Abstract
Many harmful constituents are present in e-cigarettes at much lower levels than in cigarette smoke, and the results of analysis of urinary biomarkers in e-cigarette users are consistent with these findings. However, understanding the health effects of chronic exposures to e-cigarette aerosols may require thinking beyond these comparisons. In this study, we investigated the endogenous formation of the tobacco-specific oral and esophageal carcinogen N′-nitrosonornicotine (NNN) in e-cigarette users. Salivary NNN, nornicotine, nicotine, as well as urinary tobacco biomarkers, including total NNN, were analyzed in 20 e-cigarette users, 20 smokers, and 19 nonsmokers. Nornicotine and NNN levels in e-cigarettes used by the study participants were also analyzed. The mean of NNN in saliva of e-cigarette users was 14.6 (±23.1) pg/mL, ranging from non-quantifiable (below the limit of quantitation, LOQ) to 76.0 pg/mL. In smokers, salivary NNN ranged from below LOQ to 739.0 pg/mL, with 80% of smokers having salivary NNN in the range of levels found in e-cigarette users. Consistent with a previous report, very low levels of urinary total NNN were present in only 5 out of 20 e-cigarette users (ranging from 0.001 to 0.01 pmol/mL urine). Only trace levels of NNN were found in e-cigarette liquids. Together, our findings demonstrate that NNN is formed endogenously in e-cigarette users. While the overall exposure to NNN in e-cigarette users is dramatically lower than in smokers, the known carcinogenic potency of NNN warrants further investigations into the potential consequences of its endogenous formation. Salivary NNN, rather than urinary total NNN which accounts for only 1–3% of NNN dose, should be used to monitor e-cigarette users’ exposure to this carcinogen.
Keywords: NNN, endogenous nitrosation, e-cigarette users, saliva, carcinogen biomarkers
Graphical Abstract
Introduction
N′-Nitrosonornicotine (NNN) is an important carcinogenic tobacco-specific N-nitrosamine formed via nitrosation of the tobacco alkaloids nicotine and nornicotine.1, 2 Studies in laboratory animals convincingly demonstrate the carcinogenic potency of NNN towards oral and esophageal tissues.3–8 In the most recent study in F-344 rats, chronic treatment with NNN in drinking water resulted in the formation of approximately 13 esophageal tumors per rat and approximately 8 oral tumors per rat, including tumors of tongue, oral mucosa, soft palate, epiglottis, and pharynx.8 The relevance of the laboratory animal NNN carcinogenicity findings to humans is strongly supported by the data on NNN exposure, metabolism, and cancer risk.2, 9–11 For instance, in vitro and in vivo studies provided evidence of NNN metabolic activation and DNA adduct formation in human oral and esophageal tissues.12–15 In addition, analysis of urinary total NNN (a biomarker of NNN exposure) in a prospective epidemiological study demonstrated the significant and unique role of NNN uptake in esophageal cancer risk in smokers.10, 11 The International Agency for Research on Cancer has classified NNN and the related tobacco constituent 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) as human carcinogens (Group I).9
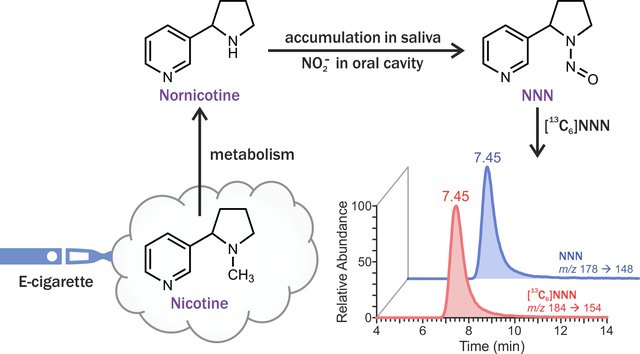
Human exposure to NNN can result from two sources: in addition to the direct intake from tobacco-containing products, this carcinogen can also be formed endogenously from nornicotine, which is a tobacco constituent and also a nicotine metabolite. For instance, co-administration of nornicotine and nitrite led to the formation of NNN in laboratory animals.16 Our group also reported on the presence of total NNN in urine of some users of oral nicotine replacement therapy (NRT) products such as nicotine gum or lozenge.17, 18 Given that NRT products do not contain NNN, the presence of urinary total NNN in users of these products indicates endogenous formation. This process is likely to occur in the oral cavity where metabolically-formed nornicotine can be excreted by salivary glands and react with nitrite, which is formed in oral cavity via the bacterial reduction of dietary nitrate.19–22 In agreement with this hypothesis, potential salivary formation of tobacco-specific N-nitrosamines, including NNN, was reported for smokeless tobacco users,23 and we recently reported that NNN can be formed from nornicotine in human saliva without deliberate addition of any other substance.24
Given the carcinogenicity of NNN, it is important to investigate the potential endogenous formation of this nitrosamine in users of electronic cigarettes (e-cigarettes) – battery-powered devices that aerosolize liquid mixtures of nicotine and flavoring agents in propylene glycol and glycerin. While their popularity has grown exponentially since they were first introduced in the U.S. in 2007, particularly among the youth and cigarette smokers,25–27 the potential long-term health outcomes of chronic exposures to e-cigarette aerosols are not well-understood. Recent literature indicates that e-cigarette aerosol chemistry is complex; however, the levels of identified constituents are generally much lower than cigarette-smoke levels of many potent toxicants and carcinogens.28–33 This is also true for NNN: while there have been initial reports on the presence of this carcinogen in e-cigarette liquids and aerosols, the detected levels are much lower than those found in cigarette smoke.28, 34, 35 Furthermore, a single previous report on the analysis of urinary total NNN, a biomarker of NNN exposure, in long-term exclusive e-cigarette users showed that this biomarker was undetectable in 21 out of 27 tested urine samples.36 However, based on our previous research, we estimate that urinary total NNN may account for not more than 3% of NNN dose17, 18, 37, 38 and thus its analysis may not be suitable for monitoring the formation of this carcinogen in the oral cavity. Therefore, we analyzed NNN in saliva of e-cigarette users. Oral carcinogenicity of NNN also makes its analysis in saliva highly relevant for the evaluation of the carcinogenic potential that may be associated with e-cigarette use.
Materials and Methods
Human subject procedures.
Subject recruitment and sample collection has been approved by the University of Minnesota Institutional Review Board (IRB Study # 0908M70881). E-cigarette users, smokers, and nonsmokers were recruited by the University of Minnesota Tobacco Research Programs. E-cigarette users were recruited if they were daily users and reported at least three months of exclusive e-cigarette use and no other tobacco use in the last 6 months. A smoker was classified as such if he/she smoked at least 10 cigarettes per day, had no regular use of nicotine replacement therapy products and no other tobacco or e-cigarette use in the last 6 months. Participants were classified as nonsmokers if they smoked less than 100 cigarettes in their lifetime and had no tobacco or e-cigarette use in the last 6 months. Eligible participants signed consent forms, completed basic demographic and tobacco use history questionnaires, and provided samples of saliva, buccal cells, urine, and blood. For the collection of oral samples (saliva and buccal cells), participants were given plastic cups and asked to rinse their mouth by rigorous swishing a mouthful of tap water for a few seconds. This was done at the beginning of the visit, under the supervision of the study coordinator, and subjects then did not drink, eat, chew gum, smoke, or use e-cigarette for at least 20 minutes, according to the routine procedure used by our and other groups. Rinsing the mouth allows for removal of food residues and constituents that come directly from cigarette smoke or e-cigarette aerosol, while waiting for 20 minutes prevents dilution of saliva with water residue after the rinse and allows for higher yields of oral cells. Saliva (2–5 mL) was collected by expectorating into a provided sterile polypropylene tube. The collected saliva and urine samples were stored at –20°C until analysis. Buccal cells and blood were also processed and stored for future analyses.
Analysis of saliva.
Analysis of NNN in saliva samples was carried out as previously described.24 Briefly, samples were mixed with [13C6]NNN (internal standard) and purified sequentially using ChemElut, OASIS MCX, and BondElut Silica cartridges. To prevent artefactual formation of NNN, ascorbic acid was added during the purification on ChemElut and OASIS MCX cartridges, when nornicotine and nitrite are present in the sample.24 Negative controls (water blanks) and positive controls (pooled smokers’ urine with established level of NNN) were included with each sample set to monitor for the potential contamination and analytical variation, respectively. The purified samples were analyzed by liquid chromatography-tandem mass-spectrometry (LC-MS/MS) monitoring for m/z 178 → 148 for NNN, and m/z 184 → 154 for [13C6]NNN. Saliva from e-cigarette users and smokers was also analyzed for nornicotine and nicotine as previously described.39 Briefly, saliva samples were diluted, mixed with stable isotope-labeled internal standards, and analyzed by LC-MS/MS monitoring m/z 149→130 for nornicotine, m/z 163→130 for nicotine, and corresponding transitions for the internal standards.
Urinary biomarkers.
For the analysis of total NNN, urine samples were treated with β-glucuronidase to convert any NNN-N-Glucuronide to free NNN,37 and then purified as described above for salivary NNN, using ascorbic acid to prevent artefactual NNN formation. The purified samples were analyzed by liquid chromatography-nanoelectrospray ionization-high resolution tandem mass-spectrometry (LC-NSI-HRMS/MS) on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Scientific, Waltham, MA) operated in the product ion scan mode. The analysis was conducted using a capillary column (75 μm i.d., 20 cm length, 15 μm orifice) created by hand packing a commercially available fused-silica emitter (New Objective, Woburn, MA) with Luna C18 bonded separation media (Phenomenex, Torrance, CA). The mobile phase consisted of 5 mM NH4OAc and CH3CN. A 5 μL injection loop was used, and the sample (2 μL) was loaded onto the capillary column with a 900 nL/min flow under the initial conditions for 6.5 min. Separation on the capillary column was performed using a linear gradient at a flow rate of 300 nL/min with increasing CH3CN from 2 to 60% over 9 min, followed by ramping to 90% CH3CN within 2 min and holding at this composition for additional 2 min. The gradient was then returned to 2% CH3CN (initial condition) in 1 min, and the system was re-equilibrated at this mobile phase composition for 6 min at 900 nL/min before next injection. The nanoelectrospray source voltage was set at 2.2 kV. The capillary temperature was 300 °C, and the S-Lens RF Level was 40%. The analysis was performed using accurate mass extracted ion chromatograms of m/z 148.0995 [C9H12N2]+ (parent ion m/z 178.1) for NNN and corresponding fragment (m/z 154.1196) for [13C6]NNN with a mass tolerance of 5 ppm. The scan events were performed using higher-energy collisional dissociation (HCD) fragmentation with a normalized collision energy of 13 units, isolation widths of 1 Da for both NNN and [13C6]NNN, and product ion spectra acquisition at a resolution of 60,000. The quantitation of NNN was based on the peak area ratio of NNN (m/z 178.1→148.0995) to [13C6]NNN (m/z 184.1→154.1196), the constructed calibration curves, and the amount of internal standard added.
Analysis of urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and cotinine was performed in a combined procedure as previously described.36 The method allows for simultaneous analysis of NNAL and cotinine by monitoring m/z 178→98 for cotinine, where m/z 178 is the [M + H]+ ion resulting from the naturally occurring [13C]cotinine. To confirm the accuracy of cotinine quantitation, a subset of urine samples from smokers and nonsmokers was diluted and re-analyzed by monitoring the regular transitions m/z 177→98 for cotinine and m/z 180→102 for [D3]cotinine (internal standard).
E-liquid analyses.
We analyzed NNN and NNK, as well as nornicotine and nicotine in 12 e-cigarette liquid varieties and one pack of disposable cartridges purchased from a retailer in the Minneapolis metropolitan area. The most popular flavors of the five available brands in the store were selected based on the store clerk’s recommendation. For each flavor and brand, we selected the highest level of nicotine (according to the label) available at the time of purchase. E-liquids were diluted with with10 mM ammonium acetate containing 5% methanol. For the disposable cartridges, their contents were removed and extracted with the same ammonium acetate-methanol mixture. Aliquots of the prepared e-liquid solutions were taken for the analysis of NNN, NNK, nornicotine, and nicotine by the standard validated methods routinely used in our laboratory.40, 41
Data analysis.
Descriptive statistics, such as mean values, standard deviations, and percentages was calculated for subject characteristics. In addition to arithmetic means and standard deviations, median values and interquartile ranges were calculated for various biomarker levels in smokers, nonsmokers and e-cigarette users. Since biomarker levels were highly right-skewed, comparisons across the groups (e-cigarette users, smokers, and nonsmokers) were done in the log scale, using a two-sample t-test. When appropriate, the levels in nonsmokers were used as reference. For statistical analysis purposes, if a biomarker level fell below the limit of quantitation (LOQ), we replaced it with the value corresponding to one half of LOQ for that biomarker. All computations were performed using SAS software, version 9.4. A p-value less than 0.05 is considered statistically significant, and if 0.05<p<0.10, the difference is considered marginally significant.
Results
Study participants.
A total of 59 subjects were recruited: 20 smokers, 20 e-cigarette users, and 19 nonsmokers. Table 1 summarizes the demographic characteristics and smoking history of the study participants. All e-cigarette users were Non-Hispanic White, 40% were female, more than 80% reported “some college or more” for their education level and were younger than smokers and nonsmokers. Three e-cigarette users reported using disposable cartridges, with the rest using refillable tank systems. The reported duration of exclusive e-cigarette use in e-cigarette users ranged from 3 to 36 months.
Table 1.
Demographics and smoking history of study participants
| Subject characteristics | E-cigarette users n=20 | Smokers n=20 | Nonsmokers n=19 |
|---|---|---|---|
|
| |||
| Age, years | 31.3 ± 12.2 | 41.9 ±11.4 | 40.6 ± 17.2 |
| Female, N (%) | 8 (40.0) | 12 (60.0) | 14 (73.7) |
| Race, N (%) | |||
| White | 20 (100.0) | 10 (50.0) | 16 (84.2) |
| Other | 0 (0.0) | 10 (50.0) | 3 (15.8) |
| Ethnicity, N (%) | |||
| Non-Hispanic | 20 (100.0) | 20 (100.0) | 19 (100.0) |
| Education, N (%) | |||
| High school graduate or less | 3 (15.8) | 9 (45.0) | 2 (10.5) |
| Some college or more | 16 (84.2) | 11 (55.0) | 17 (89.5) |
| Cigarettes per day | 18.7 ± 8.9a | 15.9 ± 4.0 | N/A |
| Age of smoking initiation, years | 15.9 ± 3.1 | 14.7 ± 3.6 | N/A |
| Time exclusive e-cigarette use, months | 12.2 ± 8.5 | N/A | N/A |
Before quitting smoking cigarettes
Biomarker levels in saliva.
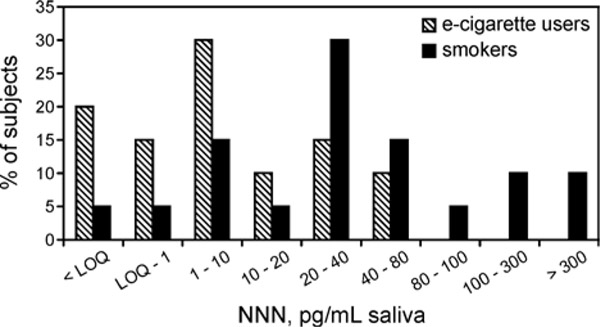
The results of salivary and urinary measurements are summarized in Table 2. Salivary NNN was found in 16 e-cigarette users, compared to 19 smokers. In e-cigarette users, salivary NNN ranged from below the LOQ (0.2 pg/mL) to 76.0 pg/mL, averaging (±SD) 14.6 ± 23.1 pg/mL saliva. In smokers, salivary NNN ranged from LOQ to 739.0 pg/mL, averaging 94.5 ± 176 pg/mL saliva. The difference between e-cigarette users and smokers was highly statistically significant (t=−2.904, p=0.006). Figure 1 illustrates the distribution of salivary NNN in e-cigarette users and smokers. Only traces of NNN were present in saliva from 5 out of 19 self-reported nonsmokers (average 0.25 ± 0.28 pg/mL saliva).
Table 2.
Biomarker levels in saliva and urine of study subjects
| Biomarker | E-cigarette users | Smokers | Nonsmokers | |
|---|---|---|---|---|
|
| ||||
| Saliva | ||||
| NNN, pg/mL | Number of samples | 20 | 20 | 19 |
| Mean ± SDa | 14.6 ± 23.1* | 94.5 ± 176 | 0.25 ± 0.28 | |
| Median (IQRb) | 2.15 (18.7) | 30.5 (57.0) | 0.10 (0.35) | |
| Range | LOQc – 76.0 | LOQ – 738.7 | LOQ – 1.0 | |
| Nornicotine, ng/mL | Number of samples | 20 | 15d | N/Ae |
| Mean ± SD | 19.4 ± 29.3 | 21.2 ± 13.7 | ||
| Median (IQR) | 11.7 (15.1) | 16.6 (18.6) | ||
| Range | 3.3 – 136.9 | 3.7 – 48.8 | ||
| Nicotine, μg/mL | Number of samples | 20 | 15d | N/A |
| Mean ± SD | 6.41 ± 13.5 | 1.19 ± 1.22 | ||
| Median (IQR) | 1.15 (6.55) | 0.73 (1.85) | ||
| Range | 0.01 – 60.8 | 0.01 – 3.80 | ||
|
| ||||
| Urine | ||||
| NNN, pmol/mL | Number of samples | 19c | 20 | 18d |
| Mean ± SD | 0.001 ± 0.002* | 0.16 ± 0.50 | 0.001 ± 0.001 | |
| Median (IQR) | 0.0001 (0.002) | 0.03 (0.003) | 0.0001 (0.000) | |
| Range | LOQ – 0.01 | LOQ – 2.2 | LOQ – 0.004 | |
| NNAL, pmol/mL | Number of samples | 19d | 19d | 18d |
| Mean ± SD | 0.07 ± 0.18* | 1.28 ± 1.04 | 0.04 ± 0.10 | |
| Median (IQR) | 0.02 (0.04) | 1.01 (1.63) | 0.01 (0.01) | |
| Range | LOQ – 0.80 | LOQ – 3.7 | LOQ – 0.45 | |
| Cotinine, nmol/mL | Number of samples | 19d | 19d | 18d |
| Mean ± SD | 17.5 ± 17.4 | 17.3 ± 10.6 | 0.32 ± 0.47 | |
| Median (IQR) | 16.8 (20.5) | 15.6 (18.3) | 0.21 (0.000) | |
| Range | 1.07 – 76.2 | LOQ – 36.2 | LOQ – 2.2 | |
IQR, interquartile range
LOQ, below the limit of quantitation: 0.2 pg/mL for salivary NNN, 0.0002 pmol/mL for urinary NNN, 0.005 pmol/mL for urinary NNAL, and 0.4 nmol/mL for urinary cotinine.
Not all saliva or urine samples were available for analysis.
N/A, not analyzed
p-value <0.05 for the comparison of log-transformed data between e-cigarette users and smokers
Figure 1.
Distribution of NNN levels in saliva of e-cigarette users and smokers
All e-cigarette users and only 15 smokers had sufficient remaining volume of saliva for the additional analyses of nornicotine and nicotine (Table 2). Saliva nornicotine levels in e-cigarette users was 19.4 ± 29.3 ng/mL (range, 3.3 to 136.9 ng/mL), and in smokers it was 21.2 ± 13.7 ng/mL (range, 3.7 to 48.8 ng/mL). The difference between e-cigarette users and smokers was not statistically significant (t=−1.270, p=0.213). Salivary nicotine levels in e-cigarette users ranged from 0.01 to 60.1 μg/mL and in smokers from 0.01 to 3.80 μg/mL, averaging 6.41 ± 13.5 μg/mL and 1.19 ± 1.22 μg/mL, respectively. The difference between e-cigarette users and smokers had borderline statistical significance (t=1.705, p=0.098).
Biomarker levels in urine.
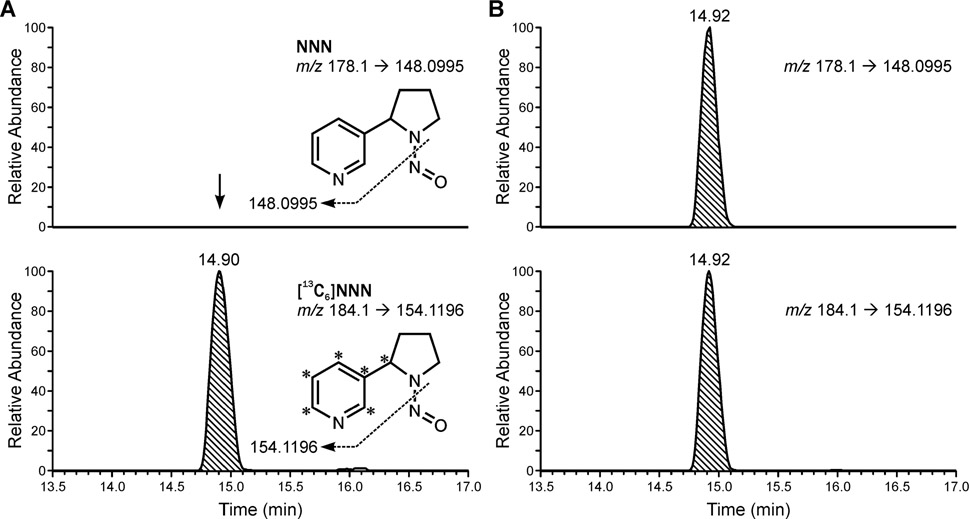
Our initial analysis of urine on the same LC-MS/MS system that was used for saliva analyses did not produce quantifiable results for urinary total NNN. We then employed a highly sensitive LC-NSI-HRMS/MS technique with an LOQ as low as 0.0002 pmol/mL. Typical chromatograms upon analysis of NNN in urine samples from an e-cigarette user and a smoker are presented in Figure 2. Urine samples were available for 19 out of 20 e-cigarette users (Table 2). Among these, 14 samples had urinary total NNN below LOQ (0.0002 pmol/mL) and the levels in the other 5 subjects ranged from 0.002 to 0.01 pmol/mL (mean was below 0.001 pmol/mL). In smokers, urinary total NNN ranged from LOQ (1 smoker) to 2.2 pmol/mL, with the mean of 0.16 ±0.5 pmol/mL. The difference between e-cigarette users and smokers was highly statistically significant (t=−7.690, p<0.0001).
Figure 2.
Typical accurate mass extracted ion chromatograms obtained upon analysis of NNN in urine samples from a (A) e-cigarette user and (B) a smoker.
Total urinary NNAL was below LOQ (0.005 pmol/mL) in six e-cigarette users, and ranged from 0.007 to 0.80 pmol/mL in the remaining 13 subjects. Average urinary total NNAL in e-cigarette users was 0.07 ± 0.18 pmol/mL, compared to 1.28 ± 1.04 pmol/mL in smokers, with the difference being highly statistically significant (t=−6.942, p<0.0001). Urinary cotinine levels in e-cigarette users and smokers were very similar, averaging 17.5 and 17.3 pmol/mL respectively (p=0.966). Among nonsmokers, eight had trace levels of total NNAL and cotinine (LOQ for cotinine 0.4 nmol/mL) in their urine, and four had either total NNAL or cotinine present at trace levels.
Constituent levels in e-cigarette liquids.
Constituent levels in e-cigarette liquids are summarized in Table 3. All e-liquids contained NNN, with levels ranging from 0.04 to 3.4 ng/mL. Similarly, NNK was present in all samples, ranging from 0.28 to 7.2 ng/mL liquid. Nornicotine levels varied widely across brands, from 0.21 to 35.1 μg/mL. In most e-liquid samples, nicotine levels were higher than those indicated on the label.
Table 3.
Levels of NNN, NNK, nornicotine, and nicotine in e-cigarette liquids analyzed in this study.
| Brand and flavor | ng/mL | Nornicotine, μg/mL | Nicotine, mg/mL | ||
|---|---|---|---|---|---|
| NNN | NNK | measured | label | ||
|
| |||||
| Black label | |||||
| Peach Perfect | 0.97 | 4.1 | 3.7 | 19.5 | 18 |
| Grapevine | 0.64 | 4.1 | 5.2 | 19.6 | 18 |
| Bohemian | |||||
| Super Melon | 2.3 | 1.7 | 13.1 | 15.1 | 12 |
| Strawberry Ice | 2.2 | 7.2 | 22.0 | 24.0 | 18 |
| The Standard | |||||
| Thumper | 1.8 | 3.5 | 1.7 | 13.2 | 12 |
| Dead Man’s Party | 3.4 | 2.4 | 2.9 | 14.1 | 12 |
| Tastypuff | |||||
| Scare Bear | 0.04 | 0.28 | 0.38 | 5.5 | 6 |
| Kiwi Lime Mint | 0.08 | 0.42 | 0.21 | 6.1 | 6 |
| TC Vape | |||||
| Absolute Berries | 1.4 | 1.9 | 18.9 | 34.8 | 24 |
| Camel | 0.82 | 2.2 | 4.1 | 35.1 | 24 |
| Menthol Tobacco | 0.55 | 2.6 | 4.0 | 29.4 | 24 |
| Marb | 1.8 | 2.5 | 19.8 | 29.4 | 24 |
| Greensmartlivinga | |||||
| Vanilla | 1.7 | 1.3 | 35.1 | 12.4 | 6 |
Disposable cartridges; constituent levels for this brand are expressed in corresponding units (ng, μg, or mg) per cartridge.
Discussion
Endogenous formation of N-nitrosamines has been convincingly demonstrated in humans.22, 42–44 In this study, we report for the first time that NNN – a potent oral and esophageal carcinogen – is formed endogenously in e-cigarette users. Sixteen out of 20 e-cigarette users analyzed here had quantifiable levels of NNN in their saliva, with the distribution of salivary NNN levels substantially overlapping between e-cigarette users and smokers (Figure 1). It is important to note that the overall exposure to NNN is much higher in cigarette smokers, who take in significant amounts of NNN from cigarette smoke, than in e-cigarette users. However, given the carcinogenic potency of NNN, and the reported remarkably strong association between NNN exposure and the risk of esophageal cancer in smokers,10 there is a need to better characterize factors that contribute to the endogenous formation of NNN and whether this process poses risks to e-cigarette users.
Nitrosation of the minor tobacco alkaloid nornicotine, is the most likely source of NNN in saliva of e-cigarette users. As a secondary amine, it is readily nitrosated to NNN in the presence of nitrite,45 and our previous studies in laboratory animals16 and humans,18 along with in vitro saliva incubation experiments24 strongly support this hypothesis. Nornicotine is a metabolite of nicotine and can potentially accumulate in saliva, similar to nicotine.19 Our analyses showed that indeed nornicotine is present in saliva of e-cigarette users (Table 2). When levels of salivary nornicotine in e-cigarette users are expressed as the percentage of nicotine in saliva of the same subjects, the values range from 0.12% to 40.6%, with the majority of e-cigarette users having this value below 1%. This is consistent with a previous report that metabolically formed nornicotine accounts for approximately 0.4% of total nicotine dose.46 The higher relative salivary nornicotine in several e-cigarette users can be attributed to either the differences in nicotine and nornicotine metabolism and/or the presence of nornicotine in e-cigarette liquids.47 Investigation of nicotine and nornicotine metabolism was beyond the scope of this study. However, we analysed nornicotine in a convenience sample of e-liquids, to provide a snapshot of the potential variation of this alkaloid across brands. Our results were in agreement with a single previous report (Table 3),47 showing that levels of nornicotine in e-cigarette liquids are generally very low and constitute approximately 0.05% of nicotine content (compared to 1–5% in tobacco).48 This suggests that nicotine metabolism is the primary source of salivary nornicotine in e-cigarette users, and the contribution of nornicotine from e-cigarettes is most likely minimal. It is important to note, however, that nornicotine levels varied approximately 175-fold across the analyzed brands in our study (Table 3) and therefore, some of commercially available e-cigarette liquids may expose users to elevated levels of this NNN precursor. The reported levels of salivary nitrite – another important component in the formation of NNN – in human saliva are approximately 1,000-fold higher than the levels of nornicotine measured in our study.49 In addition, we found significant overlap in salivary NNN levels between smokers and e-cigarette users (Figure 1) despite smokers’ exposures to high levels of nitric oxides from cigarette smoke. Therefore, nitrite is most likely not a limiting factor in the process of NNN formation in oral cavity. We also postulate that cigarette smoke-derived NNN is responsible for only a fraction of salivary NNN in smokers in our study, with the remaining amount being due to endogenous nornicotine nitrosation, which explains why the differences between smokers and e-cigarette users, while statistically significant, are not as dramatic as one would expect. This is plausible because NNN is rapidly metabolized in humans: only approximately 3% of NNN dose is excreted as unchanged NNN in smokers’ urine, and urinary NNN decreases rapidly after smoking cessation.37
Significant inter-individual variation in the levels of salivary NNN among e-cigarette users is consistent with our previous findings in nicotine replacement therapy users and in vitro saliva incubation experiments.17, 18, 24 The levels of salivary NNN measured in our smokers are also in general agreement with a previous study that reported on the levels of NNN in saliva of smokers.50 However, that study had different inclusion criteria for smokers and included individuals who smoked as few as 1 cigarette per day, occasional smokers, and also a few dual users of e-cigarettes and regular cigarettes. This is in contrast to the daily smokers of an average 18.7 cigarettes per day in our study, making it difficult to directly compare the results of the two studies. In addition, the above-referenced study reported relatively high levels of salivary NNN in nonsmokers. We notice that sample preparation in that study included a step in which hydrochloric acid, creating favorable conditions for nitrosation reactions. Therefore, it is possible that artefactual formation contributed to some of the salivary NNN measured in smokers and secondhand smoke-exposed nonsmokers in that study.
Our finding that e-cigarette users are exposed to NNN might be seen as contrasting with the previous report of undetectable or very low levels of total NNN in their urine.36 However, results of urinary biomarker analysis in our study are consistent with that report: only five out of 19 urine samples from e-cigarette users contained trace levels of NNN, as determined by using the highly sensitive LC-NSI-HRMS/MS technique with LOQ as low as 0.0002 pmol/mL (Table 2, Figure 2). Given that urinary total NNN may account for only a few percent of NNN dose, these findings are not surprising and support our initial hypothesis that the analysis of NNN exposure in e-cigarette users should be based on salivary, rather than urinary measurements. Analyses of urinary biomarkers in our study also confirmed that e-cigarette users were not smokers of regular cigarettes. In addition to low urinary NNN, only trace levels of total NNAL, a biomarker of the related carcinogen NNK, were found in some e-cigarette users. The levels of both biomarkers were not different from nonsmokers (p=1.00 for NNN and p=0.99 for NNAL). At the same time, urinary cotinine levels were similar in e-cigarette users and smokers. Together, these results confirm that salivary NNN in e-cigarette users was not from the unreported smoking of regular tobacco cigarettes.
It is important to consider alternative sources of NNN in saliva of e-cigarette users in our study. Given that nornicotine is easily nitrosated, the potential artefactual formation of NNN during sample processing is one consideration. However, we used ascorbic acid to prevent such formation of NNN during saliva processing, according to our previously established protocol.24 In addition, sample preparation procedures for saliva and urine samples were identical in our study. The virtual absence of NNN in e-cigarette users’ urine, which also contains nornicotine, rejects the possibility of artefactual NNN formation in our assay. Laboratory contamination of samples through solvents, supplies, and equipment is another concern. However, there was no NNN detected in our negative control samples included with each sample set, and the inter-day coefficient of variation for our stock of positive control samples (pooled smokers’ urine) that we include with all our NNN assays averages 9.4%. Lastly, the presence of NNN in e-cigarettes could be another potential alternative source of NNN in saliva of e-cigarette users. However, consistent with previous reports, NNN levels in e-cigarette liquids analyzed in our study were very low. For instance, the highest measured level was 3.4 ng per mL of e-liquid (Table 3), while the smoke of a single cigarette can contain over 200 ng of NNN51 – an approximately 1,000-fold difference if the levels in both products are expressed per milligram of nicotine. Therefore, salivary levels of NNN in e-cigarette users, which were comparable to those found in smokers (Figure 1), cannot be attributed to the presence of NNN in e-cigarettes.
This study has several limitations. Relatively small sample size limits our ability to generalize our findings to the general population of e-cigarette users. In addition, our data indicates that there is substantial inter-individual variation in the levels of endogenous NNN formation across e-cigarette users. The reasons for this variation are not well-understood and could potentially include such factors as oral health status, or differences in nicotine metabolism, which were not available for our analyses. The potential contribution of inter-individual differences in the rate of NNN absorption by oral mucosa and/or metabolism of the formed NNN in oral cavity is not known. Also, specific e-cigarettes/e-liquids used by the study subjects were not available in this study. Therefore, even though the results of our and other studies consistently indicate that levels of NNN are minimal in e-cigarette liquids and aerosols, we cannot dismiss or accurately quantify the potential contribution of e-cigarette-derived NNN to the amount of this carcinogen measured in saliva of e-cigarette users. Future studies in larger groups of exclusive e-cigarette users should incorporate such data to better understand the factors affecting the extent of NNN formation in the oral cavity of e-cigarette users. Lastly, we did not collect information on secondhand smoke exposure in nonsmokers or e-cigarette users. Such exposure could have been responsible for the presence of trace levels of tobacco biomarkers in some nonsmokers in our study. While secondhand smoke exposure cannot be responsible for the measured levels of salivary NNN in e-cigarette users, future studies should incorporate collecting detailed information on such exposures for a more accurate interpretation of tobacco biomarker data in e-cigarette users.
In summary, our results demonstrate that endogenous formation of the carcinogenic nitrosamine NNN occurs in oral cavity of e-cigarette users. This source of exposure to the known human carcinogen should be taken into account when the potential health impact of long-term e-cigarette use is assessed. Future studies of NNN exposure in e-cigarette users should be based on salivary, rather than urinary measurements. Given that oral cavity and esophagus are target tissues for NNN carcinogenicity, such approach is also highly relevant for the evaluation of the carcinogenic potential that may be associated with the endogenous formation of NNN in e-cigarette users.
FUNDING SOURCES
This study was supported by the National Cancer Institute of the National Institutes of Health and the Center for Tobacco Products of the Food and Drug Administration under Award Number R01CA180880. LC–MS/MS was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, supported in part by Grant CA-77598 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
ABBREVIATIONS
- E-cigarettes
electronic cigarettes
- HCD
higher-energy collisional dissociation
- LC-MS/MS
liquid chromatography-tandem mass-spectrometry
- LC-NSI-HRMS/MS
liquid chromatography-nanoelectrospray ionization-high resolution tandem mass spectrometry
- LOQ
limit of quantitation NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- NNN
N′-nitrosonornicotine
Footnotes
Conflict of interest disclosure. The authors declare no competing financial interests
References
- (1).Hecht SS, Chen CB, Hirota N, Ornaf RM, Tso TC, and Hoffmann D (1978) Tobacco specific nitrosamines: formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. J. Natl. Cancer Inst 60, 819–824. [DOI] [PubMed] [Google Scholar]
- (2).Hecht SS (1998) Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 11, 559–603. [DOI] [PubMed] [Google Scholar]
- (3).Hoffmann D, Raineri R, Hecht SS, Maronpot R, and Wynder EL (1975) Effects of N’-nitrosonornicotine and N’-nitrosoanabasine in rats. J. Natl. Cancer Inst 55, 977–981. [DOI] [PubMed] [Google Scholar]
- (4).Hecht SS, Young R, and Maeura Y (1983) Comparative carcinogenicity in F344 rats and Syrian golden hamsters of N’-nitrosonornicotine and N’-nitrosonornicotine-1-N-oxide. Cancer Lett 20, 333–340. [DOI] [PubMed] [Google Scholar]
- (5).Hecht SS, Chen CB, Ohmori T, and Hoffmann D (1980) Comparative carcinogenicity in F344 rats of the tobacco specific nitrosamines, N’-nitrosonornicotine and 4-(N-methyl-N-nitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 40, 298–302. [PubMed] [Google Scholar]
- (6).Hoffmann D, Rivenson A, Amin S, and Hecht SS (1984) Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamines in F344 rats. J. Cancer Res. Clin. Oncol 108, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hecht SS, Rivenson A, Braley J, DiBello J, Adams JD, and Hoffmann D (1986) Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Res 46, 4162–4166. [PubMed] [Google Scholar]
- (8).Balbo S, James-Yi S, Johnson CS, O’Sullivan G, Stepanov I, Wang M, Bandyopadhyay D, Kassie F, Carmella S, et al. (2013) (S)-N’-Nitrosonornicotine, a constituent of smokeless tobacco, is a powerful oral cavity carcinogen in rats. Carcinogenesis 34, 2178–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).International Agency for Research on Cancer. (2007) Smokeless tobacco and tobacco-specific nitrosamines, In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, v. 89, IARC, Lyon, FR. [PMC free article] [PubMed] [Google Scholar]
- (10).Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, and Stepanov I (2011) Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis 32, 1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Stepanov I, Sebero E, Wang R, Gao YT, Hecht SS, and Yuan JM (2013) Tobacco-specific N-nitrosamine exposures and cancer risk in the Shanghai cohort study: Remarkable coherence with rat tumor sites. Int. J. Cancer 134, 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Castonguay A, Stoner GD, Schut HAJ, and Hecht SS (1983) Metabolism of tobacco-specific N-nitrosamines by cultured human tissues. Proc. Natl. Acad. Sci., USA 80, 6694–6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Heling AK, Schutte-Borkovec K, Heppel C, Fagerstrom KO, and Richter E (2008) Pyridyloxobutylated DNA adducts in oral mucosa of nonsmokers, smokers and snuff dippers in relation to other biomarkers of exposure. Abstract presented at the 99th Annual Meeting of the American Association for Cancer Research, April 12–16, 2008, San Diego CA. Abstract nr 1883. Cancer Res 68, 1883. [Google Scholar]
- (14).Stepanov I, Muzic J, Le CT, Sebero E, Villalta P, Ma B, Jensen J, Hatsukami D, and Hecht SS (2013) Analysis of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB)-releasing DNA adducts in human exfoliated oral mucosa cells by liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem. Res. Toxicol 26, 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Schlobe D, Holzle D, Hatz D, Von Meyer L, Tricker A, and Richter E (2008) 4-Hydroxy-1-(3-pyridyl)-1-butanone-releasing DNA adducts in lung, lower esophagus and cardia of sudden death victims. Toxicology 245, 154–161. [DOI] [PubMed] [Google Scholar]
- (16).Porubin D, Hecht SS, Li ZZ, Gonta M, and Stepanov I (2007) Endogenous formation of N’-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric. Food Chem 55, 7199–7204. [DOI] [PubMed] [Google Scholar]
- (17).Stepanov I, Carmella SG, Han S, Pinto A, Strasser AA, Lerman C, and Hecht SS (2009) Evidence for endogenous formation of N’-nitrosonornicotine in some long term nicotine patch users. Nicotine Tob. Res 11, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami D, and Hecht SS (2009) Presence of the carcinogen N’-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res 69, 8236–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Rose JE, Levin ED, and Benowitz N (1993) Saliva nicotine as an index of plasma levels in nicotine patch users. Ther. Drug. Monit 15, 431–435. [DOI] [PubMed] [Google Scholar]
- (20).Jiebarth D, Spiegelhalder B, and Bartsch H (1997) N-nitrosation of medicinal drugs catalysed by bacteria from human saliva and gastro-intestinal tract, including Helicobacter pylori. Carcinogenesis 18, 383–389. [DOI] [PubMed] [Google Scholar]
- (21).Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith LM, Golden M, and Benjamin N (1995) Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med 1, 546–551. [DOI] [PubMed] [Google Scholar]
- (22).Marletta MA (1988) Mammalian synthesis of nitrite, nitrate, nitric oxides, and N-nitrosating agents. Chem. Res. Toxicol 1, 249–257. [DOI] [PubMed] [Google Scholar]
- (23).Idris AM, Nair J, Friesen M, Ohshima H, Brouet I, Faustman EM, and Bartsch H (1992) Carcinogenic tobacco-specific nitrosamines are present at unusually high levels in the saliva of oral snuff users in Sudan. Carcinogenesis 13, 1001–1005. [DOI] [PubMed] [Google Scholar]
- (24).Knezevich A, Muzic J, Hatsukami DK, Hecht SS, and Stepanov I (2013) Nornicotine nitrosation in saliva and its relation to endogenous synthesis of N’-nitrosonornicotine in humans. Nicotine Tob. Res 15, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).King BA, Alam S, Promoff G, Arrazola R, and Dube SR (2013) Awareness and ever-use of electronic cigarettes among US adults, 2010–2011. Nicotine Tob. Res 15, 1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, and Klein JD (2015) Trends in electronic cigarette use among US adults: Use is increasing in both smokers and nonsmokers. Nicotine Tob. Res 17, 1195–1202. [DOI] [PubMed] [Google Scholar]
- (27).Singh T, Arrazola RA, Corey CG, Husten CG, Neff LJ, Homa DM, and King BA (2016) Tobacco use among middle and high school students − United States, 2011–2015 Morbidity and Mortality Weekly Report 65, 361–367. [DOI] [PubMed] [Google Scholar]
- (28).Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P 3rd., and Benowitz N (2014) Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 23, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kosmider L, Sobczak A, Prokopowicz A, Kurek J, Zaciera M, Khnysak J, Smith D, and Goniewicz ML (2016) Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax 71, 376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Pankow JF, Kim K, McWhirter KJ, Luo W, Escobedo JO, Strongin RM, Duell AK, and Peyton DH (2017) Benzene formation in electronic cigarettes. PLoS One 12, e0173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Wang P, Chen W, Liao J, Matsuo T, Ito K, Fowles J, Shusterman D, Mendell M, and Kumagai K (2017) A device-independent evaluation of carbonyl emissions from heated electronic cigarette solvents. PLoS One 12, e0169811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hess CA, Olmedo P, Navas-Acien A, Goessler W, Cohen JE, and Rule AM (2017) E-cigarettes as a source of toxic and potentially carcinogenic metals. Environ. Res 152, 221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Jensen RP, Strongin RM, and Peyton DH (2017) Solvent chemistry in the electronic cigarette reaction vessel. Sci. Rep 7, 42549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Farsalinos KE, Gillman G, Poulas K, and Voudris V (2015) Tobacco-specific nitrosamines in electronic cigarettes: Comparison between liquid and aerosol levels. Int. J. Environ. Res. Public Health 12, 9046–9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schössler J, Hahn H, Kuballa T, and Lachenmeier DW (2014) Electronic cigarettes: Overview of chemical composition and exposure estimation. Tob. Induc. Dis 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kotandeniya D, Carmella SG, Ming X, Murphy SE, and Hecht SS (2015) Combined analysis of the tobacco metabolites cotinine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine. Anal. Chem 87, 1514–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Stepanov I, and Hecht SS (2005) Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol. Biomarkers Prev 14, 885–891. [DOI] [PubMed] [Google Scholar]
- (38).Hecht SS, Carmella SG, Stepanov I, Jensen J, Anderson A, and Hatsukami DK (2008) Metabolism of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone to its biomarker total NNAL in smokeless tobacco users. Cancer Epidemiol. Biomarkers Prev 17, 732–735. [DOI] [PubMed] [Google Scholar]
- (39).Harris AC, Tally L, Schmidt CE, Muelken P, Stepanov I, Saha S, Vogel RI, and LeSage MG (2014) Animal models to assess the abuse liability of tobacco products: Effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend 147, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, and Harris AC (2016) Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend 168, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yershova K, Yuan JM, Wang R, Valentin L, Watson C, Gao YT, Hecht SS, and Stepanov I (2016) Tobacco-specific N-nitrosamines and polycyclic aromatic hydrocarbons in cigarettes smoked by the participants of the Shanghai Cohort Study. Int. J. Cancer 139, 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Shepard SE, Schlatter C, and Lutz WK (1987) Assessment of the risk of formation of carcinogenic N-nitroso compounds from dietary precursors in the stomach. Food Chem. Toxicol 25, 91–108. [DOI] [PubMed] [Google Scholar]
- (43).Mirvish SS (1995) Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett 93, 17–48. [DOI] [PubMed] [Google Scholar]
- (44).Bartsch H, Ohshima H, Pignatelli B, and Calmels S (1989) Human exposure to endogenous N-nitroso compounds: quantitative estimates in subjects at high risk for cancer of the oral cavity, oesophagus, stomach and urinary bladder. Cancer Surv 8, 335–362. [PubMed] [Google Scholar]
- (45).Mirvish SS, Sams J, and Hecht SS (1977) Kinetics of nornicotine and anabasine nitrosation in relation to N’-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. J. Natl. Cancer Inst 59, 1211–1213. [DOI] [PubMed] [Google Scholar]
- (46).Benowitz NL, Jacob P, Fong I, and Gupta S (1994) Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. J. Pharmacol. Exp. Ther 268, 296–303. [PubMed] [Google Scholar]
- (47).Lisko JG, Tran H, Stanfill SB, Blount BC, and Watson CH (2015) Chemical composition and evaluation of nicotine, tobacco alkaloids, pH and selected flavors in e-cigarette cartridges and refill solutions. Nicotine Tob. Res 17, 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Stepanov I, Jensen J, Hatsukami D, and Hecht SS (2008) New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res 10, 1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Eisenbrand G, Spiegelhalder B, and Preussmann R (1980) Nitrate and nitrite in saliva. Oncology 37, 227–231. [DOI] [PubMed] [Google Scholar]
- (50).Perez-Ortuno R, Martinez-Sanchez JM, Fu M, Ballbe M, Quiros N, Fernandez E, and Pascual JA (2016) Assessment of tobacco specific nitrosamines (TSNAs) in oral fluid as biomarkers of cancer risk: A population-based study. Environ. Res 151, 635–641. [DOI] [PubMed] [Google Scholar]
- (51).Stepanov I, Knezevich A, Zhang L, Watson CH, Hatsukami DK, and Hecht SS (2012) Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: three decades of remarkable neglect by the tobacco industry. Tob. Control 21, 44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]