Abstract
Previous studies indicate that IL-17A plays an important role in mediating the intestinal microbiota and systemic metabolic functions. However, it is not known where IL-17RA signaling occurs to mediate these effects. To investigate this question, we used intestinal epithelial-specific (Il17raΔIEC) and liver-specific (Il17raΔLiver) IL-17RA knockout mice as well as littermate control mice. Our results indicate that intestinal IL-17RA signaling helps mediate systemic metabolic functions upon exposure to prolonged high-fat diet. Il17raΔIEC mice display impaired glucose metabolism, altered hormone and adipokine levels, increased visceral adiposity, and greater hepatic lipid deposition when compared with their littermate controls. We show that IL-17RA-driven changes in microbiota composition are responsible for regulating systemic glucose metabolism. Altogether, our data elucidate the importance of intestinal IL-17RA signaling in regulating high-fat diet–mediated systemic glucose and lipid metabolism.
Introduction
During recent years, the global prevalence of obesity has increased (1). Various factors, including diet, can influence obesity (1). Long-term consumption of a high-fat diet (HFD) can result in complications, including nonalcoholic fatty liver disease, by altering key metabolic tissues (2, 3). Additionally, pro-inflammatory Th17 cells and cytokines are induced under conditions that promote obesity and may mediate these disorders (4).
IL-17A is predominantly secreted from Th17, ILC3, and γδT cells and exerts protective and inflammatory functions (5). IL-17A signals via its receptor (IL-17RA/RC complex), which is expressed on a range of cells including enterocytes, hepatocytes, and adipocytes (5). Signaling by IL-17A to liver and adipose tissues is linked with steatosis, insulin resistance, and impaired glucose metabolism (2, 6).
Intestinal microbiota composition mediates host metabolic functions and weight gain (1, 3). An impaired microbiota can promote glucose intolerance and nonalcoholic fatty liver disease, and this phenotype was transmissible upon cohousing mice (7). Segmented filamentous bacteria (SFB) regulate Th17 polarization and IL-17A responses (8, 9). Furthermore, mice that specifically lack intestinal IL-17RA display impaired expression of genes that regulate the microbiota and increased colonization by SFB (10). SFB depletion is also associated with decreased IL-17A and protection from obesity-induced liver damage (11).
HFD-treated RORγt+/−, Il17−/−, and Il17ra−/− mice display greater hepatic steatosis and impaired (or similar) glucose tolerance when compared with wild-type controls (6, 11, 12). However, studies using anti–IL17A demonstrate that IL-17A may increase levels of inflammatory cytokines and aid the progression of hepatic steatosis (13). Because previous studies have solely used global cytokine or receptor knockout mice or generalized Ab–mediated cytokine neutralization, it is unclear where IL-17RA signaling occurs to mediate these effects. To address this, we used intestinal epithelium-specific and liver-specific IL-17RA knockout and littermate control mice. We demonstrate that intestinal IL-17RA signaling is specifically important for regulating systemic glucose and lipid metabolism.
Materials and Methods
Mice:
C57BL/6J, Villin-Cre (C57BL/6J background), and Albumin-Cre (C57BL/6J background) mice were purchased from The Jackson Laboratory. Generation and characterization of IL-17-floxed (Il17ra1fl/fl;Villin-cre) mice were performed as described (10). Il17ra1fl/fl;Albumin-cre mice were obtained from Dr. Jay Kolls. We placed 6-wk-old male mice on HFD (Bio Serve). All knockout and control mice were cohoused during this 6-wk period. All mice were separated according to genotype, and their weights were measured each week. Mice were housed in specific pathogen-free conditions at Stony Brook University. Studies were conducted with the approval of Stony Brook University Institutional Animal Care and Use Committee.
Glucose tolerance test:
Glucose tolerance tests (GTTs) were performed after 16 wk of HFD. Mice were placed in clean cages, weighed, and fasted for 5 h. An initial glucose reading (0 min) was obtained from a blood sample isolated from the tail. Blood glucose was measured using a Countour next (Bayer) monitor. Mice were intraperitonially injected with a body weight–specific volume (equivalent to 50% body weight) of glucose (200 ng/mL). Blood glucose was obtained 30, 60, 90, and 120 min post injection.
Cell line:
HepG2 cells were grown in 24-well plates and treated with pure media or media supplemented with 100 ng/mL IL-22 fusion protein (IL-22.Fc; Evive Biotech) or 100 ng/mL recombinant human leptin (rhLeptin; PeproTech) when growth reached 70% confluency. Cells were lysed in RLT buffer after 28 h, and RNA was isolated for RT-PCR analysis using a QIAGEN RNeasy Micro kit.
IL-22.Fc treatment:
C57BL/6J mice were i.p. injected with pure PBS or 80 μg IL-22.Fc (in PBS). Mice were euthanized after 24 h. Liver tissues were collected for RT-PCR analysis.
Antibiotics treatment:
An antibiotic mixture containing vancomycin (0.5 g/L), ampicillin (0.5 g/L), neomycin (1 g/L), and metronidazole (1 g/L) was used (14). Normal drinking water was replaced with the antibiotics starting on week 12 of HFD. Fresh antibiotics were provided every 2 d during the remaining 4 wk.
RT-PCR:
Trizol-based RNA isolation was performed for tissues. Transcription of RNA to cDNA was performed using Bio-Rad iScript. RT-PCR was performed using Bio-Rad SsoAdvanced Supermix or SYBR Green. Primer probes were used from Applied Biosystem and Integrated DNA Technologies (Supplemental Table 1).
Bacterial DNA was isolated from feces for RT-PCR and 16S rRNA sequencing. DNA was isolated using QiAMP stool DNA extraction kit. Bacterial abundance was examined using primer probes from Integrated DNA Technologies (Supplemental Table 1). The 16S rRNA sequencing was performed as described (15). The 16S rRNA data has been uploaded to the SRA database (PRJNA760519).
H&E staining:
Tissues were fixed in formalin. Paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene and rehydrated with an ethanol wash. Tissues were stained with Mayer’s hematoxylin solution (VWR International) for 2 min and washed under running tap water. A 30 s differentiation with 95% ethanol was performed. Samples were stained with eosin (VWR International) for 2 min. Slides were dehydrated in ethanol, cleared with xylene, and mounted.
Oil Red O staining:
Liver tissue was frozen in OCT compound medium. Blocks were sectioned (5 μm) and sections were dried at room temperature and stained with Oil Red O (ORO) Isopropanol Solution (Electron Microscopy Sciences) solution (3:2 ratio of ORO to H2O). Slides were washed with running tap water for 30 min, dried, and mounted. Images were acquired at 40X magnification. ImageJ was used to evaluate percent area coverage of tissue by staining.
Luminex multiplex assay:
Serum samples were collected from mice and analyzed via multiplex analysis (Luminex). The Mouse Metabolic Hormone Panel (MilliporeSigma) and Mouse Th17 Panel (MilliporeSigma) were used. Samples were run at the Stony Brook School of Medicine Genomics Core (Bio-Plex 200) and Vanderbilt University Hormone Assay and Analytical Services Core (MagPix).
Results and Discussion
Intestinal IL-17RA signaling regulates systemic glucose metabolism and tissue physiology
Because the intestine is a major site for upregulating the uptake of nutrients and mediating metabolic processes, we analyzed if intestinal IL-17RA signaling specifically influences HFD-induced disorders (16). To do so, we used Il17raΔIEC and Il17rafl/fl (littermate control) mice. Knockdown of colonic Il17ra was confirmed (Fig. 1A). Il17raΔIEC and Il17rafl/fl mice display no differences in weight gain upon HFD treatment (Fig. 1B). However, Il17raΔIEC mice display impaired glucose metabolism (Fig. 1C). Because glucose metabolism was impaired, we investigated if systemic changes could be observed in the white adipose tissue and liver. Interestingly, an increase in relative epididymal white adipose tissue (eWAT) weight was observed in Il17raΔIEC mice (Fig. 1D). We also examined transcript levels of lipid metabolism and inflammation genes of the eWAT but did not observe any differences (Supplemental Fig. 1A). H&E staining of the liver revealed greater lipid accumulation in Il17raΔIEC mice, and this was confirmed by ORO staining (Fig. 1E, 1F). Upon analyzing the expression of key inflammatory and metabolic genes in the liver, Il17raΔIEC mice displayed increased expression of Fasn, which aids in the synthesis of lipids (Supplemental Fig. 1B). Increased expression of Fasn has been observed in livers that develop steatosis, and inhibition of hepatic FASN decreases hepatic triglyceride levels and improves glucose metabolism (17, 18). Collectively, our data suggest that intestinal IL-17RA signaling may regulate systemic glucose levels and adiposity.
Figure 1.
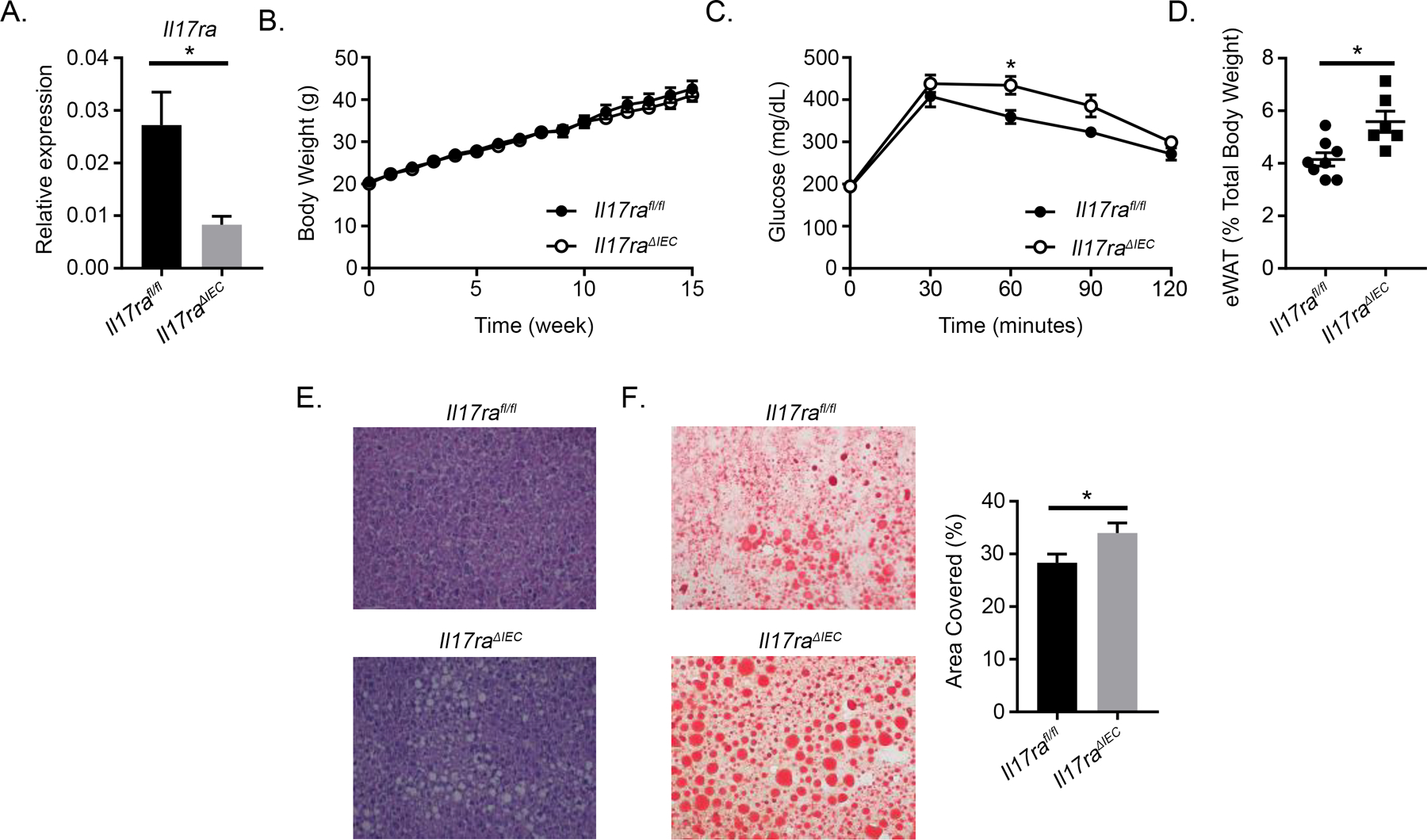
Intestinal IL-17RA mediates systemic glucose and lipid metabolism. A) RT-PCR validating knockdown of Il17ra in the distal colon (n = 6 mice per group). B) Weight gain of Il17ra1fl/fl (n = 10) and Il17raΔIEC (n = 7) mice fed HFD for 16 wk. C) GTT of HFD-fed Il17rafl/fl (n = 8) and Il17raΔIEC (n = 6) mice. D) Relative weight of eWAT after HFD. E) H&E staining of liver tissues from HFD-fed Il17rafl/fl (n = 8) and Il17raΔIEC (n = 6) mice. F) ORO staining (left) and quantification (right) of liver tissues from HFD-fed Il17rafl/fl and Il17raΔIEC mice. Data are obtained from at least two separate experiments. Error bars depict the mean ± SEM. *p < 0.05, Mann–Whitney U test and two-tailed, two-way ANOVA.
Hepatic IL-17RA signaling is not essential in regulating glucose metabolism
HFD and IL-17A promote lipid accumulation and inhibit insulin signaling in the liver, and hepatic glucose use is closely related to intestinal function (2, 11). Therefore, we examined the effects of HFD treatment on Il17raΔLiver and Il17rafl/fl mice. Knockdown of hepatic Il17ra was confirmed (Fig. 2A). HFD did not affect weight gain between Il17raΔLiver and Il17rafl/fl mice (Fig. 2B). Likewise, no differences in glucose clearance and eWAT weight were observed (Fig. 2C, 2D). Although hepatocyte ballooning was visualized via H&E staining, no differences in histological features were observed between either group (Fig. 2E). Altogether, our data suggest that hepatic IL-17RA signaling does not directly regulate systemic glucose metabolism or adiposity. Therefore, IL-17RA signaling in other sites such as the intestine may be more relevant.
Figure 2.
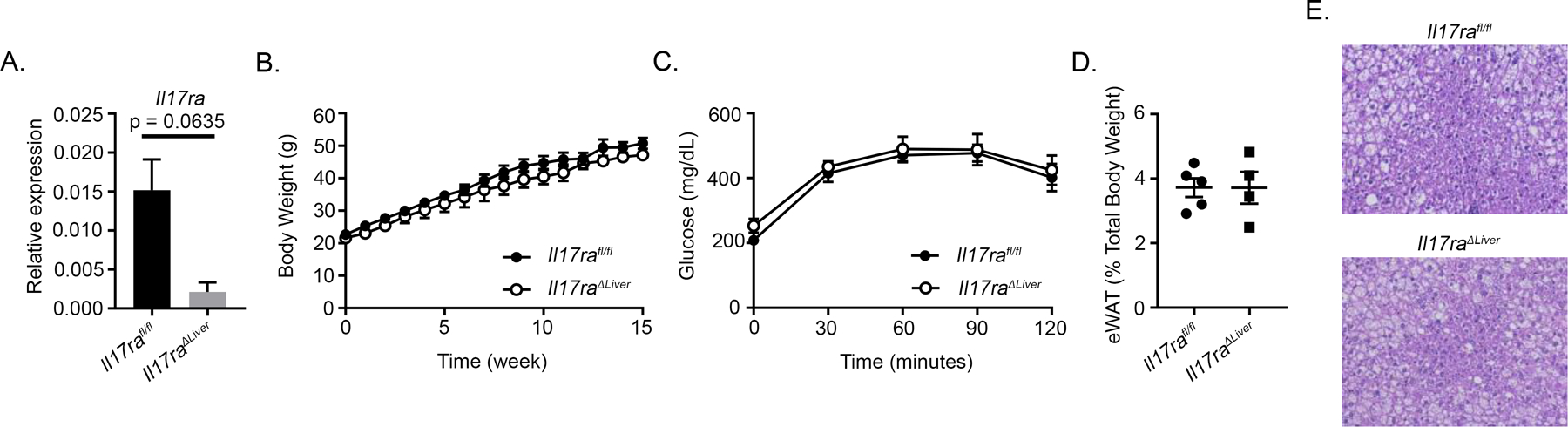
IL-17RA signaling in the liver does not mediate systemic glucose metabolism or adiposity. A) RT-PCR validating knockdown of Il17ra in the liver (n = 4–5 mice per group). B) Weight gain of HFD-fed Il17ra1fl/fl (n = 5) and Il17raΔLiver (n = 4) mice. C) GTT of Il17ra1fl/fl (n = 5) and Il17raΔLiver (n = 4) mice fed HFD for 16 wk. D) Relative weight of eWAT after mice were fed HFD for 16 wk. E) Representative H&E staining of liver tissues from Il17ra1fl/fl (n = 5) and Il17raΔLiver (n = 4) mice fed HFD for 16 wk. All data are obtained from two separate experiments. Error bars depict the mean ± SEM. Mann–Whitney U test and two-tailed, two-way ANOVA.
Altered hormone and cytokine levels are present in Il17raΔIEC mice
To characterize what factors may be promoting impaired glucose clearance in Il17raΔIEC mice, Luminex analysis was performed on serum from HFD-fed Il17raΔIEC and Il17rafl/fl mice to analyze the levels of relevant hormones. We analyzed circulating levels of connecting-peptide (c-peptide), a short amino acid sequence that maintains the proinsulin molecule, and insulin. Compared to their littermate controls, Il17raΔIEC mice displayed a trend toward having decreased levels of c-peptide and displayed significantly decreased levels of insulin (Fig. 3A) which coincides with their impaired glucose clearance. We also analyzed the levels of adipokines that are indicative of metabolic disorder. Differences in the levels of resistin were not observed, but Il17raΔIEC mice displayed decreased leptin (Fig. 3B). Finally, Luminex analysis was used to assess levels of circulating cytokines. Levels of IL-17A were below the minimum detectable range (data not shown). However, we observed increased levels of IL-22, another Th17 cytokine, in Il17raΔIEC mice (Fig. 3C). Injection of IL-22 induces hepatoprotective effects, including protecting against fatty liver (19, 20). Although leptin traditionally inhibits insulin production, IL-22 has been shown to increase insulin sensitivity, which may explain why Il17raΔIEC mice display decreased serum insulin despite displaying reduced leptin levels (20, 21). Additionally, IL-22 and leptin both activate STAT3 and initiate similar effects such as promoting wound healing, the release of antimicrobial proteins, and the secretion of mucus (22). Therefore, enhanced levels of IL-22 may be produced by Il17raΔIEC mice to compensate for lower levels of leptin. To test whether IL-22 or leptin mediate the elevated hepatic expression of Fasn in Il17raΔIEC mice, we stimulated HepG2 cells with IL-22.Fc or rhLeptin. However, we observed that neither stimulation influenced Fasn expression (Supplemental Fig. 1C). Likewise, although injection of mice with 80 μg of IL-22.Fc increased the expression of Saa1/2, an IL-22 inducible inflammatory gene, it did not influence hepatic Fasn expression (Supplemental Fig. 1D). Therefore, levels of IL-22 alone may not be sufficient to mediate Fasn expression, and additional factors during HFD may help mediate these effects. Altogether, our data suggest that intestinal IL-17RA signaling influences systemic hormone and cytokine levels.
Figure 3.
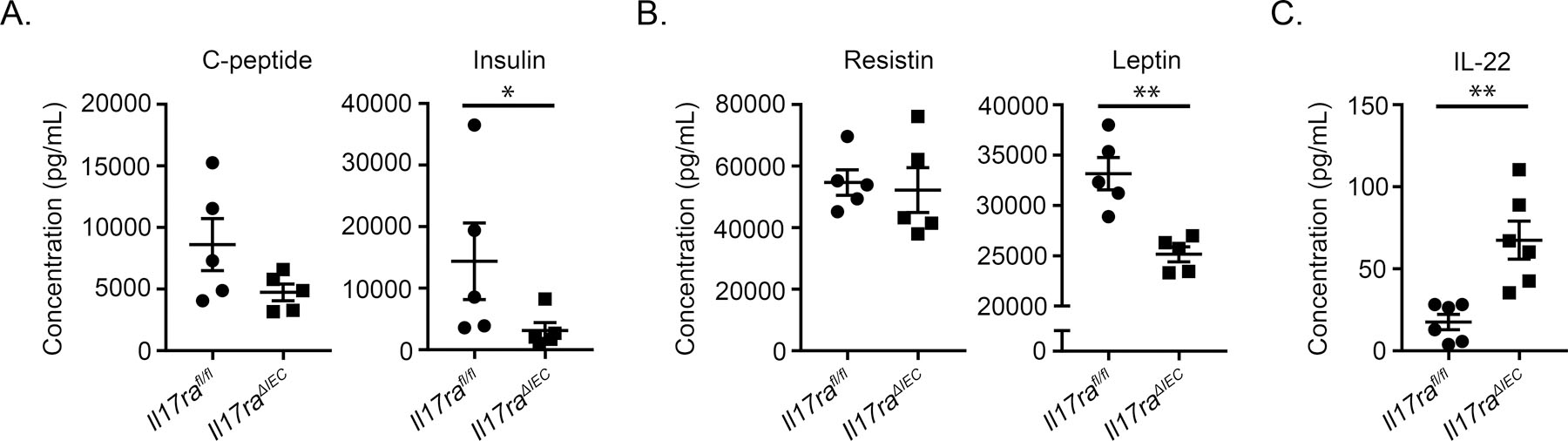
Intestinal IL-17A signaling effects systemic hormone and cytokine levels. A) Levels of serum c-peptide and insulin assessed via Luminex analysis. B) Levels of serum resistin and leptin assessed via Luminex analysis. C) Level of serum IL-22 assessed via Luminex analysis. All data are obtained from two separate experiments. Error bars depict the mean ± SEM. *p < 0.05, **p < 0.01, Mann Whitney U test.
HFD-induced microbiota changes partially drive metabolic function
Obese individuals possess a microbiota that promotes impaired glucose metabolism and increased insulin resistance, and certain commensal bacteria and bacterial metabolites may increase insulin sensitivity (23). The intestinal microbiota has also been shown to influence adipokine levels. Germ-free mice exhibit decreased serum levels of leptin than conventionalized mice when fed a chow diet, but it has also been shown that mice treated with prebiotics and fed a HFD display greater leptin sensitivity than their nonprebiotic-treated counterparts (24, 25). Therefore, we investigated whether the microbiota regulates metabolic functions of Il17raΔIEC mice.
Il17raΔIEC and Il17ra−/− mice possess increased levels of SFB, which exacerbate obesity-induced liver damage (10, 11). As expected, increased levels of SFB were detected in fecal samples of Il17raΔIEC mice before HFD treatment; interestingly, SFB levels were substantially reduced (and no longer significantly different) in both groups after prolonged HFD treatment (Fig. 4A). Because SFB have been shown to induce Th17 responses, including levels of IL-17A, this decreased level of SFB after HFD treatment may explain why serum levels of IL-17A were below the minimum levels of detection (8–10). Although 16S rRNA sequencing of fecal bacteria DNA did not reveal major phyla-level changes in microbiota composition (Supplemental Fig. 2A), family-level changes including significantly decreased levels of Sutterellaceae and Erysipelotrichaceae were observed in Il17raΔIEC mice (Fig. 4B). We also observed species-level changes in Il17raΔIEC mice, including elevated levels of Bacteroides uniformis (Supplemental Fig. 2B) which has been associated with decreased serum insulin and leptin after HFD (26).
Figure 4.
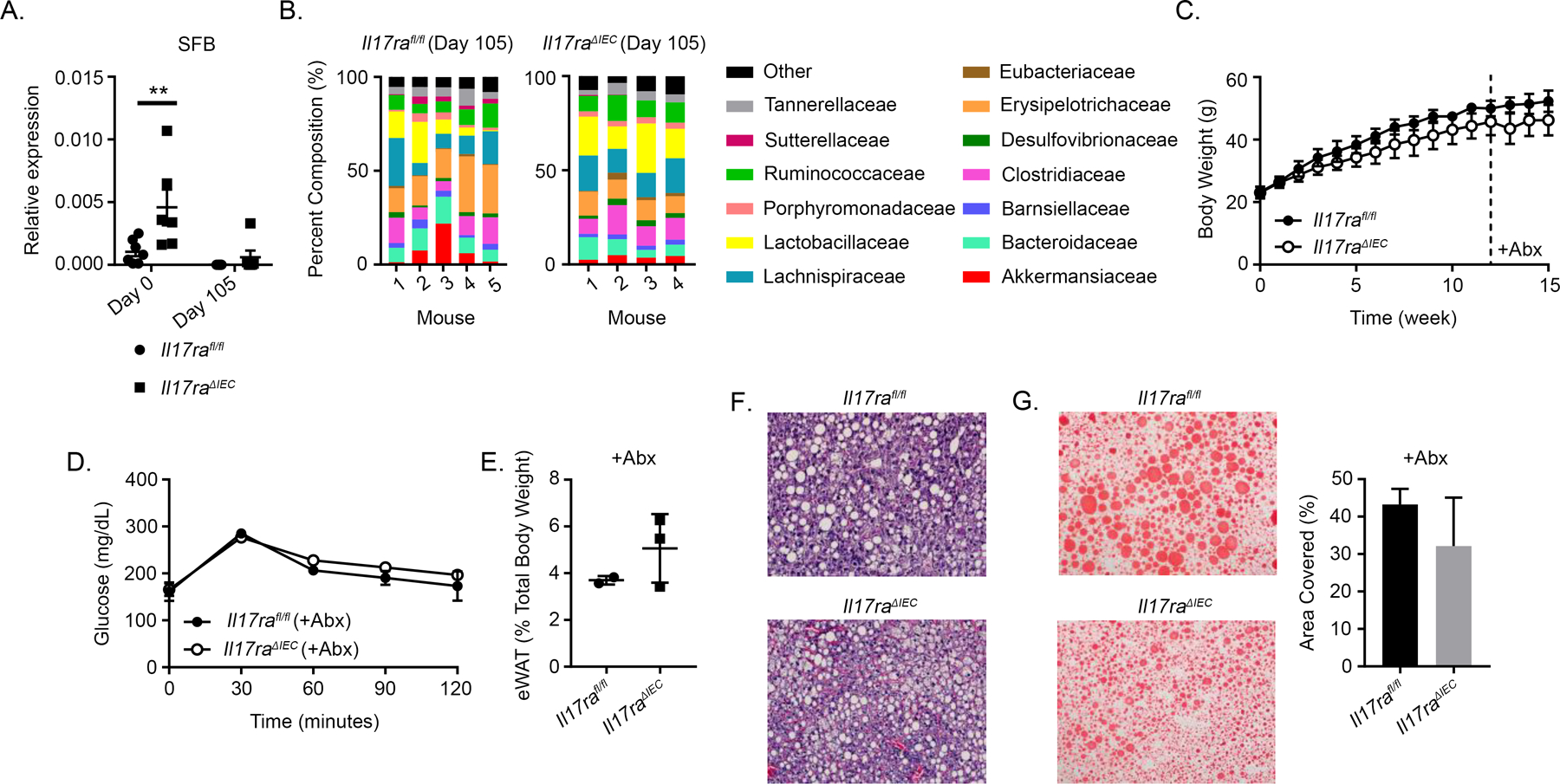
Intestinal IL-17RA signaling mediates microbiota-dependent changes in glucose metabolism. A) RT-PCR evaluation of fecal SFB levels before (day 0) and after (day 105) HFD treatment. B) Family-level 16S rRNA sequencing data collected from fecal samples. C) Weight gain of Il17rafl/fl (n = 2) and Il17raΔIEC (n = 3) mice treated with an antibiotics mixture starting week 12 of HFD. D) GTT of Il17ra1fl/fl (n = 2) and Il17raΔIEC (n = 3) mice fed HFD for 16 wk and treated with an antibiotic mixture starting on week 12. E) Relative weight of eWAT after mice were fed HFD. F) H&E staining of liver tissues from HFD- and antibiotics-treated Il17rafl/fl (n = 2) and Il17raΔIEC (n = 3) mice. G) ORO staining (left) and quantification (right) of liver tissues from HFD- and antibiotics-treated Il17rafl/fl (n = 2) and Il17raΔIEC (n = 3) mice. Data from A and B are obtained from two separate experiments. For graph A, error bars depict the mean ± SEM. For graphs C, D, E, and G (right), error bars depict the mean ± SD. **p < 0.01, Mann Whitney U test and two-tailed, two-way ANOVA.
Intestinal IL-17RA signaling regulates the microbiota by altering the expression of antimicrobial genes (10). Likewise, HFD and microbial dysbiosis have been associated with impaired intestinal barrier integrity to promote the dissemination of microbial factors that influence systemic metabolism (3). Because we detected no differences in the expression of colonic intestinal barrier or antimicrobial genes (Supplemental Fig. 2C, 2D), we analyzed if ileal IL-17RA signaling regulates gene expression after HFD. After HFD feeding for 1 wk, Il17raΔIEC mice did not display differences in ileal tight junction or Reg3-associated antimicrobial gene expression (Supplemental Fig. 2E, 2F). However, we observed decreased expression of Nox1 (produces hydrogen peroxide) and a trend towards reduced Pigr (transcytosis of secretory IgA) which have been shown to regulate the microbiota (Supplemental Fig. 2F). This coincides with data we previously published regarding naive chow diet fed Il17raΔIEC mice (10). Ileal tissues from Il17raΔIEC mice also displayed significantly decreased expression of Saa1/2 (Supplemental Fig. 2E). Although we previously observed reduced SFB colonization in the terminal ileum of naive Il17raΔIEC mice, no difference in ileal SFB levels were observed after 1 wk of HFD (Supplemental Fig. 2G). Thus, ileal IL-17RA signaling may play a greater role in regulating microbiota changes.
To characterize if IL-17A-driven microbiota changes influence systemic glucose metabolism, we placed Il17raΔIEC and Il17rafl/fl mice on HFD and treated them with an antibiotics mixture for 4 wk to deplete their microbiota. Depletion was confirmed by RT-PCR of bacterial DNA from feces (data not shown). No significant differences in weight gain were observed (Fig. 4C). As expected, microbiota depletion resulted in an overall reduction of blood glucose values. Il17raΔIEC and Il17rafl/fl mice had comparable levels of glucose (Fig. 4D). Therefore, IL-17RA signaling-driven changes in microbiota composition mediate glucose metabolism. Microbiota depletion did not greatly diminish the previously observed difference in eWAT weight, and hepatic histology did not noticeably differ between groups (Fig. 4E, 4F). ORO analysis revealed slightly less lipid droplet accumulation in the livers of antibiotics-treated Il17ra∆IEC mice (Fig. 4G). We also analyzed liver tissues for changes in metabolic genes; interestingly, while elevated expression of Fasn was present in the livers of nonantibiotics-treated Il17ra∆IEC mice, this trend was not observed after antibiotics treatment (Supplemental Fig. 3A). We also observed that the difference in serum insulin levels was eliminated between mice after antibiotics treatment, but consistent with the non-antibiotics treated group we observed a trend for decreased leptin in the antibiotics treated Il17raΔIEC mice (Supplemental Fig. 3B). This indicates that IL-17RA signaling-driven microbiota changes may also mediate systemic lipid metabolism and hormone levels.
Overall, our study elucidates the relevance of intestinal epithelial-specific IL-17RA signaling in mediating systemic metabolism via regulation of the microbiota. Furthermore, cytokines besides IL-17A may act in the intestine to regulate metabolism.
Supplementary Material
Key Points.
Intestinal IL-17RA signaling mediates systemic glucose and lipid metabolism
Hepatic IL-17RA signaling does not regulate systemic glucose metabolism
IL-17RA-driven microbiota changes influence systemic glucose metabolism
Acknowledgments
Tissue processing and embedding were performed by the Research Histology Core Laboratory at Stony Brook University. Luminex assays were conducted in part by the Stony Brook School of Medicine Genomics Core and Vanderbilt University Medical Center Hormone Assay & Analytical Services Core. The 16S rRNA analysis was conducted by MR DNA.
PK is supported by the Crohn’s and Colitis Foundation (476637), NIH R01 DK121798-01, NIH R21 AI146696, NIH R21 AI149257 and SUNY Research Foundation. SG is supported by the NIH NRSA T32 Institutional Research Training Grant (5T32AI007539-22).
Abbreviations
- c-peptide
Connecting peptide
- eWAT
Epididymal White Adipose Tissue
- GTT
Glucose Tolerance Test
- HFD
High-Fat Diet
- ORO
Oil Red O
- SFB
Segmented Filamentous Bacteria
- Abx
Antibiotics cocktail
Footnotes
Conflicts of Interest Statement
The authors have no financial conflicts of interest.
References
- 1.Pigeyre M, Yazdi FT, Kaur Y, and Meyre D 2016. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci 130: 943–986. [DOI] [PubMed] [Google Scholar]
- 2.Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, Han X, Peng Y, Chen X, Shen L, Qiu D, Li Z, and Ma X 2011. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease: IL-17 and non-alcoholic steatohepatitis. Clin. Exp. Immunol 166: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, and Burcelin R 2008. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- 4.Chehimi M, Vidal H, and Eljaafari A 2017. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med 6: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SH, and Dong C 2011. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell. Signal 23: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zúñiga LA, Shen W-J, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, and Butcher EC 2010. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol 185: 6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez J-P, Shulman GI, Gordon JI, Hoffman HM, and Flavell RA 2012. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, and Littman DR 2009. Induction of Intestinal Th17 Cells by Segmented Filamentous Bacteria. Cell 139: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YK, Menezes JS, Umesaki Y, and Mazmanian SK 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci 108: 4615–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar P, Monin L, Castillo P, Elsegeiny W, Horne W, Eddens T, Vikram A, Good M, Schoenborn AA, Bibby K, Montelaro RC, Metzger DW, Gulati AS, and Kolls JK 2016. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 44: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harley ITW, Stankiewicz TE, Giles DA, Softic S, Flick LM, Cappelletti M, Sheridan R, Xanthakos SA, Steinbrecher KA, Sartor RB, Kohli R, Karp CL, and Divanovic S 2014. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatol. Baltim. Md 59: 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garidou L, Pomié C, Klopp P, Waget A, Charpentier J, Aloulou M, Giry A, Serino M, Stenman L, Lahtinen S, Dray C, Iacovoni JS, Courtney M, Collet X, Amar J, Servant F, Lelouvier B, Valet P, Eberl G, Fazilleau N, Douin-Echinard V, Heymes C, and Burcelin R 2015. The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORγt and Controls Metabolic Disease. Cell Metab 22: 100–112. [DOI] [PubMed] [Google Scholar]
- 13.Xu R, Tao A, Zhang S, and Zhang M 2013. Neutralization of interleukin-17 attenuates high fat diet-induced non-alcoholic fatty liver disease in mice. Acta Biochim. Biophys. Sin 45: 726–733. [DOI] [PubMed] [Google Scholar]
- 14.Greer RL, Dong X, Moraes ACF, Zielke RA, Fernandes GR, Peremyslova E, Vasquez-Perez S, Schoenborn AA, Gomes EP, Pereira AC, Ferreira SRG, Yao M, Fuss IJ, Strober W, Sikora AE, Taylor GA, Gulati AS, Morgun A, and Shulzhenko N 2016. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat. Commun 7: 13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudino SJ, Beaupre M, Lin X, Joshi P, Rathi S, McLaughlin PA, Kempen C, Mehta N, Eskiocak O, Yueh B, Blumberg RS, van der Velden AWM, Shroyer KR, Bialkowska AB, Beyaz S, and Kumar P 2020. IL-22 receptor signaling in Paneth cells is critical for their maturation, microbiota colonization, Th17-related immune responses, and anti-Salmonella immunity. Mucosal Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiela PR, and Ghishan FK 2016. Physiology of Intestinal Absorption and Secretion. Best Pract. Res. Clin. Gastroenterol 30: 145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorn C, Riener M-O, Kirovski G, Saugspier M, Steib K, Weiss TS, Gäbele E, Kristiansen G, Hartmann A, and Hellerbrand C 2010. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol 3: 505–514. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu M, Singh SB, Wang J, Chung CC, Salituro G, Karanam BV, Lee SH, Powles M, Ellsworth KP, Lassman ME, Miller C, Myers RW, Tota MR, Zhang BB, and Li C 2011. Antidiabetic and antisteatotic effects of the selective fatty acid synthase (FAS) inhibitor platensimycin in mouse models of diabetes. Proc. Natl. Acad. Sci 108: 5378–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, Ruan X, Huang H, Wang Z, Huang Z, Huang Y, Yan X, and Chen Y 2010. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J. Hepatol 53: 339–347. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, and Ouyang W 2014. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514: 237–241. [DOI] [PubMed] [Google Scholar]
- 21.Seufert J 2004. Leptin Effects on Pancreatic B-Cell Gene Expression and Function. Diabetes 53: S152–S158. [DOI] [PubMed] [Google Scholar]
- 22.Mackey-Lawrence NM, and Petri WA 2012. Leptin and mucosal immunity. Mucosal Immunol 5: 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JET, Bloks VW, Groen AK, Heilig HGHJ, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JBL, and Nieuwdorp M 2012. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 143: 913–916.e7. [DOI] [PubMed] [Google Scholar]
- 24.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, and Gordon JI 2004. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, and Cani PD 2011. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 60: 2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauffin Cano P, Santacruz A, Moya Á, and Sanz Y 2012. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PloS One 7: e41079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


