Abstract
Axillary lymphadenopathy ipsilateral to the vaccination site has been clinically and radiologically reported after administration of COVID-19 vaccines. This can be an important diagnostic dilemma, particularly in cancer patients who are being staged or re-staged, as this benign entity may mimic metastasis, cause unnecessary biopsies and changes in therapy. Here we present a breast cancer patient and a patient with squamous cell carcinoma of the head and neck, who had already received the first two doses of mRNA type COVID-19 vaccines before, now presenting with new hypermetabolic reactive lymphadenopathy on FDG PET/CT after the third booster dose.
Keywords: Covid 19, vaccine, vaccination, booster, lymphadenopathy, mRNA
Abbreviations: RAL, Reactive ipsilateral axillary lymphadenopathy; FDA, The United States Food and Drug Administration; CDC, The Center for Disease Control
Introduction
The United States Food and Drug Administration (FDA) has approved the use of Pfizer-BioNTech COVID-19 vaccine on August 23, 2021 (FDA Approves First COVID-19 Vaccine 2021). The other most commonly used vaccine, Moderna, received emergency use authorization on December 18, 2020 (Moderna COVID-19 Vaccine 2021). As of September 4, 2021, 52.3% of the USA population was fully vaccinated, and 9.3% received one dose of vaccination (Covid vaccine data tracker 2021). Recently, both vaccines have been approved for a booster dose in certain immunocompromised individuals to help decrease COVID-19 infection and death. According to the Center for Disease Control (CDC), 955,000 people received a booster dose after approval [(Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals 2021; Jr, 2021)]. At the time we were writing this paper, on October 11, 2021, FDA approved the emergency use authorization for the Pfizer-BioNTech COVID-19 Vaccine single booster dose, to be administered at least six months after completion of the first two doses in individuals 65 years of age and older; individuals 18 through 64 years of age at high risk of severe COVID-19; and individuals 18 through 64 years of age whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19, such as health care workers, teachers, daycare staff, grocery workers and those in homeless shelters or prisons. (FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations 2021). CDC also recommends an additional dose of mRNA COVID-19 vaccine at least 28 days after the second dose of Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 Vaccine in people with moderately to severely compromised immune systems (COVID-19 Vaccines for Moderately to Severely Immunocompromised People 2021).
Reactive ipsilateral axillary lymphadenopathy (RAL) has been clinically and radiologically very well reported after administration of COVID-19 vaccines (Local Reactions 2021; Local Reactions 2021, 10, Mehta et al., 2021, Michal Eifer, 2021, Özütemiz et al., 2021, Becker et al., 2021, Mortazavi, 2021, D Cohen et al., 2021, Eshet et al., 2021, D Cohen et al., 2021, Adin et al., 2021, Schapiro et al., 2021). This can be an important diagnostic dilemma in oncology patients with all imaging modalities, and this is particularly a problem in staging or re-staging imaging since this benign entity may mimic metastasis, cause unnecessary biopsies, and changes in therapy (Özütemiz et al., 2021, Becker et al., 2021).
We commonly observed RAL in vaccine recipients after the initial two doses with mRNA type vaccines, particularly in the first half of 2021. Now, we have started to observe a new surge of COVID-19 vaccine-induced RAL coinciding with the initiation of the third booster dose in immunocompromised patients. To our knowledge, RAL after the third booster has not been described before. In this report, we present two cases with RAL after COVID-19 vaccine booster dose.
Case series
An institutional review board approved this Health Insurance Portability and Accountability Act–compliant retrospective case series. Written, informed consent for publication of each case was obtained from the patients. Cases were observed on 9.2.2021 and 9.3.2021.
Case 1: 62-year-old woman with a history of metastatic breast cancer. The patient presented with left knee pain in late 2014, and an MRI demonstrated a bone tumor in the left distal femur. The patient underwent surgical resection of the lytic lesion with internal fixation. Pathology revealed metastatic undifferentiated ER+/HER2- adenocarcinoma of the breast. This was followed by a PET/CT scan which then showed a 2.1 cm spiculated mass in the medial left breast and scattered metastatic bone lesions. The patient received radiotherapy targeted to the left distal femur and started chemotherapy with letrozole and was stable for five years under treatment with letrozole. Unfortunately, she developed a radiation-related high-grade osteosarcoma of the left distal femur in January 2020 and started neoadjuvant chemotherapy with doxorubicin and cisplatin, and continued treatment with letrozole. Later, on follow-up PET/CT in January 2021, she was found to have an FDG avid right scapula lesion which was biopsy-proven to be metastatic breast lesion (Fig. 1 ). She started treatment with fulvestrant and abemaciclib; however, abemaciclib was later substituted with palbociclib secondary to rash. Later, the patient received the first dose of Moderna COVID-19 vaccination on March 15th and the second dose on April 12th, 2021. A PET/CT obtained between two doses on April 1st, 2021, showed positive response to treatment in the scapular lesion while there were two ovoid-shaped hypermetabolic lymph nodes in the right axilla. These were interpreted as vaccine-induced RAL as they disappeared in the follow-up PET/CT in July 2021 (Fig. 1). Despite a favorable response to treatment regarding the known bone metastasis, this new PET/CT showed a growing hypermetabolic calcified nodule in the left lung, which was concerning for osteosarcoma metastasis. The decision was restaging of the lung disease with PET/CT to confirm its oligometastatic status. The most recent PET/CT from September 2nd, 2021 demonstrated new hypermetabolic right axillary lymph nodes (14 mm - maxSUV: 5.23, 15 mm - maxSUV 3.55, MaxSUVmediastinum:2.8). The metabolic activity of the right scapular lesion was further decreased, while the hypermetabolic calcified lesion was slightly bigger in size (Fig. 1). The result of a questionnaire before the PET/CT revealed that she received her third dose of Moderna COVID-19 vaccine one day before the PET/CT. Based on lack of other sites of osteosarcoma metastasis, the solitary lung metastasis was resected. The pathology confirmed an osteosarcoma diagnosis for the lung nodule.
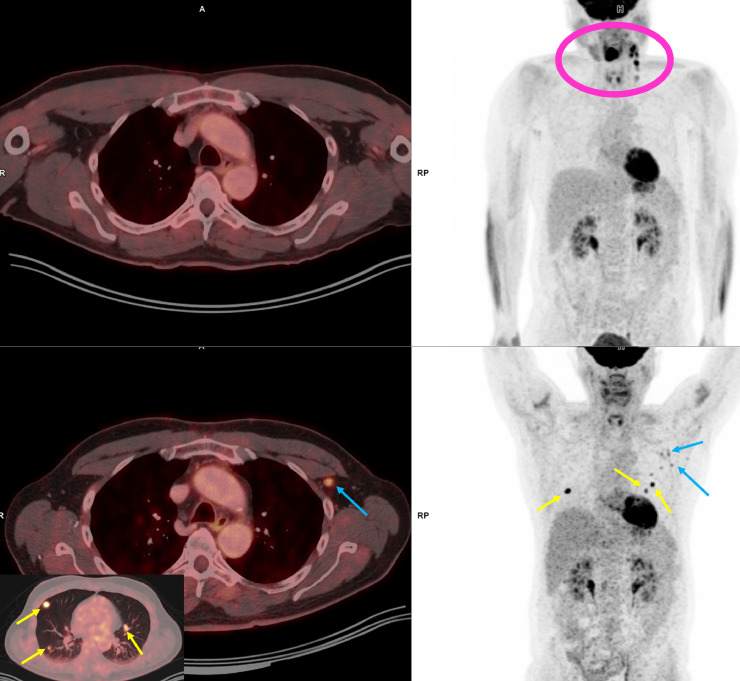
Fig. 2.
62-year-old man with a history of squamous cell cancer. The upper row is the PET/CT from 2019, which was obtained for cancer staging. The fused PET/CT axial image at the axilla level is unremarkable. MIP image on the right shows hypermetabolic tongue cancer and metastatic left cervical lymphadenopathy (pink circle). The lower row is the PET/CT obtained in early September in 2021, 1 day after the third dose of COVID-19 vaccine targeted to the left arm. Yellow arrows show hypermetabolic lung nodules, consistent with metastasis, and new hypermetabolic left axillary lymph nodes (blue arrows). Bilateral symmetric uptake in the deltoid muscles in the lower right MIP image was considered physiologic muscle uptake.
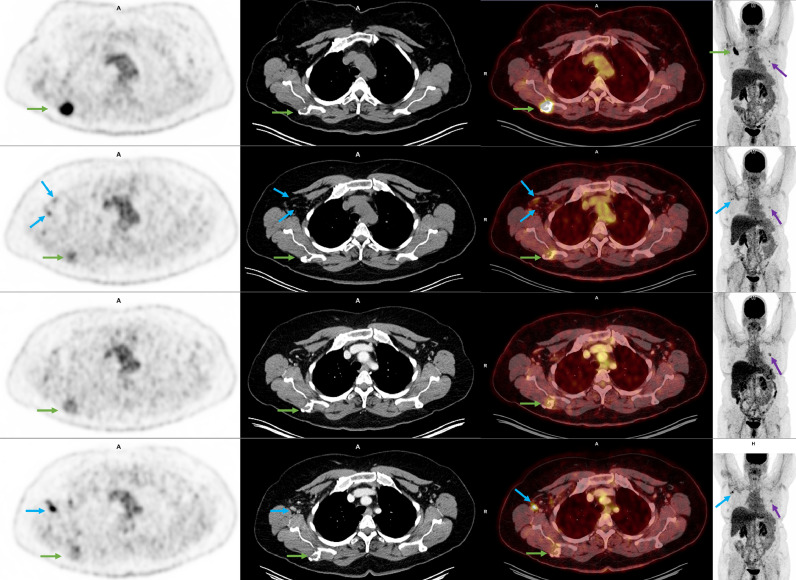
Fig. 1.
62-year-old woman with a history of metastatic breast cancer. Each row represents PET/CT exams from top to bottom, dating from January, April, July, and September 2021. Each column represents axial PET, CT, fused PET/CT, and coronal MIP images from left to right. Initially, in January, the patient presented with a right scapular hypermetabolic bone lesion (green arrows), which later biopsy-proven as metastasis from breast cancer. MIP images show a mildly metabolic new nodule in the left lung (purple arrow). In April, two weeks after COVID-19 vaccine, the scapular lesion improved (green arrows) following treatment with chemotherapy, but there were new hypermetabolic right axillary lymph nodes (blue arrows). Lung nodule was stable (purple arrow). In July, the lymphadenopathy completely resolved, consistent with vaccine-related reactive lymphadenopathy, and there was further decreased size and metabolic activity of the scapular lesion (green arrow). The lung lesion was slightly more conspicuous (purple arrow). In September, while under chemotherapy, the right scapular lesion was more improved (green arrows). However, the known left lung nodule was increased in size (purple arrow), and there was new right axillary hypermetabolic lymphadenopathy (blue arrows). Chart review showed that the patient had her third vaccine one day ago; thus lymph nodes were more compatible with vaccine-induced reactive lymph nodes. Wedge resection of the lung nodule confirmed osteosarcoma metastasis.
Case 2: 62-year-old man with history of squamous cell cancer of the tongue status post treatment with radiation and prostate cancer status post treatment with prostatectomy/radiation, presented with exertional dyspnea in June 2021. A coronary CT angiography was ordered for symptoms, which showed minimal non-obstructive coronary artery disease in August 2021 but also revealed multiple pulmonary nodules with concern of malignancy. Subsequent PET/CT on September 3rd, 2021 showed multiple hypermetabolic pulmonary nodules (Largest is 20 mm - maxSUV: 6.54, MaxSUVmediastinum:1,5) along with new hypermetabolic left axillary lymph node clusters (Largest is 10 mm – maxSUV: 2.7). The activity in the lymph nodes was not as high as the lung nodules, and further investigation of the electronic medical records revealed that the patient received three Pfizer COVID-19 vaccines on 3/2021, 4/2021, and 9/2021 (2 days before PET/CT), respectively. Based on imaging and history, the axillary lymph nodes were considered benign and reactive, but the lung nodules were highly suspicious for malignancy, and due to history of two separate cancers, the patient was referred for lung nodule biopsy.
Discussion
We have highlighted two cases with RAL secondary to the third booster dose of mRNA type COVID-19 vaccines. In the first case, there were two ongoing cancers which demonstrated mixed response to treatment, where the metastatic breast cancer was responding to treatment while the osteosarcoma was progressing. On top of all, there was new development of axillary lymphadenopathy, which can be highly challenging for the oncologist and radiologist. Similarly, in the second patient, there was history of two previously treated cancers with new hypermetabolic lung nodules and ipsilateral axillary lymph nodes. Despite the prior story of routine COVID-19 vaccination, the radiologist checked for vaccination status and found out that a third booster dose was administered to explain the lymphadenopathy. This led to a decision of biopsy of lung nodules. If that information was not sought, the clinician and radiologist might have preferred doing a biopsy of axillary lymph nodes as they are relatively easier and safer for biopsy.
With the start of worldwide COVID-19 vaccine campaigns, RAL secondary to COVID-19 vaccine has become a routine consideration in oncologic imaging. Initially, the radiology community made recommendations, including planning radiology scans in oncologic follow-up cases prior to vaccination, injection of the vaccine in the contralateral extremity in certain cancers, for example, breast cancer or melanoma, delaying follow-up imaging after vaccination up to 6 weeks, preparing pre-scan questionnaires regarding vaccination status, and using ultrasonography in the follow up of these RALs (Becker et al., 2021, Grimm et al). This being said, the value of timely imaging may outweigh the issue of spurious detection of vaccination-related RAL. Eshet et al. later showed that RAL could still persist within 7–10 weeks after the vaccination using PET/CT imaging in 30% of the cases (Eshet et al., 2021). Moreover, Cohen et al. reported that the RAL occurred more frequently and with higher intensity in PET/CTs after the second dose with Pfizer vaccine (D Cohen et al., 2021). Therefore, with the addition of third booster doses, clinicians and radiologists should prepare themselves for these reactive lymph nodes on imaging, even after 60 days.
Many cancer patients may still have concerns about these hypermetabolic and enlarged lymph nodes after reading the radiology report. It should be clearly stated that these are benign and transient, and the clinician should take the necessary time to explain the finding in order to avoid unnecessary stress and request for biopsy. One recent study suggests that having hypermetabolic lymph nodes after vaccination may reflect a robust immune response, which may be beneficial. Cohen et al. reported a high correlation between the presence of hypermetabolic lymph nodes after COVID-19 vaccine and serologic antibody testing after vaccination. In contrast, the rate of hypermetabolic lymph nodes was significantly lower in patients who recently received anti-CD20 antibody therapy in hematologic malignancies, which directly affects humoral immunity. Overall, their paper suggests that the presence of hypermetabolic lymph nodes is, in fact, a sign of effective humoral immunity (D Cohen et al., 2021).
Overall, a third booster campaign is about to start, and we have already begun to see its effects in routine oncologic imaging practice. Radiologists, oncologists, and internists should be aware of this phenomenon, and the booster dose history should be meticulously evaluated in each patient before image interpretation with the knowledge that it may occur quickly as it was present in 1–2 days post-vaccination. Finally, imaging that is needed should not be delayed, and it is important for the treating physicians and the patients to understand that lymphadenopathy ipsilateral to the vaccination site is likely to be vaccine-related and can occur even after a third vaccination.
Consent statement
An institutional review board approved this Health Insurance Portability and Accountability Act–compliant retrospective case series. Written, informed consent for publication of each case was obtained from the patients.
Footnotes
Authors have nothing to disclose related to this article.
No funding was received.
References
- FDA Approves First COVID-19 Vaccine. [ https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine ]. Accessed in 09/06/ 2021.
- Moderna COVID-19 Vaccine. [ https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/moderna-covid-19-vaccine.]. Accessed in 09/06/ 2021.
- Covid vaccine data tracker. [ https://ourworldindata.org/covid-vaccinations?country=OWID_WRL ]. Accessed in 09/06/ 2021.
- Coronavirus (COVID-19) Update: FDA Authorizes Additional Vaccine Dose for Certain Immunocompromised Individuals. [ https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised.]. Accessed in 09/06/ 2021.
- Jr BL. Nearly 1 million Covid booster shots have already been administered in the U.S., CDC data shows.[ https://www.cnbc.com/2021/08/31/cdc-nearly-1-million-covid-booster-shots-have-already-been-administered-in-us.html ]. Accessed in 09/06/ 2021.
- FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations [ https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations ]. Accessed in 10/11/ 2021.
- COVID-19 Vaccines for Moderately to Severely Immunocompromised People. [ https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html ]. Accessed in 10/11/ 2021.
- Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: moderna COVID-19 Vaccine.[ https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html ]. Accessed in 01/24/ 2021.
- Local Reactions, Systemic Reactions, Adverse Events, and Serious Adverse Events: pfizer-BioNTech COVID-19 Vaccine.[ https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html ]. Accessed in 01/24/ 2021.
- Lars Grimm SD, Basak Dogan, Brandi Nicholson, Brian Dontchos, Emily Sonnenblick HM, JoAnn Pushkin, John Benson, Katia Dodelzon, Neha Modi, Roger Yang VD, Vidushani Perera. SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination.[ https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-recommendations-for-managing-axillary-adenopathy-post-COVID-vaccination.pdf ]. Accessed in
- Mehta N, Sales RM, Babagbemi K, Levy AD, McGrath AL, Drotman M, et al. Unilateral axillary Adenopathy in the setting of COVID-19 vaccine. Clin. Imaging. 2021;75:12–15. doi: 10.1016/j.clinimag.2021.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michal Eifer YE. Imaging of COVID-19 Vaccination at FDG PET/CT. Radiology. 2021;299:E248. doi: 10.1148/radiol.2020210030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker AS, Perez-Johnston R, Chikarmane SA, Chen MM, El Homsi M, Feigin KN, et al. Multidisciplinary Recommendations Regarding Post-Vaccine Adenopathy and Radiologic Imaging: radiology Scientific Expert Panel. Radiology. 2021;300:E323–E3E7. doi: 10.1148/radiol.2021210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi S. COVID-19 Vaccination-Associated Axillary Adenopathy: imaging Findings and Follow-Up Recommendations in 23 Women. AJR Am. J. Roentgenol. 2021:1–2. doi: 10.2214/AJR.21.25651. [DOI] [PubMed] [Google Scholar]
- Cohen D, Krauthammer SH, Wolf I, Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1854–1863. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshet Y, Tau N, Alhoubani Y, Kanana N, Domachevsky L, Eifer M. Prevalence of Increased FDG PET/CT Axillary Lymph Node Uptake Beyond 6 Weeks after mRNA COVID-19 Vaccination. Radiology. 2021;300:E345–E3E7. doi: 10.1148/radiol.2021210886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, Hazut Krauthammer S, Cohen YC, Perry C, Avivi I, Herishanu Y, et al. Correlation between BNT162b2 mRNA Covid-19 vaccine-associated hypermetabolic lymphadenopathy and humoral immunity in patients with hematologic malignancy. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3540–3549. doi: 10.1007/s00259-021-05389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adin ME, Isufi E, Kulon M, Pucar D. Association of COVID-19 mRNA Vaccine With Ipsilateral Axillary Lymph Node Reactivity on Imaging. JAMA Oncol. 2021;7:1241–1242. doi: 10.1001/jamaoncol.2021.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özütemiz C, Krystosek LA, Church AL, Chauhan A, Ellermann JM, Domingo-Musibay E, et al. Lymphadenopathy in COVID-19 Vaccine Recipients: diagnostic Dilemma in Oncologic Patients. Radiology. 2021;300:E296–E300. doi: 10.1148/radiol.2021210275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro R, Moncayo VM, Meisel JL. Case report of lymph node activation mimicking cancer progression: a false positive F18 FDG PET CT after COVID-19 vaccination. Curr. Probl. Cancer Case Rep. 2021;4 doi: 10.1016/j.cpccr.2021.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]