Graphic abstract
Keywords: Ultrasound, Edible film, Protein structure, Pea protein, Water vapor permeability
Highlights
-
•
US induced unfolding of PPI and changed its secondary structure.
-
•
US increased surface hydrophobicity and decreased surface charge of PPI.
-
•
PPI structural changes induced by US improved properties of PPI-film.
-
•
US decreased cracks and protein aggregates at the PPI-film surface.
-
•
US-treated films are better in transparency, tensile strength and moisture barrier.
Abstract
Pea protein is a promising alternative to animal-based protein and the interest in its application in food industry has been rapidly growing. In this study, pea protein isolates (PPI) were used to form protein-based edible films and the effect of ultrasound treatment on the structure of PPI and the structural, optical, mechanical and physicochemical properties of PPI-films were investigated. Ultrasound induced unfolding of PPI and exposed interior hydrophobic groups to protein surface while both PPI dissociation and formation of large aggregates were observed, as confirmed by measuring intrinsic emission fluorescence, surface hydrophobicity, surface charge, and particle size distribution and polydispersity index, respectively. FE-SEM showed that ultrasound decreased the cracks and protein aggregates at the surface of PPI-film. The film structure was also investigated by FTIR, which showed peak shift in amide I and II region and noticeable difference of protein secondary structure as affected by ultrasound. As a result of such structural changes caused by ultrasound, the properties of PPI-films were improved. Results showed that ultrasound greatly improved the film transparency, significantly increased film tensile strength but not elongation at break, and decreased moisture content and water vapor permeability of the film. This study provided structural data as evidence for utilizing ultrasound technique to develop PPI-films with improved optical, mechanical and water barrier properties.
1. Introduction
Currently, the most common materials that are used to form films for food packaging purpose are those from petroleum origin including polyethylene, polypropylene, polystyrene, polyamide, and polyethylene terephthalate [1]. These polymers are preferred by the food packaging industry because they can form films that have desired optical, mechanical, and barrier properties, that is, high transparency, mechanical strength, and resistance to moisture and gas (e.g., oxygen and carbon dioxide). However, these compounds are all synthetic ones, presenting great threats to both consumers and the environment [2]. Because of this, there is an urgent need for natural and biodegradable materials that can form food packaging films with comparable properties to the synthetic ones. Proteins are good examples of such material that can form edible films for food packaging. For example, a lot of work has been done in the past decade to develop edible films based on whey, soy, and peanut proteins [3], [4], [5].
In recent years pea protein has received special attention from the food industry as a promising alternative to proteins from animal sources. Because of the growing demand for plant-based food choices from consumers, there are opportunities not only in developing pea protein as foods but also in exploring new applications for pea proteins. Edible film is one of such applications owing to the natural, plant-based, and biodegradable nature of pea proteins. However, developing pea protein films requires more research because pea protein is known to have poorer functionalities comparing to proteins from other sources such as whey and soy protein. For example, Stone et al. reported significant better water holding, foaming, and emulsifying properties of whey protein than pea protein [6]. Similarly, gels formed by pea protein have been noted to be weaker than that of soybean protein [7]. These suggest that the film-forming properties of pea protein may not match that of other proteins as well. In fact, developing pea protein film with desirable properties is still a challenge today since little progress has been made during the last decade. The earliest study on developing pea protein film can be traced back to the year of 1998 when Gueguen et al. reported the influence of plasticizer type on the hydrophobic characteristic and tensile strength of pea protein films [8]. The same group also reported fabrication of pea protein films using polyols with different chain lengths as the plasticizer [9]. Later, Choi and Han [10], [11] reported the influences of plasticizer concentration and heat treatment on the tensile strength, elastic modulus, elongation and water vapor permeability properties of pea protein films. More recently, Kowalcyzk et al. published a series of papers which studied the influence of plasticizer type and concentration, pH, heat treatment and lipid incorporation on various properties of PPI-film [12], [13], [14]. One additional contribution of Kowalcyzk et al. was that they tried to incorporate lipid compounds into pea protein film to improve the film properties [14]. In the meantime, the above pioneer studies mainly aimed for successful fabrication of pea protein films, whereas the application value of pea protein films is still limited because of their inferior film properties as compared to that of whey protein films. Therefore, there is a need for strategies to further improve the properties of these pea protein films for potential applications in the future.
High-intensity ultrasound generally refers to ultrasound (US) with an intensity higher than 1 W/cm2 and frequencies between 20 and 500 kHz [15]. It is a nonthermal food processing technique that is known to modify protein structure and thus improve several protein functionalities such as solubility, gelling, foaming and emulsifying properties [16]. Until now, high-intensity ultrasound has also been used to modify the film properties of whey [17], gluten [18] and peanut [5], but insights into its effect on the pea protein film have not been reported. More importantly, although the improved film properties by ultrasound treatment, as reported in previous studies, was often attributed to ultrasound altering the protein structures, yet there has not been a study that investigated both protein structures and the resultant film properties. Therefore, the objective of this study was to systematically investigate whether ultrasound technique could be used to meet the challenge of improving the properties of pea protein films. The effect of ultrasound on pea protein structure and thus the structural, optical, mechanical and physicochemical properties of the resultant pea protein films were investigated.
2. Materials and methods
2.1. Materials
Pea protein isolate (PPI) in powder form was donated by Roquette America Inc (Geneva, IL, USA). PPI contains 80% protein, 7% moisture, 6% lipids, 4% ash and 3% carbohydrates. 8-anilino-1-naphthalenesulfonic acid (ANS) was purchased from Sigma (St. Louis, MO, USA). All other chemicals and reagents used in this study were of analytical grade and purchased from VWR (Chicago, IL, USA). All solutions were prepared using ultrapure water (Barnstead Nanopure ultrapure water system, Thermo Scientific, MA, USA).
2.2. High-intensity ultrasound treatment of PPI
PPI dispersions (10.0%, w/v) were obtained by adding PPI powder to water under stirring for 4 h at 25 °C before overnight storage at 4 °C. Protein solution was then treated with high-intensity ultrasound using an ultrasound processor (Q Sonica, Q700, USA) at different amplitude (40% and 80%) for different ultrasonication time (0, 10 and 20 min). The diameter of the probe was 12.7 mm, and the ultrasonication was performed at pulse duration of on-time 5 s and off-time 3 s. An ice bath was used during ultrasonication to avoid overheating of the samples. The PPI solution without ultrasound application was used as control. The total energy delivered to the sample was displayed on the screen of ultrasonic processor. The acoustic power density (W/cm2) was calculated by dividing the total energy delivered to the sample by ultrasound time and probe area, which was summarized in Table 1. After ultrasound treatment, part of the protein solutions were lyophilized (VirTis Benchtop K) for structure characterization of PPI (Section 2.3), while the remaining solutions were used for fabrication and characterization of PPI-based films (Section 2.4).
Table 1.
Sample name abbreviations and ultrasound power density for each treatment.
| Treatment | Amplitude (%) | Time (min) | Total energy (J) | Acoustic power density (W/cm2) |
|---|---|---|---|---|
| Control | 0 | 0 | 0 | 0 |
| US410 | 40 | 10 | 30,147 | 31.15 |
| US420 | 40 | 20 | 58,963 | 30.46 |
| US810 | 80 | 10 | 54,698 | 56.52 |
| US820 | 80 | 20 | 107,216 | 55.39 |
2.3. Characterization of PPI structure
For the following three measurements in this section, the concentration of PPI solution was based on protein content. For this purpose, PPI solubility was firstly measured using Bradford dye-binding method according to our previous paper with no modifications [19].
2.3.1. Particle size and ζ-potential
Lyophilized PPI was dissolved in water at 0.5 mg protein/mL. The PPI solution was then diluted 50-fold before being subjected to measurements of particle size distribution, particle size (reported as Z average), and polydispersity index by a Zetasizer (Nano ZS90, Malvern Instruments, Malvern, UK). For ζ-potential measurement, the same instrument was used with disposable capillary cells (DTS1070, Malvern Instruments).
2.3.2. Intrinsic emission fluorescence spectroscopy
Protein solutions (0.15 mg protein/mL) were prepared in 10 mM phosphate buffer (pH 7.0). Intrinsic emission fluorescence spectra of various PPI samples were determined by a fluorometer (Horiba JY FluoroMax-4, Horiba Co., Japan). Protein solutions were excited at 290 nm, and emission spectra were recorded from 305 to 450 nm at a constant slit of 5 nm for both excitation and emission.
2.3.3. Surface hydrophobicity (H0)
The surface hydrophobicity of PPI was measured by the fluorescence method as described in our previous study [19]. Briefly, protein solutions (0.05, 0.10, 0.15, 0.20 and 0.25 mg protein/mL) were prepared in 10 mM phosphate buffer (pH 7.0). Then, 4 mL of each protein solution was added with 20 µL of ANS (8 mM in the same phosphate buffer). The fluorescence intensity was measured at 390 nm (excitation) and 470 nm (emission) (Horiba JY FluoroMax-4, Horiba Co., Japan). The initial slope of fluorescence intensity versus protein concentration calculated by linear regression analysis was used to represent the protein surface hydrophobicity.
2.4. Characterization of PPI-based films
2.4.1. Film formation
Film forming solution was prepared by adding glycerol (5 wt%) into the PPI solutions (10 wt%) so that the protein/plasticizer ratio is 2:1. After stirring and degassing, 6 g of the film forming solution was poured onto Petri dishes (10 cm in diameter), followed by drying at 25 °C for 24 h. Dried films were manually peeled off. The dried films were then conditioned at 55% relative humidity at 25 °C for 3 days before performing the following measurements. Film thickness was determined using a digital micrometer (VWR, US) with five measurements at random spots of each film. The average film thickness was 0.17 mm and no significant differences were found.
2.4.2. Surface morphology by filed emission scanning electron microscopy (FE-SEM)
Film pieces were coated by a Cressington Carbon Coater (Cressington Scientific Intruments Ltd., Watford, UK). A field emission scanning electron microscope (FEI Nova 400 nanoSEM, FEI Company, Hillsboro, US) was then used to observe sample morphologies. An accelerating voltage of 10 kV was used. Micrographs with magnification of × 20,000 were obtained for comparison.
2.4.3. Structural characterization of films by FTIR-ATR
Film pieces were measured by a Fourier transform infrared spectrometer (FTIR) system coupled to an attenuated total reflectance attachment (ATR) (JASCO 6800, JASCO, Japan). The films were scanned from 4000 to 400 cm−1 with a resolution of 4 cm−1 at 64 scans. Before the scanning of each sample, the background was collected. The acquired spectra were then processed by noise elimination and baseline correction. Second derivatives and Gaussian curve fitting were obtained using Origin (OriginLab Corp., MA, US).
2.4.4. Optical properties
The color properties of PPI-films were measured by a colorimeter (LabScan XE, HunterLab, VA, US). A white and a black standard plate was used for calibration before measurements. HunterLab color parameters, L*, a* and b*, were obtained from EasyMatch QC software. The opacity of films were determined as film absorbance at 600 nm, measured by a spectrophotometer, divided by film thickness (0.17 mm) [20].
2.4.5. Mechanical properties
Tensile strength (TS) and elongation at break (EAB) were measured by a TA.XTplus texture analyzer (Stable Micro System, Godalming, UK) [21]. Briefly, pre-conditioned films were cut into strips of 6 cm × 3 cm and fixed by two grips that were 30 mm apart vertically. Films were then stretched at a speed of 2 mm/s until break and the force and distance were recorded. TS (MPa) was calculated by dividing the maximum load for breaking film (N) by the film cross-sectional area (mm2); EAB (%) was calculated by dividing the distance between grips at the moment of rupture by the initial distance between grips and multiply by 100. The average of four measurements (two films with two stripes from each) were reported as results.
2.4.6. Moisture content and total soluble matter
The moisture content and total soluble matter were determined according to Munoz et al. with no modifications [22]. In brief, the moisture content of the film was determined by the weight loss after oven-drying at 105 °C for 24 h. After oven-drying, the dry matter content of the film was also obtained. The same oven-dried films were then solubilized in water for 24 h, and then the undissolved films were collected, redried, and weighed. The total soluble matter was calculated as the difference between the weight of initial dry matter content and the undissolved films.
2.4.7. Water vapor permeability (WVP)
The water vapor permeability was measured according to ASTM, 2000 [23]. Glass cups containing silica gel beads were sealed by films and then placed in a desiccator that was conditioned with distilled water. The weight gains of cups were recorded every 2 h for the first 8 h and then every 8 h for another 24 h. The water vapor permeability was calculated as:
, where is the weight gain of cups over time in g; l is film thickness in mm; t is time in h; A is the exposed film area in m2; is the partial vapor pressure difference of the atmosphere with silica gel and pure water.
2.5. Statistical analysis
All measurements were performed on triplicate samples unless otherwise noted above. The means were analyzed by analysis of variance (ANOVA) using SPSS 25.0 (SPSS Inc., IL, US) and the differences between mean values were compared using Duncan test with a significance level of p < 0.05.
3. Results and discussion
3.1. Effect of ultrasound on the structural properties of PPI
3.1.1. Particle size and particle size distribution
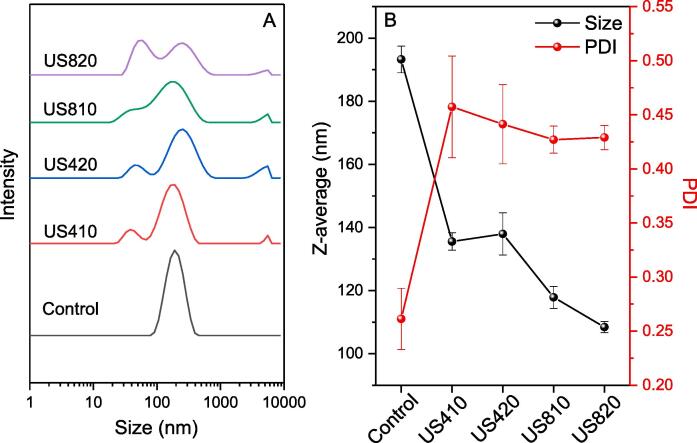
Due to the high energy input from ultrasound treatment, the protein particles in the solution could undergo disassociation and/or aggregation. Such disassociation and/or aggregation is useful information in this study because it affects the degree of protein–protein interactions during the formation of the film network in later steps. The particle size of PPI as affected by high intensity ultrasound treatment was measured by dynamic laser scattering. The particle size distribution is shown in Fig. 1A, while Fig. 1B shows both mean value (Z average) and the polydispersity index (PDI), which describes the distribution width. It was found that the untreated sample (control) showed a unimodal distribution, supported by a smaller PDI value, while ultrasound induced broader size distribution in all four treatment groups with a significantly larger PDI value. Also, all ultrasound-treated samples exhibited three peaks, with one major peak at the similar size as the control, a second peak at about 60 nm, and a third minor peak at the micron range. The average particle size of PPI was also significantly decreased from 193.3 to 108.4 nm by ultrasound treatment. Such decreased particle size is not surprising since during ultrasound treatment, the proteins which exist as aggregates in the solution would dissociate into smaller yet still soluble aggregates under the forces of cavitation [24]. This also suggested that the protein intermolecular forces, and thus the tertiary structure, were disrupted so that there would be a chance for more hydrophobic groups to be exposed to the environment. On the other hand, the larger size aggregates at the micron level could be formed due to reaggregation of the smaller aggregates probably through noncovalent interactions. This is supported by similar findings in protein isolates from whey, soy and black bean [25], [26], [27]. However, how such a change could influence the properties of protein-based films has not been reported.
Fig. 1.
The particle size distribution (A), particle size in Z average (left y axis, B), and polydispersity index (right y axis, B) of pea protein isolates as affected by ultrasound. For sample name abbreviations refer to Table 1.
3.1.2. Intrinsic fluorescence emission
To further confirm the conformational change of PPI caused by ultrasound treatment we then measured the intrinsic fluorescence spectra of all samples, of which the tryptophan residues fluoresce depending on the folding of the protein tertiary structure. Fig. 2 shows that ultrasound treatment greatly lowered the fluorescence intensity of PPI. This data indicated both an increased amount of tryptophan chromophores being exposed to the polar solvent (water in this case) and increased intermolecular interactions among these molecules [28]. Another finding from Fig. 2 is that the wavelength of maximum fluorescence intensity (λmax) shifted from 344 nm for the control to longer wavelengths (up to 350 nm) after ultrasound treatment. This again indicated that sonication disrupted the intact tertiary structure of PPI because such a shift was due to the tryptophan residues moving to the outside of protein molecules and being exposed to the polar microenvironment [29].
Fig. 2.
The intrinsic fluorescence emission spectra of pea protein isolates as affected by ultrasound. For sample name abbreviations refer to Table 1.
3.1.3. Surface hydrophobicity and surface charge
Because of the exposure of more hydrophobic groups, as demonstrated above, it was thus reasonably to expect the surface properties of PPI was also altered by the ultrasound treatment. Therefore, we further confirmed the surface hydrophobicity (Fig. 3A) and surface charge (Fig. 3B) of all samples. Fig. 3A provides direct evidence of the amount of hydrophobic amino acids residues that are exposed to protein surface. As expected, it was observed that the ultrasound treatment significantly increased the surface hydrophobicity of PPI as compared to the control. The highest surface hydrophobicity was found for US820, while no significant difference was found among US410, US420 and US810. The increased H0 is evidence of elevated exposure of not just tryptophan, which was measured by intrinsic fluorescence in Fig. 2, but also other hydrophobic domains of the protein peptides that were originally buried inside the protein hydrophobic core and other non-polar side chains of amino acids such as valine, leucine, isoleucine, phenylalanine, etc. This increase in H0, again, proved the unfolding and dissociation of PPI as affected by ultrasound treatment. In the meantime, it should be noted that data in Fig. 1A suggests the formation of large aggregates in our study, which, in theory, could protect the hydrophobic regions of the proteins, and therefore decreases H0 [26]. However, our result in Fig. 3A shows that the net effect of ultrasound treatment was to increase H0, meanning that the hydrophobic groups of PPI were more likely to be exposed than protected during this process.
Fig. 3.
The surface hydrophobicity (A) and ζ-potential (B) of pea protein isolates as affected by ultrasound. Different letters indicate significant difference (P < 0.05). For sample name abbreviations refer to Table 1.
The ζ-potential of PPI as affected by ultrasound treatment is shown in Fig. 3B. ζ-potential measures protein surface charge which is a net result of all available ionizable groups (e.g., carboxyl and amino groups) at the protein surface. In this study, it was found that the negative charge of PPI was substantially reduced by ultrasound treatment, from −40.7 mV for the control to −31.9 mV for the group treated at 80% amplitude for 20 min. In a similar study on black bean protein isolates, authors attributed the decreased absolute ζ-potential value to the formation of aggregates [26]. This could also be the case in our study as supported by the peaks in Fig. 1A that shows the formation of larger PPI aggregates. In addition, since ζ-potential is a value of the net charge, it could also be explained by the fact that more nonpolar, thus non-charged, groups were exposed to the protein surface after ultrasound treatments. The finding that sonication decreased the negative charge on PPI surface is important in this study because it indicates that, since all PPI carries charge of the same direction, the decrease of their magnitude means there would be less electrostatic repulsion among protein molecules, which could further influence the formation of film network in later steps.
Altogether, due to the effects of ultrasound on the conformation, dissociation and aggregation, surface hydrophobicity and the electrostatic interaction among protein molecules, it is thus reasonably to expect that ultrasound treatment would also influence many functional properties of PPI-based edible films.
3.2. Effect of ultrasound on the properties of PPI-based film
3.2.1. Surface morphology
The surface morphology of PPI films was examined using FE-SEM. It should be noted that when using SEM to investigate the surface morphology of films, magnifications ranging from × 50 to × 2,000 have all been reported in previous publications [4], [18], [21], but no obvious difference was observed for our films within this range. Fig. 4 shows the micrographs of PPI films as influenced by US at × 20,000 magnification. It was found that the untreated film (control) showed valleys with several non-continuous cracks. Also, protein aggregates were in presence at the surface. By comparison, no cracks were observed in all US-treated films while the non-smooth surface with valleys could still be found in all US-treated films although to a lesser extent for films treated by 80% amplitude (US810 and US820). The disappearance of cracks in the US-treated films indicated that the structural changes induced by US tended to form a more intact film with stronger intermolecular interactions within the film network. This could contribute to superior mechanical properties of the films. In the meantime, protein aggregates were only found at the surface of US410 film among all US-treated films but in smaller sizes than the control. Interestingly, although US410 and US420 treatments applied similar acoustic power density (Table 1), the total energy delivered by US420 was higher, which might explain the disappearance of protein aggregates upon film formation. This comparison suggested that there could be an energy threshold instead of powder density threshold that influenced the breakdown of protein aggregates.
Fig. 4.
Filed emission scanning electron microscopy (FE-SEM) micrographs of pea protein isolates films. For sample name abbreviations refer to Table 1.
3.2.2. FTIR spectra
The effect of ultrasound on the protein structure within the film was investigated by FTIR spectra (Fig. 5A). It was observed that the peak in the amide I region (1600–1700 cm−1) was slightly shifted, from 1631 cm−1 for the control to 1627 cm−1 for all US-treated films. Likewise, the peak in amide II region (1500–1600 cm−1) shifted from 1540 cm−1 for the control to 1536 cm−1 for the treatment groups. Such changes indicated the unfolding of protein molecules within film network [30]. When studying quinoa protein/chitosan blended film, Vera et al., 2020 also reported similar changes, which were claimed to be related to flexion vibration frequencies of intra and intermolecular hydrogen bonding as affected by cavitation during ultrasound [31]. As for the amide III region, no peak differences were observed among all groups. In addition, since peak characteristics in amide I region are commonly related to protein secondary structure, we then investigated the second derivative of FTIR spectra as affected by ultrasound treatment (Fig. 5B-F). Assignment of peaks to each protein secondary structure was illustrated in Figue5B and C in accordance with previous work [32]. Table 2 shows that US caused slight increase of β-sheet and considerable increase of random coils. Also, α-helix and anti-parallel β-sheet were significantly decreased, while β-turn remained unaffected. US treatment time and amplitude did not affect all secondary structures except for β-sheet and α-helix. Because the structures of β-sheet, α-helix and random coils are maintained by different patterns of hydrogen bond, the changes demonstrated in Table 2 indicated that US treatment rearranged the hydrogen bonding of the PPI within the film network, which suggested that US could cause different protein–protein and protein-glycerol interactions of the film network. It should be noted that currently there is no agreement on the effect of ultrasound on protein secondary structures. For example, Jiang et al. [26] and Yang et al. [33] reported increased β-sheet and decreased α-helix in black bean protein and rice protein, respectively. On the contrary, Hu et al. [34] reported no effects of US on soybean β-conglycinin and glycinin, while decreased β-sheet and increased α-helix induced by US treatment has been reported in edible fly protein [35]. These mixed results could be due to differences in the native protein and US conditions. In our study, FTIR revealed protein unfolding as well as changes in hydrogen bonding and secondary structure of PPI within the film, suggesting different intra and intermolecular forces of the PPI film network as affected by ultrasound treatment.
Fig. 5.
The Fourier transform infrared spectra (A), and second derivatives of amide I region (1600–1700 cm−1) (B-F) of pea protein isolates films. For sample name abbreviations refer to Table 1.
Table 2.
The secondary structure of pea protein isolates as affected by ultrasound.*
| Treatment | β-sheet | α-helix | β-turn | anti-parallel β-sheet | Random coil |
|---|---|---|---|---|---|
| Control | 56.5 ± 0.4a | 21.0 ± 0.1c | 9.1 ± 0.2 | 9.5 ± 0.2b | 3.8 ± 0a |
| US410 | 58.0 ± 0.2bc | 15.2 ± 0.3b | 8.8 ± 0.3 | 7.2 ± 0.1a | 10.7 ± 0.1b |
| US420 | 57.4 ± 0.2b | 15.4 ± 0.2b | 9.1 ± 0.3 | 7.2 ± 0.1a | 10.8 ± 0.2b |
| US810 | 57.5 ± 0.1b | 15.5 ± 0.3b | 9.0 ± 0.2 | 7.2 ± 0.1a | 10.8 ± 0.1b |
| US820 | 58.6 ± 0.1c | 14.6 ± 0.1a | 9.2 ± 0.3 | 7.0 ± 0.2a | 10.6 ± 0.1b |
*Different letters indicate significant difference within the same column (P < 0.05). No significant difference was found for β-turn.
3.2.3. Optical properties
Optical properties are important because they greatly influence consumers’ acceptance of the films alone and also the food products that are wrapped. In this study, the color parameters (Fig. 6A) and opacity (Fig. 6B) were measured to assess the optical properties of PPI-based films as affected by ultrasound treatment. Fig. 6A shows that ultrasound had no obvious impact on the L* value (light vs dark). All films had a* values near zero (−0.23 to −0.45), indicating a balanced color between green and red. While ultrasound decreased a* significantly, the magnitude was so subtle (from −0.23 to −0.45) that such a change could not be perceived by human eyes. The positive b* values indicate the yellow color of all films, which were not significantly affected by ultrasound treatment. In previous studies on peanut protein-based film [5] and whey protein concentrate-based film [36], no significant effects of ultrasound on the color parameters were found either. However, as shown in Fig. 6B, the opacity of the PPI-based film was significantly reduced by ultrasound while no differences were found among different treatments. The inlet of Fig. 6B also shows the visual appearances of the control and ultrasound-treated film, which demonstrated the improvement in transparency. The photo of US410 was shown here as an example of the treatment groups because no visual differences could be observed among different US-treated groups. The better transparency of the treatment groups is considered as a great benefit for the development of edible films because it is favored by consumers. One explanation of the improved transparency might involve carbohydrates. During ultrasound treatment, it was likely that the starch granules that caused opacity were destroyed so that the overall transparency of the film was improved. For example, it has been reported that high power ultrasound could destroy the crystalline region of starch granules and also improve corn starch granules solubility [37]. Another reason of the improved transparency was the enhanced solubility of ultrasound-treated PPI. In this study, the film forming solutions contained 10 wt% PPI and it was known that ultrasound could improve PPI solubility in the solution [24], so it was expected that the untreated sample had more non-dissolved particles. FE-SEM micrographs (Fig. 4) also supported this explanation as the control films contained large aggregates at the film surface that could reflect visible light, thus being more opaque.
Fig. 6.
The Hunterlab L*, a* and b* (A), and opacity (B) of pea protein isolates films as affected by ultrasound. Different letters indicate significant difference (P < 0.05). Figure B inlet is the visual appearance of control and US410 films. For sample name abbreviations refer to Table 1.
3.2.4. Mechanical properties
The mechanical properties of PPI-based films were characterized by measuring tensile strength and elongation at break, which are listed in Table 3. It was found that the control had the lowest tensile strength value (0.61 MPa), which was increased (up to 1.48–2.19 MPa) for treatment groups. This result is not unexpected because of the PPI structural changes induced by ultrasound, which were characterized in Section 3.1—ultrasound disrupts PPI structure, exposes hydrophobic side chains to protein surface and reduces intermolecular electrostatic repulsion. FE-SEM micrographs (Fig. 4) also supported this stronger tensile strength as the cracks at the film surface of the control were disappeared after US treatment. Generally, during the drying of the protein solutions/dispersions, cohesive films would form when protein chains associate with each other through hydrogen bonding, covalent bonding and ionic and hydrophobic interactions; therefore, in films prepared from the treated PPI, unfolded side chains could associate through enhanced covalent bonding and hydrophobic interactions, thereby forming more stable networks. In the meantime, the reduced intermolecular electrostatic repulsion could also play a role during this process. One supporting evidence is from Kowalczyk & Baraniak who found that increasing the negative charges of non-denatured proteins at pH 11.0, thereby electrostatic repulsion, prevented the formation of homogenous protein network [12]. Likewise, Gennadios et al. noted that at pH 12 no soybean protein film could be formed due to strong repulsive forces between protein chains that prevented protein intermolecular association, and thus the film formation [38].
Table 3.
The tensile strength (TS), elongation at break (EAB), moisture content (MC), total soluble matter (TSM), and water vapor permeability (WVP) of pea protein isolates films as affected by ultrasound.*
| Treatment | TS (MPa) | EAB (%) | MC (%) | TSM (%) | WVP (g mm h−1 mm−2 kPa−2) |
|---|---|---|---|---|---|
| Control | 0.61 ± 0.04a | 68.86 ± 12.75 | 28.61 ± 0.51b | 35.73 ± 0.25 | 0.61 ± 0.02b |
| US410 | 1.48 ± 0.14b | 86.29 ± 14.25 | 24.49 ± 0.31a | 36.05 ± 1.24 | 0.52 ± 0.04a |
| US420 | 1.61 ± 0.14bc | 82.42 ± 17.22 | 24.28 ± 0.76a | 34.52 ± 1.18 | 0.49 ± 0.04a |
| US810 | 1.82 ± 0.21bc | 77.49 ± 17.27 | 25.56 ± 0.93a | 36.09 ± 0.37 | 0.53 ± 0.02a |
| US820 | 2.19 ± 0.24c | 88.93 ± 10.57 | 24.69 ± 0.26a | 36.87 ± 1.64 | 0.51 ± 0.03a |
*Different letters indicate significant difference within the same column (P < 0.05). No significant difference was found for EAB and TSM.
In addition, ultrasound treatment did not affect the elongation at break of PPI-based films significantly (Table 3). This finding indicated that, when evaluating the film mechanical properties, tensile strength and elongation at break are not always correlated, which could be supported by reviewing several previous studies. For example, Mehyar et al. reported that addition of pea starch or carnauba wax into whey protein film reduced both tensile strength and elongation [39]; Kowalczyk et al. found that increasing pH from 7 to 9 increased both tensile strength and elongation of PPI-based film [13]. By contrast, Gul et al. noted that ultrasound treatment decreased tensile strength but increased elongation at break of hazelnut protein film [21], whereas Vera et al. reported increased tensile strength but decreased elongation of quinoa protein/chitosan blended film as affected by ultrasound treatment [31]. Moreover, similarly as our data, it was found that changing the ratio of mucilage to whey protein concentrate at pH 7 resulted in decreased tensile strength but elongation remained unaffected [22]. In general, film mechanical properties are not only influenced by protein network but also plasticizers. When added into a polymer system such as protein solution, plasticizers can increase the free volume within the network formed by polymer chains, resulting in increased freedom for chains movements and rotation [40]. Because of this, plasticizers are commonly added to a polymer to improve its flexibility and stretchability. In this study, it was likely that elongation was largely influenced by plasticizer, which was same (50 wt% of PPI) for all samples.
3.2.5. Physicochemical properties
The moisture content, total soluble matter and water vapor permeability of PPI-films were listed in Table 3. The untreated PPI-film had a moisture content of 28.61%, which was decreased significantly after ultrasound treatment. This could be due to the increased hydrophobicity of PPI structure (Fig. 2, Fig. 3) so that less water molecules were retained within the protein network during film formation. In addition, the decreased moisture content as affected by ultrasound could also be due to the promoted formation of protein cross-linking induced by ultrasound, thereby denser and more compact films that retain less moisture. In the meantime, because denser films were formed, it is expected that the thickness of these films would be decreased. This theory was cited by several studies [4], [36]. However, it should be noted that the film thickness in our samples remained unaffected.
Another unexpected finding in our study was that the control and treated films showed no significant difference of total soluble matter (Table 3). This result is contrary to several previous publications. Cruz-Diaz et al., 2019 reported significant increase in solubility of whey protein concentrates-based film as affected by ultrasound [36]. They believed this was because ultrasound induced exposition of hydrophilic groups outside protein molecules and an increase in charged groups of proteins, which improved interaction among water and protein molecules. However, no data of surface charge was reported in their study while our data (Fig. 3B) showed the opposite—less charged groups were resulted by ultrasound treatment. The different findings could be due to different native protein (whey and pea protein) composition and conformation, thereby protein network in the film. Additionally, our result is different from C.-C. Liu et al., 2004 who found increased film solubility of peanut protein film after ultrasound treatment [5]. In their study, they mentioned that the ultrasound-treated films were difficult to peel off and no intact films could be obtained whereas in our experiment all films could be easily peeled off, so it was likely that the intermolecular forces holding up the protein network was significantly different between our study and theirs, which explained why the effect of ultrasound was not consistent across different studies. Theoretically, higher protein surface hydrophobicity leads to lower solubility; however, it should be noted that although ultrasound increases surface hydrophobicity, it does not always mean protein solubility would be decreased. For example, S. Jiang et al., 2017 reported increased surface hydrophobicity of PPI powder, as well as increased solubility, but this increase was believed owing to formation of soluble-aggregates during ultrasound treatment [24]. In our study where PPI was used to form film, it was likely that the effect of increased surface hydrophobicity counteracted that of increased soluble-aggregates, which explained why no significant differences were found.
Table 3 also shows the water vapor permeability of PPI-film, which was significantly reduced by US. Significant difference was also observed within the treatment groups with higher amplitude but not time leading to lower WVP values. Low WVP values are preferred since edible films are supposed to limit moisture transfer between the food products and their surrounding atmosphere. Generally, protein films are poor barriers to moisture because of their predominantly hydrophilic nature. Addition of hydrophobic compounds (e.g. fatty acids and wax) to protein films are known to improve this [14], [41]. In our experiments, we proved that US could also help preventing the moisture transfer across the films by increasing the protein surface hydrophobicity (Fig. 3A).
4. Conclusions
In this study, the effects of high intensity ultrasound treatment on PPI structure and film properties of PPI-films were studied. It was found that ultrasound treatments generated proteins of smaller particle size on average, while both dissociation and aggregation into larger aggregates at micro level were observed. Ultrasound was confirmed to alter protein tertiary and secondary structures, causing protein unfolding which induced more hydrophobic groups to the protein surface while decreasing electrostatic repulsion. Ultrasound also eliminated the cracks and decreased protein aggregates at the film surface. These identified structural changes were used as evidence to support the improved PPI-film properties. It was found that ultrasound treatment did not influence film thickness, total soluble matter or elongation at break, but the ultrasound-treated films had better transparency, tensile strength and water barrier properties.
CRediT authorship contribution statement
Jingjing Cheng: Investigation, Formal analysis, Validation, Writing – original draft. Leqi Cui: Conceptualization, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kumar S., Mukherjee A., Dutta J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020;97:196–209. doi: 10.1016/j.tifs.2020.01.002. [DOI] [Google Scholar]
- 2.Qi R., Jones D.L., Li Z., Liu Q., Yan C. Behavior of microplastics and plastic film residues in the soil environment: a critical review. Sci. Total Environ. 2020;703:134722. doi: 10.1016/j.scitotenv.2019.134722. [DOI] [PubMed] [Google Scholar]
- 3.Gounga M.E., Xu S.-Y., Wang Z. Whey protein isolate-based edible films as affected by protein concentration, glycerol ratio and pullulan addition in film formation. J. Food Eng. 2007;83(4):521–530. [Google Scholar]
- 4.Jiang J., Xiong Y.L., Newman M.C., Rentfrow G.K. Structure-modifying alkaline and acidic pH-shifting processes promote film formation of soy proteins. Food Chem. 2012;132(4):1944–1950. doi: 10.1016/j.foodchem.2011.12.030. [DOI] [Google Scholar]
- 5.Liu C.-C., Tellez-Garay A.M., Castell-Perez M.E. Physical and mechanical properties of peanut protein films. LWT-Food Sci. Technol. 2004;37(7):731–738. [Google Scholar]
- 6.Stone A.K., Karalash A., Tyler R.T., Warkentin T.D., Nickerson M.T. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res. Int. 2015;76:31–38. doi: 10.1016/J.FOODRES.2014.11.017. [DOI] [Google Scholar]
- 7.Shand P.J., Ya H., Pietrasik Z., Wanasundara P.K.J.P.D. Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem. 2007;102(4):1119–1130. doi: 10.1016/j.foodchem.2006.06.060. [DOI] [Google Scholar]
- 8.Gueguen J., Viroben G., Noireaux P., Subirade M. Influence of plasticizers and treatments on the properties of films from pea proteins. Ind. Crops Prod. 1998;7:149–157. doi: 10.1016/S0926-6690(97)00043-5. [DOI] [Google Scholar]
- 9.Viroben G., Barbot J., Mouloungui Z., Guéguen J. Preparation and characterization of films from pea protein. J. Agric. Food Chem. 2000;48(4):1064–1069. doi: 10.1021/jf9813891. [DOI] [PubMed] [Google Scholar]
- 10.Choi W.-S., Han J.H. Physical and mechanical properties of pea-protein-based edible films. J. Food Sci. 2001;66(2):319–322. [Google Scholar]
- 11.Choi W.S., Han J.H. Film-forming mechanism and heat denaturation effects on the physical and chemical properties of pea-protein-isolate edible films. J. Food Sci. 2002;67(4):1399–1406. [Google Scholar]
- 12.Kowalczyk D., Baraniak B. Effects of plasticizers, pH and heating of film-forming solution on the properties of pea protein isolate films. J. Food Eng. 2011;105(2):295–305. doi: 10.1016/j.jfoodeng.2011.02.037. [DOI] [Google Scholar]
- 13.Kowalczyk D., Gustaw W., Świeca M., Baraniak B. A study on the mechanical properties of pea protein isolate films. J. Food Process. Preserv. 2014;38(4):1726–1736. doi: 10.1111/jfpp.12135. [DOI] [Google Scholar]
- 14.Kowalczyk D., Gustaw W., Zieba E., Lisiecki S., Stadnik J., Baraniak B. Microstructure and functional properties of sorbitol-plasticized pea protein isolate emulsion films: effect of lipid type and concentration. Food Hydrocoll. 2016;60:353–363. doi: 10.1016/j.foodhyd.2016.04.006. [DOI] [Google Scholar]
- 15.Guimarães J.T., Balthazar C.F., Scudino H., Pimentel T.C., Esmerino E.A., Ashokkumar M., Freitas M.Q., Cruz A.G. High-intensity ultrasound: a novel technology for the development of probiotic and prebiotic dairy products. Ultrason. Sonochem. 2019;57:12–21. doi: 10.1016/J.ULTSONCH.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi N.K. Opportunities and challenges in application of ultrasound in food processing. Crit. Rev. Food Sci. Nutr. 2011;51(8):705–722. doi: 10.1080/10408391003770583. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee R., Chen H., Wu J. Milk protein-based edible film mechanical strength changes due to ultrasound process. J. Food Sci. 1996;61(4):824–828. [Google Scholar]
- 18.Marcuzzo E., Peressini D., Debeaufort F., Sensidoni A. Effect of ultrasound treatment on properties of gluten-based film. Innov. Food Sci. Emerg. Technol. 2010;11(3):451–457. doi: 10.1016/j.ifset.2010.03.002. [DOI] [Google Scholar]
- 19.Cui L., Kimmel J., Zhou L., Chen B., Rao J. Improving the functionality of pea protein isolate through co-spray drying with emulsifying salt or disaccharide. Food Hydrocoll. 2021;113 [Google Scholar]
- 20.Lee J.H., Lee J., Bin Song K. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015;46:208–215. doi: 10.1016/j.foodhyd.2014.12.020. [DOI] [Google Scholar]
- 21.Gul O., Saricaoglu F.T., Besir A., Atalar I., Yazici F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018;41:466–474. doi: 10.1016/j.ultsonch.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Muñoz L.A., Aguilera J.M., Rodriguez-Turienzo L., Cobos A., Diaz O. Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. J. Food Eng. 2012;111(3):511–518. doi: 10.1016/j.jfoodeng.2012.02.031. [DOI] [Google Scholar]
- 23.ASTM, Standard methods for water vapor transmission of material (E96-00), in: Annu. B. Am. Stand. Test. Methods, Philadelphia, 2000.
- 24.Jiang S., Ding J., Andrade J., Rababah T.M., Almajwal A., Abulmeaty M.M., Feng H. Modifying the physicochemical properties of pea protein by pH-shifting and ultrasound combined treatments. Ultrason. Sonochem. 2017;38:835–842. doi: 10.1016/j.ultsonch.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 25.Arzeni C., Martínez K., Zema P., Arias A., Pérez O.E., Pilosof A.M.R. Comparative study of high intensity ultrasound effects on food proteins functionality. J. Food Eng. 2012;108(3):463–472. doi: 10.1016/j.jfoodeng.2011.08.018. [DOI] [Google Scholar]
- 26.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. doi: 10.1016/j.foodres.2014.04.022. [DOI] [Google Scholar]
- 27.Huang L., Ding X., Li Y., Ma H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019;279:114–119. doi: 10.1016/j.foodchem.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 28.Shen L., Tang C.H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012;48(1):108–118. doi: 10.1016/j.foodres.2012.03.006. [DOI] [Google Scholar]
- 29.Miriani M., Iametti S., Bonomi F., Corredig M. Structural changes of soy proteins at the oil–water interface studied by fluorescence spectroscopy. Colloids Surfaces B Biointerfaces. 2012;93:41–48. doi: 10.1016/j.colsurfb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu W., Zhang Z.Q., Liu C.M., Xie M.Y., Tu Z.C., Liu J.H., Liang R.H. The effect of dynamic high-pressure microfluidization on the activity, stability and conformation of trypsin. Food Chem. 2010;123(3):616–621. doi: 10.1016/j.foodchem.2010.04.079. [DOI] [Google Scholar]
- 31.Vera A., Tapia C., Abugoch L. Effect of high-intensity ultrasound treatment in combination with transglutaminase and nanoparticles on structural, mechanical, and physicochemical properties of quinoa proteins/chitosan edible films. Int. J. Biol. Macromol. 2020;144:536–543. doi: 10.1016/j.ijbiomac.2019.12.120. [DOI] [PubMed] [Google Scholar]
- 32.Gao H., Ma L., Li T., Sun D., Hou J., Li A., Jiang Z. Impact of ultrasonic power on the structure and emulsifying properties of whey protein isolate under various pH conditions. Process Biochem. 2019;81:113–122. doi: 10.1016/j.procbio.2019.03.012. [DOI] [Google Scholar]
- 33.Yang X., Li Y., Li S., Oladejo A.O., Ruan S., Wang Y., Huang S., Ma H. Effects of ultrasound pretreatment with different frequencies and working modes on the enzymolysis and the structure characterization of rice protein. Ultrason. Sonochem. 2017;38:19–28. doi: 10.1016/j.ultsonch.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 34.Hu H., Cheung I.W.Y., Pan S., Li-Chan E.C.Y. Effect of high intensity ultrasound on physicochemical and functional properties of aggregated soybean β-conglycinin and glycinin. Food Hydrocoll. 2015;45:102–110. doi: 10.1016/j.foodhyd.2014.11.004. [DOI] [Google Scholar]
- 35.Mintah B.K., He R., Dabbour M., Xiang J., Jiang H., Agyekum A.A., Ma H. Characterization of edible soldier fly protein and hydrolysate altered by multiple-frequency ultrasound: Structural, physical, and functional attributes. Process Biochem. 2020;95:157–165. [Google Scholar]
- 36.Cruz-Diaz K., Cobos Á., Fernández-Valle M.E., Díaz O., Cambero M.I. Characterization of edible films from whey proteins treated with heat, ultrasounds and/or transglutaminase. Application in cheese slices packaging. Food Packag. Shelf Life. 2019;22:100397. doi: 10.1016/j.fpsl.2019.100397. [DOI] [Google Scholar]
- 37.Jambrak A.R., Herceg Z., Šubarić D., Babić J., Brnčić M., Brnčić S.R., Bosiljkov T., Čvek D., Tripalo B., Gelo J. Ultrasound effect on physical properties of corn starch. Carbohydr. Polym. 2010;79:91–100. doi: 10.1016/j.carbpol.2009.07.051. [DOI] [Google Scholar]
- 38.Gennadios A., Brandenburg A.H., Weller C.L., Testin R.F. Effect of pH on properties of wheat gluten and soy protein isolate films. J. Agric. Food Chem. 1993;41(11):1835–1839. [Google Scholar]
- 39.G.F. Mehyar, K. Al-Ismail, J.H. Han, G.W. Chee, Characterization of edible coatings consisting of pea starch, whey protein isolate, and carnauba wax and their effects on oil rancidity and sensory properties of walnuts and pine nuts, J. Food Sci. 77 (2012). https://doi.org/10.1111/j.1750-3841.2011.02559.x. [DOI] [PubMed]
- 40.R. Sothornvit, J.M. Krochta, Plasticizers in edible films and coatings, in: Innov. Food Packag., Elsevier, 2005: pp. 403–433.
- 41.Fabra M.J., Talens P., Chiralt A. Tensile properties and water vapor permeability of sodium caseinate films containing oleic acid–beeswax mixtures. J. Food Eng. 2008;85:393–400. doi: 10.1016/j.jfoodeng.2007.07.022. [DOI] [Google Scholar]