Abstract
Background
Post-COVID-19 Olfactory impairment has a negative impact on quality of life. The Sniffin Sticks test 12 items (SST-12) can be used in quick olfactory disorders screening. Its evaluation in a post-covid-19 situation was the main objective of this work.
Methods
All patient impaired with a post-COVID olfactory loss were included while consulting to the ENT department. The clinical examination included an olfaction recovery self-assessment (VAS), a nasofibroscopy, a quality of life (QoL) assessment, the complete Sniffin’ Sticks Test (SST), and the SST-12.
Results
Among the 54 patients included, 92% (n = 50) were correctly screened as olfactory impaired by SST-12. We report excellent correlations between SST-12 and SST (rho (52) = 0.98, p < 0.001), QoL(rho(52) = 0.33 p = 0.016), or VAS (rho(52) = 0.49, p < 0.001) assessments.
Conclusions
SST-12 is a quick and reliable tool to screen large-scale population of post-COVID-19 olfactory impaired patients and could be used in a general daily clinical practice.
Keywords: Olfactory dysfunction, Screening, Sniffin sticks test 12 items, Covid-19, Olfaction
Introduction
The onset of a sudden partial (hyposmia) or total (anosmia) loss of smell is now recognized as highly predictive of SARS-COV-2 infection [1]. A long-lasting anosmia can lead to a quality of life [2] impairment, bad diet habits [3], changes in social relations [4, 5] psychiatric disorders, such as depression [6], anxiety, or anorexia [7] and its nutritional consequences [8], and finally cognitive impairment [4]. Thus, olfactory disorders have to be diagnosed and managed especially as 12 [9] months after the COVID-19 infection, 30% of patients retain an olfactory complaint and require attention.
Although there are different ways to assess a patient's ortho and retro-olfaction [10, 11], only 50% of ENTs assess the olfactory disorders on an anamnesis, and 10% assess through psychophysical tests [12]. Olfaction is most often evaluated by subjective self/hetero questionnaires with a significant variability of the results and a probable underestimation [12], given the poorer olfactory perception before 20 years and after 50 [13, 14]. Complete psychophysical olfactory tests, with assessment of odor threshold, odor discrimination, and odor identification, are the gold standard [12] and allow to specify the olfactory disorder [15]. The most used in Europe is the Sniffin’ sticks test® (SST) [12, 16] that includes an odor Threshold detection (T), an odor Discrimination (D), and an odor Identification (I) tests. However, these psychophysical tests are expensive and take a long time (between 30 and 60 min)) [10, 11], thus making their daily clinical use difficult. It therefore seems important to look for other olfactory tests that are faster (≤ 5 min) and accessible to specialists, but also to general practitioners.
The Sniffin’ Sticks Test 12 items (SST-12) is an olfactory screening test in the form of a 4-min identification test allowing, according to its authors, to detect anosmia and hyposmia with comparable measurement reliability other similar scent screening tests [17–19]. It can also be used laterally (one nostril tested independently of the other).
Seeing that it has been demonstrated that identification impairment is predominant in post-COVIDs [2, 20], the objective of this study was to assess the potential value of SST-12 in screening and characterizing a persistent post-COVID-19 olfactory disorder in a daily practice.
Material and methods
Population
This study was approved by the institutional review board of the Nice University Hospital (CNIL number: 412) and registered with a ClinicalTrials.gov number (ID: NCT04799977). Since March 2020, we prospectively enrolled patients at ENT division of Nice University Hospital from March 2020 to May 2021. All were contaminated by COVID-19 with persistent olfactory disorders more than 6 weeks (2 to 14 months). Patients where mainly self-referred or referred by general practitioners or colleagues. Patients had either a RT-PCR-proven SARS-CoV-2 diagnosis or a CT-proven SARS-CoV-2 diagnosis secondarily confirmed by serology. We retrospectively extracted patients’ demographic data and clinical features, including nasofibroscopy, subjective taste impairment, visual analog scale (VAS) for the subjective assessment of olfactory recovery (ranging from 0 to 100%), diet habits modifications (over consumption of salt and sugar), and olfactory quality of life. Total and subdomains SST [21–23] score results were systematically assessed.
Sniffin’ sticks test 12 items
Olfaction diseases SST-12 test has been validated in 2001 by Hummel et al. [19]. This 4-min screening psychophysical test is an odor identification test based on 12 from the 16 odors being sniffed during the identification subdomain part of the original SST.
The original SST identification odors set include peppermint, orange, fish, leather, rose, cloves, coffee, pineapple, licorice, anise, lemon, banana, cinnamon, apple, turpentine, and garlic. During the identification SST test, subjects were blindfolded. Sixteen odorant sticks were presented once, separated by an interval of at least 20 s to prevent olfactory desensitization. Each stick presentation was accompanied by a written list containing the correct odorant and 3 semantic distractors. Retrospectively, results from all odors set but apple, turpentine, garlic and anise were summed up to the SST-12 global score, as previously described [19]. We defined a normosmia (SST-12 ≥ 11), an hyposmia (10 > SST-12 > 6), or an anosmia (SST-12 ≤ 6) based on normative values assessed from more than 1200 patients assessed with SST and olfactive evoked potential for anosmic and hyposmic ones [19]. Apple, turpentine, and garlic have been removed from the SST-12 because identified by less than 55% of its normosmic validation cohort [19]. Anise was removed too because of being too similar to liquorice. With a reproducibility kappa coefficient of 0.77, the diagnosis agreement can be considered as “good” (Altman, 1991). Although olfactory abilities decreased at extreme ages, SST-12 can be used before the age of 10 and after the age of 80.
Olfactory quality of life
The Questionnaire of Olfactory Disorders (QOD) is a widely validated tool related to olfaction quality of life evaluation [24]. Fifty-two items are reported in the original version regarding negative and positive social impacts of olfactory loss [25], but only negative statements subdomain of QOD(QOD-NS) [26] has been shown to be more consistent with SST results [27]. Moreover, being shorter than the original QOD, QOD-NS is more suitable for daily clinical practice [28, 29], increasing the response rate and reducing patient's mental burden while using it [28]. In order to improve QOD-NS, Mattos et al. [28] developed an even shorter version (Short-QOD-NS) with 7 most consistent questions related to social aspect (n = 3), eating (n = 2), anxiety (n = 1), and annoyance (n = 1) following an olfactory loss. Being now validated [30] in French [31], Short-QOD-NS was used this study allowing to evaluate olfactory quality of life with a score ranging from 0 to 21 (21 means there is no disorder).
Statistical analysis
Quantitative variables are presented as mean (SD) and qualitative variables as frequency and percentage. The degree of accordance between the SST and the SST-12 in patients’ categorization was calculated using Cohen’s Kappa coefficient. Sensitivity and specificity of the SST-12 compared to the SST in classifying patients as anosmic were also reported. To verify whether patients who increased their consumption of salt and sugar had lower SST, SST-12, and Short-QOD-NS scores compared to those who did not, we employed Mann–Whitney U tests. Chi2 tests were employed to explore links between self-reported taste disorders and the presence of an increased salt and sugar consumption. To investigate correlations between subjective reports (VAS), odor identification disorders (based on the SST and SST-12), and QOD, we performed bivariate correlation analyses. As data were not normally distributed (as suggested by Kolmogorov–Smirnov test), non-parametric Spearman’s correlations were employed. All tests were considered significant for alpha < 0.05.
Results
Demographic and clinical features
We included fifty-four patients from the ENT department of Nice University Hospitals (CHU) complaining about olfactory loss, 5.4 ± 3.1 months after a COVID-19 infection. Clinical features and demographic data are reported in Table 1. Forty-three of patients were male (n = 23), with a mean age of 39.9 ± 13.9 years. 17 patients (31.5%) received a COVID-19 dedicated treatment, but no one did olfactory training before. All patients had a mild illness form.
Table 1.
Demographic and clinical characteristics
| n | % | |
|---|---|---|
| Total | 54 | 100 |
| COVID-19 testing | ||
| Molecular PCR test | 46 | 85.2 |
| Chest CT | 11 | 20.4 |
| Serology (antibody test) | 16 | 29.6 |
| COVID-19 dedicated treatment | ||
| Oral corticosteroids | 6 | 11.1 |
| Nasal corticosteroids | 4 | 7.4 |
| Inhaled corticosteroids | 2 | 3.7 |
| Azithromycin alone | 7 | 13.0 |
| Hydroxychloroquine alone | 1 | 1.9 |
| Azithromycin + Hydroxychloroquine | 3 | 5.5 |
| Amoxicillin alone | 1 | 1.9 |
| Amoxicillin + Azithromycin | 2 | 3.7 |
| Others (vitamins, zinc) | 5 | 9.3 |
| Self-reported olfactory disorders | ||
| Hyposmia | 4 | 7.4 |
| Anosmia | 49 | 90.7 |
| Parosmia | 11 | 20.4 |
| Phantosmia | 13 | 24.1 |
| Taste disorders | 49 | 90.7 |
| Retro-olfaction alone | 35 | 64.8 |
| Retro-olfaction + taste | 12 | 22.2 |
| Taste alone | 1 | 1.8 |
CT computerized tomography, PCR polymerase chain reaction
Retrospective olfactory and taste complains screening results
Subjective, psychophysical, and quality of life tests results relatives to the loss of smell are reported in Table 2. On the day of consultation, patients reported to have recovered only 33.9 ± 25.6% of their olfaction (ranging from 0 to 90%). 90.7% of the patients (n = 49) reported taste disorders divided among retro-olfaction (food flavors) alone (64.8%, n = 35), retro-olfaction associated to taste (22.2%, n = 12; 16.7% concerning sweet and salty, 11.1% concerning sour and bitter), or taste alone (1.8%, n = 1 concerning sweet and salty). 45.5% of patients (20 out of the 44 who responded to the question) reported that they increased their consummation of salt, and 20.5% (9 out of 44) that they increased their consummation of sugar. In terms of self-reported symptoms, 90.7% of the patients (n = 49) reported to suffer from anosmia, 7.4% (n = 4) from hyposmia, 20.4% (n = 11) from parosmia, and 24.1% (n = 13) from phantosmia.
Table 2.
Visual analog scale (VAS), Sniffin’ sticks tests, and short version of questionnaire of olfactory disorders (Short-QOD-NS) results
| Mean | SD | |
|---|---|---|
| VAS (subjective % of olfactory recovery) | 33.9 | 25.6 |
| Sniffin’ Sticks test—scores | ||
| Threshold detection | 4.7 | 4.0 |
| Discrimination | 10.3 | 3.1 |
| Identification | 9.4 | 3.9 |
| Short-QOD-NS—total | 11.1 | 5.0 |
| Short-QOD-NS—Eating | 3.1 | 2.2 |
| Short-QOD-NS—Anxiety | 2.1 | 1.0 |
| Short-QOD-NS—Annoyance | 1.0 | 1.1 |
| Short-QOD-NS—Social | 4.7 | 2.5 |
SD standard deviation
Categorization of patients based on the results of the olfactory tests is presented in Table 3. The global results (TDI) of the Sniffin’ Sticks Test (SST) suggested that 24.1% (n = 13) of the patients could be classified as normosmic (TDI ≥ 30.75), 53.7% (n = 29) as hyposmic (16.25 ≤ TDI ≤ 30.5), and 22.2% (n = 12) as functional anosmic (TDI ≤ 16). Based on the SST-12, 14.8% (n = 8) of the patients could be classified as normosmic (SST-12 ≥ 11), 48.1% (n = 26) as hyposmic (6 < SST-12 < 10), and 37% (n = 20) as functional anosmic (SST-12 ≤ 6). Interestingly, patients who increased their consummation of salt showed lower SST (U = 112.5, p = 0.003) and SST-12 (U = 121, p = 0.005) scores compared to the patients who did not increase salt usage (20.0 ± 8.8 vs. 27.9 ± 7.7, and 5.7 ± 3.5 vs. 8.5 ± 2.2, respectively). The same result was found for patients who increased their consummation of sugar, that showed lower SST (U = 73.5, p = 0.014) and SST-12 (U = 62, p = 0.005) scores compared to the other patients (26.2 ± 8.0 vs. 17.1 ± 8.4, and 4.6 ± 2.9 vs. 6.9 ± 2.9, respectively). Interestingly, patients who increased their consummation of sugar had a lower Short-QOD-NS alimentary subscale 1.4 ± 1.8 vs. 3.5 ± 2.0; U = 66.5, p = 0.006). The self-reported presence of taste disorders did not show any significant link with the presence of an increased consummation of salt (Chi2 = 0.74, p = 389) or sugar (Chi2 = 1.13, p = 287).
Table 3.
Categorization of subjects based on the Sniffin’ Sticks Test scores (SST), Sniffin’ Sticks Test 12 items scores (SST-12), and inter-test reliability (Kappa)
| N = 54 | Normosmic N (%) | Hyposmic N (%) | Anosmic N (%) |
|---|---|---|---|
| SST | 13(24.1) | 29(53.7) | 12(22.2) |
| SST-12 | 8(14.8) | 26(48.1) | 20(37.0) |
| Correct categorization | 4(7.4) | 17(31.5) | 12(22.2) |
| False positive | 0(0) | 9(16.7) | 8(14.8) |
| False negative | 4(7.4) | 0(0) | 0(0) |
| Cohen’s Kappa | 0.242 | 0.224 | 0.654 |
SD standard deviation
Taking SST as the gold standard, on 54 patients, 61% (n = 33) were classified in the same category by the SST-12 patients. SST-12 misdiagnosed 4 patients as normosmic (7.4%), 8 as anosmic (14.8%), and 9 as hyposmic (16.7%). Importantly, all the patients who were diagnosed as anosmic by the SST were also detected by the SST-12. Accordingly, Cohen’s Kappa coefficient revealed a week agreement between the two tests in classifying patients as normosmic (Kappa = 0.24) and hyposmic (Kappa = 0.22), but a strong agreement in classifying patients as anosmic (Kappa = 0.65). The sensitivity and specificity of the SST-12, compared to the SST score, are reported in Table 4 and suggest that a score of 6 is the cut-off that maximizes the combination between specificity (100%) and sensitivity (81%) in detecting anosmic patients. The presence of taste disorders did not affect the type of errors of the SST-12 compared to the SST.
Table 4.
Sensitivity and specificity of the different cut-off scores of the SST-12 compared to the SST in classifying patients as anosmic
| SST-12 score | Sensitivity | Specificity |
|---|---|---|
| 0 | 1.000 | 0.00 |
| 1 | 1.000 | 0.17 |
| 2 | 1.000 | 0.25 |
| 3 | 0.98 | 0.33 |
| 4 | 0.93 | 0.42 |
| 5 | 0.88 | 0.75 |
| 6 | 0.81 | 1.00 |
| 7 | 0.69 | 1.00 |
| 8 | 0.55 | 1.00 |
| 9 | 0.38 | 1.00 |
| 10 | 0.19 | 1.00 |
| 11 | 0.07 | 1.00 |
| 12 | 0.00 | 1.00 |
The cut-off of 6, which maximizes the sensitivity/specificity ratio, is reported in bold
Correlations between self-reported olfactory recovery, SST, SST-12 score, and Short-QOD-NS
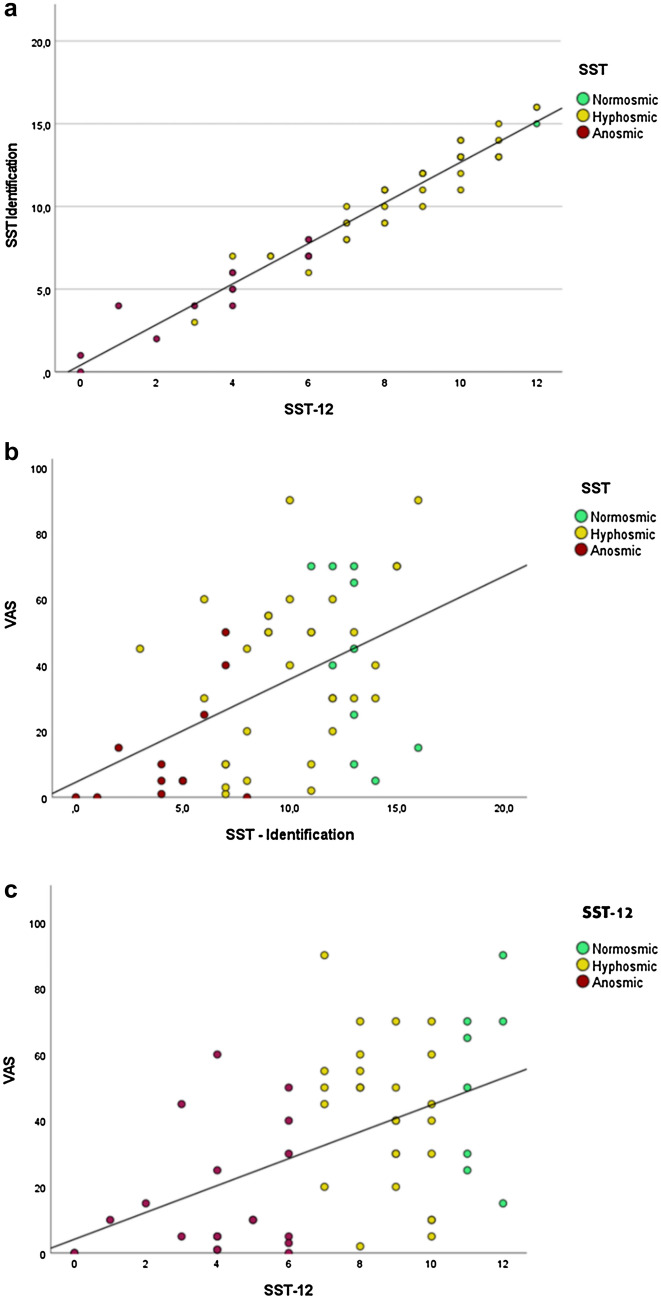
VAS scores were 45 ± 24% (range 5–70%), 38 ± 25% (range 1–90%), and 13 ± 16% (range 0–50%) for, respectively, normosmic, hyposmic, and anosmic patients, based on the SST. Based on the SST-12, VAS scores were 52 ± 26%, 42 ± 22%, and 16 ± 19% for, respectively, normosmic, hyposmic, and anosmic patients. An almost perfect correlation between scores at the SST identification score and SST-12 was found (rho(52) = 0.98, p < 0.001), confirming that the SST-12 can assess odor identification as well as the SST. We observed positive correlation between VAS recovery (%) and the identification scores for both the SST (rho(52) = 0.47, p < 0.001) and the SST-12 scores (rho(52) = 0.49, p < 0.001), testifying that the two scales were equally correlated to self-reported disorders. These results are reported in Fig. 1.
Fig. 1.
Correlations between a SST-12 and SST identification score, b percentage of subjective olfactory recovery (VAS) and SST, and c percentage of subjective olfactory recovery (VAS) and SST-12
Olfactory quality of life—We found a significant positive correlation between the SST global score and the global (rho(52) = 0.30, p = 0.027) and social Short-QOD-NS subscales (rho(52) = 0.38, p = 0.041). Concerning identification scores, a significant correlation was found between SST-I and the global Short-QOD-NS (rho(52) = 0.29, p = 0.036), between SST-I and the Short-QOD-NS eating subscale (rho(52) = 0.29, p = 0.036), between SST-12 and the global Short-QOD-NS (rho(52) = 0.33 p = 0.016), its eating (rho(52) = 0.32 p = 0.017), and anxiety (rho(52) = 0.29 p = 0.032) subscales. The only significant correlation with the SST discrimination score was with the anxiety subscale (rho(52) = 0.27, p = 0.048). No significant correlation between SST threshold scale and Short-QOD-NS was found. The VAS score showed positive correlations with the global Short-QOD-NS (rho(52) = 0.39, p = 0.003) and with the Short-QOD-NS social (rho(52) = 0.31, p = 0.022) and eating (rho(52) = 0.40, p = 0.003) subscales.
Discussion
This is the first study that evaluates quantitatively the efficiency of the SST-12 to screen for post-COVID-19 olfactory disorders, and specially to identify post-COVID-19 anosmic patients.
Screening for olfactory disorders is important because, in addition to allowing to set up appropriate care for patients, it helps prevent the occurrence of consequences of long-term anosmia, like an alteration in the quality of life [2], bad diet habits [3], changes in social relations [4], psychiatric disorders, such as depression [6], anxiety, or anorexia [7], its nutritional consequences [8], and finally cognitive impairment [4]. Although a subjective olfactory complaint (80% anosmia, 20% hyposmia) is now a very frequent symptom of a COVID-19 infection [32] affecting 70 to 85% of patients [33, 34], only 21% of clinicians use psychophysical olfactory tests to characterize this olfactory complaint [35].
The present study shows the reliability of SST-12 in screening for post-COVID-19 olfactory disorders, and in particular anosmia. Among diagnostic errors, only 7% (n = 4) of hyposmic patients would have been considered normosmic by SST-12. The other differences in scores between the SST-12 and the SST do not modify the purpose of the screening, which is to perform or have performed complete olfactory tests in the event of an abnormality detected. In this way, all but 4 patients (92%) would have been correctly screened using the SST-12. All the post-COVID-19 anosmic patients at the SST were correctly screened by the SST-12 as evidenced by the "good" correlation coefficient (0.61 ≤ Kappa ≤ 0,80—Altman 1991). Full identification subdomain test of SST, with 16 items, is often used in COVID-19 olfactory complain assessment [34, 36, 37] but, although already validated [38], remains longer, and uses garlic, turpentine, apple, and anis which are poorly identified by normosmic patients and raising the risk of overestimating the olfactory impairment [19]. SST-12 thus seems to be perfectly suited to face the challenge of large cohort quick (four minutes [19]) screening potentially impaired with olfactory disorder, especially concerning general practitioners.
The total SST-12, as SST, was significantly correlated (p < 0.001) with odor complaint (VAS—Fig. 1) which reflects persistent post-COVID-19 olfactory impairment, i.e., mainly identification disorders rather than threshold or discrimination [2] as we published previously. Post-COVID-19 olfactory disorders show unique psychophysical characteristics consistent with a central olfactory impairment [2, 15]. In a population of 34 patients deprived of their olfaction for about 6 months after COVID-19 and presenting a persistent odor complaint (VAS), we previously highlighted a significantly predominant, and gradually worsening, odor identification impairment [2], as Ianuzzi et al. [20] underlined a lack of identification recovery in their 30 patients study. Unlike the SST, the SST-12 only assesses the identification of odors and thus seems more suited to detect an identification disorder than the SST, which adds to the identification score, a score for discrimination, and perception of the odor threshold. As shown in previous post-COVID-19 olfactory impairment studies [2, 36], the SST interpretation can conclude to a global normosmia even if patients actively complain about their olfaction. The full SST (i.e., threshold, discrimination, and identification global score) might therefore not be used as a gold standard in the post-COVID-19 odor evaluation given that some patients, early [37] and at a distance [2] from SARS-COV-2 infection, may be incorrectly classified as normosmic on SST despite olfactory complain [2].
In this study we found that olfactory complain (VAS) was significantly linked to an SST or SST12 impairment, justifying the no need to a psychophysical screening test to take social distancing and barrier measures in case of acute olfactory disorders in COVID-19 pandemic times. Moreover, 7% of hyposmic patients could be missed with such a test. This is especially true since such a screening test poses a contamination risk to the examiner. However, at a distance from acute infection, SST-12 could be helpful to screen post-COVID-19 olfactory disorders. In case of a complaining person, as our results suggest, a complete psychophysical olfactory test might be directly performed as an olfactory complain is highly correlated with an impaired SST and SST-12. But in case of a non-complaining, or olfactory impairment unaware, post-COVID-19 patient, SST-12 could prevent negative consequences of unknown olfactory disorders, especially quality of life [39] and metabolic impairments.
Indeed, smell loss causes a significant deterioration in the quality of life [39]. QOD and specifically QOD-NS [26, 39] are often used for ENT olfaction assessment but could be a problem in clinical research as patients’ mental burden could be important. The Mattos et al. [28] short version has been chosen in this work as it shows a strong correlation with QOD-NS total and subdomain-specific scores. Less time consuming, it better fits with the constraints of the routine clinical assessment. In our study, Short-QOD-NS results underlined the quality of life impairment of an olfactory loss and show a significant correlation with SST, SST-12, and VAS results, specifically when identification is impaired, as we published in a previous work [2]. Short-QOD-NS subscales analysis showed mainly that eating subscale is always significantly impaired. This could explain that 45.5 and 20.5% (n = 44) of post-COVID-19 patients increased, respectively, their daily diet salt and sugar intake. As previously published, salt and sugar intake increased concerned near 30% of COVID-19 patients [40], especially young women. Our results suggest that these bad diet habits could concern in fact olfactory impaired post-COVID-19 patients, especially anosmic ones (SST-12 ≤ 6) being deprived of their original food tastes and trying to enhance it whatever the way. Interestingly, there is no significant relation, otherwise only with the SST-12 score, between the risk of bad diet habits and subjective olfactory complain, underlining the benefits of using SST-12. The sugar intake is also concerned as COVID-19 could basically raise blood glucose and HbA1c levels [41], which has to be monitored after hospital discharge. It is a major public health concern as post-COVID-19 olfaction disorders recovery time is still uncertain and long-term salt and sugar intake could increase, respectively, blood pressure [42] and type 2 diabetes [43] onset and so cardiovascular risk. In case of SST-12 screened anosmia, a not to change daily use of salt and sugar advice must be added to the patient consultation.
Despite these interesting results, this study suffers from some limitations. The main limitation is our cohort size (54 patients), with no follow-up reported, who spontaneously consulted our university hospital, which represents the risk of a recruitment bias. The small sample size and the high number of subjective variables in this study (VAS, SST, SST-12, sugar and salt consumption) may have contributed to a limited strength of correlations (rho(32) MAX = 0.49), and therefore, our results cannot be directly generalized to all patients with a post-COVID olfactory disorder and should be verified in a larger prospective cohort study. Patients recruitment at different times from their post-COVID-19 olfactory loss introduces heterogeneity in the analysis, as many cases recover over time [44], but allowed us to evaluate SST-12 to many different impaired patients. Even if it could be a bias, we have chosen not to exclude patients who took corticosteroids because of weak evidences of its usefulness on olfactory recovery after a post-viral olfactory loss, especially in COVID-19 [45].
Conclusion
The SST-12 is an olfactory psychophysical test suitable for quick screening an olfactory sequelae post-COVID-19. Its use in the context of screening for a long olfactory COVID could be used for the implementation of personalized general practice management of olfactory disorders and the prevention of psychological and metabolic consequences.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerkin RC, Ohla K, Veldhuizen MG, et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses. 2020;2021(46):1–12. doi: 10.1093/chemse/bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vandersteen C, Payne M, Dumas L-E, et al. Persistent olfactory complaints after COVID-19: a new interpretation of the psychophysical olfactory scores. Rhinol Online. 2021;4(14):66–72. doi: 10.4193/RHINOL/21.010. [DOI] [Google Scholar]
- 3.Aschenbrenner K, Hummel C, Teszmer K, et al. The influence of olfactory loss on dietary behaviors. Laryngoscope. 2008;118(1):135–144. doi: 10.1097/MLG.0b013e318155a4b9. [DOI] [PubMed] [Google Scholar]
- 4.Valsamidis K, Printza A, Constantinidis J, Triaridis S. The impact of olfactory dysfunction on the psychological status and quality of life of patients with nasal obstruction and septal deviation. Int Arch Otorhinolaryngol. 2020;24(02):e237–e246. doi: 10.1055/s-0040-1701269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schablitzky S, Pause BM. Sadness might isolate you in a non-smelling world: olfactory perception and depression. Front Psychol. 2014;5:2. doi: 10.3389/fpsyg.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur K, Choi JS, Zheng M, Shen J, Wrobel B. Association of alterations in smell and taste with depression in older adults. Laryngoscope Investig Otolaryngol. 2018;3(2):94–99. doi: 10.1002/lio2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life-an updated review. Chem Senses. 2014;39(3):185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 8.Nordin S. Food for the ageing population. Elsevier; 2009. Sensory perception of food and ageing; pp. 73–94. [Google Scholar]
- 9.Boscolo-Rizzo P, Guida F, Polesel J, et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur Arch Oto-Rhino-Laryngol. 2021;2:0123456789. doi: 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doty RL. Office procedures for quantitative assessment of olfactory function. Am J Rhinol. 2007;21(4):460–473. doi: 10.2500/ajr.2007.21.3043. [DOI] [PubMed] [Google Scholar]
- 11.Su B, Bleier B, Wei Y, Wu D. Clinical implications of psychophysical olfactory testing: assessment, diagnosis, and treatment outcome. Front Neurosci. 2021;15:1–12. doi: 10.3389/fnins.2021.646956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol J. 2017;54(26):1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 13.Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Oto-Rhino-Laryngology. 2019;276(3):719–728. doi: 10.1007/s00405-018-5248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sorokowska A, Schriever VA, Gudziol V, et al. Changes of olfactory abilities in relation to age: odor identification in more than 1400 people aged 4 to 80 years. Eur Arch Oto-Rhino-Laryngology. 2015;272(8):1937–1944. doi: 10.1007/s00405-014-3263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitcroft KL, Cuevas M, Haehner A, Hummel T. Patterns of olfactory impairment reflect underlying disease etiology. Laryngoscope. 2017;127(2):291–295. doi: 10.1002/lary.26229. [DOI] [PubMed] [Google Scholar]
- 16.Sorokowska A, Oleszkiewicz A, Minovi A, Konnerth CG, Hummel T. Fast screening of olfactory function using the Q-sticks test. ORL. 2019;81(5–6):245–251. doi: 10.1159/000500559. [DOI] [PubMed] [Google Scholar]
- 17.Doty RL, Marcus A, William LW. Development of the 12-item cross-cultural smell identification test(CC-SIT) Laryngoscope. 1996;106(3):353–356. doi: 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Jackman AH, Doty RL. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope. 2005;115(12):2209–2212. doi: 10.1097/01.mlg.0000183194.17484.bb. [DOI] [PubMed] [Google Scholar]
- 19.Hummel T, Rosenheim K, Konnerth C-G, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110(10):976–981. doi: 10.1177/000348940111001015. [DOI] [PubMed] [Google Scholar]
- 20.Iannuzzi L, Salzo AE, Angarano G, et al. Gaining back what is lost: recovering the sense of smell in mild to moderate patients after COVID-19. Chem Senses. 2020;45(9):875–881. doi: 10.1093/chemse/bjaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odour identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22(1):39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Allis TJ, Leopold DA. Smell and taste disorders. Facial Plast Surg Clin North Am. 2012;20(1):93–111. doi: 10.1016/j.fsc.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Rumeau C, Nguyen DT, Jankowski R. Comment tester l’olfaction avec le Sniffin’ Sticks test®. Ann françaises d’Oto-rhino-laryngologie Pathol Cervico-faciale. 2016;133(3):183–186. doi: 10.1016/j.aforl.2015.04.010. [DOI] [Google Scholar]
- 24.Brämerson A, Nordin S, Bende M. Clinical experience with patients with olfactory complaints, and their quality of life. Acta Otolaryngol. 2007;127(2):167–174. doi: 10.1080/00016480600801357. [DOI] [PubMed] [Google Scholar]
- 25.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Oto-Rhino-Laryngology. 2005;262(3):231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 26.Mattos JL, Schlosser RJ, DeConde AS, et al. Factor analysis of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8(7):777–782. doi: 10.1002/alr.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope. 2012;122(7):1450–1454. doi: 10.1002/lary.23349. [DOI] [PubMed] [Google Scholar]
- 28.Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2019;9(10):1144–1150. doi: 10.1002/alr.22392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smeets MAM, Veldhuizen MG, Galle S, et al. Sense of smell disorder and health-related quality of life. Rehabil Psychol. 2009;54(4):404–412. doi: 10.1037/a0017502. [DOI] [PubMed] [Google Scholar]
- 30.Han P, Su T, Qin M, Chen H, Hummel T. A systematic review of olfactory related questionnaires and scales. Rhinol J. 2020;59(2):2. doi: 10.4193/Rhin20.291. [DOI] [PubMed] [Google Scholar]
- 31.Leclercq C, Chiesa-Estomba CM, Horoi M, et al. Validity and reliability of the french short version of the questionnaire of olfactory disorders-negative statements (sQOD-NS) Ear, Nose Throat J. 2021;2:2. doi: 10.1177/01455613211032004. [DOI] [PubMed] [Google Scholar]
- 32.Gane SB, Kelly C, Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinol J. 2020;58(3):299–301. doi: 10.4193/Rhin20.114. [DOI] [PubMed] [Google Scholar]
- 33.Lechien JR, Chiesa-Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck. 2020;42(7):1583–1590. doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le Bon SD, Pisarski N, Verbeke J, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Oto-Rhino-Laryngology. 2021;278(1):101–108. doi: 10.1007/s00405-020-06267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechien JR, Cabaraux P, Chiesa-Estomba CM, et al. Psychophysical olfactory tests and detection of COVID-19 in patients with sudden onset olfactory dysfunction: a prospective study. Ear, Nose Throat J. 2020;99(9):579–583. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 38.Mueller CA, Grassinger E, Naka A, Temmel AFP, Hummel T, Kobal G. A self-administered odor identification test procedure using the ‘Sniffin’’ Sticks"’. Chem Senses. 2006;31(6):595–598. doi: 10.1093/chemse/bjj064. [DOI] [PubMed] [Google Scholar]
- 39.Shu CH, Lee PO, Lan MY, Lee YL. Factors affecting the impact of olfactory loss on the quality of life and emotional coping ability. Rhinol J. 2011;49(3):337–341. doi: 10.4193/Rhino10.130. [DOI] [PubMed] [Google Scholar]
- 40.Rolland B, Haesebaert F, Zante E, Benyamina A, Haesebaert J, Franck N. Global changes and factors of increase in caloric/salty food intake, screen use, and substance use during the early COVID-19 containment phase in the general population in France: survey study. JMIR Public Heal Surveill. 2020;6(3):e19630. doi: 10.2196/19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, Wu C, Wang X, Yu J, Sun Z. The impact of COVID-19 on blood glucose: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2020;11(October):1–8. doi: 10.3389/fendo.2020.574541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;2017:4. doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lean MEJ, Te Morenga L. Sugar and type 2 diabetes. Br Med Bull. 2016;120(1):43–53. doi: 10.1093/bmb/ldw037. [DOI] [PubMed] [Google Scholar]
- 44.Hopkins C, Surda P, Vaira LA, et al. Six month follow-up of self-reported loss of smell during the COVID-19 pandemic. Rhinol J. 2020;11:2. doi: 10.4193/Rhin20.544. [DOI] [PubMed] [Google Scholar]
- 45.Huart C, Philpott CM, Altundag A, et al. Systemic corticosteroids in coronavirus disease 2019 (COVID-19)-related smell dysfunction: an international view. Int Forum Allergy Rhinol. 2021;11(7):1041–1046. doi: 10.1002/alr.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]