Abstract
Background and aims
Molnupiravir is a newer oral antiviral drug that has recently been tested in COVID-19. We aim to conduct a systematic review of literature to find out the efficacy and safety of molnupiravir in patients with COVID-19.
Methods
We systematically searched the electronic database of PubMed, MedRxiv and Google Scholar from inception until October 15, 2021, using MeSH keywords. Ongoing trials of molnupiravir in COVID-19 were additionally searched from the ClinicalTrials.Gov and ctri.nic.in/Clinicaltrials. We retrieved all the available granular details of phase 1 to 3 studies of molnupiravir in COVID-19. Subsequently we reviewed the results narratively.
Results
Two phase 1 double-blind, randomized, placebo-controlled (DBRPC) studies of molnupiravir showed that 1600 mg daily dose is safe and tolerable, without any serious adverse events up to 5.5 days. One phase 2 DBPRC study found significantly lower time to clearance (RNA negativity) with molnupiravir 800 mg twice daily compared to the placebo (log-rank p value = 0.013) in mild to moderate COVID-19. Interim report of one phase 3 DBRPC study in non-hospitalized COVID-19 found a significant reduction in the risk of hospital admission or death by 50% (p = 0.0012). However, no significant benefit was observed with molnupiravir in the later stage of moderate to severe COVID-19.
Conclusion
Molnupiravir is first oral antiviral drug to demonstrate a significant benefit in reducing hospitalization or death in mild COVID-19 and could be an important weapon in the battle against SARS-CoV-2. However, its role in moderate to severe COVID-19 is questionable and more studies are needed.
Keywords: Molnupiravir, EIDD-2801, MK-4482, COVID-19, SARS-CoV-2
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has led to a substantial increase in morbidity and mortality worldwide. Even though adequate vaccines are now available and newer ones are also under development for the prevention of severe COVID-19, effective antiviral drugs to combat this disease are currently lacking. While some of the drugs have recently been given an emergency use authorization (EUA) for COVID-19, all of these pharmaceutical agents have to be administered in hospital settings. Therefore, the development of a simple oral antiviral agent has been an elusive goal since the beginning of this pandemic.
Molnupiravir (Emory Institute of Drug Development-2801 [EIDD-2801]/MK-4482) is one upcoming oral drug which seems promising. This oral agent was developed by Drug Innovation Ventures at Emory University, later was acquired by Ridgeback therapeutics in partnership with Merck & Co, USA. In general, antiviral drugs tested so far usually terminate the elongation of RNA-chain by targeting the viral polymerases but such antivirals have not shown a very promising role in treatment of SARS-CoV-2 infections because of exonucleolytic proof-reading activity that can remove mis-incorporated nucleotides from the nascent RNA. Both molnupiravir and remdesivir (GS-5734) targets RNA-dependent RNA-Polymerase (RdRp) enzyme used by the corona virus for transcription and replication of its viral RNA genome [1,2]. While remdesivir a nucleoside analog that stalls the RdRp and thus circumvents proof reading, molnupiravir has a unique mechanism of action pretty similar to favipiravir. Notably, favipiravir was tried in the early part of pandemic without much success. Remdesivir was granted an EUA by the US Food Drug Administration (FDA), however in some studies it failed to show the expected efficacy, the reason as to why WHO did not recommend it. Moreover, it can only be administered via intravenous route in in-hospital settings which has its own limitations.
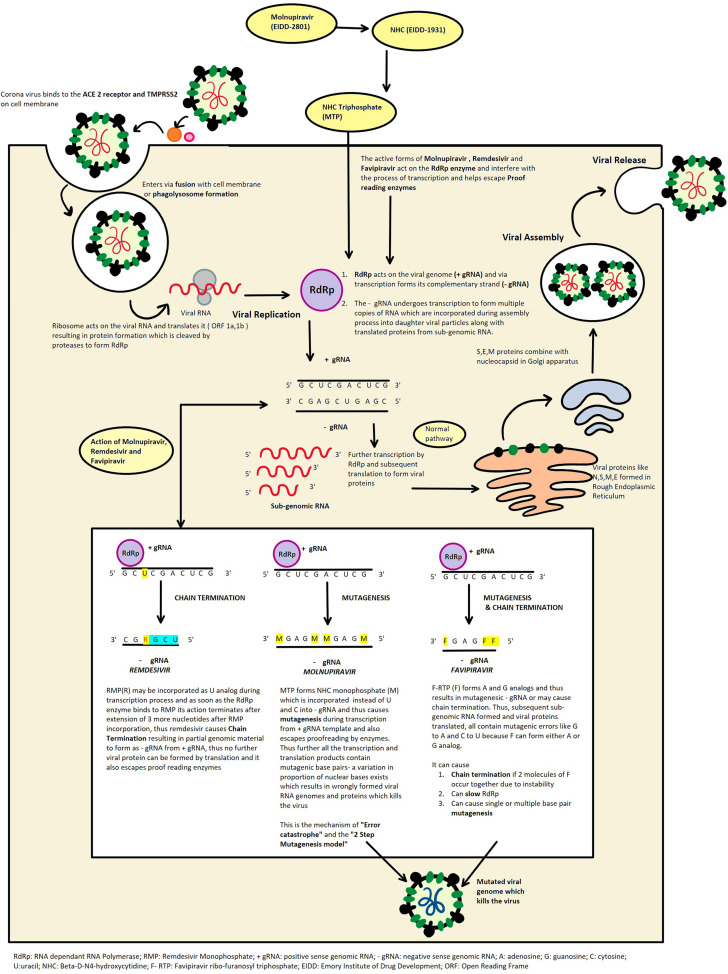
Molnupiravir initially emerged as a possible treatment of influenza viruses, encephalitic alphaviruses like Venezuelan, Eastern and Western equine encephalitic viruses due to its significant inhibitory effect in cell cultures [3,4]. It appears to work by the mechanism of “error catastrophe” which is essentially based on the concept that by increasing the rate of mutation in the viral genome beyond a biologically tolerable threshold it will become lethal to the virus and lead to its extinction [5,6]. The broad-spectrum antiviral activity of this drug is attributed to its 2-step mutagenesis mechanism. Molnupiravir is an isopropyl ester prodrug, which is cleaved in plasma by host esterases to an active nucleoside analog β-D-N4-hydroxycytidine (NHC) or EIDD-1931 [5]. This active form of the drug is distributed to various tissues and subsequently converted to its corresponding 5′-triphosphate (NHC triphosphate or MTP). This then targets the RdRp which is virally encoded and competitively inhibits the cytidine and uridine triphosphates and incorporates M instead. The RdRp uses the NHC triphosphate as a substrate instead of the cytidine and uridine triphosphates and then incorporates either A or G in the RdRp active centers forming stable complexes and thus escaping proof reading by the synthesis of a mutated RNA [7,8]. Kabinger et al. [7] confirmed with structural studies about the formation of M-G and M-A base pairs in the active center of RdRp and after cryo-EM density interpretation assumed that one stable tautomer predominates in each case, that is, amino-M tautomer forms a base pair with G and the imino-M tautomer forms a base pair with A and do not impair the RdRp progression [6]. Thus, the 2-step mutagenesis can be summarized as follows- in the first step, RdRp synthesizes negative strand genomic RNA(-gRNA) by using positive strand genomic RNA(+gRNA) as a template. Following this, in the second step, +gRNA or sub genomic RNA is synthesized using M-containing RNA as template. The M containing RNA in the -gRNA causes mutation in +gRNA and sub-genomic RNA subsequently formed resulting in mutagenesis which is lethal to the virus [5,6]. Fig. 1 illustrates the mechanism of action (schematic representation) of molnupiravir against SARS-CoV-2 and its comparison with remdesivir and favipiravir. These mutations are also produced in the host cell (mammalian DNA) which raises concerns regarding its interference with vaccination, and its potential carcinogenic and teratogenic effects which are theoretically possible with mutagenic drugs [9]. However, it might be less likely because of its proposed short-term use – twice daily for 5 days. It is also interesting to note that RNA synthesis in hepatitis C polymerase or RNA polymerase of respiratory syncytial virus is not seen with NHC triphosphate [10].
Fig. 1.
Mechanism of action (schematic representation) of molnupiravir against SARS-CoV-2 (works by inducing mutagenesis in viral RNA) as compared with remdesivir (works by stalling RdRp in turn causing chain termination of newly formed RNA strand) and favipiravir (works by slowing/stalling RdRp causing chain termination or inducing mutagenesis or both).
Earlier, molnupiravir had illustrated in vitro activity in human airway epithelial cell culture against SARS-CoV-2. Improvement in pulmonary function and decline in viral titer were noted in mice infected with SARS-CoV-2 that were administered molnupiravir [6]. Wahl et al. [11] demonstrated via in vivo studies on human lung-only mice (LoM) that EIDD 2801 dramatically inhibited the replication of SARS-CoV-2. Also, the drug demonstrated reduced viral shedding and inflammatory infiltrates in nasal lavages and adequate humoral antiviral response in ferret model of influenza [12]. Additionally, molnupiravir demonstrated an inhibitory effect on the replication of SARS-CoV-2 in Syrian hamster model when it was commenced 12 h before or after experimental infection [13]. Thus, further trials in humans were conducted for SARS-COV-2 infections. In this systematic review, we aimed to provide clinical data with molnupiravir through phase 1 to 3 studies conducted in patients with COVID-19.
2. Methods
A systematic literature search was conducted in the electronic database of PubMed, MedRxiv and Google Scholar from inception until October 15, 2021, using MeSH keyword “COVID-19”, “SARS-CoV-2”, AND “Molnupiravir”, “EIDD-2801”, “MK-4482”. Details of all the studies that reported outcomes with molnupiravir in people with COVID-19 so far, were retrieved. Cross references related to this topic were also retrieved. An additional search was made in the data base of ClinicalTrials.gov and ctri.nic.in/Clinicaltrials for ongoing study of molnupiravir in COVID-19 in USA and India, respectively. Data available in grey literature as a top-line or interim result were also retrieved. Two authors independently checked the veracity of data. Here, we report the synthesis from the results of studies conducted with molnupiravir in COVID-19.
3. Results
Overall, 48, 11 and 436 articles were found in the database of PubMed, MedRxiv, and Google Scholar, respectively. A total of 7 and 12 studies on molnupiravir were found on ClinicalTrials.gov and ctri.nic.in/Clinicaltrials, respectively. After exclusion of repetitions, review articles, commentaries, perspectives and experimental studies, we found following human studies that have reported the results from phase 1 to 3 studies including completed, incomplete, and several ongoing trials.
3.1. Phase 1 studies
First double-blind, randomized-controlled Phase 1 trial (NCT04392219) on healthy volunteers (n = 130) showed that molnupiravir was well tolerated and there was a dose proportional pharmacokinetics following administration. After oral administration, the prodrug molnupiravir is rapidly cleaved to its active form EIDD-1931 with a median time of maximum observed concentration of 1–1.75 h. The geometric half-life was found to be nearly 1 h with an apparent slower elimination phase following high single dose or multiple doses, however no accumulation was noted after multiple dosing. No decrease in overall absorption was noted in the fed state although there was some decrease in the rate of absorption during fed state. A range of 50–800 mg twice daily dosing for 5.5 days and a single dose up to 1600 mg was found to be safe and well tolerated. Unlike other nucleoside analogs and natural nucleosides that are generally actively secreted by the kidney, very little EIDD-1931 was detected in urine which might be attributed to the metabolism of EIDD-1931 to cytidine and uridine. Regarding formulation, in all parts of the study, capsules of molnupiravir were used except for single ascending doses where an oral solution was used for flexibility in dose escalation. For tolerability, a greater proportion of patients in placebo arm had higher adverse events compared to molnupiravir in both single (43.8% vs. 35.4%, respectively) and multiple ascending doses (50.0% vs. 42.9%, respectively) arm. While headache was the most frequently reported adverse event in single ascending doses study (placebo arm had proportionately higher headache compared to molnupiravir [18.8% vs. 12.5%, respectively]), diarrhea was the most frequently reported adverse event noted in multiple ascending dose study (7.1% each in placebo and molnupiravir). No clinically significant abnormality in either laboratory or vital signs or electrocardiography were seen, and neither were any serious adverse events noted. Only one subject discontinued because of mild truncal maculopapular pruritic rash following 800 mg twice daily doses of molnupiravir which the investigator thought was related to the drug [14].
An open label randomized controlled but a small (n = 18) phase Ib/IIa trial- AGILE (NCT04746183) was conducted at the Royal Liverpool and Broadgreen Clinical Research Facility using Bayesian approach. Adult patients with RT-PCR confirmed SARS-CoV-2 infection within 5 days of symptom onset were included and randomized to either standard of care (SOC, n = 6) or 300, 600 and 800 mg doses of molnupiravir twice daily for 5 days by oral route (n = 4 in each arm). The primary outcome was to study the dose limiting toxicity (DLT) while secondary outcome included evaluation of safety and clinical progression. The primary outcome of DLT up to 7-days was modelled on Bayesian dose-toxicity Mozgunov-model with a priori assumed toxicity risk of 10% in SOC arm. A dose was judged unsafe or unacceptably toxic if the probability of DLT is 30% or greater over the controls. All (4/4, 100%) patients receiving 300 and 600 mg, 1/4 (25%) patients receiving 800 mg, and 5/6 patients (83%) receiving SOC were found to have mild adverse events. This finds highest dose of 800 mg twice daily had a 0.9% probability of having 30% excess toxicity over the controls, and therefore molnupiravir was considered safe and well tolerated, with a plasma concentration within the target range [15].
3.2. Phase 2 studies
A double-blind, randomized-controlled, multicentric, phase 2a trial (MK-4482-006) was conducted (NCT04405570, n = 202) to evaluate the safety and tolerability in patients with mild to moderate COVID-19. Twice daily oral doses of 200 mg, 400 mg and 800 mg molnupiravir for 5 days were administered in the test arm vs. placebo, after the randomization. While time to clearance of viral RNA in nasopharyngeal swabs (tested by RT-PCR) was the primary endpoint, secondary outcomes of the trial included evaluation of time to infectious viral elimination from nasopharyngeal swabs and median RNA change from baseline on days 3, 5 and 7. Assessment of activity, safety and tolerability was made for 28 days after initiation of the study. Collection of nasopharyngeal swabs was done on day 1 (baseline) and days 3,5,7,14 and day 28, and antiviral activity (both quantitative and qualitative) was evaluated using RT-PCR as well as after viral isolation method using Vero C1008 cell culture method on days 2 and 5 post inoculation. Time to clearance (RNA negativity) - the primary outcome - was significantly reduced in the molnupiravir 800 mg arm twice daily compared to the placebo (log-rank p value = 0.013, median: 14 days). Moreover, reduction in time to clearance of viral RNA was also greater and significant when compared to placebo (median: 14 days vs. 27 days; p value = 0.01). It was noted that viral isolation was significantly lower in participants receiving 800 mg twice daily molnupiravir compared to placebo (1.9% vs. 16.5% viral isolation, respectively; p = 0.02) on Day 3. On Day 5 no viral isolation was noted from patients receiving 400 or 800 mg twice daily molnupiravir versus 11.1% viral isolation in participants receiving placebo (p = 0.03). Proportion of subjects who achieved SARS-CoV-2 negativity by the end of the study was 92.5%, 78.7%, 91.3% for 800 mg, 400 mg, 200 mg twice daily molnupiravir, respectively and 80.3% for placebo. Regarding tolerability, overall, very few, low grade adverse events were noted in this study and was found to be lowest in molnupiravir 800 mg twice daily group. Headache, insomnia and increased levels of alanine aminotransferase (ALT) were the only adverse events reported by more than 4 participants and, 5% and 8.1% of molnupiravir and placebo groups, respectively showed grade 3 level of adverse events. Two (2/140, 1.4%) as compared to one (1/62, 1.6%) adverse event led to discontinuation in molnupiravir and placebo groups respectively. No hematological or dose related trend in clinical chemistry data was found. Treatment was discontinued in 4 patients due to serious adverse events. This includes one placebo administered patient who had hypoxia, two patients from 400 mg molnupiravir group who had decreased oxygen saturation and cerebrovascular accident, and one patient from 800 mg molnupiravir group who had acute respiratory failure. One death was reported in a patient from placebo group suffering from hypoxia due to COVID-19 outside the 28-day time window [16]. Another double-blind phase 2 trial (NCT04405739, n = 96) evaluating The Safety of EIDD-2801 and Its Effect on Viral Shedding of SARS-CoV-2 (END-COVID Study) is currently under progress [17].
3.3. Phase 3 studies
The phase 3 double-blind, randomized study (MOVe-OUT) that was planned to assess efficacy and safety of molnupiravir in 1850 non-hospitalized adult (18 years or older) participants with COVID-19 (NCT04575597, MK-4482-002) has recently been stopped by the independent data safety monitoring board due to excessive benefit in active treatment arm compared to the placebo. The inclusion criteria of this study included a confirmed SARS-CoV-2 infection with sample collection ≤5 days prior to the day of randomization having negative serological tests in response to recent or prior infection, having initial onset of signs/symptoms attributable to COVID-19 for ≤5 days prior to the day of randomization and at least 1 of the following sign/symptom attributable to COVID-19 on the day of randomization - having mild or moderate COVID-19 and having at least 1 characteristic or underlying medical condition associated with an increased risk of severe illness (obesity, an age of over 60 years, diabetes, and heart disease) from COVID-19. Additionally, males either agreed to abstain from heterosexual intercourse or to use contraception during the intervention period and for at least 4 days after the last dose of study intervention. Females must be having negative pregnancy test or not breastfeeding, and either agree to abstain from heterosexual intercourse or must agree to use contraception during the intervention period and for at least 4 days after the last dose of study intervention. Exclusion criteria included currently hospitalized or expected to need hospitalization for COVID-19 within 48 h of randomization, on dialysis, or having reduced estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 by the Modification of Diet in Renal Disease (MDRD) equation, having any of the following conditions: human immunodeficiency virus (HIV), history of hepatitis B virus (HBV) or hepatitis C virus (HCV) with cirrhosis, end-stage liver disease, hepatocellular carcinoma, aspartate aminotransferase (AST) and/or ALT) >3X upper limit of normal and low platelet count <100,000/μL. The primary outcome of this trial included – i) % of participants who are hospitalized (all cause hospitalization ≥24 h of acute care) and/or die (death due to any cause) in a time frame of 29 days, ii) % of participants with an adverse event (AE) in a time frame up to ∼7 months, iii) % of participants who discontinued study intervention due to an AE in a time frame up to 6 days [18].
The interim analysis (n = 775) of this phase 3 study (NCT04575597) showed a significant reduction in risk of hospital admission or death by 50% (p = 0.0012) at day 29. Study found 7.3% of patients (28/385) on molnupiravir as compared to 14.1% of patients on placebo (53/377) had either been admitted to hospital or died, and no death was reported in the molnupiravir group as compared to 8 deaths in the placebo group on day 29. Moreover, the efficacy of molnupiravir was unaffected by the SARS-CoV-2 variant (gamma, delta or mu), the time of onset of symptoms and with the underlying risk factors. The incidence of any adverse events (35% vs. 40% in molnupiravir vs. placebo arm, respectively) and drug-related adverse events (12% vs. 11% in molnupiravir vs. placebo arm, respectively) were found to be similar in both the molnupiravir and placebo arm. However, fewer people in the molnupiravir arm discontinued treatment as compared to the placebo arm (1.3% vs. 3.4%, respectively) [19,20]. Other phase 3 double-blind, randomized study (MOVe-IN, NCT04575584) that was planned to assess efficacy and safety of molnupiravir in 304 hospitalized adult (18 years or older) participants with COVID-19 (MK-4482-001) was terminated following an interim analysis of data that found it is unlikely to demonstrate a clinical benefit in hospitalized patients [21,22]. Nonetheless, a large (n = 1,332) phase 3, multicentric, randomized, double-blind, placebo-controlled study (NCT04939428) is currently evaluating the efficacy and safety of molnupiravir for the prevention of COVID-19 in adults (MOVe-AHEAD) residing with a person with COVID-19, with a hypothesis that molnupiravir would be superior to placebo in preventing laboratory-confirmed COVID-19 infection through day 14 [23].
Meanwhile, few interim result of phase 3 trials conducted with molnupiravir in mild (upper respiratory tract symptoms and or fever without shortness of breath or hypoxia) and moderate (pneumonia with no signs of severe disease but with presence of clinical feature of dyspnea and or hypoxia, fever, cough including SpO2 ≤ 93% [range 90–93%] on room air, respiratory rate ≥24 per minute) COVID-19 in Indian patients (age between 18 and 60 years of age), were announced by the different Indian pharmaceutical companies recently. Hetero Labs Limited, Hyderabad, India announced the interim results on July 9, 2021, from their open-label, randomized, multicenter clinical trial (CTRI/2021/05/033739) of 741 mild COVID-19 patients in which the efficacy and safety of molnupiravir 800 mg (4 × 200 mg) every 12 h (twice daily) for 5 days plus SOC vs. SOC (control arm) alone within 5 days of onset of symptoms were evaluated. The interim results from this study of mild COVID-19 patients (n = 741) revealed – i) earlier clinical improvement (2-point decrease in WHO Clinical Progression Scale) in molnupiravir vs. SOC at day 5 (63.4% vs. 22.3%, respectively; p < 0.0001), day 10 (79.0% vs. 49.5%, respectively; p < 0.0001) and day 14 (81.6% vs. 73.2%, respectively; p = 0.02), ii) faster time to clinical improvement in molnupiravir vs. SOC (median time: 8 days vs. 12 days, respectively; p = 0.0001), iii) higher RT-PCR negativity (all p < 0.0001) in molnupiravir vs. SOC at day 5 (77.4% vs. 26.1%, respectively), day 10 (94.0% vs. 57.2%, respectively) and day 14 (97.0% vs. 85.2%, respectively), iv) significantly fewer hospital admissions in molnupiravir vs. SOC alone (1.9% vs. 6.2% respectively; p = 0.003) over 14 days. No death reported in either group. Nausea, diarrhea and headache were most common side effect reported – all with mild severity. No one discontinued from the trial [24]. Collectively, this interim report from the ongoing phase 3, open-label, randomized clinical trial from India showed a significantly lower hospitalization in molnupiravir recipients compared to SOC alone arm in people with mild COVID-19 and with no obvious drug-induced adverse events, although no difference in death rate was observed between the two arms.
Another interim report from the phase 3 trial of molnupiravir in mild COVID-19 patients from India was announced by Optimus pharma (CTRI/2021/06/033992) on July 21, 2021. The first interim result on 353 patients has shown a higher RT-PCR negativity in molnupiravir arm vs. SOC alone (78.3% vs. 48.4%, respectively; p = not reported) on day 5 [25]. In a personal communication, Optimus Pharma noted a significant increase in RT-PCR negativity with molnupiravir vs. SOC alone at day 5 (77.4% vs. 51.5%, p < 0.0001) and day 10 (99.5% vs. 69.5% respectively; p < 0.0001), although no difference noted at day 14 (99.5% vs. 98.5%; p = 0.62) in a second interim analysis of 403 patients. Faster clinical improvement (at least one-point improvement from baseline on WHO ordinal scale) was also observed in molnupiravir vs. SOC at both day 5 (79.0% vs. 51.3%, respectively; p < 0.0001) and day 10 (97.8% vs. 82.3% respectively; p < 0.0001). However, no difference was noted at day 14. There has been 1 hospitalization in molnupiravir arm compared to 3 in SOC, and 6.5% subjects in molnupiravir arm had adverse events compared to 8.9% in SOC group. Serious adverse events were reported in 1 subject in molnupiravir arm compared to 3 in SOC. Collectively, this interim report from an ongoing open-label, randomized clinical trial from India also found a faster clinical and viral recovery (RT-PCR negativity) with molnupiravir compared to SOC alone, without any obvious drug-related adverse events in mild COVID-19. Several other ongoing studies of molnupiravir in mild COVID-19 by other Indian pharmaceutical companies such as Aurobindo pharma, Telangana, India (CTRI/2021/07/034588); NATCO pharma, Hyderabad, India (CTRI/2021/05/033693); MSN Laboratories, Hyderabad, India (CTRI/2021/05/033904); BDR Pharmaceuticals, Mumbai, India (CTRI/2021/06/034130); Dr. Reddys Lab, Hyderabad, India (CTRI/2021/06/033938) and Stride Pharma, Bengaluru, India (CTRI/2021/06/034015) are yet to report their results. Notably, all these trials of molnupiravir in mild COVID-19 have been originally planned for a total of 1218–1220 patients, as per the discretion of Drug Controller General of India (DCGI) [26].
Trials of molnupiravir in people with “moderate” COVID-19 (SpO2 ≤ 93% [range 90–93%] on room air) that was conducted by MSN Lab, India (CTRI/2021/05/033864) and Aurobindo Pharma, India (CTRI/2021/08/035424) has been stopped, arguably due to no benefit [27]. Argument of futile results in these trials have been hypothesized to inclusion of sicker patients in Indian studies which would have been otherwise deemed “severe” rather than “moderate” COVID-19, since an SpO2 of <93% is considered “severe” COVID-19 in US based trials, as per the USFDA definition. However, studies of Hetero Labs, India (CTRI/2021/05/033736) and BDR Pharmaceuticals, India (CTRI/2021/06/034220) is still undergoing with molnupiravir vs. SOC in people with moderate COVID-19. Table 1 summarizes published, unpublished (interim results), stopped and ongoing studies with molnupiravir in COVID-19.
Table 1.
A. Published and unpublished (interim results), B. Stopped, and C. Ongoing studies with molnupiravir in COVID-19.
| Study, First author | CTRL/CTRI identifier, Eponyms | N; Types of study | Severity of COVID-19 | Arms (n) | Results | Side effects | Strength/Limitations |
|---|---|---|---|---|---|---|---|
| A. Published and unpublished interim results | |||||||
| Phase 1 studies | |||||||
| Painter et al.14 | NCT04392219 | 130; DBRPC | NA |
|
A range from 50 to 800 mg twice daily dosing for 5.5 days and a single dose up to 1600 mg was found to be safe and well tolerated | A greater proportion of patients in placebo arm had higher adverse events compared to molnupiravir in both single (43.8% vs. 35.4%, respectively) and multiple ascending doses (50.0% vs. 42.9%, respectively) arm. While headache was the most frequently reported adverse event in single ascending doses study (placebo arm had proportionately higher headache compared to molnupiravir [18.8% vs. 12.5%, respectively]), diarrhea was the most frequently reported adverse event noted in multiple ascending dose study (7.1% each in placebo and molnupiravir). | Peer reviewed, Published |
| Khoo et al.15 | NCT04746183, AGILE | 18; R, OL | Mild or moderate |
|
Primary outcome – dose-limiting toxicity | All (4/4, 100%) patients receiving 300 and 600 mg, 1/4 (25%) patients receiving 800 mg, and 5/6 patients (83%) receiving SOC were found to have mild adverse events. This finds highest dose of 800 mg twice daily had a 0.9% probability of having 30% excess toxicity over the controls, and therefore molnupiravir was safe and well tolerated with a plasma concentration within the target range. | Peer reviewed, Published |
| Phase 2 studies | |||||||
| Fischer et al.16 | NCT04405570 | 202; DBRPC | Mild or moderate |
|
Time to clearance (RNA negativity) - the primary outcome - was significantly reduced in the molnupiravir 800 mg arm twice daily compared to the placebo (log-rank p value = 0.013, median: 14 days). Moreover, reduction in time to clearance of viral RNA was also greater and significant when compared to placebo (median: 14 days vs. 27 days; p value = 0.01). | Very few adverse events were noted in this study and was found to be lowest in molnupiravir 800 mg twice daily group. Headache, insomnia and increased levels of alanine aminotransferase (ALT) were the only adverse events reported by more than 4 participants and 5% and 8.1% of molnupiravir and placebo groups, respectively showed grade 3 level of adverse events. Two (2/140, 1.4%) as compared to one (1/62, 1.6%) adverse event led to discontinuation in molnupiravir and placebo groups respectively | Peer reviewed, Published |
| Phase 3 studies | |||||||
| – | NCT04575597, MOVe-OUT18-20 | 775; DBRPC | Mild to moderate with 1 risk factor | Study found 7.3% of patients (28/385) on molnupiravir as compared to 14.1% of patients on placebo (53/377) had either been admitted to hospital or died suggesting a significant reduction in the risk of hospital admission or death by 50% (p = 0.0012) at day 29. No death was reported in the molnupiravir group as compared to 8 deaths in the placebo group on day 29. | The incidence of any adverse events (35% vs. 40% in molnupiravir vs. placebo arm, respectively) and drug-related adverse events (12% vs. 11% in molnupiravir vs. placebo arm, respectively) were found to be similar in both molnupiravir and placebo arm. Fewer people in the molnupiravir arm discontinued treatment as compared to the placebo arm (1.3% vs. 3.4%, respectively). | Limitations: Interim report, not peer reviewed and unpublished. | |
| – | CTRI/2021/05/03373924 | 741; OL, R, PC | Mild |
|
i) Earlier clinical improvement (2-point decrease in WHO Clinical Progression Scale) in molnupiravir vs. SOC at day 5 (63.4% vs. 22.3%, respectively; p < 0.0001), day 10 (79.0% vs 49.5%, respectively; p < 0.0001) and day 14 (81.6% vs. 73.2%, respectively; p = 0.02). ii) Faster time to clinical improvement in molnupiravir vs. SOC (median time 8 days vs. 12 days, respectively; p = 0.0001). iii) Higher RT-PCR negativity (all p < 0.0001) in molnupiravir vs. SOC at day 5 (77.4% vs. 26.1%, respectively), day 10 (94.0% vs. 57.2%, respectively) and day 14 (97.0% vs. 85.2%, respectively). iv) Significantly fewer hospital admissions in molnupiravir vs. SOC alone (1.9% vs. 6.2% respectively; p = 0.003) over 14 days. No death reported in either group. |
Nausea, diarrhea and headache were most common side effect reported – all with mild severity. No one discontinued from the trial. | Limitations: Interim report, not peer reviewed and unpublished. Trial still undergoing. |
| – | CTRI/2021/06/03399225 | 353- FIA, OL, R, OC 403- SIA; OL, R, PC |
Mild |
|
i) The first interim results on 353 patients have shown higher RT-PCR negativity in molnupiravir arm vs. SOC alone (78.3% vs. 48.4%, respectively; p = not reported) on day 5. ii) Second interim results from 403 patients showed – a. significant increase in RT-PCR negativity with molnupiravir vs. SOC alone at day 5 (77.4% vs. 51.5%, p < 0.0001) and day 10 (99.5% vs. 69.5% respectively; p < 0.0001), although no difference noted at day 14 (99.5% vs. 98.5%; p = 0.62). b. Faster clinical improvement (at least one-point improvement from baseline on WHO ordinal scale) was also observed in molnupiravir vs. SOC at both day 5 (79.0% vs. 51.3%, respectively; p < 0.0001) and day 10 (97.8% vs. 82.3% respectively; p < 0.0001). However, no difference was noted at day 14. c. There have been 1 hospitalization in molnupiravir arm compared to 3 in SOC. |
6.5% subjects in molnupiravir arm had adverse events compared to 8.9% in SOC group. Serious adverse events were reported in 1 subject in molnupiravir arm compared to 3 in SOC. | Limitations: Interim report, not peer reviewed and unpublished. Trial still undergoing. |
| B. Stopped phase 3 studies due to futility | |||||||
| – | NCT04575584, MOVe-IN21, 22 | 304; DBRPC | Severe, Hospitalized | NR | Stopped due to futility | – | Limitations: Interim report, not peer review and unpublished. |
| – | CTRI/2021/05/03386427 | NA; OL, R, PC | Moderate | NR | Stopped due to futility | – | Limitations: Interim report, not peer review and unpublished. |
| – | CTRI/2021/08/03542427 | 100; OL, R, PC | Moderate | NR | Stopped due to futility | – | Limitations: Interim report, not peer review and unpublished. |
| B. Ongoing phase 2 and 3 studies | |||||||
| – | NCT04405739, END-COVID17 | 96; DBRPC | Mild to moderate but hospitalized |
|
Assessing virological clearance. | Being assessed | – |
| – | NCT04939428, MOVe-AHEAD23 | 1332; DBRPC | Non-COVID |
|
Assessing prevention with molnupiravir as a post-exposure prophylaxis. | Being assessed | First prevention trial |
| – | CTRI/2021/05/03373626 | 1282, OL, R, PC | Moderate |
|
Assessing proportion of clinical improvement at day 14 as a primary outcome. Secondary outcome assessment includes proportion of clinical improvement at day 28, mortality rate at day 28; viral negativity at day 10, day 14 and day 28. | Being assessed | – |
| – | CTRI/2021/06/03422026 | 1282, OL, R, PC | Moderate |
|
Assessing proportion of clinical improvement at day 14 as a primary outcome. Secondary outcome assessment includes proportion and time to clinical improvement at day 28, mortality rate at day 28; viral load at day 10, day 15 and day 28. | Being assessed | – |
| – | CTRI/2021/07/03458826 | 1220; OL, R, PC | Mild |
|
Assessment of rate of hospitalization# from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; and proportion of clinical improvement at day 10, day 14 and day 28. | Being assessed | – |
| – | CTRI/2021/05/03369326 | 1218; OL, R, PC | Mild |
|
Assessment of rate of hospitalization# from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; mortality rate at day 14; RT-PCR negativity at day 10 and day 15; time to clinical improvement at day 14; and proportion of clinical improvement at day 10, day 14 and day 28. | Being assessed | – |
| – | CTRI/2021/05/03390426 | 1218; OL, R, PC | Mild |
|
Assessment of rate of hospitalization# from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; proportion of clinical improvement at day 10, day 14 and day 28; time to clinical improvement up to day 14; and change in viral load up to EOT. | Being assessed | – |
| – | CTRI/2021/06/03413026 | 1218; OL, R, PC | Mild |
|
Assessment of rate of hospitalizationb from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; proportion of clinical improvement at day 10, day 14 and day 28; time to clinical improvement up to day 14; and mortality rate at day 14 and day 28. | Being assessed | – |
| – | CTRI/2021/06/03393826 | 1218; OL, R, PC | Mild |
|
Assessment of rate of hospitalization# from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; proportion of clinical improvement at day 10, day 14 and day 28; time to clinical improvement up to day 14; rate of viral negativity at day 10, day 15 and day 28; and mortality rate at day 14 and day 28. | Being assessed | – |
| – | CTRI/2021/06/03401526 | 1220; OL, R, PC | Mild |
|
Assessment of rate of hospitalization# from randomization up to day 14 as a primary outcome. Secondary outcome assessment includes rate of hospitalization up to day 28; proportion of clinical improvement at day 10, day 14 and day 28; time to clinical improvement up to day 14; and mortality rate at day 14 and day 28 | Being assessed | – |
Completed the trial.
Hospitalization is defined as hospital admission for >24 h with RR ≥ 24/minute and SpO2 ≤ 93% in room air requiring O2 supplementation; DBRPC: Double-blind, randomized, placebo-controlled; OL: Open-label; R: Randomized; PC; Placebo-controlled; SOC: Standard of care; MOLNU: Molnupiravir; PBO; Placebo; RT-PCR: Reverse transcriptase polymerase chain reaction; NA: Not applicable/available; NR; Not retrievable; BID: Twice daily; FIA: First interim analysis; SIA: Second interim analysis.
4. Discussion
Summarily, molnupiravir is a broad spectrum, directly acting oral antiviral agent that acts on the RdRp enzyme and by competing with uridine and cytidine triphosphate substrates finally leads to incorporation of A and G forming stable complexes in the active RdRp center leading to mutagenesis, escaping proof reading. Molnupiravir aims to stop viral replication by its 2-step mutagenesis model and “error catastrophe” mechanisms. In other words, it mutates the virus to kill itself. Molnupiravir is required to be given only for a short term (5 days) via oral route, easier to be administered in outpatient department and hence has better compliance. The advantage for a drug like molnupiravir is that it can be produced at a larger scale and doesn't require cold transportation, nor it requires in-hospital settings for its administration, unlike other EUA approved drugs for COVID-19. Available data so far suggests that molnupiravir has been well tolerated and found to be safe without any major adverse events in phase 1, 2 and 3 clinical trials, at least in short-terms. Given in mild to moderate (SpO2 >93% on room air as per US-based definition) COVID-19 within ≤5 days of symptoms, it can drastically decrease the disease progression by reducing hospitalization and/or death as observed in the interim report of MOVe-OUT study. These emerging findings may hint that molnupiravir may prove to be a global game changer in the battle against SARS-CoV-2. As an oral antiviral drug, it would be an incredible asset.
However, it is still premature to predict whether it would really work as Thor's hammer – as the name suggests – given the minimal evidence available at the moment, and majorly the futility of molnupiravir in moderate (SpO2 90–93% on room air) to severe COVID-19. Importantly, since absolute risks were reduced to 7.3% from 14.1% in placebo arm, number needed to treat (NNT) to prevent one hospitalization or death is 14.7. This means on an average; 15 patients would have to receive molnupiravir treatment instead of placebo for one additional patient of not needing hospitalization or to die. This also suggests that the drug needs to be very safe and affordable for widespread use. Second, molnupiravir has to be used too early (≤5 days from symptom onset) before someone is deemed ill enough when it won't work. Issue remains with asymptomatic individuals and how early to start their treatment. Third, we still lack any study that is evaluating the role of molnupiravir in breakthrough infections following vaccination especially since many of the counties will soon have a reasonably good proportion of vaccinated people. Fourth, further studies need to be done to establish its interference with available vaccines. From the safety perspective, theoretically, while its mutagenic potential on one hand is a boon for reducing the viral RNA, it might turn into a bane by inducing mutations in the virus further leading to an increased resistance. At least one study [9] in animal cell cultures found mutations in cells treated with molnupiravir and recommends to assess mutagenic potential and potential genotoxic side effects in vivo, focusing on rapidly dividing cells. Skepticism about safety still remains given the fact that the participants in the trial had to maintain abstinence or use contraception for the fear of birth defect if they conceived. Although this appears to be highly unlikely with the short-term use of 5 days. Moreover, since the mutations occur randomly it's difficult for the virus to evolve resistance. Furthermore, Merck has conducted a comprehensive nonclinical program Big Blue and PIG-a which were designed to provide a robust measure of a drug or chemical's ability to induce mutations in vivo and found molnupiravir neither mutagenic nor genotoxic in in vivo mammalian systems [22]. In any case, USFDA should not have given permission to conduct human trials had they suspected any ounce of doubt. Finally, cost effectiveness and accessibility for all is a major issue that needs to be dealt with and effect of molnupiravir with other therapies for COVID-19 needs to be examined. The major limitation of this systematic review is absence of even a single peer-reviewed published phase 3 study at the moment-precluding any conclusion. However, jury is still out to call molnupiravir a “magic” pill. We would be curiously waiting for the complete results from the 10 ongoing studies in India (8 studies in mild COVID-19, 2 studies in moderate COVID-19) and much awaited international prevention trial of molnupiravir - MOVe-AHEAD. The caveat for Indian studies lies with the fact that all of them are open-label though randomized.
Funding
No funding.
Authorship
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship and take responsibility for the integrity of the work. They confirm that this paper will not be published elsewhere in the same form, in English or in any other language, including electronically.
Author's contribution
AKS conceptualized, searched the literature; AS and RS wrote the first draft; AKS, AS, RS and AM edited the final draft. All authors agreed mutually to submit for publication.
Declaration of competing interest
Nothing to declare for all authors.
References
- 1.Cannalire R, Cerchia C, Beccari AR, et al. Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: state of the art and future opportunities. J Med Chem. DOI: 10.1021/acs.jmedchem.0c01140. [DOI] [PMC free article] [PubMed]
- 2.Subissi L., Posthuma C.C., Collet A. One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities. Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):E3900–E3909. doi: 10.1073/pnas.1323705111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crotty S., Cameron C.E., Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci USA. 2001;98:6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agostini M.L., et al. Small-molecule antiviral β-d-N4-hydroxycytidine inhibits a proofreading-intact coronavirus with a high genetic barrier to resistance. J Virol. 2019;93:24. doi: 10.1128/JVI.01348-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toots M., Yoon J.-J., Hart M., Natchus M.G., Painter G.R., Plemper R.K. Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model. Transl Res. 2020;218:16–28. doi: 10.1016/j.trsl.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheahan T.P., Sims A.C., Zhou S., et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020 Apr 29;12(541) doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabinger F., Stiller C., Schmitzová J., et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021 Sep;28(9):740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon C.J., Tchesnokov E.P., Schinazi R.F., Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J Biol Chem. 2021 Jul;297(1):100770. doi: 10.1016/j.jbc.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S., Hill C.S., Sarkar S., et al. β-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells. J Infect Dis. 2021 Aug 2;224(3):415–419. doi: 10.1093/infdis/jiab247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon J.J., et al. Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.00766-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahl A., Gralinski L.E., Johnson C.E., et al. SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801. Nature. 2021 Mar;591(7850):451–457. doi: 10.1038/s41586-021-03312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox R.M., Wolf J.D., Plemper R.K. Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat Microbiol. 2021 Jan;6(1):11–18. doi: 10.1038/s41564-020-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenke K., Hansen F., Schwarz B., et al. Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model. Nat Commun. 2021;12:2295. doi: 10.1038/s41467-021-22580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Painter W.P., Holman W., Bush J.A., et al. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob Agents Chemother. 2021 Mar 1;65(5) doi: 10.1128/AAC.02428-20. e02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoo S.H., Fitzgerald R., Fletcher T., et al. Optimal dose and safety of molnupiravir in patients with early SARS-CoV-2: a Phase I, open-label, dose-escalating, randomized controlled study. J Antimicrob Chemother. 2021 Aug 27 doi: 10.1093/jac/dkab318. [Online ahead of print] dkab318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer W., Eron J.J., Holman W., et al. Molnupiravir, an oral antiviral treatment for COVID-19. medRxiv. 2021 Jun 17 doi: 10.1101/2021.06.17.21258639. Preprint. 2021.06.17.21258639. [DOI] [Google Scholar]
- 17.The safety of molnupiravir (EIDD-2801) and its effect on viral shedding of SARS-CoV-2 (END-COVID) - full text view - ClinicalTrials.gov (Last accessed on October 20, 2021).
- 18.Efficacy and safety of molnupiravir (MK-4482) in non-hospitalized adult participants with COVID-19 (MK-4482-002) - full text view - ClinicalTrials.gov. (Last accessed on October 20, 2021).
- 19.Mahase E. Covid-19: molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ. 2021 Oct 4;375:n2422. doi: 10.1136/bmj.n2422. [DOI] [PubMed] [Google Scholar]
- 20.Merck and Ridgeback's investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate covid-19 in positive interim analysis of phase 3 study. Oct 2021. https://www.merck.com/news/merckand-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-riskof-hospitalization-or-death-by-approximately-50-percent-compared-toplacebo-for-patients-with-mild-or-moderat [Google Scholar]
- 21.Efficacy and safety of molnupiravir (MK-4482) in hospitalized adult participants with COVID-19 (MK-4482-001) - full text view - ClinicalTrials.gov. (Last accessed on October 20, 2021).
- 22.Merck and Ridgeback biotherapeutics provide update on progress of clinical development program for molnupiravir, an investigational oral therapeutic for the treatment of mild-to-moderate COVID-19 - Merck.com. (Last accessed on October 20, 2021).
- 23.Study of MK-4482 for prevention of coronavirus disease 2019 (COVID-19) in adults (MK-4482-013) - full text view - ClinicalTrials.gov (Last accessed on October 20, 2021).
- 24.Press_Release_Molnupiravir_Interim_Clinical_Results_Final_090721.pdf (heteroworld.com). (Last accessed on October 20, 2021).
- 25.Optimus announces interim clinical results from phase III clinical trials of molnupiravir conducted in India – ThePrint. (Last accessed on October 20, 2021).
- 26.Search result, clinical trials registry - India (CTRI). CTRI (Last accessed on October 20, 2021).
- 27.Two Indian drugmakers to end trials of generic Merck pill for moderate COVID-19 | Reuters. (Last accessed on October 20, 2021).