Introduction
Cutaneous lupus erythematosus (CLE) and lichen planus (LP) are considered skin disease processes with distinct pathogenic mechanisms, clinical features, histologic findings, and disease courses. However, there exists a rare disorder termed CLE/LP overlap syndrome that has been inconsistently reported in the literature due to the absence of clear diagnostic criteria, with most reports involving cases with coexisting or overlapping clinical and histologic features of CLE and LP.1, 2, 3 In this article, we report 2 cases of CLE/LP overlap syndrome, propose a diagnostic criterion for CLE/LP overlap syndrome, and briefly review the literature and classify previously reported cases under our proposed criterion.
Case reports
Case 1
A 62-year-old African American woman presented with a 2-year history of groin lesions. A thorough drug history was taken. Previous treatments included nystatin cream, clotrimazole lotion, cephalexin, and fluconazole, without improvement. Physical examination revealed confluent, macerated plaques in the groin with surrounding hyperkeratosis and adherent whitish material. Biopsy demonstrated a psoriasiform lichenoid dermatitis with superficial and deep perivascular infiltrate with numerous plasma cells and no dermal mucin, which was thought to represent markedly inflamed LP. The antinuclear antibody (ANA) titer was high (>1:640), with a speckled pattern. She was given a short prednisone taper (40 mg daily for 3 days, 30 mg daily for 3 days, and 20 mg daily for 3 days), but her lesions persisted over the next several months. In addition, white lacy discoloration and focal ulcerations developed on her buccal mucosa. A biopsy of a new shin lesion (Fig 1) demonstrated marked hyperkeratosis with focal parakeratosis, and superficial and deep perivascular and periadnexal inflammatory infiltrate with frequent plasma cells but an absent lichenoid pattern (Fig 2). This was considered more suggestive of hypertrophic chronic CLE than LP. The ANA test was repeated, showing a titer of 1:320. An extractable nuclear antigen (ENA) panel revealed the presence of anti-Ro(SS-A) antibodies and anti-La(SS-B) antibodies. An anti–double-stranded DNA antibody test was negative. Because the strong serologic markers of lupus erythematosus were unquestionable, direct immunofluorescence (DIF) was not performed to substantiate the diagnosis of CLE versus LP. Liver function tests were unremarkable, and hepatitis testing was not performed. She was given a presumptive diagnosis of atypical subacute CLE with positive anti-Ro(SS-A) and anti-La(SS-B) serology. The patient was started on hydroxychloroquine 200 mg twice daily and topical fluocinonide 0.05% ointment twice daily as needed. She was also restarted on a slow prednisone taper (30 mg daily alternating with 20 mg daily for 2 weeks, then 20 mg daily) to decrease the local inflammatory response around the skin lesions. Unfortunately, she was lost to follow-up at this point.
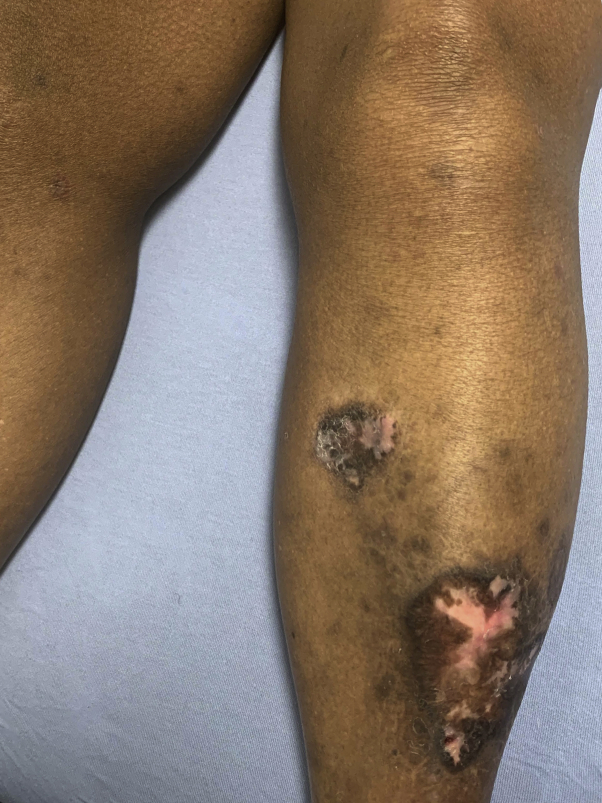
Fig 1.
Hypertrophic pink plaques with peripheral hyperpigmentation on the left shin.
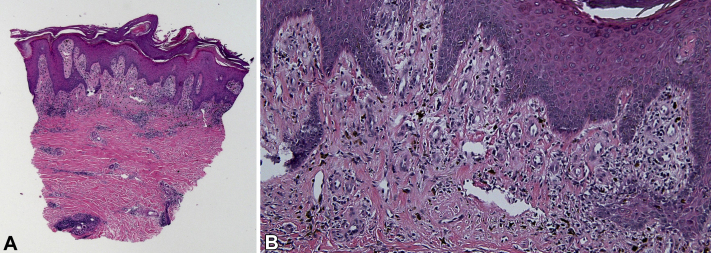
Fig 2.
Punch biopsy with features of cutaneous lupus erythematosus. A, Histology. Epidermal hyperplasia with a lichenoid inflammatory infiltrate, pigmentary incontinence, slight dermal fibrosis, and deep perivascular and periadnexal inflammatory infiltrate. B, Histology. Along the dermoepidermal junction, the sparse interface inflammatory process can be appreciated along with pigmentary incontinence and slight dermal fibrosis. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×20; B, ×100.)
A decade later, she returned to seek care for extensive atrophic lesions with hypertrophic, hyperpigmented borders involving her groin, vulva, legs, arms, and hands (Fig 3). Two repeat biopsies showed lichen planus-like features, including compact hyperkeratosis, irregular epidermal hyperplasia, and lichenoid interface dermatitis with a saw-toothed rete pattern (Fig 4). Hepatitis C testing at this time was negative. Methotrexate 5 mg weekly was initiated with a prolonged prednisone taper (60 mg daily for 5 days, 40 mg daily for 5 days, 30 mg daily for 5 days, 20 mg daily for 5 days, 20 mg daily alternating with 15 mg daily for 2 weeks, 20 mg daily alternating with 10 mg daily for 2 weeks, 20 mg daily alternating with 5 mg daily for 2 weeks, and 20 mg every other day). However, after repeat ANA and ENA panels again revealed an elevated titer (>1:640) and the presence of anti-Ro(SS-A) and anti-La(SS-B) antibodies, hydroxychloroquine was restarted at 400 mg daily. At the time of publication, she remained on hydroxychloroquine 400 mg daily and prednisone 10 mg daily, and her methotrexate had been steadily increased to 15 mg weekly. She reported some improvement with therapy at her 3-month follow-up, including decreased itching and mild regression of skin lesions.
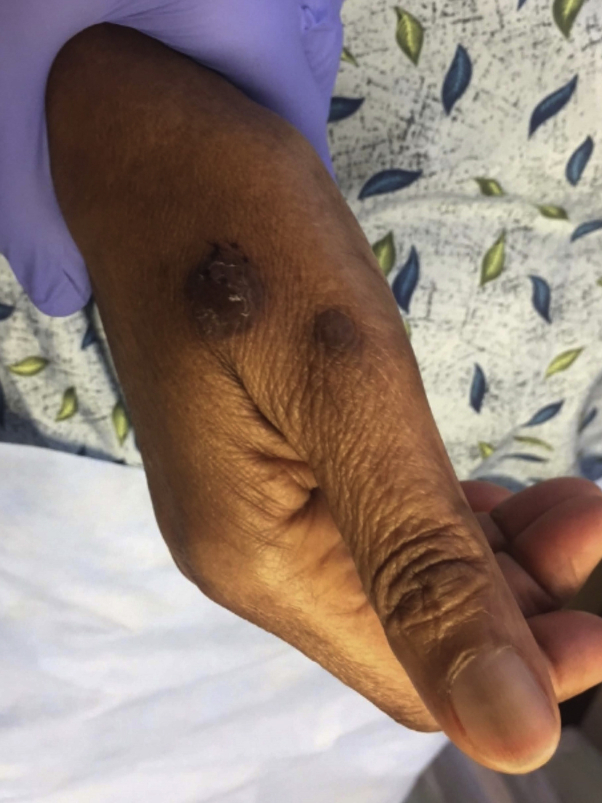
Fig 3.
Violaceous-to-brown flat-topped plaque and papule with fine adherent scale on the right hand.
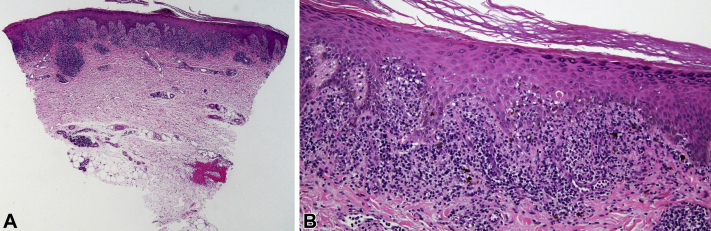
Fig 4.
Punch biopsy with features of lichen planus. A, Histology. Dense lichenoid infiltrate which is very lichen planus-like. There is a sparse deep inflammatory component around an eccrine coil. B, Histology. Along the dermoepidermal junction, the band-like nature of the inflammatory infiltrate can be appreciated, along with features reminiscent of lichen planus: saw-toothing of rete ridges, hypergranulosis, scattered necrotic keratinocytes, and blurring of the dermoepidermal junction. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×20; B, ×100.)
Case 2
A 54-year-old African American woman presented with a 1-year history of ulcerating groin lesions that subsequently spread to her mouth, buttocks, and feet. A biopsy done by an outside provider was read by 2 pathologists, with one reporting LP and the other chronic CLE. The first report described a lichenoid interface infiltrate of lymphocytes at the junction of a hyperplastic epidermis with degenerative changes along the dermoepidermal junction, including the presence of colloid bodies and subepidermal clefting. Mild superficial and deep perivascular and focal periadnexal inflammatory infiltrates were present. DIF was negative for IgA, IgG, immunoglobulin M, and fibrinogen. The second report was concisely labeled psoriasiform interface dermatitis. Prior laboratory testing revealed a negative ANA and positive ribonucleoprotein and antichromatin antibodies. A thorough drug history was obtained. She had been treated with hydroxychloroquine 200 mg twice daily, dapsone 50 mg daily, and a short prednisone taper (initiated by an outside provider with unknown dose), with mild improvement according to the patient's report. On presentation to our clinic, her examination was significant for multiple hyperkeratotic, hyperpigmented plaques on the buttocks with overlying desquamation, ulcerating confluent hyperpigmented plaques in the inguinal folds that spared the labia majora, multiple ulcerations with hyperpigmentation on the feet, and ulcerations on the hard palate and buccal mucosa. She was continued on hydroxychloroquine 200 mg twice daily, dapsone 50 mg daily, and prednisone taper to completion, and started on triamcinolone 0.1% ointment. Her ANA titer was high (>1:640) with a speckled pattern, and the ENA panel was strongly positive for antiribonucleoprotein antibodies. Liver function tests were unremarkable, and hepatitis testing was not performed. At the 2-week follow-up, plaquenil was decreased to 100 mg twice daily because monitoring labs showed elevated creatinine (1.3 mg/dL). The elevated creatinine was later evaluated by Nephrology, who felt it was likely due to chronic nonsteroidal antiinflammatory drug use, which was discontinued, and a repeat creatinine level at 3 months was normal. There was no other evidence of internal organ involvement to suggest systemic lupus erythematosus. At her 2-month follow-up with Dermatology, clinical improvement was noted, as evidenced by patient-reported relief of symptoms in the areas involved and observed decrease in the local inflammatory response. Dapsone was discontinued. She was continued on hydroxychloroquine 200 mg daily, and her topical corticosteroid therapy was increased to clobetasol 0.05% ointment twice daily as needed. She did not return for follow-up.
Discussion
The diagnosis of CLE is based on clinical history, physical examination, laboratory studies, serologic markers, and histologic features.4 The diagnosis of LP is also based on clinical history, physical examination, and histologic features, but unlike CLE, there are no serologic markers or autoantibodies in LP.5 CLE/LP overlap syndrome is a rare disorder characterized by features of both disease processes. Clear diagnostic criteria for this syndrome do not currently exist. This has led to inconsistent reporting in the literature, with most reports including cases with coexisting or overlapping clinical and histologic features of CLE and LP.1, 2, 3 Table I details our proposed diagnostic criterion for CLE/LP overlap syndrome, which divides cases into “classic” and “possible” CLE/LP overlap syndrome. Specifically, we propose the definition of “classic” CLE/LP overlap syndrome as cases that have mixed clinical features of CLE and LP, histologic features of LP (with or without features of CLE), and any positive serologic markers of CLE (positive ANA with titers ≥1:80 on HEp-2 cells, ENA antibodies, anti–double-stranded DNA antibodies, or antiphospholipid antibodies).6, 7, 8 Our definition of “possible” CLE/LP overlap syndrome includes the same clinical and histologic features as “classic” CLE/LP overlap syndrome; however, it is used for cases with negative ANA (titers <1:80) and negative serologic tests. DIF is not needed for diagnosis but may be helpful and, when performed, may demonstrate features of both CLE and LP.
Table I.
Proposed diagnostic criterion for cutaneous lupus erythematosus/lichen planus overlap syndrome
| Classification | Clinical features | Histologic features | Serologic findings | DIF |
|---|---|---|---|---|
| Classic CLE/LP overlap syndrome | Mixed clinical features of CLE and LP (eg, well-demarcated erythematous to violaceous scaly papules and plaques). | Features of LP with or without features of CLE. Features of LP include hyperkeratosis without parakeratosis, hypergranulosis, irregular saw-tooth acanthosis, vacuolar degeneration of the basal cell layer, pigment incontinence, and colloid bodies. Features of CLE include hyperkeratosis, vacuolar degeneration of the basal cell layer, follicular plugging, superficial and deep perivascular and periadnexal lymphoid infiltrate, interstitial mucin, and accentuation of the basement membrane. |
Any positive serologic test:
|
DIF may be helpful but is not needed for diagnosis. If performed, it may demonstrate features of both CLE and LP. In CLE, DIF reveals granular deposition of immunoglobulin (IgG, IgA, and IgM) and complement along the DEJ and around hair follicles and IgM staining of colloid bodies. In LP, DIF reveals colloid bodies in the papillary dermis that stain for complement and immunoglobulins (especially IgM) and fibrin in a fibrillar pattern along the DEJ. |
| Possible CLE/LP overlap syndrome | Same as above. | Same as above. | Negative ANA (titers <1:80) and negative serologic tests. Serial testing recommended. | Same as above. |
ANA, Antinuclear antibody; CLE, cutaneous lupus erythematosus; DEJ, dermoepidermal junction; DIF, direct immunofluorescence; dsDNA, double-stranded DNA; ENA, extractable nuclear antigen; LP, lichen planus; RNP, ribonucleoprotein.
To test our proposed criterion, a review of the existing literature was performed using a PubMed search for “lichen planus lupus erythematosus overlap syndrome,” which yielded 34 results (similar to the results of Schmitz et al2). Sixteen of these publications were relevant and applicable. Several additional cases were identified in the course of reviewing these publications and their references.9, 10, 11, 12 In total, 38 cases of CLE/LP overlap syndrome were identified.1, 2, 3,9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Of these 38 cases, 12 were classified as “classic,” 21 were classified as “possible,” and 5 had incomplete workup and thus were unable to be classified as CLE/LP overlap syndrome according to our criterion. Demographic information, clinical features, histologic features, immunofluorescence, serologic findings, laboratory findings, treatments, and treatment responses in these cases are summarized in Table II.
Table II.
Summary of previously reported cases of cutaneous lupus erythematosus/lichen planus overlap syndrome
| Case | Demographics | Clinical features | Histologic features and immunofluorescence | Serologic and laboratory findings | Treatment and response | Applying our criteria |
|---|---|---|---|---|---|---|
| Lospinoso et al1 | 36 yo; AA; F | Thick scaling scarring plaques on trunk and extremities. Recurrent spontaneous ulcerations on ankles and feet. Mild discoid scarring and fixed serpiginous white streaks on lips. |
Hyperkeratosis and hypergranulosis with irregular saw-tooth acanthosis. Lichenoid lymphocytic infiltrate with interface changes, colloid bodies, and pigment incontinence. Perieccrine inflammation with occasional plasma cells and eosinophils and an increase in dermal mucin. DIF revealed fibrin along the DEJ in a band-like pattern and IgM+ cytoid bodies. |
(+) ANA (1:160), speckled pattern. Anti-ENA, anti-dsDNA, C3, C4, CH50, Hep B, and Hep C serologies, and CMP unremarkable. |
Topical corticosteroid → NI 4-wk prednisone taper → Healing of the ulcerations and resolution of pain. Subsequent flare treated with prednisone taper and acitretin 50 mg daily → Resolution of hyperkeratotic and ulcerative lesions. |
Classic overlap |
| Schmitz et al2 | 70 yo; M | Erythematous plaques with scale on scalp and ears. Numerous well-demarcated erythematous plaques on chest, abdomen, back, and inguinal folds. Solitary thick annular lichenified plaque on erythematous base with silvery scale on mid-lower portion of the back. Annular and centrifugal erythematous papules and plaques without scale on extremities. Cheilitis with multiple erosions of lips and white lacy plaques on tongue. |
Lower lip, chest, lower portion of the back—hyperkeratosis, patchy lichenoid in filtrate, and many necrotic keratinocytes consistent with LP. DIF in a later biopsy revealed granular deposition of C3 and IgM at the BMZ, correlating to an evolving lupus band. |
(+) ANA, elevated anti–SS-A (>8.0 IU), (+) anticardiolipin IgM and IgA, borderline anti-histone antibody, low C3 (49.6). Anti-Smith, anti-dsDNA, anti–smooth muscle, anti-RNP, rheumatoid factor, C4, and CH50 unremarkable. |
Topical corticosteroid, phototherapy, antipruritics → NI Hydroxychloroquine 200 mg twice daily with short-term bursts of oral prednisone during disease flares in the first 3 mo of treatment → Treatment response not available. |
Classic overlap |
| Nagao and Chen3 | 53 yo; F | Violaceous erythema with faint whitish streaks (Wickham's striae) surrounding the nasal cavity and on the upper lip. Slightly atrophic erythema with fine scales on the cheeks and neck, and white lacework on the buccal mucosa. Indurated erythema on bilateral palms. |
Right cheek—thinning of the epidermis, liquefaction degeneration, and a patchy inflammatory infiltrate consisting of mononuclear cells around blood vessels and skin appendages in the dermis consistent with discoid LE. Lip—wedge-shaped areas of hypergranulosis and a band-like mononuclear infiltrate at the papillary dermis consistent with LP. Neck—follicular plugging, a band-like lymphocytic infiltration, and patchy mononuclear cell infiltrates in the dermis. Eosinophilic colloid bodies (cytoid bodies) were present at the DEJ. DIF (neck) showed deposits of IgG, IgM, and C3 forming a granular pattern in a line along the BMZ and clustering of IgG- and IgM- positive cytoid bodies at the DEJ. |
(+) ANA (1:40), speckled and homogenous staining pattern. (+) anti-dsDNA. C3 and C4 slightly decreased. CBC, ESR, LFTs, routine urine tests WNL. |
0.1% tacrolimus ointment → Satisfactorily suppressed the active LE, LP, and overlapping lesions but did not eradicate the skin lesions. | Classic overlap |
| Smirnov et al13 | 42 yo; White; F | Bilateral erythematous, atrophic, and verrucous papules coalescing into annular and polycyclic plaques of the bilateral arms. Scaling, atrophic, pink papules and plaques on the face, ears, lateral and posterior aspects of the neck and chest, with sparing of postauricular and submental locations. |
Squamatized epithelia with irregular, exaggerated acanthosis, overlying rounded parakeratosis, accompanied by a lichenoid and vacuolar interface at the DEJ accompanied by wedge-shaped hypergranulosis, irregular jagged rete peg alteration, and a perieccrine lymphocytic infiltrate, with BM thickening demonstrated by PAS staining. DIF revealed fine granular IgG deposition along the BM and within keratinocyte nuclei in the lower one-third portion of the epidermal strata, granular IgM with cytoid body staining, C3, C5b-9, and weaker IgA deposition along the epidermal and adnexal BMs, with shaggy fibrinogen staining. |
(+) ANA (1:640). | Not reported. | Classic overlap |
| Patil et al14 | 40 yo; F | Multiple erythematous to depigmented atrophic scaly plaques with peripheral hyperpigmentation on extensors of both upper and lower extremities and back. Buccal erosions with scalloped borders and diffuse cheilitis. 4 mo prior patient experienced multiple violaceous scaly papules and plaques on the upper portion of the trunk and extremities, with oral mucosal involvement. |
Buccal mucosa—subepidermal cleft with dense lymphohistiocytic infiltrate hugging the DEJ Atrophic plaque (back)—compact hyperkeratosis, wedge-shaped hypergranulosis and basal cell degeneration with interface lymphocytic infiltrate suggestive of LP. Features of LE, including deep perivascular and periappendageal infiltrate with mucin. DIF (back) revealed granular BMZ band staining positive for IgM and C3, with colloid bodies in the papillary dermis staining positive for IgM, IgA, C3 and epidermal ANA staining with IgG. |
(+) ANA (1:1000), speckled pattern. Anti-dsDNA and antihistone, HBsAg, anti-HCV, and VDRL negative. ESR elevated (40 mm/h). Microcytic hypochromic anemia. Raised T3, T4 with normal TSH. |
Oral prednisolone in tapering doses over 3 mo, oral dapsone, and topical mometasone furoate 0.1% cream → NI Photoprotection, oral chloroquine 250 mg twice daily, topical mometasone furoate 0.1% cream in the morning and topical tacrolimus 0.1% ointment at night for skin lesions, and topical tacrolimus 0.03% at night for oral lesions → NI at 1 mo. Added methotrexate 10 mg weekly → Lesions gradually healed with hypopigmentation and oral erosions completely resolved after 1 mo of therapy. |
Classic overlap |
| Grabbe and Kolde9 | 84 yo; White; F | Erythematous, slightly scaling ovoid plaques with mild central atrophy and a raised, livid red peripheral border surrounded by a halo of increased brownish pigmentation predominantly located on the face, lower portion of the back, thighs, and arms. These lesions tended to confluence, leaving reticular postinflammatory hyperpigmentation. Violaceous lichenoid papules and plaques with a shiny whitish surface and Wickham's striae, sometimes also in a reticular pattern. Occasional discrete scaling or superficial ulceration was detectable. Lower lip and buccal mucosa showed areas of reticular leukoplakia and a small aphthous-like ulcer. Scalp and eyebrows demonstrated mild diffuse nonscarring alopecia. |
SCLE-like lesions—epidermal atrophy, hydropic degeneration of the basal keratinocytes, discrete thickening of the BM, patchy perivascular lymphocytic infiltrates, and edema of the papillary dermis. DIF (SCLE-like lesions) contained granular deposits of IgM and C3 at the DEJ and fibrinogen in the papillary dermis. LP-like lesions—orthohyperkeratosis and hypergranulosis, focal degeneration of the basal layer, a band-like lymphocytic infiltrate in the papillary dermis with focal epidermotropism, some eosinophilic colloid bodies and dermal melanophages. DIF (LP-like lesions) revealed fibrinogen deposits along the BMZ, extending to the papillary dermis, and low amounts of IgM within scattered colloid bodies. In some biopsy specimens, alterations typical of both LP and SCLE were observed adjacent to each other. |
(+) ANA (1:1000), homogeneous pattern, anti-Ro(SS-A), anti-Sm Abs, RF, and slightly elevated antimicrosomal Abs. Anti-U1RNP, anti-Scl-70, anti-La/SS-B, anti-histones, anti-centromere, anti-dsDNA negative. C3, C4, and CH50 WNL. ESR (59 mm/h), CRP, fibrinogen elevated. Normocytic, normochromic anemia, thrombocytopenia, lymphocytopenia. |
Topical corticosteroids → NI Hydroxychloroquine 400 mg daily → Marginal improvement at 10 wk. Hydroxychloroquine reduced to 200 mg daily. Dosage was subsequently adjusted to maintain CSA serum concentrations between 80 and 100 ng/mL → Inflammatory lesions improved significantly over a 12-wk course, leaving postinflammatory reticular hyperpigmentation and discrete yellow-gray skin atrophy. |
Classic overlap |
| Komori et al15 | 59 yo; F | Erythematous papules on left dorsal aspect of the hand, soles, and trunk. Oral ulcers with no pain and nonscarring alopecia were also observed. Lesions later spread to right forearm. |
Hand—perivascular infiltration of lymphocytes and dermal mucinosis. Upper extremity—band-like lymphocyte infiltration was present in the papillary dermis with necrotic keratinocytes in the epidermis. Dermal perivascular infiltration of lymphocytes and dermal mucinosis was evident in the same view. |
(+) ANA (1:1280). (+) anti-dsDNA (24 IU/mL). Leukopenia. |
Topical corticosteroid (0.05% clobetasol propionate) and platelet inhibitors (300 mg/d of sarpogrelate hydrochloride and 60 μg/d of beraprost sodium) → Pain was ameliorated dramatically, but skin lesions spread to right forearm. | Classic overlap |
| Camisa et al16—case 5 | 33 yo; AA; F | Plaques with central atrophy, hyperpigmentation, scaling, and elevated violaceous borders: face, ears, scalp, extensor surfaces of arms, chest (associated with large keloid). | Focal parakeratosis; admixture of cell-rich and cell-poor band-like lymphocytic infiltrate; focal hypergranulosis and “saw-tooth” rete pegs; vacuolar degeneration of basal layer with occasional colloid body; abundant incontinent pigment; extravasated erythrocytes in dermal papillae; perivascular and periadnexal lymphocytic infiltrates. DIF revealed cytoid bodies: IgG, IgA, IgM, C4, fibrinogen, linear; BMZ: IgM, continuous granular. |
(+) ANA (1:320), speckled pattern. (+) RF (1:20.) |
Topical fluocinonide ointment → improvement. | Classic overlap |
| Tursen et al17 | 34 yo; F | Violaceous, thickened, scaly lesions on scalp, arms, malar, and auricular regions. Buccal and lip mucosa showed white papules with a reticular pattern. Erythematous, well-defined, mildly raised plaque on the left lower eyelid involving 1/3 of the lateral portion. |
Eyelid—Hydropic degeneration of the basal layer, moderate lymphocytic inflammation and melanophages consistent with late-phase lichenoid dermatitis. Intermediate immunohistochemical staining with IgG, consistent with LP. Oral mucosa, lower lip—epithelium showed lichenoid hyperplasia and band-like lymphocytic infiltration. Nasolabial sulcus, scalp, auricle—epidermal atrophy, follicular plugging, perivascular lymphocytic infiltration and vacuolar alteration, consistent with DLE. Strong immunostaining with IgG in the BMZ also consistent with DLE. |
(+) ANA. (+) anti-dsDNA. ESR elevated (30 mm/h). CBC, IgG, IgA, IgM, C3, and C4 levels WNL. |
Hydroxychloroquine 200 mg orally twice daily → Eyelid, mucosal, and skin lesions improved dramatically within 2 wk. Therapy was gradually tapered over 6 mo and the patient remained free from symptoms. |
Classic overlap |
| Kiyani and Shahroz10 | 42 yo; F | Bilateral, white, lace-like (lichenoid) striations on the buccal mucosa with pigmentation on the gingiva and buccal mucosa. | Buccal mucosa—infiltration of chronic inflammatory cells through the BM of the oral epithelium. Mild, band-like lymphocytic infiltrate in superficial connective tissue. DIF was negative for deposition of IgG, IgM, IgA, or C3 at the BMZ. |
(+) ANA. (+) anti-dsDNA. |
Hydroxychloroquine 400 mg and prednisone 60 mg for 1 mo → Symptoms improved and prednisone was gradually tapered. At 18-mo follow-up, oral LP was unaffected in appearance; SLE symptoms were under control with maintenance dose of 200 mg of hydroxychloroquine and 5 mg of prednisone. |
Classic overlap |
| Sekar et al18 | 35 yo; F | Multiple hyperpigmented and annular polycyclic plaques present on the trunk and both limbs. Few erosive and crusted plaques were seen on the forearms and lower portions of the legs. Oral cavity showed palatal erosions with hyperpigmentation. |
Epidermis with hyperkeratosis, follicular plugging, and basal vacuolar damage. Few Civatte bodies were seen in the epidermis and papillary dermis. Papillary dermis was edematous and showed mucin deposits with perivascular lymphocytic infiltrate and melanophages. DIF showed linear deposits of fibrinogen at the BMZ. |
(+) ANA. (+) anti-Ro. Lupus anticoagulant, anti-cardiolipin, VDRL, HIV ELISA negative. CBC, LFTs, and renal function WNL. |
Oral steroids and hydroxychloroquine → Lesions subsided. | Classic overlap |
| Romero et al19—case 3 | 72 yo; AA; F | Erythematous atrophic patches with hypopigmentation and hyperpigmentation and telangiectasia: face. | Cell-rich pattern predominant but atrophy and follicular plugging in some areas. IF revealed ovoid bodies: IgG, IgM, C3. |
(+) ANA (1:80), speckled pattern. | Not individually reported. See case 1. |
Classic overlap |
| Van der Horst et al11—case 1 | 45 yo; F | LE-like lesions consisting of circumscribed, livid red, partly atrophic, partly indurated patches, usually with follicular hyperkeratosis and scaling, occasionally with telangiectatic spots on back, breasts, arms. Lichenoid papules on wrists. Verrucous lesions on forearms. Mucosal lesions in mouth. |
Forearm—HP showed more LP than LE, DIF showed LP. Mouth—HP showed more LP than LE, DIF showed LP. |
(+) ANA. Anti-dsDNA negative. Complement profile WNL. Elevated serum gamma globulin. ESR 18 mm/h. |
Not individually reported. In all patients, prior treatment—topical steroids → NI Some treatments included topical steroids, antimalarial drugs, hydroxychloroquine, and prednisone. |
Possible overlap (ANA titer not reported) |
| Demirci et al20 | 26 yo; M | Erythematous, slightly scaly, irregularly bordered, infiltrated large plaques with central atrophy on the back. Butterfly-type rash involving the nose and malar region. Erythema on the ears and neck with sparing of the retroauricular region. Widespread violaceous lichenoid papules on the upper and lower extremities, but no mucosal or nail involvement. |
Back—thinning of the epidermis, basal layer, vacuolar degeneration, perifollicular chronic inflammatory infiltrates, and deposition of mucin in the dermis consistent with subacute CLE. DIF revealed deposits of immunoglobulin (IgM>IgG, IgA) and C3 forming a granular pattern (feature of LE) and linear fibrinogen deposition at the BMZ (feature of LP). Dorsum of the hand—hypergranulosis, with a band-like mononuclear infiltrate at the DEJ, consistent with LP. |
ANA, anti-dsDNA, anti-Ro(SS-A), anti-La(SS-B), anti-Sm negative. C3, C4 levels WNL CBC, ESR, routine urine tests WNL except for mild elevations of ALT and AST. |
Mometasone furoate 0.1% cream applied twice daily for 2 wk → Rapid improvement of the lesions was seen at the end of the second week | Possible overlap |
| Shahzadi et al12 | 24 yo; F | Well-defined, 2- to 4-cm plaques with hyperpigmented borders and depressed, hypopigmented, scaly center and cicatricial alopecia on the scalp. Lips showed scaly, violaceous, and atrophic plaques. Buccal mucosae revealed bluish lace-like pattern and ulceration. Diffuse, soft swelling on front of neck, moving on deglutition. |
Skin—epidermal atrophy, follicular plugging, basal cell vacuolar degeneration, periappendageal infiltrate, and interface dermatitis. Oral mucosa—parakeratosis, hypergranulosis, acanthosis, and interface dermatitis. DIF was not performed because of lack of facility. |
ANA, anti-dsDNA negative. C3, C4 levels WNL. TSH elevated (9.03), free T4 WNL. |
Oral prednisolone 40 mg and tab thyroxine 50 μg daily → Improvement in mucocutaneous lesions and thyroid functions within 6 wk. | Possible overlap |
| Inalöz et al21 | 50 yo; F | Patches of alopecia with some follicular plugging. Erythematous butterfly facial rash predominantly involving the nose and malar region. Patchy hypopigmentation on the back and hands consistent with vitiligo. |
Scalp (multiple biopsies) —periadnexal chronic inflammatory infiltrate and basal cell layer hydropic degeneration consistent with LE. DIF negative for LE bands, but revealed linear fibrinogen deposition at the BMZ. Deposits of IgM and IgA in cytoid bodies noted in dermal papillae. Fluorescent cytoid bodies demonstrated a tendency to cluster in groups. Overall, consistent with LP. |
Autoantibody screens, including anticardiolipins, were negative. Mild iron-deficiency anemia. ESR, urea, electrolytes, LFTs, glucose, and urine tests WNL. |
Topical hydrocortisone for facial butterfly rash → Resolution with residual small hypopigmented areas. Topical potent corticosteroids for scarring alopecia → Little overall benefit. |
Possible overlap |
| De Jong and Van De Kerkhof22 | 49 yo; M | Well-demarcated erythematous squamous plaques, some showing atrophy and Wickham's striae, on the back, arms, hands, and feet. Lesions on hands and arms showed violaceous erythema. The palmar eruption was partly erosive, with some papular and hyperkeratotic changes. Slight hyperpigmentation of the lesions on the back. |
Back—partly atrophic epidermis with hydropic degeneration of the basal layer, with hyperkeratosis, slight parakeratosis, and Civatte bodies within the epidermis. In the papillary dermis there was a perivascular, mainly lymphocytic, inflammatory infiltrate that showed a perifollicular localization. The granular layer of the epidermis was of variable thickness. IF showed granular IgM deposition at the DEJ with some C3 deposits at the DEJ, but no reaction for IgG, IgA, or C1q. |
ANA, rheumatoid factor negative. Elevated ESR (30 mm/h). Other routine blood tests and biochemical studies WNL. |
Prior treatment: Topical clobetasol propionate → NI Acitretin 35 mg daily in addition to continuance of topical steroids → Acitretin increased to 50 mg daily → After 12 wk, the lesions had flattened and some clearance was noted. Acitretin dose reduced to 17.5 mg daily → Lesions remained in remission. |
Possible overlap |
| Jamison et al23 | 53 yo; F | Circinate lesions with erythematous borders, some with central atrophy and postinflammatory hyperpigmentation and hypopigmentation, primarily involving the hands, but also the face, neck, chest, back, anterior thigh areas, arms, and intergluteal fold. The left palm was involved with erosive lesions with some impetiginization. |
Skin—hyperkeratosis, vascular degeneration of the basal cell layer, absence of follicular plugging, and a dense, band-like lymphocytic and histiocytic infiltration of the upper portion of the dermis. Consistent with LP. IF revealed deposition of clumps of IgG, IgM, and fibrinogen at the DEJ, but no C3 was detected. Consistent with either LP or DLE. |
ANA, ENA, anti-DNA negative. ESR elevated (76-82 mm/h) |
Prednisone 40 mg every other day → Poor clinical response → Changed to prednisone 20 mg daily (1 wk later). Chloroquine 250 mg daily and topical applications of glucocorticoids were also administered → Lesions on soles and thighs resolved, and partial resolution of hand lesions was observed. |
Possible overlap |
| Romero et al19—case 1 | 41 yo; AA; F | Livid red–to–violet atrophic ulcerative patches with telangiectasia and bullae: acral extremities, trunk, face, nails. | Cell-rich and cell-poor patterns equally admixed; band-like infiltrate in some specimens with thickened BM and liquefaction degeneration in others; numerous colloid bodies. IF revealed ovoid bodies: IgG, IgM. |
ANA negative | Not individually reported. Generally, poorly responsive to therapy, which consisted of topical and intralesional corticosteroids and systemic medications in a few patients, including corticosteroids, antimalarials, and immunosuppressive drugs. |
Possible overlap |
| Romero et al19—case 2 | 49 yo; AA; M | Verrucoid atrophic erythematous to violaceous plaques with telangiectasia: palms, soles, dorsal aspects of the hands and feet, nails, trunk, penis. | Cell-rich and cell-poor patterns admixed; thickened BM in some and lichenoid cell-rich response in others. IF revealed ovoid bodies: IgG, IgM, C3; linear band on one occasion: IgG, IgM. |
ANA negative. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 4 | 45 yo; AA; F | Atrophy and telangiectasia of proximal nailfolds and nail beds with loss of nail plates—nails only | Cell-rich pattern predominant with liquefaction degeneration and necrosis of keratinocytes, but BM thickening and basal layer hypertrophy in others. IF revealed ovoid bodies: IgG, IgM. |
ANA negative | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 5 | 40 yo; AA; F | Atrophic erythematous to violaceous patches with hypopigmentation of distal phalanges and nail beds and loss of nail plates; lower lip and oral mucosa also involved. | Cell-rich pattern predominant, but with atrophy, liquefaction degeneration, telangiectasia, and numerous plasma cells. IF revealed ovoid bodies: IgG, IgM, C3. |
ANA negative | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 6 | 46 yo; AA; M | Erythematous atrophic areas with poikiloderma, mild follicular plugging, and nail dystrophy: Nails, extremities, lip, face, scalp; oral mucosa also involved. | Cell-rich and cell-poor patterns equally admixed with band-like infiltrate in some areas, and BM thickening with few cells in others. IF revealed ovoid bodies: IgG, IgM, C3. |
(+) ANA (1:40), homogeneous pattern. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 7 | 23 yo; White; M | Atrophic, erythematous areas with hypopigmentation, telangiectasia, and anonychia: nail beds only | Cell-rich pattern predominant, but with atrophy, hyperkeratosis, and BM thickening in several areas; numerous plasma cells. IF revealed ovoid bodies: IgG. |
ANA negative. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 8 | 58 yo; White; M | Atrophic, erythematous to violaceous areas with hypopigmentation, telangiectasia, scaling: dorsal aspect of the hands, fingers, nails, extremities, neck. | Cell-poor pattern predominant, with thickened BM, dermal fibrosis, and telangiectasia. IF revealed ovoid bodies: IgG; linear band on one occasion: IgG, IgM, C3. |
ANA negative. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 9 | 44 yo; AA; F | Erythematous atrophic plaques: arms, face, trunk. | Cell-poor pattern predominant, but cell-rich in some areas of papillary dermis and in perifollicular connective tissue. IF revealed ovoid bodies: IgM; linear band on one occasion: IgG, IgM, C3. |
(+) ANA (1:40), homogeneous pattern. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 10 | 63 yo; White; M | Atrophic violaceous to erythematous scaling plaques: dorsal aspect of the hands, proximal aspect of the nailfolds, fingers, face, scalp. | Cell-rich and cell-poor patterns admixed with smudgy BM and thickening of blood vessel walls. IF revealed ovoid bodies: IgG, IgM; linear band on one occasion: IgG, IgM, C3. |
ANA negative. | Not individually reported. See case 1. |
Possible overlap |
| Romero et al19—case 11 | 63 yo; AA; M | Atrophic violaceous scaling, plaques: face. | Cell-rich pattern predominant, but with some areas showing thickened BM. IF revealed ovoid bodies: IgG, IgM, C3. |
ANA negative. | Not individually reported. See case 1. |
Possible overlap |
| Camisa et al16—case 1 | 63 yo; White; F | Violaceous papules and plaques: palms, soles, dorsal aspect of the hands, extensor surfaces of arms, knees, nailfolds, and nail beds; erosions of fingertips, lips, and buccal mucosa also involved. | Orthohyperkeratosis; cell-rich, band-like lymphocytic infiltrate with subepidermal clefts and bullae; many subepidermal colloid bodies; hypergranulosis and “saw-tooth” rete pegs. DIF revealed cytoid bodies: IgG, IgA, IgM, clustered: BMZ: negative. |
ANA negative. (+) RF (1:40). | Low-dose systemic and topical corticosteroids → Symptomatic improvement. | Possible overlap |
| Camisa et al16—case 2 | 43 yo; AA; F | Atrophic plaques with central hyperpigmentation, follicular plugging: face, scalp with alopecia | Orthohyperkeratosis with one follicular plug; cell-poor band-like lymphocytic infiltrate, vacuolar degeneration of basal layer, focal thickening of BM, and a few colloid bodies; subepidermal clefts; abundant incontinent pigment; dense lymphocytic infiltrate around eccrine glands extending into subcutis. DIF revealed cytoid bodies: IgM, linear; BMZ: IgM, focal, granular; fibrinogen, thick, shaggy. |
ANA, RF negative. | Hydroxychloroquine | Possible overlap |
| Camisa et al16—case 3 | 57 yo; White; F | Violaceous atrophic scaling plaques: extensor surfaces of forearms, “V” of neck, nailfolds. | Hyperparakeratosis and epidermal atrophy; cell-poor band-like lymphocytic infiltrate, vacuolar degeneration of basal layer with many intraepidermal and subepidermal colloid bodies; extravasated erythrocytes and incontinent pigment; perivascular and periadnexal lymphocytic infiltrates. DIF revealed cytoid bodies: IgA, IgM, linear; BMZ: fibrinogen, thick, shaggy. |
ANA negative. (+) RF. | Low-dose systemic and topical corticosteroids → Symptomatic improvement. | Possible overlap |
| Camisa et al16—case 4 | 68 yo; White; F | Livid red–to–violaceous verrucoid plaques—extensor surfaces of arms, dorsal aspects of the hands. | Orthohyperkeratosis with prominent follicular plugging; admixture of cell-rich and cell-poor band-like lymphocytic infiltrate; vacuolar degeneration of basal layer with thickened BM and many colloid bodies; solar elastosis. DIF revealed cytoid bodies—IgM, linear; BMZ—fibrinogen, thick, shaggy. |
(+) ANA (1:40), diffuse pattern RF negative. |
Topical fluocinonide cream and hydroxyzine hydrochloride → Improvement | Possible overlap |
| Camisa et al16—case 6 | 58 yo; White; F | Erythematous to violaceous papules and plaques; face, dorsal aspects of hands and forearms (scaling), “V” of neck; lips involved. | Orthohyperkeratosis; cell-rich band-like lymphocytic infiltrate; focal hypergranulosis, vacuolar degeneration of basal layer, and scattered intraepidermal colloid bodies. DIF revealed cytoid bodies: negative; BMZ: negative. |
ANA negative. RF not done. |
Betamethasone valerate cream → Improvement | Possible overlap |
| Zhang et al24 | 27 yo; F | Atrophic, erythematous, and hyperpigmented plaques involving the scalp, face, and extremities. Multiple flares with extensive ulceration of hands and feet. |
Not reported. | Not reported. | Topical corticosteroid, griseofulvin, dapsone, azathioprine, hydroxychloroquine, cyclophosphamide, cyclosporine, isotretinoin, erythromycin, beta-carotene, methotrexate, and photochemotherapy with psoralen and ultraviolet A → NI Prednisone → Effective, but with significant side effects. Thalidomide 100 mg alternating with 50 mg daily → Ulcers and facial rash cleared within 1 mo; no significant new eruptions for 19 y on this medication. 8 y into treatment trial of mycophenolate mofetil due to concerns of long-term use of thalidomide → Disease flared and thalidomide was restarted. |
Incomplete workup |
| Chopra et al25 | 45 yo; F | Well-defined, scaly plaques showing central atrophy and erythematous vesicular borders over both the dorsa of feet and buttocks and follicular popular lesions over buttocks and lumbar area. 6 mo later developed erythema over the butterfly area of face. |
Foot—hyperkeratosis, parakeratosis, acanthosis, and elongation of rete ridges, with sharpening of rete ridges giving sawtoothed appearances in some places. DEJ showed linear band-like round cell infiltration. Reticular dermis showed perivascular infiltrate and in places there was massive round cell infiltration. |
No ANA reported Routine blood tests initially WNL. 6 mo later, anemic (HGB 8.5 g/dL) with leukopenia (3500 cells per microliter). |
Topical corticosteroid → NI No further treatment reported. |
Incomplete workup |
| Abreu-Velez et al26 | 59 yo; White; F | Multiple well-defined flat papulosquamous scaly violaceous plaques, mostly in the forearms, in the anterior neck, scalp, ears, shoulders, elbows, ears, buttocks, hands, fingers, and under the chin. Ulcerated plaques on the palate inside the mouth. |
Skin—unremarkable epidermis. Mild focal interface infiltrate of lymphocytes and histiocytes. Within the dermis, a superficial and deep perivascular and periadnexal infiltrate of lymphocytes, histiocytes, and plasma cells was observed. PAS stain showed mild reinforcement of the BMZ of the DEJ. Suggestive of LP/LE overlap. DIF revealed deposits of IgG, IgM, C3, and fibrinogen in a granular pattern at the BMZ of the DEJ and deposits of IgA surrounding the papillary dermal blood vessels. IgG and C3 were also present at the BMZ of the sebaceous glands. |
No ANA reported Routine blood tests WNL. |
Topical triamcinolone steroid cream 3 times daily, prednisone 40 mg taper, and mouth washes → Good response. | Incomplete workup |
| Van der Horst et al11—case 3 | 29 yo; M | LE-like lesions on face, forearms. Lichenoid papules on wrists. Mucosal lesions in mouth. |
Mouth—HP and DIF showed LP. |
No ANA reported. (+) Rheumatoid factors. Anti-dsDNA negative. ESR 32 mm/h. Marginal thrombocytopenia. |
Not individually reported. See case 1. |
Incomplete workup |
| Van der Horst et al11—case 6 | 41 yo; M | LE-like lesions on scalp. Lichenoid papules on penis. Mucosal lesions in mouth. |
Mouth—HP and DIF not done. |
No ANA reported. (+) Rheumatoid factors. Anti-dsDNA negative. Elevation of alkaline phosphatase and GGT. ESR 13 mm/h. |
Not individually reported. See case 1. |
Incomplete workup |
AA, African American; Abs, antibodies; ALT, alanine aminotransferase; ANA, antinuclear antibody; AST, aspartate aminotransferase; BM, basement membrane; BMZ, basement membrane zone; CBC, complete blood cell count; CH50, 50% hemolyzing dose of complement; CLE, cutaneous lupus erythematosus; C1q, complement 1q; CMP, comprehensive metabolic panel; CRP, C-reactive protein; CSA, cyclosporine A; DEJ, dermoepidermal junction; DIF, direct immunofluorescence; DLE, discoid lupus erythematosus; dsDNA, double-stranded DNA; ELISA, enzyme-linked immunosorbent assay; ENA, extractable nuclear antigen; ESR, erythrocyte sedimentation rate; F, female; GGT, gamma-glutamyl transferase; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; Hep, hepatitis; HGB, hemoglobin; HP, histopathology; IF, immunofluorescence; Ig, immunoglobulin; LE, lupus erythematosus; LFT, liver function test; LP, lichen planus; M, male; NI, no improvement; PAS, periodic acid–Schiff; RF, rheumatoid factor; RNP, ribonucleoprotein; Scl-70, scleroderma-70; SCLE, subacute cutaneous lupus erythematosus; SLE, systemic lupus erythematosus; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone; U1RNP, U1 ribonucleoprotein; VDRL, venereal disease research laboratory; WNL, within normal limits; yo, years old.
Our hope is that this proposed criterion will benefit future research by helping to identify cases of this rare disorder and by generating discourse among clinicians to publish their own thoughts and experiences regarding appropriate criteria for and treatment of CLE/LP overlap syndrome. There are several limitations to our proposed criterion. First, many of the clinical and histologic features of CLE and LP intrinsically overlap, which may lead to the overcalling of purely CLE cases as CLE/LP overlap syndrome. Second, many cases of chronic CLE are seronegative, which means that these cases would not be included in our definition of “classic” CLE/LP overlap syndrome; however, these cases may be captured by our proposed “possible” cases of overlap. Ultimately, further studies will be needed to elucidate the pathogenesis and most effective treatments for CLE/LP overlap syndrome.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Lospinoso D.J., Fernelius C., Edhegard K.D., Finger D.R., Arora N.S. Lupus erythematosus/lichen planus overlap syndrome: successful treatment with acitretin. Lupus. 2013;22(8):851–854. doi: 10.1177/0961203313492243. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz S., Vatanchi M., Alapati U. Seven-year itch: a perplexing case of lichen planus-lupus erythematosus overlap syndrome. Dermatol Online J. 2018;24(9) 13030/qt40g268t4. [PubMed] [Google Scholar]
- 3.Nagao K., Chen K.R. A case of lupus erythematosus/lichen planus overlap syndrome. J Dermatol. 2006;33(3):187–190. doi: 10.1111/j.1346-8138.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- 4.Okon L.G., Werth V.P. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27(3):391–404. doi: 10.1016/j.berh.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Cleach L., Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366(8):723–732. doi: 10.1056/NEJMcp1103641. [DOI] [PubMed] [Google Scholar]
- 6.Weedon D. 3rd ed. Elsevier; 2010. Weedon's Skin Pathology. [Google Scholar]
- 7.Mutasim D.F., Adams B.B. Immunofluorescence in dermatology. J Am Acad Dermatol. 2001;45(6):803–822. doi: 10.1067/mjd.2001.117518. [quiz: 822] [DOI] [PubMed] [Google Scholar]
- 8.Carrizosa A.M., Elorza F.L., Camacho F.M. Antinuclear antibodies in patients with lichen planus. Exp Dermatol. 1997;6(1):54–56. doi: 10.1111/j.1600-0625.1997.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Grabbe S., Kolde G. Coexisting lichen planus and subacute cutaneous lupus erythematosus. Clin Exp Dermatol. 1995;20(3):249–254. doi: 10.1111/j.1365-2230.1995.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 10.Kiyani A., Shahroz N. Synchronous presentation of systemic lupus erythematosus and oral reticular lichen planus. JAAD Case Rep. 2018;4(2):135–137. doi: 10.1016/j.jdcr.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Horst J.C., Cirkel P.K., Nieboer C. Mixed lichen planus-lupus erythematosus disease: a distinct entity? Clinical, histopathological and immunopathological studies in six patients. Clin Exp Dermatol. 1983;8(6):631–640. doi: 10.1111/j.1365-2230.1983.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 12.Shahzadi N., Khurshid K., Pal S.S. Lichen planus/lupus erythematosus overlap with hypothyroidism: a case report. J Pak Assoc Dermatol. 2008;18:238–240. [Google Scholar]
- 13.Smirnov B., Bowles A.A., Strasswimmer J.M., Nousari C.H. Lupus erythematosus lichen planus overlap syndrome mimicking squamous cell carcinoma. J Clin Aesthet Dermatol. 2019;12(9):36–38. [PMC free article] [PubMed] [Google Scholar]
- 14.Patil P., Nayak C., Tambe S., Das D. Lupus erythematosus-lichen planus overlap syndrome in an HIV-infected individual. Int J STD AIDS. 2016;27(12):1117–1122. doi: 10.1177/0956462415618109. [DOI] [PubMed] [Google Scholar]
- 15.Komori T., Otsuka A., Honda T., Kaku Y., Kabashima K. A case of chilblain lupus erythematosus with lupus erythematosus/lichen planus overlap syndrome. J Eur Acad Dermatol Venereol. 2017;31(9):e424–e425. doi: 10.1111/jdv.14239. [DOI] [PubMed] [Google Scholar]
- 16.Camisa C., Neff J.C., Olsen R.G. Use of indirect immunofluorescence in the lupus erythematosus/lichen planus overlap syndrome: an additional diagnostic clue. J Am Acad Dermatol. 1984;11(6):1050–1059. doi: 10.1016/s0190-9622(84)70258-1. [DOI] [PubMed] [Google Scholar]
- 17.Tursen U., Oz O., Ikizoglu G., Kaya T.I., Dusmez D. A case of lichen planus-lupus erythematosus overlap syndrome with eyelid involvement. Eur J Ophthalmol. 2002;12(3):244–246. doi: 10.1177/112067210201200314. [DOI] [PubMed] [Google Scholar]
- 18.Sekar C.S., Rai R., Karthika N., Laila A. Scle-lp overlap syndrome. Indian J Dermatol. 2011;56(2):209–210. doi: 10.4103/0019-5154.80420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R.W., Nesbitt L.T., Jr., Reed R.J. Unusual variant of lupus erythematosus or lichen planus. Clinical, histopathologic, and immunofluorescent studies. Arch Dermatol. 1977;113(6):741–748. [PubMed] [Google Scholar]
- 20.Demirci G.T., Altunay I.K., Sarıkaya S., Sakiz D. Lupus erythematosus and lichen planus overlap syndrome: a case report with a rapid response to topical corticosteroid therapy. Dermatol Rep. 2011;3(3):e48. doi: 10.4081/dr.2011.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inalöz H.S., Chowdhury M.M., Motley R.J. Lupus erythematosus/lichen planus overlap syndrome with scarring alopecia. J Eur Acad Dermatol Venereol. 2001;15(2):171–174. doi: 10.1046/j.1468-3083.2001.00223.x. [DOI] [PubMed] [Google Scholar]
- 22.De Jong E.M., Van De Kerkhof P.C. Coexistence of palmoplantar lichen planus and lupus erythematosus with response to treatment using acitretin. Br J Dermatol. 1996;134(3):538–541. [PubMed] [Google Scholar]
- 23.Jamison T.H., Cooper N.M., Epstein W.V. Lichen planus and discoid lupud erythematosus. Overlap syndrome associated with cryoglobulinemia and hypocomplementemia. Arch Dermatol. 1978;114(7):1039–1042. [PubMed] [Google Scholar]
- 24.Zhang L., Au S., Aronson I.K. Successful long-term thalidomide therapy for discoid lupus erythematosus-lichen planus overlap syndrome. Dermatol Online J. 2014;20(10) 13030/qt73r7492v. [PubMed] [Google Scholar]
- 25.Chopra A., Bahl R.K., Puri R.K., Gill S.S. Lichen planus and lupus erythematosus overlap syndrome. Indian J Dermatol Venereol Leprol. 1996;62(2):110–111. [PubMed] [Google Scholar]
- 26.Abreu-Velez A.M., Brown V.M., Howard M.S. Antibodies to piloerector muscle in a patient with lupus-lichen planus overlap syndrome. N Am J Med Sci. 2010;2(6):276–280. [PMC free article] [PubMed] [Google Scholar]