Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in Wuhan, China, in late 2019, and the first identified case in Japan was in January 2020.1 Later, the number of new cases began to increase exponentially in the middle of February, leading to a nationwide spread.2 , 3 Since then, Japan has faced five waves of the pandemic, each bigger than the last. The National Center for Geriatrics and Gerontology (NCGG) is one of the national centers for advanced and specialized medicine composed of a hospital and a research institute located in Aichi Prefecture, central Japan. Due to its roles in geriatric medicine, the center accepts many elderly patients, with a higher risk of mortality due to COVID-19. To investigate the cumulative SARS-CoV-2 infection rate among workers in the NCGG over time, we designed a repeated cross-sectional study to examine SARS-CoV-2 antibodies. We have previously reported the results of the baseline survey, a low seroprevalence of SARS-COV-2 (0.32%) in June 2020 (after the first wave).4 In this paper, we report the results of the second survey using serum collected in June 2021(during and after the fourth wave).
Out of 632 employees who participated in the 2020 survey, 556 agreed to participate in the second survey (participation rate, 88.0%). In June 2021, we asked the participants who provided written informed consent to answer an electronic questionnaire on sociographic and COVID-19-related factors. The institutional review board of the ethics and conflicts of interest committee approved this study (No, 1481).
We measured the antibodies against SARS-CoV-2 nucleocapsid (N) and spike (S) protein to analyze the prevalence of naturally infected SARS-CoV-2. At the in-house laboratory, we performed chemiluminescence enzyme immunoassays with Sysmex SARS-CoV-2 N-IgG and S-IgG kits, which detect Immunoglobulin G (IgG) against N and S proteins, respectively. We also used Abbott SARS-CoV-2 assay to detect N protein-specific IgG. Additionally, electrochemiluminescence immunoassays were performed using the Roche Elecsys Anti-SARS-CoV-2 RUO system to detect total IgG-antibodies of N protein at the laboratory of SRL Inc. (Tokyo, Japan). Those who showed positive results in two or more tests were considered seropositive. Data were analyzed using SPSS for Windows (version 27.0, IBM). The difference between the baseline and the second-year surveys was examined with McNeamer's test. All statistical tests were two-sided, and a pvalue < 0.05 was considered statistically significant.
The total participant characteristics, the difference in the experiences related to COVID-19, and prevalence of seropositive cases against SARS-CoV-2 between the baseline and the second survey are summarized in Table 1 . Of 556 participants, the mean ± SD age was 42.0 ± 11.4 years, and 63.3% were female. The clinical staff, including doctors, nurses, and allied health care professionals, was 63.8%. Approximately 90% of the participants were vaccinated during the period between February 2020 and June 2021. Most of the participants (n = 451) completed the vaccination (mRNA vaccine, Pfizer-BioNTech) twice almost one month before the second survey.
Table 1.
Prevalence of anti-SARS-CoV-2 at the initial and the follow-up survey.
| n | % | ||||||
|---|---|---|---|---|---|---|---|
| Total | 556 | 100.0 | |||||
| Female | 352 | 63.3 | |||||
| Age (years) | |||||||
| < 30 | 120 | 21.6 | |||||
| 30–39 | 125 | 22.5 | |||||
| 40–49 | 177 | 31.8 | |||||
| ≥ 50 | 134 | 24.1 | |||||
| Mean age = 42.0 ± 11.4. | |||||||
| Job | Doctor | 53 | 9.5 | ||||
| Nurse | 148 | 26.6 | |||||
| Allied health care professional | 154 | 27.7 | |||||
| Clerical and administrative staff | 71 | 12.8 | |||||
| Researcher | 119 | 21.4 | |||||
| Other | 5 | 0.9 | |||||
| Missing | 6 | 1.1 | |||||
| Engagement in Covid-19 related work | |||||||
| Experienced | 167 | 30.0 | |||||
| Never | 333 | 59.9 | |||||
| Missing | 56 | 10.1 | |||||
| Vaccination | |||||||
| Done | 471 | 84.7 | |||||
| Not yet | 29 | 5.2 | |||||
| No answer | 56 | 10.1 | |||||
| Baseline (2020) | Second year (2021) | ||||||
| n | % | n | % | p-value* | |||
| Symptom indicative of Covid-19 | |||||||
| High fever | 13 | 2.3 | 27 | 4.9 | 0.01 | ||
| Severe fatigue | 24 | 4.3 | 30 | 5.4 | 0.52 | ||
| Dyspnea | 7 | 1.3 | 8 | 1.4 | 1.00 | ||
| Loss of sense of taste or smell | 1 | 0.2 | 1 | 0.2 | 1.00 | ||
| Close contact with patient with Covid-19 | 13 | 2.3 | 23 | 4.1 | 0.02 | ||
| History of PCR testing for SARS-CoV-2 | 101 | 18.2 | 121 | 21.8 | < 0.001 | ||
| History of Covid-19 confirmed by PCR testing | 2 | 0.4 | 2 | 0.4 | 1.00 | ||
| IgG anti-SARSCoV-2 antibody | |||||||
| Roche | N-IgG ≥ 1.0 COI | 2 | 0.36 | 4 | 0.72 | 0.22 | |
| Abbott | N-IgG ≥ 1.4 S/CO | 4 | 0.72 | 11 | 1.98 | 0.50 | |
| Sysmex | N-IgG ≥ 10.0 SU/ml | 2 | 0.36 | 3 | 0.54 | 1.00 | |
| S-IgG ≥ 10.0 SU/ml | 1 | 0.18 | 521 | 93.7 | <0.001 | ||
Covid-19, Coronavirus disease 2019; IgG, Immunoglobulin G; PCR, Polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
McNeamer test.
Even though the prevalence of those who reported having polymerase chain reaction (PCR) test for SARS-CoV-2 increased from 18.2 to 21.8%, the number of positive results were unchanged. Regarding anti-N protein antibodies, no significant difference was observed between the baseline and the second survey in all tests. Conversely, the positive rate of S-IgG evaluated by Sysmex's test largely increased from 0.18% (n = 1) to 93.7% (n = 521). Among those who reported to have taken vaccination (n = 474), 99.4% were positive on S-IgG.
Three participants met the criteria of seropositivity in the second survey, giving a seroprevalence of 0.54% (95% CI: 0.11–1.57). Two of them (male, ≥ 50 and female, ≥ 50) showing positive on all three anti-N antibody tests were all clinical workers. Both of them were diagnosed with COVID-19 using a PCR test. However, one participant (male, 30 s), a non-clinical worker, was positive on the Abbott's and the Sysmex's N-tests but negative on Roche's tests in both the 2020 baseline and in the second survey. He reported no history of PCR testing, common cold-like symptoms, or close contact with patients with SARS-CoV-2 indicating asymptomatic infection. Seven participants were positive only on one of the N-tests: five on Abbott's test and two on Roche's test.
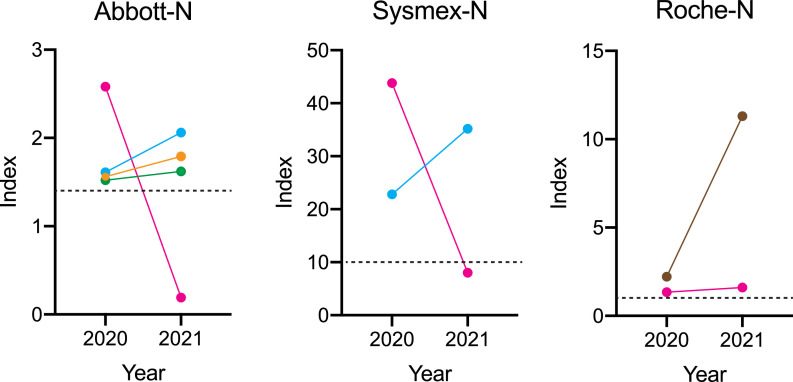
Five participants showed positive at least one of the N-tests in the baseline survey. All of them were positive in at least one test in the second survey. A two-point measurement of the antibody titers in the 2020 baseline and 2021 s survey was shown in Fig. 1 . The seroprevalence was maintained, except for one's (female, ≥ 50) Abbott's and Sysmex's N-tests.
Fig. 1.
Transition of the level of anti-N protein in seropositive participants. Index for each test was plotted for the participants who were positive in the 2020 survey. The dots and lines with the same color indicate the same individuals. Positive values are indicated in dotted lines.
The present study indicates that the antibody against the specific epitopes of the N protein obtained through natural infection is possibly maintained for at least one year. Therefore, previous infection with SARS-CoV-2 could be estimated using antibody prevalence against N protein even in individuals with vaccination. A survey of the general population conducted by the Ministry of Health, Labor and Welfare of Japan, which used similar assays as this study, revealed that seroprevalence in December 2020 was 0.54% in Aichi, where NCGG is located.5 Antibody prevalence of this study (0.54%) is almost equivalent to the 2021 survey. Also, we confirmed that mRNA vaccination is highly effective to increase anti-SARS-CoV-2- spike-binding antibody level.
In conclusion, a regular survey of seroprevalence using multiple tests is effective to identify COVID-19 spread and monitor infection control measures in facilities.
Data availability statement
Not applicable.
Funding
This work was supported by the Japan Health Research Promotion Bureau Research Fund (2020-B-09).
Declarations of Competing Interest
None
Acknowledgments
The authors thank Shuji Nakamura, Megumi Banno, Hiroyuki Fujikawa, and Yukari Kido for their technical assistance.
References
- 1.Furuse Y., Ko Y.K., Saito M., Shobugawa Y., Jindai K., Saito T., et al. Epidemiology of COVID-19 outbreak in Japan, January-March 2020. Jpn J Infect Dis. 2020;73:391–393. doi: 10.7883/yoken.JJID.2020.271. [DOI] [PubMed] [Google Scholar]
- 2.Karako K., Song P., Chen Y., Tang W., Kokudo N. Overview of the characteristics of and responses to the three waves of COVID-19 in Japan during 2020-2021. BioSci Trends. 2021;15:1–8. doi: 10.5582/bst.2021.01019. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto S., Tanaka A., Oshiro Y., Ishii M., Ishiwari H., Konishi M., et al. Seroprevalence of SARS-CoV-2 antibodies in a national hospital and affiliated facility after the second epidemic wave of Japan. J Infect. 2021;88:237–279. doi: 10.1016/j.jinf.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikimi A., Kojima M., Watanabe K., Watanabe A., Yasuoka M., Oshima H., et al. Seroprevalence of antibodies against SARS-CoV-2 among workers in a national research institute and hospital in Central Japan. GHM Open. 2021;1:40–42. [Google Scholar]
- 5.Ministry of Health and Labour J. Results of a survey on SARS-CoV-2 antibody in the general population in Japan as of December 2020. 2020 [cited 2021 March 15]; Available from: https://www.mhlw.go.jp/content/000734482.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.