P. glauca-engelmannii [[24], [25], [26],28,30,38,39]. The results show that in general, TIBs offers an effective system for both proliferation and maturation of commercially important conifer somatic embryos including A. nordmanniana [33] and P. abies [26,39].
Different models of TIBs have been utilized for proliferation and maturation of conifer SE cultures with varying success. In previous TIB studies with P. abies SE cultures [26,39,40], proliferation rates were not measured as the goals were to increase the yields of mature embryos by the use of TIBs. However, in A. nordmanniana SE cultures both proliferation rate and yield of embryo were measured to almost three times higher in TIBs than on control plates [33]. A TIB designed for micropropagation (Plantform, Hjärup, Sweden) tested with several cell lines of Norway spruce resulted in overall lower success rates for maturation in the TIB than on solid medium [39,40] whereas the TIB specifically designed for conifer SE (and also used in the present study) showed two times higher yields of mature embryos [26].
Such differences may be related to the designs of the TIB where one major difference lays in the location of the liquid culture medium. In some TIBs like Plantform [40], RITA® [41], MATIS® (CIRAD, Montpellier, France), the liquid culture medium is kept inside the same container as the plant material, whereas in the TIB (Bioautomaton Systems Inc., Atlanta, GA, USA) [42] used in this study, the medium is kept in a separate container from the plant material. Furthermore, the airspace to culture area ratio differ between bioreactor models also adding to the creation of different culture environments. For example, the maturation yields of Coffea arabica [43], Musa [44] and Prunus [1] cultured in the MATIS TIBs were much higher than in the RITA TIBs being the smaller version of the same model TIB and with a lower surface-to-air ratio.
SE plant production from hybrid larch have been previously demonstrated by using solid medium for proliferation, maturation and germination [10,11]. High FW increase of up to ten times was shown after one week of culture on solid medium of some REVE-VERT cell lines [10] . This is higher than in the present study where the two REVE-VERT cell lines studied showed only two-fold fresh weight increase after one week on solid medium. The embryo yields on solid medium obtained in this study was also considerably lower ranging from 130 to 300 than previously shown for the related cell lines producing 200 to 1400 embryos per gram starting fresh weight [10]. It could be argued that the reason for the difference in yields is due to an assumed ‘ageing’ effect of the cultures due to prolonged in vitro cultivation as has been reported for other conifers such as Pinus pinaster [45,46] but no such ‘ageing’ effect was detected when studied previously in two of the REVE-VERT lines [10]. However, another possible ageing effect could come from the longer-term in vitro cultivation related to the difference in the overall time from initiation of the cell lines to the experiments (three to five years for the first experiments in 2009 and 12- 14 years for the current study). There could also be a negative effect from the air transportation of the SE cultures from France to Sweden. In the present study, the FW increase in TIB was however almost twice as high compared to the FW increase on solid medium in Petri plates. The yield of cotyledonary embryos was also almost double in TIBs compared to Petri plates, indicating the potential for improved growth and development using TIBs also for SE cultures of larch.
In the present study, SE cultures of hybrid larch were successfully proliferated, matured and germinated in TIBs. The main reason for the lack of success when using fully immersed suspension cultures for maturation of conifer PEMs appears to be the loss of polarity of the PEMs that has been shown to be a requirement for response to the maturation treatment [47]. The advantages of a TIB system for supporting formation of polarized PEMs structures were shown here by double staining of TIB-cultured hybrid larch PEMs (Fig. 5C).
Inoculum density is in general an important factor influencing the productivity of in vitro cultures e.g. cell suspension, tissues and organ cultures, and somatic embryos [17,48]. The relation between the initial inoculum density and numbers and quality of somatic embryos formed in the culture has been well documented in cultures of carrot (Daucus carota) [49] and studied in other species e.g. Cocos nucifera [50]. Inhibitory effects on the SE process from compounds released to the culture medium at high culture densities were demonstrated in Daucus carota and Larix leptolepis [51], [52], [53], [54] and Coffea arabica [55,56]. From the present study, an initial inoculum density above 1 g per TIB resulted in a lower yield of cotyledonary embryos under the culture conditions used. This suggests that there is a threshold value for PEM inoculum density to obtain optimal somatic embryo formation in hybrid larch. However, it is possible that higher inoculum densities can be supported by subculture to fresh maturation medium during the maturation process.
In conifers, a common problem during germination is shoot formation associated with poor root formation [57,58] as was also observed in the present study by the higher rate of shoot compared to root formation (Table 1). Germination of cotyledonary conifer somatic embryos are usually on solid medium horizontally or sometimes inserted into the solid medium. There are to date no reports on germination of conifer somatic embryos supported by liquid medium in TIBs. The benefits from liquid medium in a TIB system has however previously been demonstrated for germination of non-coniferous woody plants like Psidium guajava [59], Coffea arabica [60], Bactris gasipaes [61], Theobroma cacao [62], Elaeis guineensis [63] and Hevea brasiliensis [64]. Furthermore, the quality of the plantlets was reported to be better when developed in TIBs than in conventional culture systems in terms of shoot development of Bactris gasipaes [61], root development in Hevea brasiliensis [64] and overall plant development in Crescentia cujete [65]. In this study, of the four germination culture systems tested for enhancing the quantity and quality of germination of hybrid larch somatic embryos, the highest germination rates were obtained in solid cultures when considering both shoot- and root development respectively. However, both shoot- and root development were significantly better in the horizontal TIB system compared to both the vertical TIB and the two traditional solid medium systems indicating that conifer somatic embryo germination benefit from liquid culture medium equally to other woody species. Successful germination based on liquid medium open for possibilities to utilize methods for automation and scale up also of the germination process [34].
Rooting and adapting to ex vitro conditions are important key steps for in vitro based propagation. A well-developed root will support faster and better acclimatization. The results from germination in the vertical TIB used in this study suggest that with further optimizations of culture procedures, cost-effective, automated production of planted SE plantlets is possible [34,66]. Furthermore, the results showed that roots formed in the vertical TIB are more straight compared to the horizontal TIB systems (Fig 5J, L). Such straight plantlet without the frequently occurring angle between root and shoot in conifer somatic plantlets provide easy handling and transfer of plantlets to the substrate in ex vitro condition also for manual planting.
5. Conclusions
The TIB system was demonstrated to be a potential method for proliferation, maturation and germination of hybrid larch embryogenic cultures. In the present study, the yields of cotyledonary embryos on solid medium in plates were relatively lower than what was previously shown for the same cell lines, however the yield of cotyledonary embryos in the TIB system was still relatively and significantly higher. Furthermore, although the germination yield in the TIB system was lower than on solid medium, the quality of embryos was higher with better root- and shoot development. The germination condition can likely be improved to increase the germination yield by optimization of immersion frequency and light quality and intensity.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
CRediT authorship contribution statement
Kim-Cuong Le: Conceptualization, Investigation, Validation, Formal analysis, Visualization, Writing – original draft. Beata Dedicova: Conceptualization, Project administration. Sofie Johansson: Conceptualization, Project administration. Marie-Anne Lelu-Walter: Writing – review & editing, Project administration. Ulrika Egertsdotter: Conceptualization, Supervision, Resources, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to acknowledge Dr. Sonali Ranade from UPSC, Umeå, Sweden, for taking the pictures and reviewing this article. This work was supported by a grant from the Kempe Foundation of Sweden.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2021.e00684.
Appendix. Supplementary materials
References
- 1.Vidal N., Sánchez C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019;19:896–915. doi: 10.1002/elsc.201900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klimaszewska K., Hargreaves C., Lelu-Walter M.A., Trontin J.F. Methods Mol. Biol. Humana Press Inc.; 2016. Advances in conifer somatic embryogenesis since year 2000; pp. 131–166. [DOI] [PubMed] [Google Scholar]
- 3.Wu H.X. Benefits and risks of using clones in forestry–a review. Scand. J. For. Res. 2019;34:352–359. [Google Scholar]
- 4.K.-.A. Högberg, S. Varis, Vegetative propagation of Norway spruce: experiences and present situation in Sweden and Finland, Veg. Propag. For. Trees/Eds. Yill-Sung Park. Jan M. Bonga Heung-Kyu Moon. (2016).
- 5.M.-.A. Lelu-Walter, C. Teyssier, V. Guérin, L. Pâques, Vegetative propagation of larch species: somatic embryogenesis improvement towards its integration in breeding programs, Veg. Propag. For. Trees. (2016).
- 6.Lelu-Walter M.A., Thompson D., Harvengt L., Sanchez L., Toribio M., Pâques L.E. Somatic embryogenesis in forestry with a focus on Europe: state-of-the-art, benefits, challenges and future direction. Tree Genet. Genomes. 2013;9:883–899. doi: 10.1007/s11295-013-0620-1. [DOI] [Google Scholar]
- 7.Shmakov V.N., Konstantinov Y.M. Somatic embryogenesis in Larix: the state of art and perspectives. Vavilov J. Genet. Breed. 2020;24:575. doi: 10.18699/VJ20.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lelu-Walter M.-.A., Klimaszewska K., Miguel C., Aronen T., Hargreaves C., Teyssier C., Trontin J.-.F. Somatic embryogenesis for more effective breeding and deployment of improved varieties in Pinus spp.: bottlenecks and recent advances. Somat. Embryog. Fundam. Asp. Appl. 2016:319–365. [Google Scholar]
- 9.Lelu M.A., Bastien C., Klimaszewska K., Ward C., Charest P.J. An improved method for somatic plantlet production in hybrid larch (Larix × leptoeuropaea): part 1. Somatic embryo maturation. Plant Cell. Tissue Organ Cult. 1994;36:107–115. [Google Scholar]
- 10.Lelu-Walter M.A., Pâques L.E. Simplified and improved somatic embryogenesis of hybrid larches (Larix × eurolepis and Larix × marschlinsii). Perspectives for breeding. Ann. For. Sci. 2009;66 doi: 10.1051/forest/2008079. [DOI] [Google Scholar]
- 11.Kraft A., Kadolsky M. Step Wise Protoc. Somat. Embryog. Important Woody Plants. Springer; 2018. Hybrid larch (Larix × eurolepis Henry) pp. 149–158. [Google Scholar]
- 12.Kim S.J., Dewir Y.H., Moon H.K. Large-scale plantlets conversion from cotyledonary somatic embryos of Kalopanax septemlobus tree using bioreactor cultures. J. Plant Biochem. Biotechnol. 2011;20:241–248. [Google Scholar]
- 13.Maruyama T.E., Hosoi Y. Step Wise Protoc. Somat. Embryog. Important Woody Plants. Springer; 2018. Protocol for somatic embryogenesis in Japanese black pine (Pinus thunbergii Parl.) and Japanese red pine (Pinus densiflora Sieb. et Zucc.) pp. 229–241. [Google Scholar]
- 14.Egertsdotter U. Plant physiological and genetical aspects of the somatic embryogenesis process in conifers. Scand. J. For. Res. 2019;34:360–369. doi: 10.1080/02827581.2018.1441433. [DOI] [Google Scholar]
- 15.Ho T.-.T., Lee K.-.J., Lee J.-.D., Bhushan S., Paek K.-.Y., Park S.-.Y. Adventitious root culture of Polygonum multiflorum for phenolic compounds and its pilot-scale production in 500L-tank. Plant Cell, Tissue Organ Cult. 2017;130:167–181. [Google Scholar]
- 16.Paek K.-.Y., Murthy H.N., Zhong J.-.J. Springer; 2014. Production of Biomass and Bioactive Compounds Using Bioreactor Technology. [Google Scholar]
- 17.Monja-Mio K.M., Herrera-Alamillo M.Á., Robert M.L. Somat. Embryog. Fundam. Asp. Appl. Springer; 2016. Somatic embryogenesis in temporary immersion bioreactors; pp. 435–454. [Google Scholar]
- 18.Robert M.L., Herrera-Herrera J.L., Herrera-Herrera G., Herrera-Alamillo M.Á., Fuentes-Carrillo P. Plant Cell Cult. Protoc. Springer; 2006. A new temporary immersion bioreactor system for micropropagation; pp. 121–129. [DOI] [PubMed] [Google Scholar]
- 19.Berthouly M., Etienne H. Liq. Cult. Syst. Vitr. Plant Propag. Springer; 2005. Temporary immersion system: a new concept for use liquid medium in mass propagation; pp. 165–195. [Google Scholar]
- 20.Lyam P.T., Musa M., Jamaleddine Z., Okere U.A., Odofin W.T. The potential of temporary immersion bioreactors (TIBs) in meeting crop production demand in Nigeria. J. Biol. Life Sci. 2012;3:66–86. [Google Scholar]
- 21.Egertsdotter U., Ahmad I., Clapham D. Automation and scale up of somatic embryogenesis for commercial plant production, with emphasis on conifers. Front. Plant Sci. 2019;10:109. doi: 10.3389/fpls.2019.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tautorus T.E., Lulsdorf M.M., Kikcio S.I., Dunstan D.I. Bioreactor culture of Picea mariana Mill.(black spruce) and the species complex Picea glauca-engelmannii (interior spruce) somatic embryos. Growth parameters. Appl. Microbiol. Biotechnol. 1992;38:46–51. [Google Scholar]
- 23.T.E. Tautorus, D.I. Dunstan, Scale up of embryogenic plant suspension cultures in bioreactors, Somat. Embryog. Woody Plants. (1995).
- 24.Attree S.M., Pomeroy M.K., Fowke L.C. Production of vigorous, desiccation tolerant white spruce (Picea glauca [Moench.] Voss.) synthetic seeds in a bioreactor. Plant Cell Rep. 1994;13:601–606. doi: 10.1007/BF00232929. [DOI] [PubMed] [Google Scholar]
- 25.Ingram B., Mavituna F. Effect of bioreactor configuration on the growth and maturation of Picea sitchensis somatic embryo cultures. Plant Cell. Tissue Organ Cult. 2000;61:87–96. [Google Scholar]
- 26.Mamun N.H.A., Aidun C.K., Egertsdotter U. Improved and synchronized maturation of Norway spruce (Picea abies (L.) H. Karst.) somatic embryos in temporary immersion bioreactors. Vitr. Cell. Dev. Biol. 2018;54:612–620. doi: 10.1007/s11627-018-9911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valdiani A., Hansen O.K., Johannsen V.K., Nielsen U.B. An efficient bioreactor platform for scaling up the proliferation of Nordmann fir's (Abies nordmanniana) somatic embryos. Int. J. Environ. Sci. Technol. 2020;17:1425–1438. [Google Scholar]
- 28.Choudhury H., Tandon P. Non-destructive assessment of growth performance of embryogenic suspension culture of Pinus kesiya (Royle ex. Gord.) in shake-flask and self-designed bubble bioreactor and successful regeneration of plantlets from the culture systems. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014;84:771–777. [Google Scholar]
- 29.Aidun C.K., Egertsdotter E.M.U. Fluidics-based automation of clonal propagation via somatic embryogenesis: sE-fluidics system. Proc. IUFRO Work. Party. 2012;2:25–28. [Google Scholar]
- 30.Businge E., Trifonova A., Schneider C., Rödel P., Egertsdotter U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of Eucalyptus, birch and fir. Forests. 2017;8 doi: 10.3390/f8060196. [DOI] [Google Scholar]
- 31.Gorbatenko O., Hakman I. Desiccation-tolerant somatic embryos of Norway spruce (Picea abies) can be produced in liquid cultures and regenerated into plantlets. Int. J. Plant Sci. 2001;162:1211–1218. [Google Scholar]
- 32.Becwar M.R., Nagmani R., Wann S.R. Initiation of embryogenic cultures and somatic embryo development in loblolly pine (Pinus taeda) Can. J. For. Res. 1990;20:810–817. [Google Scholar]
- 33.Businge E., Trifonova A., Schneider C., Rödel P., Egertsdotter U. Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of Eucalyptus, birch and fir. Forests. 2017;8:196. doi: 10.3390/f8060196. [DOI] [Google Scholar]
- 34.Aidun C.K., Egertsdotter U. Step Wise Protoc. Somat. Embryog. Important Woody Plants. Springer; 2018. SE Fluidics System; pp. 211–227. [DOI] [Google Scholar]
- 35.Gupta P.K., Holmstrom D. Protoc. Somat. Embryog. Woody Plants. Springer; 2005. Double staining technology for distinguishing embryogenic cultures; pp. 573–575. [Google Scholar]
- 36.Rosvall O., Bradshaw R.H.W., Egertsdotter U., Ingvarsson P.K., Wu H. Using Norway spruce clones in Swedish forestry: introduction. Scand. J. For. Res. 2019;34:333–335. [Google Scholar]
- 37.Sun H., Aidun C.K., Egertsdotter U. Possible effect from shear stress on maturation of somatic embryos of Norway spruce (Picea abies) Biotechnol. Bioeng. 2011;108:1089–1099. doi: 10.1002/bit.23040. [DOI] [PubMed] [Google Scholar]
- 38.Tautorus T.E., Lulsdorf M.M., Kikcio S.I., Dunstan D.I. Nutrient utilization during bioreactor culture, and maturation of somatic embryo cultures ofPicea mariana andPicea glauca-engelmannii. Vitr. Cell. Dev. Biol. 1994;30:58–63. [Google Scholar]
- 39.S. Välimäki, L. Paavilainen, M. Tikkinen, F. Salonen, S. Varis, T. Aronen, Production of Norway spruce embryos in a temporary immersion system (TIS), Vitr. Cell. Dev. Biol. (2020) 1–10.
- 40.F. Salonen, S. Varis, T. Aronen, From Petri dishes to bioreactors–First experiences on optimization of Norway spruce SE-process for bioreactors, in: Proc. 4th Int. Conf. IUFRO Unit 2.09. 02 “Development Appl. Veg. Propag. Technol. Plant. For. to Cope with a Chang. Clim. Environ. Sept. 19-23, 2016. La Plata, Arg, ag, 2017.
- 41.Alvard D., Cote F., Teisson C. Comparison of methods of liquid medium culture for banana micropropagation. Plant Cell. Tissue Organ Cult. 1993;32:55–60. [Google Scholar]
- 42.Escalona M., Lorenzo J.C., González B., Daquinta M., González J.L., Desjardins Y., Borroto C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999;18:743–748. [Google Scholar]
- 43.Etienne H., Bertrand B., Georget F., Lartaud M., Montes F., Dechamp E., Verdeil J.-.L., Barry-Etienne D. Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects. Tree Physiol. 2013;33:640–653. doi: 10.1093/treephys/tpt034. [DOI] [PubMed] [Google Scholar]
- 44.Bello-Bello J.J., Cruz-Cruz C.A., Pérez-Guerra J.C. A new temporary immersion system for commercial micropropagation of banana (Musa AAA cv. Grand Naine) Vitr. Cell. Dev. Biol. 2019;55:313–320. [Google Scholar]
- 45.Breton D., Harvengt L., Trontin J.F., Bouvet A., Favre J.M. Long-term subculture randomly affects morphology and subsequent maturation of early somatic embryos in maritime pine. Plant Cell. Tissue Organ Cult. 2006;87:95–108. doi: 10.1007/s11240-006-9144-9. [DOI] [Google Scholar]
- 46.Klimaszewska K., Noceda C., Pelletier G., Label P., Rodriguez R., Lelu-Walter M.A. Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.) Vitr. Cell. Dev. Biol. - Plant. 2009;45:20–33. doi: 10.1007/s11627-008-9158-6. [DOI] [Google Scholar]
- 47.Filonova L.H., Bozhkov P.V., von Arnold S. Developmental pathway of somatic embryogenesis in Picea abies as revealed by time-lapse tracking. J. Exp. Bot. 2000;51:249–264. doi: 10.1093/jexbot/51.343.249. [DOI] [PubMed] [Google Scholar]
- 48.González-Cabrero N., Ruiz-Galea M., Alegre J., Toribio M., Celestino C. Growth, morphology and maturation ability of Pinus pinea embryogenic suspension cultures. Plant Cell, Tissue Organ Cult. 2018;135:331–346. [Google Scholar]
- 49.Halperin W. Population density effects on embryogenesis in carrot-cell cultures. Exp. Cell Res. 1967;48:170–173. doi: 10.1016/0014-4827(67)90292-3. [DOI] [PubMed] [Google Scholar]
- 50.Kong E.Y.Y., Biddle J., Foale M., Adkins S.W. Cell suspension culture: a potential in vitro culture method for clonal propagation of coconut plantlets via somatic embryogenesis. Ind. Crops Prod. 2020;147 [Google Scholar]
- 51.Kobayashi T., Higashi K., Sasaki K., Asami T., Yoshida S., Kamada H. Purification from conditioned medium and chemical identification of a factor that inhibits somatic embryogenesis in carrot. Plant Cell Physiol. 2000;41:268–273. doi: 10.1093/pcp/41.3.268. [DOI] [PubMed] [Google Scholar]
- 52.Umehara M., Ogita S., Sasamoto H., KAMADA H. Inhibitory factor (s) of somatic embryogenesis regulated suspensor differentiation in suspension culture of Japanese Larch (Larix leptolepis Gordon) Plant Biotechnol. 2004;21:87–94. [Google Scholar]
- 53.Umehara M., Ogita S., Sasamoto H., Koshino H., Asami T., Fujioka S., Yoshida S., Kamada H. Identification of a novel factor, vanillyl benzyl ether, which inhibits somatic embryogenesis of Japanese larch (Larix leptolepis Gordon) Plant Cell Physiol. 2005;46:445–453. doi: 10.1093/pcp/pci041. [DOI] [PubMed] [Google Scholar]
- 54.Umehara M., Ogita S., Sasamoto H., Koshino H., Nakamura T., Asami T., Yoshida S., Kamada H. Identification of a factor that complementarily inhibits somatic embryogenesis with vanillyl benzyl ether in Japanese larch. Vitr. Cell. Dev. Biol. - Plant. 2007;43:203–208. doi: 10.1007/s11627-006-9016-3. [DOI] [Google Scholar]
- 55.Loyola-Vargas V.M., Avilez-Montalvo J.R., Avilés-Montalvo R.N., Márquez-López R.E., Galaz-Ávalos R.M., Mellado-Mojica E. Somat. Embryog. Fundam. Asp. Appl. Springer; 2016. Somatic Embryogenesis in Coffea spp; pp. 241–266. [Google Scholar]
- 56.Nic-Can G.I., Galaz-Ávalos R.M., De-la-Peña C., Alcazar-Magaña A., Wrobel K., Loyola-Vargas V.M. Somatic embryogenesis: identified factors that lead to embryogenic repression. A case of species of the same genus. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0126414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montalbán I.A., Moncaleán P. Rooting of Pinus radiata somatic embryos: factors involved in the success of the process. J. For. Res. 2019;30:65–71. doi: 10.1007/s11676-018-0618-5. [DOI] [Google Scholar]
- 58.J. Bonga, Y.-.S. Park, C. Ding, What technical improvements are needed to achieve industrial application of conifer somatic embryogenesis?, Clonal Trees Bioeconomy Age Oppor. Challenges. (2018).
- 59.Kosky R.G., Perozo J.V., Valero N.A., Peñalver D.A. the Rita® Temporary Immersion System and On Semisolid medium, in: Liq. Cult. Syst. Vitr. Plant Propag. Springer; 2005. Somatic embryo germination of Psidium guajava L; pp. 225–229. [Google Scholar]
- 60.Barbon R., Hien N.T., Capote A., de Feria M., Quiala E., Pérez A. Effect of the inoculation density in Coffea arabica L. cv.’Caturra rojo'somatic embryos germination in RITA® Temporary Immersion System. Biotecnol. Veg. 2014;14:91–97. [Google Scholar]
- 61.Heringer A.S., Steinmacher D.A., Fraga H.P.F., Vieira L.N., Montagna T., Quinga L.A.P., Quoirin M.G.G., Jiménez V.M., Guerra M.P. Improved high-efficiency protocol for somatic embryogenesis in Peach Palm (Bactris gasipaes Kunth) using RITA® temporary immersion system. Sci. Hortic. (Amsterdam). 2014;179:284–292. [Google Scholar]
- 62.Niemenak N., Saare-Surminski K., Rohsius C., Ndoumou D.O., Lieberei R. Regeneration of somatic embryos in Theobroma cacao L. in temporary immersion bioreactor and analyses of free amino acids in different tissues. Plant Cell Rep. 2008;27:667–676. doi: 10.1007/s00299-007-0497-2. [DOI] [PubMed] [Google Scholar]
- 63.Sumaryono I.R., Kasi P.D., Ginting G. Growth and differentiation of embryogenic callus and somatic embryos of oil palm (Elaeis guineensis Jacq.) in temporary immersion system. Indones. J. Agric. 2008;1:109–114. [Google Scholar]
- 64.Etienne H., Lartaud M., Michaux-Ferrière N., Carron M.-.P., Berthouly M., Teisson C. Improvement of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.) using the temporary immersion technique. Vitr. Cell. Dev. Biol. 1997;33:81–87. [Google Scholar]
- 65.Murch S.J., Liu C., Romero R.M., Saxena P.K. In vitro culture and temporary immersion bioreactor production of Crescentia cujete. Plant Cell. Tissue Organ Cult. 2004;78:63–68. [Google Scholar]
- 66.Aidun C.K., Integrated germination method and device, Applicant-Georgia Tech Research Corporation. Patent publication no. WO2016098083 A1, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.