Abstract
Shiga toxigenic Escherichia coli (STEC) strains are a diverse group of organisms associated with severe gastrointestinal and systemic diseases in humans. Within the STEC family, eae-positive STEC strains, particularly those belonging to serogroups O157 and O111, appear to have greater virulence for humans. However, in spite of being eae negative, STEC strains belonging to serogroup O113 have frequently been associated with cases of severe STEC disease, including hemolytic-uremic syndrome (HUS). We have developed a modified multiplex PCR assay for detection of STEC strains belonging to these three serogroups in cultures of feces by using primers specific for portions of the genetic loci (rfb) encoding biosynthesis of the respective O antigen. These primers direct amplification of PCR products of 259, 406, and 593 bp for serogroups O157, O111, and O113, respectively. The assay was validated by testing 40 previously characterized STEC strains, with 100% agreement. It also detected STEC strains of the appropriate genotype in primary fecal cultures from 13 patients with HUS or bloody diarrhea. Thirty other primary fecal cultures from patients without evidence of STEC infection were negative.
Shiga toxigenic Escherichia coli (STEC) strains are an important cause of gastrointestinal disease in humans, particularly since such infections may result in life-threatening sequelae such as hemolytic-uremic syndrome (HUS) (12, 15, 21). It has been recognized for a number of years that STEC strains causing human disease may belong to a broad range of O serogroups (12). However, a subset of these (particularly O157 and O111) appear to be responsible for the majority of serious cases (those complicated by HUS) (11, 12, 21). These STEC strains have the capacity to produce attaching and effacing lesions on intestinal mucosa, a property mediated by the outer membrane protein intimin. Intimin is encoded by the eae gene, which is part of a pathogenicity island termed the locus for enterocyte effacement (6, 7). However, production of intimin is not essential for pathogenesis, because a number of sporadic cases of HUS are caused by eae-negative STEC strains (21). One of the most commonly reported eae-negative STEC serogroups associated with human disease is O113 (particularly serotype O113:H21) (12, 21). Indeed, 2 of the 12 STEC strains originally isolated from patients with HUS in the landmark study of Karmali et al. (13) belonged to this serotype. We have reported in an accompanying paper that an O113:H21 STEC strain was also responsible for a cluster of three HUS cases in Adelaide, South Australia, in 1998 (20), the first such report for an eae-negative STEC strain.
The capacity to rapidly determine whether a patient with diarrhea is infected with STEC is extremely important from both the clinical and epidemiological viewpoints. Early knowledge of the infecting STEC serogroup is also valuable, because it may provide an indication that clusters of cases could have a common cause and may also enable microbiological investigations of suspected foods to be targeted at a particular serogroup. We have previously described a multiplex PCR assay for genes within the O-antigen biosynthesis loci (rfb) from E. coli O111 and O157 (17). We routinely use this assay, in combination with another specific for Shiga toxin 1 (stx1), Shiga toxin 2 (stx2), eae, and plasmid-encoded hemolysin (EHEC [enterohemorrhagic E. coli]-hlyA) genes, for direct detection and characterization of STEC in crude fecal culture extracts (17). Over the last 5 years, O113 has been the third-most-prevalent STEC serogroup associated with cases of HUS in South Australia. Thus, expansion of this assay to include serogroup O113 is likely to be a useful adjunct in the diagnosis of STEC disease and in epidemiological studies. We have recently isolated and sequenced the entire rfb locus of E. coli O113 (18), and in the present study, we have used this information to design O113-specific primers, which have been incorporated into a multiplex PCR assay for direct detection of STEC serogroups O111, O113, and O157.
O113-specific PCR.
The rfb region of E. coli O113 contains nine genes which may be cotranscribed. Comparison with sequence databases identified candidate genes for four glycosyl transferases, an O-acetyl transferase, an O-unit flippase, and an O-antigen polymerase, as well as copies of galE and gnd (18). A portion of the O113 O-antigen polymerase gene (wzy) was the preferred target for a serogroup-specific PCR assay, because it shows the lowest degree of sequence homology with any genes submitted to GenBank (18). Moreover, the fact that the O-antigen polymerase must exhibit absolute specificity for both the oligosaccharide repeat unit and the type of glycosidic linkage formed during polymerization renders the existence of homologous sequences in other organisms extremely unlikely. The O113-specific PCR primers used were 5′-AGCGTTTCTGACATATGGAGTG-3′ and 5′-GTGTTAGTATCAAAAGAGGCTCC-3′ (designated O113F and O113R, respectively). These direct amplification of a 593-bp portion of the O113 wzy gene (nucleotides 3690 to 4282 in the published sequence [18]).
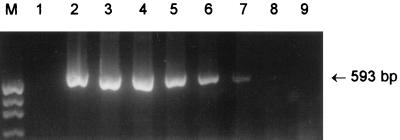
Crude DNA extracts were then prepared from 40 STEC strains in our collection, as described previously (19). O113:H21 STEC strains included five clinical isolates from South Australia (strains 97MW1, 98NK2, and 98BN1) and New Zealand (strains 1183 and 3848; kindly provided by Jenny Bennett) and a food isolate (strain MW10) from South Australia. The other STEC strains tested included serogroups O157 (5 isolates); O111 (7 isolates); O26 and OX3 (2 isolates each); O48, O91, O98, O123, O128, O141, and O159 (1 isolate each); and 11 STEC strains which were O nontypeable. Samples (2 μl) of each extract were amplified in 50-μl reaction mixtures containing 200 μM deoxynucleoside triphosphates (dNTPs), approximately 250 nM each primer (O113F and O113R, as well as the O111- and O157-specific primers designated O111F, O111R, O157F, and O157R [17]), and 1 U of Taq polymerase (Boehringer Mannheim, Mannheim, Germany) in a mixture of 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 2 mM MgCl2, 0.1% gelatin, 0.1% Tween 20, and 0.1% NP-40. Samples were subjected to 35 PCR cycles, each consisting of 1 min of denaturation at 95°C and 2 min of annealing at 65°C for the first 10 cycles, with decreases in decrements to 60°C by cycle 15, and 1.5 min of elongation at 72°C, with increases in increments to 2.5 min from cycles 25 to 35. PCR mixtures were electrophoresed on 2% agarose gels and stained with ethidium bromide. Extracts from all six O113 STEC isolates yielded 593-bp PCR products (Fig. 1, lanes 2 to 7), whereas extracts from the O111 and O157 STEC strains yielded 406- and 259-bp PCR products, respectively (results for three strains of each serogroup are shown in Fig. 1, lanes 8 to 13). Extracts from the 22 remaining STEC strains were PCR negative (results not presented).
FIG. 1.
Analysis of reference STEC strains by multiplex PCR. Lanes: M, DNA size markers (pUC19 DNA digested with HpaII; fragment sizes visible are 501/489, 404, 331, 242, 190, 147, and 111 bp); 1, negative control; 2 to 7, O113:H21 STEC strains MW10, 98NK2, 98BN1, 97MW1, 1183, and 3848, respectively; 8 to 10, O111:H− STEC strains 96RO1, 95JB1, and PH, respectively; 11 to 13, O157:H− STEC strains 96GR1, 96/0629, and 95SF2, respectively. The expected mobilities for the various serogroup-specific PCR products are also indicated.
Sensitivity of O113-specific PCR.
To assess the sensitivity of the O113-specific PCR, a fresh overnight broth culture of E. coli K-12 was spiked with serial 10-fold dilutions of a broth culture of the O113:H21 STEC strain 98NK2. Extracts of these samples were then subjected to the multiplex PCR assay (Fig. 2). A 593-bp PCR could still be seen in the sample that contained 106-fold-diluted STEC culture (equivalent to <102 STEC CFU per assay), but not in the sample containing 107- or 108-fold dilutions.
FIG. 2.
Sensitivity of O113-specific PCR. A culture of E. coli K-12 was spiked with serial 10-fold dilutions of a culture of O113:H21 STEC strain 98NK2, and extracts of these samples were then subjected to the rfb-specific PCR assay. Lanes: M, DNA size markers (pUC19 DNA digested with HpaII; fragment sizes visible are 501/489, 404, 331, and 242 bp); 1, negative control (unspiked E. coli K-12 extract); 2 to 9, extracts of E. coli K-12 culture spiked with 101-, 102-, 103-, 104-, 105-, 106-, 107-, and 108-fold-diluted 98NK2 cultures, respectively. The expected mobility for the O113-specific PCR product is also indicated.
Analysis of primary fecal cultures.
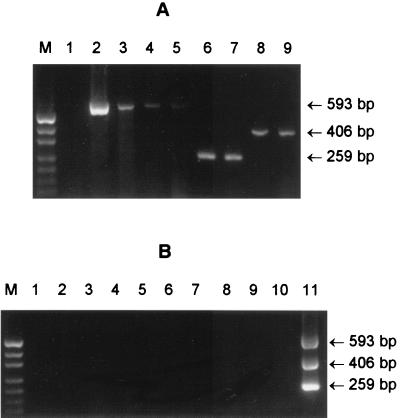
To demonstrate the diagnostic utility of the assay, crude DNA extracts of primary fecal cultures from 22 patients with HUS or bloody diarrhea were examined. Each of these samples had previously tested positive by PCR for the presence of stx1 and/or stx2 genes. Three of the original fecal cultures had yielded an O113 STEC isolate, while five yielded O111, and four yielded O157 STEC isolates. All but one of the remainder yielded STEC belonging to other serogroups. Extracts from all three O113 culture-positive samples yielded a 593-bp PCR product (Fig. 3A, lanes 2 to 4). One of these was from a patient with HUS, one was from a patient with diarrhea complicated by microangiopathic hemolytic anemia and thrombocytopenia, and the third was from a patient with bloody diarrhea. A previously culture negative, but stx2-positive sample from a patient with HUS who had serological evidence of O113 infection (20) was also positive for the O113-specific sequences by PCR (Fig. 3A, lane 5). The extracts from fecal cultures which yielded O157 or O111 STEC isolates also were positive for the appropriate serogroup by PCR, generating 259- or 406-bp PCR products, respectively. (Results for two extracts for each serogroup are shown in Fig. 3A, lanes 6 to 9.) No PCR products were observed for any of the nine other stx-positive extracts from cultures which yielded STEC isolates belonging to other serogroups (results not presented). A total of 30 fecal cultures from patients with diarrhea which had tested negative by PCR for stx were also tested in the serogroup-specific PCR assay. As expected, all of these extracts were also negative in the O111/O113/O157 PCR assay (results for 10 of these are shown in Fig. 3B).
FIG. 3.
Multiplex PCR analysis of primary fecal cultures. (A) Crude DNA extracts of stx-positive primary fecal cultures analyzed by using the rfb-specific PCR assay. Lanes: M, DNA size markers (pUC19 DNA digested with HpaII; fragment sizes visible are 501/489, 404, 331, 242, 190, 147, and 111 bp); 1, negative control; 2 to 4, extracts from three patients with culture-proven O113:H21 STEC infection; 5, extract from an stx2-positive, but culture-negative HUS patient with serological evidence of O113 infection; 6 and 7, extracts from patients with culture-proven O157:H− STEC infection; 8 and 9, extracts from patients with culture-proven O111:H− STEC infection. (B) Crude DNA extracts of stx-negative primary fecal cultures analyzed by using the rfb-specific PCR assay. Lanes: M, DNA size markers; 2 to 10, extracts of stx-negative primary fecal cultures; 11, positive control (pooled DNA extracts from reference O157, O111, and O113 STEC strains). The expected mobilities for the various serogroup-specific PCR products are also indicated.
When tested in our previously described multiplex PCR assay for STEC virulence factor genes (17), the O113-positive fecal culture extracts were all positive for stx2 and EHEC-hlyA; three of the four O157-positive samples were positive for stx2, eae, and EHEC-hlyA; all of the O111-positive and one of the O157-positive samples were positive for stx1, stx2, eae, and EHEC-hlyA. Of the samples yielding STEC isolates belonging to other serogroups, four were O26, and all of these were positive for stx1, eae, and EHEC-hlyA. The remaining five samples yielded isolates which were all eae negative; one was serogroup O23, while the others were nontypeable. In all of the above cases, the multiplex PCR profile of the crude extracts matched that of the STEC strain isolated from that sample (results not presented).
Discussion and conclusions.
PCR is generally considered to be the most sensitive means of determining whether a fecal specimen or a food sample contains STEC (21). Although direct extracts of feces or foods can be used as templates for PCR, the best results are usually obtained by testing extracts of primary broth cultures (2, 9, 19, 21). Broth enrichment serves two purposes: inhibitors in the sample are diluted, and bacterial growth increases the number of copies of the target sequence. Detection of either stx1 or stx2 genes confirms the presence of STEC, but valuable additional information about the infecting strain can be obtained by testing for the presence of genes encoding putative accessory virulence factors, such as intimin or the plasmid-encoded hemolysin (10, 17, 22). Sequence differences between the eae genes from O157 STEC and other eae-positive serogroups such as O111 have also been used to develop PCR assays capable of recognizing STEC belonging to these serogroups (8, 14). However, in both studies, the O157 eae primers also reacted with E. coli O55, an enteropathogenic E. coli serogroup closely related to O157 STEC. Furthermore, not all O111 STEC strains tested reacted with the O111 eae primers (14). The availability of sequence data for the rfb regions of E. coli O111 and O157 (1, 4) has recently enabled development of a multiplex PCR assay with absolute specificity for these two important STEC serogroups (17).
O113 was one of the first STEC serogroups to be associated with HUS (13) and is among the most common of the eae-negative STEC types isolated from cases of human disease (12, 21). This is in spite of the fact that the incidence of infections with STEC serogroups other than O157 may be underestimated. Such strains cannot be recognized by culture on sorbitol MacConkey agar and require more sophisticated diagnostic strategies (21). O113 STEC strains are also prevalent in cattle (3, 5, 16), and so there is ample scope for their entry into the human food chain. In the present study, we have designed PCR primers based on the wzy gene from the O113 rfb locus. These have been combined with the primers specific for regions of the O111 and O157 rfb loci in a multiplex format. The various primers were designed such that the serogroup-specific PCR products differ in size (259, 406, and 593 bp for O157, O111, and O113, respectively), and so can be readily distinguished by agarose gel electrophoresis. The specificity of the O113 primers was confirmed by testing DNA extracted from a wide range of STEC strains of a known serogroup, as well as by testing extracts of crude fecal cultures from diarrhea patients without evidence of STEC infection. The utility of this assay for direct detection of O113 sequences in primary fecal cultures from patients infected with O113 STEC was also confirmed. The O113-specific PCR assay was also very sensitive, and a culture spiked with 106-fold-diluted O113 STEC (i.e., STEC comprised 0.0001% of the total flora) generated a PCR product which was visible on an ethidium bromide-stained agarose gel.
The trivalent serogroup-specific multiplex PCR assay described in this paper is clearly a useful adjunct to our previously described PCR for stx1, stx2, eae, and EHEC-hlyA (17). Collectively, these two assays can provide comprehensive information about the genotype of an infecting STEC strain within 24 h of receipt of a specimen. Detection of a similar PCR profile in crude fecal extracts from more than one patient within a given period of time may provide the earliest (albeit circumstantial) evidence for a link between cases consistent with a common source outbreak. Confirmation of an outbreak would ultimately depend on isolation and genotyping of the causative STEC, but this may take weeks. Moreover, given the sensitivity of PCR screens, there is a likelihood that a proportion of genuine STEC PCR-positive specimens will not yield an isolate even after heroic efforts. However, knowledge of the serogroup of the infecting STEC strain obtained by multiplex PCR analysis would greatly increase the probability of isolating an STEC strain, because it would enable the deployment of immunomagnetic enrichment techniques using beads coated with antibodies to the respective O antigen.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Bastin D A, Reeves P R. Sequence analysis of the O antigen gene (rfb) cluster of Escherichia coli O111. Gene. 1995;164:17–23. doi: 10.1016/0378-1119(95)00459-j. [DOI] [PubMed] [Google Scholar]
- 2.Begum D, Jackson M P. Direct detection of Shiga-like toxin-producing Escherichia coli in ground beef using the polymerase chain reaction. Mol Cell Probes. 1995;9:259–264. doi: 10.1016/s0890-8508(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 3.Beutin L, Geier D, Steinrück H, Zimmermann S, Scheutz F. Prevalence and some properties of verotoxin (Shiga-like toxin)-producing Escherichia coli in seven different species of healthy domestic animals. J Clin Microbiol. 1993;31:2483–2488. doi: 10.1128/jcm.31.9.2483-2488.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilge S S, Vary J C, Jr, Dowell S F, Tarr P I. Role of the Escherichia coli O157:H7 O side chain in adherence and analysis of an rfb locus. Infect Immun. 1996;64:4795–4801. doi: 10.1128/iai.64.11.4795-4801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanco M, Blanco J E, Blanco J, Mora A, Prado C, Alonso M P, Mourino M, Madrid C, Balsalobre C, Juarez A. Distribution and characterization of faecal verotoxin-producing Escherichia coli (VTEC) isolated from healthy cattle. Vet Microbiol. 1997;54:309–319. doi: 10.1016/s0378-1135(96)01292-8. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 7.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y K, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 8.Gannon V P J, D’Souza S, Graham T, King R K, Rahn K, Read S. Use of the flagellar H7 gene as a target in multiplex PCR assays and improved specificity in identification of enterohemorrhagic Escherichia coli strains. J Clin Microbiol. 1997;35:656–662. doi: 10.1128/jcm.35.3.656-662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gannon V P J, King R K, Kim J Y, Thomas E J G. Rapid and sensitive method for detection of Shiga-like toxin-producing Escherichia coli in ground beef using the polymerase chain reaction. Appl Environ Microbiol. 1992;58:3809–3815. doi: 10.1128/aem.58.12.3809-3815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gannon V P J, Rashed M, King R K, Thomas E J G. Detection and characterization of the eae gene of Shiga-like toxin-producing Escherichia coli using polymerase chain reaction. J Clin Microbiol. 1993;31:1268–1274. doi: 10.1128/jcm.31.5.1268-1274.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin P M. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R I, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press; 1995. pp. 739–761. [Google Scholar]
- 12.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 14.Louie M, De-Azavedo J, Clarke R, Borczyk A, Lior H, Richter M, Brunton J. Sequence heterogeneity of the eae gene and detection of verotoxin-producing Escherichia coli using serotype-specific primers. Epidemiol Infect. 1994;112:449–461. doi: 10.1017/s0950268800051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orden J A, Ruiz-Santa-Quiteria J A, Cid D, Garcia S, Sanz R, de la Fuente R. Verotoxin-producing Escherichia coli (VTEC) and eae-positive non-VTEC in 1–30-days-old diarrhoeic dairy calves. Vet Microbiol. 1998;63:239–248. doi: 10.1016/s0378-1135(98)00218-1. [DOI] [PubMed] [Google Scholar]
- 17.Paton A W, Paton J C. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998;36:598–602. doi: 10.1128/jcm.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton, A. W., and J. C. Paton. Molecular characterization of the locus encoding biosynthesis of the lipopolysaccharide O-antigen of Escherichia coli serotype O113. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Paton A W, Paton J C, Goldwater P N, Manning P A. Direct detection of Escherichia coli Shiga-like toxin genes in primary fecal cultures by polymerase chain reaction. J Clin Microbiol. 1993;31:3063–3067. doi: 10.1128/jcm.31.11.3063-3067.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paton A W, Woodrow M C, Doyle R M, Lanser J A, Paton J C. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic-uremic syndrome. J Clin Microbiol. 1999;37:3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt H, Beutin L, Karch H. Molecular analysis of the plasmid-encoded hemolysin of Escherichia coli O157:H7 strain EDL 933. Infect Immun. 1995;63:1055–1061. doi: 10.1128/iai.63.3.1055-1061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]