Abstract
Oropharyngeal squamous cell cancer (OPSCC) is now the most common site of head and neck squamous cell cancer. Despite the focus on treatment deintensification in clinical trials, little is known about the preferences, experiences and needs of patients with OPSCC when deciding between surgery and radiation therapy as primary treatment with curative intent. In this qualitative study, pre-treatment and post-treatment oropharyngeal cancer patients were recruited to take part in one-on-one interviews (n = 11 pre-treatment) and focus group discussions (n = 15 post-treatment) about treatment decision-making. Recordings were transcribed and assessed for emergent themes using framework analysis. From the one-on-one interviews and focus group discussions with OPSCC patients, fourteen themes were identified. Participants expressed alarm at diagnosis, decisional conflict, and a variety of roles in decision-making (physician-controlled, shared, and autonomous). Decisions were driven by the perceived recommendation of the treatment team, a desire for physical (surgical) tumor removal, fear of adverse effects of treatment, and patient-specific values. Although participants felt well-informed by their treating physicians, they identified a need for additional patient-centered information. Participants were critical of the poor quality of information available on the internet, and acknowledged the advantage of hearing the experiences of post-treatment patients. The experiences identified herein may be used to guide patient-centered communication during patient counseling and to inform interventions designed to support patients’ needs at diagnosis, ultimately helping to implement high-quality, patient-centered care.
Keywords: Head and neck cancer, Oropharyngeal cancer, Decision-making, Qualitative, Patient-centered care, Communication
Introduction
The incidence of oropharyngeal squamous cell cancer (OPSCC) is rising, due to human papillomavirus (HPV) [1]. The growing population of patients with OPSCC that are expected to have excellent long-term survival has inspired investigation of deintensified treatment regimens aiming to preserve oncologic outcomes while reducing long-term toxicity [2]. Transoral surgery, intensity-modulated radiation therapy and immunotherapy offer deintensification strategies. While treatment paradigms evolve, little is known regarding the values, needs, and treatment preferences of this relatively new patient population [3].
With some exceptions, for patients with OPSCC curative therapy entails either radiation therapy or surgery, with or without adjuvant treatment [4]. Retrospective studies to date have found no significant difference in survival and oncologic outcomes among primary therapies, [5] and prospective data on the long-term functional outcomes for these treatment options is only beginning to emerge [6].
As a result, patients with OPSCC face a situation of clinical equipoise, that is, a choice between treatments with similar outcomes. The burden of this deliberation potentially generates unique decisional needs in this often anxious population [7]. For some, anxiety at diagnosis is related to the fact that their cancer was caused by a sexually transmitted infection (HPV) [8–10]. It is important to understand the needs of these patients in order to inform shared decision-making, which in turn can improve engagement, patient satisfaction, [11] quality of life, [12,13] and health outcomes [14].
Qualitative research methods, which explore how people perceive and experience the world, have been used to describe patient decision-making in other cancer sites [15–20]. This qualitative study describes the experiences and needs of patients making decisions regarding primary treatment for their OPSCC.
Material and methods
Patients from the Johns Hopkins Hospital and Greater Baltimore Medical Center in Baltimore, Maryland were recruited to join this qualitative research study. These institutions adhere to current National Comprehensive Cancer Network guidelines for OPSCC treatment [4]. Surgery is typically transoral robotic resection with concurrent neck dissection.
The study consisted of one-on-one, semi-structured interviews and focus group discussions with OPSCC patients to identify experiences of treatment decision-making. Recruitment was performed using purposive sampling of patients attending outpatient visits in the Departments of Otolaryngology-Head and Neck Surgery and Radiation Oncology and through peer networking. Eligibility criteria for the interviews included incident diagnosis of OPSCC amenable to both primary surgery and primary radiation therapy, and completed consultations with a radiation oncologist and a head and neck surgeon. If a full course of chemoradiotherapy would definitely be recommended after surgery, these patients were not considered eligible as chemoradiation was likely recommended instead. Interviews with the group of “pre-treatment” patients were conducted in person or by phone prior to the start of treatment. A questionnaire was completed during the interviews collecting patient characteristics, and preferred decisional style as measured by the Control Preferences Scale [21]. For the focus group discussions, OPSCC survivors who had completed treatment between one and five years prior to data collection (“post-treatment” patients) were eligible. Focus groups took place in a private room on a hospital campus. Interview and focus group prompts (Supplement) were developed based on guidance provided in the Ottawa Hospital Research Institute Patient Decision Aid Development Online Training [22]. Oneon-one interviews and focus groups were led by an otolaryngology—head and neck surgery resident (MW, whose affiliation was disclosed to participants), audio-recorded and transcribed verbatim. Written informed consent was obtained from each participant. Institutional review boards at both institutions approved the study protocol.
Prior to analysis, data were evaluated to ensure they met the qualitative recommendations for content analysis [23]. Unlike quantitative analysis, which requires large samples, the goal of this study was to achieve thematic saturation, which occurs when the collection of new data offers no additional insight [24]. Each transcript was thematically hand-coded by two researchers with training in qualitative research methods (MW and DL) and cross-checked for agreement about the application of the codes. Themes were organized into overarching domains and compiled with representative quotations, which were edited for readability.
Results
Eleven interviews (n = 11) and three focus group discussions (n = 15; range 4–7 participants per group) were held between March 2018- September 2019. The interviews lasted a median of 32 min (range 22–39), and each focus group was around 90 min. Characteristics of the participants are described in Table 1.
Table 1.
Characteristics of oropharyngeal cancer patients.
| Characteristic | Pre-treatment patients N = 11 n(%) | Post-treatment patients N = 15 n(%) |
|---|---|---|
| N = 11 | N = 15 | |
| n(%) | n(%) | |
| Age: median (IQR) | 62 (56–64) | 64 (60–66.5) |
| Sex | ||
| Female | 3 (27) | 2 (13) |
| Male | 8 (73) | 13 (87) |
| Race/ethnicity | ||
| Caucasian/non-Hispanic | 11 (10 0) | |
| Marital status | ||
| Married/ Partnered | 10 (91) | 14 (93) |
| Divorced | 0 (0) | 1 (7) |
| Single/never married | 1 (9) | 0 (0) |
| “How difficult is it for you to meet the monthly payments on your family’s bills?” | ||
| Not at all difficult | 9 (82) | |
| A little difficult | 1 (9) | |
| Prefer not to answer | 1 (9) | |
| Employment status | ||
| Employed | 5 (55) | |
| Disabled and not working | 1 (0) | |
| Retired and not working | 4 (36) | |
| Highest level of education attained | ||
| High school diploma | 2 (18) | |
| College degree | 3 (27) | |
| University graduate degree | 6 (54) | |
| AJCC 7th ed. overall stagea | ||
| I–II | 1 (9) | 1(7) |
| III–IV | 10 (91) | 12 (80) |
| Unknown | 0 | 2 (13) |
| HPV tumor status | ||
| Positive | 10 (91) | 15 (100) |
| Negative | 1 (9) | 0 (0) |
| Primary treatment | ||
| CRT | 8 (73) | 9 (60) |
| Surgery | 1 (9) | 0 (0) |
| Surgery + RT | 1 (9) | 5 (33) |
| Surgery + CRT | 1 (9) | 1 (7) |
| Months after treatment end: median (IQR) | 30 (19.5–36.5) | |
Empty cells denote data were not collected or do not apply.
Abbreviations: IQR, interquartile range; AJCC, American Joint Committee on Cancer; Ed, Edition; RT, radiotherapy; CRT, chemoradiotherapy
According to AJCC 8th edition overall staging, pre-treatment group included 10 stage 1 (91%) and 1 stage II (9%).
Decisional style and choice predisposition
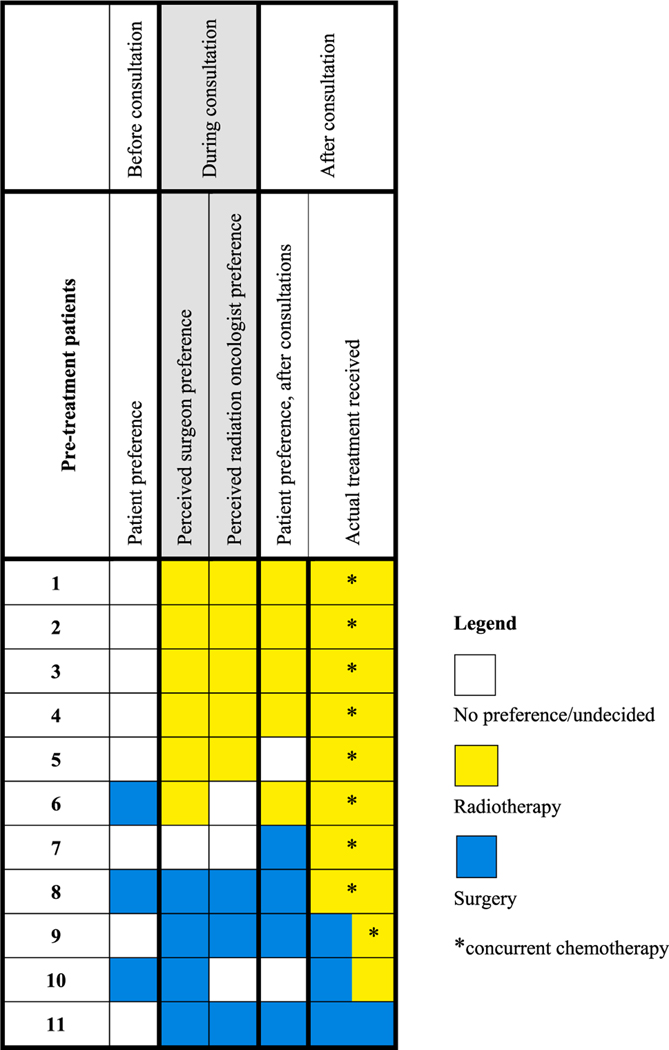
Among interviewed pre-treatment patients, most preferred a shared approach with their physician (Table 2). Choice disposition collected before and after specialty consultation, as well as patient-perceived treatment preference of the specialists, are displayed alongside actual treatment received in Fig. 1. In all cases (n = 8; 100%) where the patient perceived a physician preference during consultations, the patient perceived the preference of the surgeon and radiation oncologist to be the same, and perceived specialist preferences were in turn the same as the patient’s post-consultation treatment preference.
Table 2.
Decision-making characteristics of pre-treatment oropharyngeal cancer patients.
| Characteristic | N = 11 |
|---|---|
| n(%) | |
| Control Preferences Scale | |
| 1. I prefer to make the final treatment decision | 1 (9) |
| 2. I prefer to make the final treatment decision after seriously considering my doctor’s opinion | 4 (36) |
| 3. I prefer that my doctor and I share responsibility for deciding which treatment is best | 4 (36) |
| 4. I prefer that my doctor makes the final treatment decision, but seriously considers my opinion | 2 (18) |
| 5. I prefer that my doctor makes the final decision | 0 (0) |
| Sources of information accessed besides consultation with treatment team | |
| Other specialist doctors | 9 (82) |
| Other cancer post-treatments | 8 (73) |
| Internet | 8 (73) |
| Hours spent on internet searching diagnosis/treatment: median (IQR) | 10 (3–15) |
Abbreviations: IQR, interquartile range
Fig. 1.
Concordance of treatment preferences and actual treatment received among pre-treatment oropharyngeal cancer patients.
Domains of decisional experiences
Major themes relevant to treatment decision-making that emerged in the interviews and focus groups relevant to treatment decision-making were organized into four domains: (1) emotional response (2) roles in decision-making (3) other factors influencing treatment decision-making, and (4) informational needs. All themes were present among both pre- and post-treatment groups (Table 3).
Table 3.
Domains and themes of treatment decision-making experiences among oropharyngeal cancer patients (pre-treatment and post-treatment).
| Domain | Theme | Representative Quotes |
|---|---|---|
| Emotional response | Alarm at cancer diagnosis | “It’s like being shot in the head; like hearing somebody was in a terrible car accident or something” – pre-treatment “You can have all kinds of things wrong with you in your life but once that ‘C’ word is mentioned it’s like another world” – post-treatment |
| Decisional conflict | “I think that what is more stressful is trying to determine what is best for me and my family … and I’m not sure I have all the tools to do that” – pre-treatment “They want decisions to come from the individuals. You know? And that’s hard” – post-treatment |
|
| Roles in decision-making | Trust in the treatment team | “As far as what treatments to have, I don’t have any knowledge of how to treat cancer and I’m at [X hospital], so I feel like they know what they’re doing, for sure, and I know it, so I’m going to go with what they say” – pre-treatment “I think it would be foolish not to go along with the professional that’s giving you that advice. So of course, you’re going to go with whatever they say. They’re the cancer specialists. They know what they’re doing” – post-treatment |
| Shared decision with doctor/team | “[the doctor] is the one that gave me two choices and made me start to think about which avenue I was going to take” – pre-treatment “After doing some background research on my own and discussing it with my medical oncologist and with the surgeon, I decided to go not with the radiation and surgery, but with chemotherapy in addition” – post-treatment |
|
| Shared decision with spouse | “[my wife and I] discuss things in a fair amount of detail. That’s the primary part. I mean, she’s the other half of the relationship” – pre-treatment “My wife and I shared that decision” – post-treatment | |
| Ultimately decisional autonomy | “Ultimately, it’s my decision” – pre-treatment “My husband attended the decision-making meetings, though it was ultimately my decision as to which route to take” – post-treatment |
|
| Other factors influencing treatment decision-making | Desire for physical tumor removal | “I really wanted the option of doing surgery and getting rid of it” – pre-treatment “Get it out of there right now. Not tomorrow. Not next week. Don’t zap it out of there. Let’s go. Get it out” – post-treatment |
| Fear of adverse effects of treatment | “The surgery has real complications and risks and recovery periods” – pre-treatment “Incredible pain; loss of swallowing; loss of taste and likely dental problems” – pre-treatment “I’m going crazy in my head thinking, oh my gosh, they’re going to still have to take a part of my tongue out and everything else” – post-treatment “The thing that scared me the most was the dry mouth, which I still have today and losing your taste” – post-treatment “I didn’t want no part to chemo to be honest with you … sitting there six hours and that stuff’s going in your brain” – post-treatment |
|
| Possible need for adjuvant therapy obviates advantage of surgery | “Now [the treatment team is] talking about 60 percent chance of, if they do the surgery, they’ll still have to do a full course of radiation and chemo. That starts making the surgery less attractive as an option than the other ones.” – pre-treatment “even if [the doctor] were able to remove it surgically I would still need chemo and radiation. So, they felt that based on that, it would be better just for me to begin chemo and radiation” – post-treatment |
|
| Patient-specific values | “I chose the surgery because it was a one-time thing” – pre-treatment “I’m a musician and it’s important that I get to hopefully play. And the idea of surgery where margins were slight and then that could maybe affect my ability to play—that’s up there. But for someone else, maybe they just couldn’t stand the idea of the chemotherapy or something that was toxic to their body” – post-treatment |
|
| Informational needs | Felt generally well-informed | “I felt like I had an adequate amount of information” – pre-treatment “They explained everything” – post-treatment |
| Patient-centered information would be helpful | “I think the simplicity goes a long way, and it takes away the scary nature of some complicated words that, in laymen’s terms, need to be laid out to the patient because the fear of the unknown of what some terminology mean” – pre-treatment “the other thing I wish I had known more about was the chemotherapy” – post-treatment “one of the things they didn’t really talk about going into it was how your whole … shoulder and pec… just collapse” – post-treatment “how do you measure units of radiation? What are they called? What does it mean? That would be extremely helpful to have that information right up front” – post-treatment |
|
| Internet is a poor resource for information-gathering | “Probably … don’t [search for] it. You get a lot of misinformation that way. Talk with your doctor and figure it out” – pre-treatment “I knew I had time but I found it fairly frustrating to jump on the internet to say, ‘let me get a little information on it’ because you were just bombarded with junk” – post-treatment |
|
| Advantage of hearing from post-treatment survivors | “the majority of doctors … who treat cancer may have not had it themselves. And so, they can talk to the cure process of it, the treatment plans and how those all execute. But talking to someone who’s gone through it and can tell you day to day, the challenges they’ve experienced, it allows you to prepare better than someone without that knowledge or opportunity.” – pre-treatment “there’s enough folks out there that would be willing to share their experience. So that would be something I think would be very beneficial to someone that is scared to death of going through it” – post-treatment |
Domain 1: Emotional response
Almost all participants expressed alarm in response to their cancer diagnosis, conveying initial shock (“your world kind of stops”; “I was shocked”; “[my wife and I] went ballistic”), using language that was fatalistic (“I’m done, I’m sunk”; “I’m toast”; “I got the big C … woe is me, the end of my life”), and discussing feeling the imminence of death (“I am afraid of death … I have a lovely wife and two kids”). A few participants described making financial arrangements for their family. Alarm was heightened by the fact that participants generally felt well when seeking evaluation (“I didn’t expect it to be cancer”; “I’ve been healthy for 64 years”; “no weight loss, no discomfort, no problems swallowing”), and felt the severity of the diagnosis did not match their perception of good health. The prognosis of OPSCC was only occasionally mentioned as relief (“once I knew the cancer was highly treatable, I knew I could move forward and do it”).
Decisional conflict, defined as personal perceptions of uncertainty in treatment decision-making, [25] was frequently conveyed. Participants described feeling as if they were “in a bind”, referring to the decision as “terribly unfair” and “horrible… because you’re potentially making a go/no go decision with your life”. This conflict was contextually related to time pressure to make treatment decisions, perceived patient ownership of the ultimate decision, and difficulty focusing after having recently received a cancer diagnosis: “I felt like I had just 200 dB of sound and music or just distraction noise in [my] mind because all these emotions are running through”.
Domain 2: Roles in decision-making
Complex and perhaps contradictory roles in treatment decision-making were articulated, often by the same participants.
Trust in the treatment team was highly prevalent, often illustrated by deference (“I’m going to do whatever they tell me to do”; “I think it would be foolish not to go along with the professional that’s giving you that advice”; “honestly there weren’t any decisions to be made because I was just going to go along with whatever the doctor said”). In addition to deference, many participants described a decision that was shared with the doctor/treatment: “[the doctor] and I made the decision together to do surgery”; “It was something [the doctor and I] came up with mutually”. Another dominant theme was shared decision-making with a spouse: “it was really my wife and myself”; “we’ve come to every decision jointly”; “my wife and I both decided”.
Despite following the doctors’ recommendations and shared decision-making with doctors and a spouse, participants felt ultimate decisional autonomy (“ultimately it was really my decision”; “It’s not your risk. It’s my risk”; “it’s 100 percent up to me to make my own decision”). This autonomy was viewed as positive (“it was good because … I was being given options and information on the options and [I was] asked to make a decision”) or daunting, “so the surgeon said, ‘it’s your choice,’ which … I actually would have preferred being told”.
Domain 3: Other factors influencing treatment decision-making
Desire for physical tumor removal dominated the discussion regarding surgery (“get it out”). One participant compared the feeling to having a bug land on one’s arm as a child. This perceived clear advantage to surgical extirpation was expressed even among those who had decided to pursue upfront radiotherapy.
Fear of adverse effects of treatment, detected for all treatment modalities, was a main driver of decision-making: “when they say your carotid artery, well, you focus on that being, in your mind, being cut and then that’s it”; “there’s the anxiety that you don’t wake up”; “incredible pain; loss of swallowing; loss of taste and likely dental problems”; “I had actually pursued surgery just to avoid it. That’s how fearful I am of the radiation, chemotherapy treatment”; “the damages of chemo are known to me personally from seeing them”. These reactions were as often derived from knowing persons or public figures who had experienced cancer treatment as from the side effects of treatment discussed during clinical consultation. Occasionally, the adverse effects mentioned were misinformation (“I know with the chemo, you know, I’m going to lose my hair”; I’ve heard about some negative consequences [of radiation] … Three Mile Island, to Hiroshima, to Ukraine”). Related to avoiding additional treatment, a prevailing theme was that in select cases, the possible need for adjuvant therapy obviates the advantage of surgery: “I could just have the radiation, chemotherapy without the surgery. It seems like … why would I have the surgery?”.
Patient-specific values apart from desire for tumor removal or concerns about adverse treatment effects were raised as drivers of decision-making. A “gut feel” toward a particular treatment was often cited. Surgery was repeatedly perceived as the “quicker” option, making it desirable from a timeline perspective, that in some cases related to transportation. One participant described how the possible limitation of arm movement after neck dissection was perturbing given the participant’s avid interest in martial arts, whereas another expressed concern that transoral resection may limit the participant’s ability to play wind instruments.
Domain 4: Informational needs
When informational needs were explored, almost all participants described feeling generally well-informed (“I had enough facts”; “I’ve got as much information as there is to get”). This was contextually tied with an overall positive and appreciative feeling toward the treating physicians (“I feel like you guys are doing a great job”). On further discussion, however, there was a global acknowledgement that patient-centered information would be helpful (“I think it’s real helpful to have a lay perspective”): some medical information delivered in the consultation was in language that was difficult to understand (“what would be good is a glossary of terms and terminology and better patient understanding of what things mean and simplifying things”), incomplete (“I guess the doctors are only supposed to tell you so much. But there’s questions we probably have that, to us, are important and to them it’s miniscule because they’re smarter than we are”), or overwhelming (“I can’t remember anything I was told. I was told so much”).
When exploring other sources of treatment information participants had accessed, it became clear that the internet was a poor resource for information-gathering. Participants reported misinformation: “I did read some stuff that had me passed away in five years. So, I just don’t believe that stuff”; “there was a cesspool of information out there”. Suspecting this to be the case, other participants abstained from using the internet: “you can get any answer you want and certainly be scared”; “I chose not to go online and get sucked into that vat of information”. Most interviewees acknowledged use of the internet to search for their diagnosis and treatment. The median amount of time spent on the internet was 10 hours (Table 2).
Participants strongly expressed the advantage of hearing from post-treatment survivors. Some participants spoke from personal experience (“I went to a support group meeting … before I started all of the procedures and I found that invaluable because I happened to see people that looked like this that had been through it and that was amazing”; “that was very helpful to know that this [survivor] went back to his life in relatively the same fashion he had been before”). Other participants spoke of the theoretical benefit it would provide: (“to be able to pick someone’s brain … that’s traveled that path, I think is a great idea”; “I want to talk someone who’s been through this. It’s a little scary so it would be good to talk to someone in order to prepare”).
Discussion
This is the first study to qualitatively capture the decisional experiences and needs of patients with OPSCC making treatment decisions. As patients who are surgical candidates are given a choice between primary treatments for their cancer, treatment choice is considered to be “preference-sensitive”. That is, decisions are based off of patient experiences during and outside of their consultations with the treatment team, representing ideal conditions for shared decision-making [26]. As treatment paradigms for OPSCC evolve, it is paramount that efforts are made toward patient-centered care, which is viewed as a component of quality care by the Institute of Medicine [27], the Affordable Care Act, [28] and the otolaryngology—head and neck surgery community [29–31].
Discrepant to the treatment teams’ understanding that the prognosis for most patients with OPSCC is excellent, participants strongly feared mortality at diagnosis. This is consistent with our prior report that most head and neck cancer patients are very concerned about their mortality, especially those with HPV-associated cancer [7]. Our finding suggests that emphasis should be placed on prognosis during oncologic consultations and that adequate time be provided between sharing a diagnosis and providing complex medical information.
Importantly, patients may have difficulty expressing their values and needs due to fears regarding prognosis. In some cases, our study participants felt ill-equipped to decide on treatment. There is evidently a need for support to help patients clarify and communicate their preferences and values and to ensure congruency between these and the chosen treatment. Apart from a desire for cure and survival, treatment-related priorities tend to vary widely among patients [7].
Data from head and neck cancer [32] and other cancer sites [15,33,34] shows that patients place substantial weight on their physician’s recommendation. Our study corroborates this finding as many patients acknowledged their choice was based on the treatment team’s recommendation, and choice preference was concordant between the patient and the treatment team whenever a physician preference was perceived (Fig. 1). Just as predominant as physician-controlled decision-making, however, was the feeling that ultimate decisional responsibility lay with the patient. These dichotomous decisional roles are in contrast to the results of the Control Preferences Scale, [21] in which all but one interviewed participant preferred a degree of shared decision-making with their doctor (Table 2). Indeed, many participants felt they had arrived at their decision through a shared approach with their doctor and spouse. These findings reflect that patient decision-making is complex and roles are often unclear even to the patient.
Medical ethics literature sheds some light on the complex nature of patient decision-making, emphasizing models that describe how the decision is made rather than who makes the decision. Though respect for patient autonomy is widely held as a core principle of bioethics, enabling autonomy first requires delivery of relevant healthcare information, the selection of which is necessarily paternalistic [35]. In fact, some argue that paternalism is inseparable from the practice of medicine: clinical training necessarily prepares physicians to counsel and guide patients to make decisions that align with their best health outcome, rather than deliver a formulary of facts [36]. Though the physician consultations were not examined as part of this study, it is likely that for uncaptured reasons (disease characteristics or perceived patient values), a physician treatment preference was at times emphasized during consultation in a demonstration of protective paternalism. In response, the patients felt their autonomy was respected not necessarily out of freedom of decisional action to choose a primary modality (positive autonomy), but more as a freedom of refusal (negative autonomy) had they disagreed with the expert recommendation. Regardless, it is encouraging that if protective paternalism did play a role in our study, it was consistent across disciplines, implying there was a valid reason for the perceived treatment recommendation.
An encouraging takeaway is that most participants both preferred and recalled shared decision-making. A recent large-scale survey of colorectal and lung cancer patients found that those who recalled physician-controlled decision-making rated their physician’s communication and quality of care lower, regardless of the patient’s stated preferred role in decision-making [37]. It may be that patient involvement in decision-making to some degree is important even among patients that prefer a physician-controlled approach.
The desire for physical (surgical) tumor removal detected among our study population is a robust theme in decision literature describing other cancer sites, [38–40] most notably breast cancer [19]. Though most patients viewed surgery as favorable for this reason, treatment choice was ultimately upfront radiation therapy if patients perceived they would likely require adjuvant chemoradiotherapy. At our study institutions, we emphasize avoidance of trimodality (surgery, radiation therapy, and chemotherapy) treatment where possible, with the understanding that additional modalities of therapy have a multiplicative effect on toxicity [41].
Similarly, avoiding modality-specific toxic treatment effects was a main driver of treatment decision-making. Notably, these fears were derived from personal encounters of other people’s cancer treatment as often as from the risks of treatment discussed in physician consultations. This finding highlights the power of social or public narratives of cancer treatment whether these narratives describe oropharyngeal cancer treatment or not. One way in which the experiences of other people would clearly be considered helpful to our study population is connecting newly diagnosed patients with survivors of oropharyngeal cancer who have completed treatment. Related to this, participants expressed a desire for vetted, patient-centered information rather than information derived from internet searches. These needs were voiced together with a feeling that adequate information had been provided by their doctor, suggesting that patients need to hear a layperson perspective to gather a different type of information than their doctors should provide, rather than to fill informational gaps left by their doctors.
The findings described herein reflect patient experiences at two hospitals in Maryland and may not be generalizable to patient experience at institutions located elsewhere. In addition, our analysis describes perspectives from a primarily male and white non-Hispanic group. Although this is reflective of the population of patients with oropharyngeal cancer, our findings may not represent the experiences of patients from other demographic strata. An important limitation to the study is the focus group population and setting. The post-treatment patients were not selected on the basis of eligibility for both primary surgery and radiation, and the majority of patients in that group received primary chemoradiotherapy. Furthermore, the data collected in the group setting was subject to groupthink. Despite these limitations, the fact that all decisional themes were detected among post-treatment and pre-treatment survivors provides validity to our findings.
In conclusion, patients with OPSCC experience distress related to their anticipated mortality and treatment decisions. Although patients describe multiple roles in decision-making and largely defer to their doctors’ recommendations, they ultimately feel responsibility for their decision. Preference for surgery or radiation therapy is primarily motivated by avoidance of toxic treatment effects and desire for physical (surgical) tumor removal, and there is a need for patient-centered information separate from the adequate information provided by the treatment team. Findings from this study will be used to inform patient-centered communication during counseling and to guide interventions designed to support patients at diagnosis, contributing to the provision of patient-centered, high-quality care.
Supplementary Material
Acknowledgements
We are grateful to the participants who took part in this study. We acknowledge with special gratitude the efforts of Dorothy Gold, MSW, and Barbara Messing, SLP-CCC and Director, at the Milton J. Dance, Jr Head & Neck Cancer Center in facilitating this study. Thank you to the Milton J. Dance Endowment for awarding funding to this project.
Funding/support
This study was supported by the John R. Saunders, MD Research Award, the National Institute of Dental and Craniofacial Research [grant number R35DE026631], the National Institute on Deafness and Other Communication Disorders [grant number 5T32DC000027-29], and the National Cancer Institute [grant number T32CA0093140].
Role of the funder/sponsor
The funders had no role in the design and conduct of the study.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.oraloncology.2020.105044.
References
- [1].Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bigelow EO, Seiwert TY, Fakhry C. Deintensification of treatment for human papillomavirus-related oropharyngeal cancer: current state and future directions. Oral Oncol 2020;105:104652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Windon MJ, D’Souza G, Fakhry C. Treatment preferences in human papillomavirus-associated oropharyngeal cancer. Future Oncol 2018;14:2521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].National Comprehensive Cancer Network. Head and Neck Cancers (Version 2. 2017). [DOI] [PubMed] [Google Scholar]
- [5].Sinha P, Karadaghy OA, Doering MM, Tuuli MG, Jackson RS, Haughey BH. Survival for HPV-positive oropharyngeal squamous cell carcinoma with surgical versus nonsurgical treatment approach: a systematic review and meta-analysis. Oral Oncol 2018;86:121–31. [DOI] [PubMed] [Google Scholar]
- [6].Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol 2019;20(10):1349–59. [DOI] [PubMed] [Google Scholar]
- [7].Windon MJ, D’Souza G, Faraji F, et al. Priorities, concerns, and regret among patients with head and neck cancer. Cancer 2019;125(8):1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].D’Souza G, Zhang Y, Merritt S, et al. Patient experience and anxiety during and after treatment for an HPV-related oropharyngeal cancer. Oral Oncol 2016;60:90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taberna M, Inglehart RC, Pickard RKL, et al. Significant changes in sexual behavior after a diagnosis of human papillomavirus-positive and human papillomavirus-negative oral cancer. Cancer 2017;123(7):1156–65. [DOI] [PubMed] [Google Scholar]
- [10].Milbury K, Rosenthal DI, El-Naggar A, Badr H. An exploratory study of the informational and psychosocial needs of patients with human papillomavirus-associated oropharyngeal cancer. Oral Oncol 2013;49(11):1067–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stacey D, Paquet L, Samant R. Exploring cancer treatment decision-making by patients: a descriptive study. Curr Oncol 2010;17(4):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kashaf MS, McGill E. Does shared decision making in cancer treatment improve quality of life? A systematic literature review. Med Decis Making 2015;35(8):1037–48. [DOI] [PubMed] [Google Scholar]
- [13].Street RL, Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making 1997;17(3):298–306. [DOI] [PubMed] [Google Scholar]
- [14].Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med 2004;2(6):595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sattar S, Alibhai SMH, Fitch M, Krzyzanowska M, Leighl N, Puts MTE. Chemotherapy and radiation treatment decision-making experiences of older adults with cancer: a qualitative study. J Geriatr Oncol 2017;9:47–58. [DOI] [PubMed] [Google Scholar]
- [16].Jen WY, Yoong J, Liu X, Tan MSY, Chng WJ, Chee YL. Qualitative study of factors affecting patient, caregiver and physician preferences for treatment of myeloma and indolent lymphoma. Patient Prefer Adherence 2020;14:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schwartz RM, Gorbenko K, Kerath SM, et al. Thoracic surgeon and patient focus groups on decision-making in early-stage lung cancer surgery. Future Oncol 2017;14:151–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Owens OL, Estrada RM, Johnson K, et al. ‘I’m not a chance taker’: a mixed methods exploration of factors affecting prostate cancer treatment decision-making. Ethn Health 2019;1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dicks E, Roome R, Chafe J, et al. Factors influencing surgical treatment decisions for breast cancer: a qualitative exploration of surgeon and patient perspectives. Curr Oncol 2019;26(2):216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raphael DB, Ter Stege JA, Russell NS, Boersma LJ, van der Weijden T. What do patients and health care professionals view as important attributes in radiotherapy decisions? Input for a breast cancer patient decision aid. Breast 2020;49:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Degner L, Sloan J, Venkatesh P. The control preferences scale. CJNR 1997;29(3):21–43. [PubMed] [Google Scholar]
- [22].O’Conner A, Stacey D, Boland L. Ottawa decision support tutorial. The Ottawa Hospital Research Institute. https://decisionaid.ohri.ca/ODST/odst.php. Published 2015. [accessed September 30, 2017]. [Google Scholar]
- [23].Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks, CA: SAGE Publications; 2002. [Google Scholar]
- [24].Hairston TK, Links AR, Harris V, et al. Evaluation of parental perspectives and concerns about pediatric tonsillectomy in social media. JAMA Otolaryngol Head Neck Surg 2019;145(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].O’Connor A. Validation of a decisional conflict scale. Med Decis Making 1995;15:25–30. [DOI] [PubMed] [Google Scholar]
- [26].Elwyn G, Frosch D, Rollnick S. Dual equipoise shared decision making: definitions for decision and behaviour support interventions. Implement Sci 2009;4:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Levit L, Balogh E, Nass S, Ganz PA. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- [28].The Patient Protection and Affordable Care Act (PPACA). Pub. L. No. 111–148, 124 Stat. 119.
- [29].Ikeda AK, Hong P, Ishman SL, Joe SA, Randolph GW, Shin JJ. Evidence-based medicine in otolaryngology part 7: introduction to shared decision making. Otolaryngol Head Neck Surg 2018;158(4):586–93. [DOI] [PubMed] [Google Scholar]
- [30].Pynnonen MA, Hawley ST. A patient-centered approach to clinical practice guidelines in otolaryngology. Otolaryngol Head Neck Surg 2014;150(6):910–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vila PM, Schneider JS, Piccirillo JF, Lieu JE. Understanding quality measures in otolaryngology-head and neck surgery. JAMA Otolaryngol Head Neck Surg 2016;142(1):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shuman AG, Larkin K, Thomas D, et al. Patient reflections on decision making for laryngeal cancer treatment. Otolaryngol Head Neck Surg 2017;156(2):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smitt MC, Heltzel M. Women’s use of resources in decision-making for early-stage breast cancer: results of a community-based survey. Ann Surg Oncol 1997;4(7):564–9. [DOI] [PubMed] [Google Scholar]
- [34].Showalter T, Mishra M, Bridges J. Factors that influence patient preferences for prostate cancer management options: a systematic review. Patient Prefer Adher 2015;9:899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sjöstrand M, Eriksson S, Juth N, Helgesson G. Paternalism in the name of autonomy. J Med Philos 2013;38(6):710–24. [DOI] [PubMed] [Google Scholar]
- [36].Drolet BC, White CL. Selective Paternalism. Virtual Mentor 2012;14(7):582–8. [DOI] [PubMed] [Google Scholar]
- [37].Kehl KL, Landrum MB, Arora NK, et al. Association of actual and preferred decision roles with patient-reported quality of care shared decision making in cancer care. JAMA Oncol 2015;1(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiang T, Stillson CH, Pollack CE, et al. How men with prostate cancer choose specialists: a qualitative study. J Am Board Fam Med 2017;30(2):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nickel B, Brito JP, Moynihan R, Barratt A, Jordan S, McCaffery K. Patients’ experiences of diagnosis and management of papillary thyroid microcarcinoma: a qualitative study. BMC Cancer 2018;18(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hewitt L, Powell R, Zenginer K, et al. Patient perceptions of the impact of treatment (surgery and radiotherapy) for soft tissue sarcoma. Sarcoma 2019;2019:9581781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trotti A, Pajak TF, Gwede CK, et al. TAME: development of a new method for summarising adverse events of cancer treatment by the Radiation Therapy Oncology Group. Lancet Oncol 2007;8(7):613–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.