Abstract
SARS-CoV-2 infection can impact the physical, cognitive, mental health of patients, especially in those recovered in intensive care units. Moreover, it was proved that the effects of the virus may persist for weeks or months. The term long-COVID or post-COVID syndrome is commonly used for indicating a variety of physical and psychological symptoms that continue after the resolution of the acute phase. This narrative review is aimed at providing an updated overview of the impact of physical, cognitive, and psychological health disorders in COVID-19 survivors, by summarizing the data already published in literature in the last year. Studies cited were found through PubMed searches. We also presented an overview of the post-COVID-19 health consequences on three important aspects: nutritional status, neurological disorders, and physical health. Moreover, to activate a correct health planning policy, a multidisciplinary approach for addressing the post- COVID-19 issue, has been proposed. Finally, the involvement of health professionals is necessary even after the pandemic, to reduce expected post-pandemic psychosocial responses and mental health disorders.
Keywords: COVID-19 pandemic, Public health, Neurocognitive disorders, Intensive care units
Introduction
Clinical manifestations of COVID-19 vary from asymptomatic forms to self-limiting conditions, up to severe manifestations featuring respiratory and multi-organ involvement [1–6]. Epidemiological data reveal that up to 20% of COVID-19 patients progress to a severe condition that requires hospitalization [7]. Among those who are hospitalized, up to one-quarter need intensive care unit (ICU) admission, making them more vulnerable to secondary pneumonia, cardiac injury, sepsis, kidney injury, and neurologic disorders [8].
The term ‘Long COVID’ or ‘Post-COVID’ is commonly used to describe an array of signs and symptoms that are present after acute COVID-19. The UK’s National Institute for Health and Care Excellence described the Long COVID as “ongoing symptomatic COVID-19” (symptoms between 4 and 12 weeks) and “Post-COVID syndrome” when symptoms lasting longer than 12 weeks [9]. Recently the Long COVID has been recognized by the World Health Organization (WHO) as an international healthcare concern and an “emergency-use” ICD code has been issued [10]. The study of the long-term outcome of patients discharged from ICU revealed significant disabilities, collectively known as post-intensive care syndrome (PICS) [2], affecting physical, cognitive, and psychological health. PICS includes symptoms like generalized weakness, memory disturbances, poor concentration, depression, anxiety, and post-traumatic distress disorder (PTSD) [2].
PICS incidence can be quite high: affecting up to 60% of the patients for what concerns cognitive and psychological symptoms and between 25 and 60% for what concerns neuromuscular disorders [11–13]. Furthermore, the PICS can last many years, affecting the health-related quality of life (HR-QoL) and the ability to return to work [14, 15]. Patients who survive acute distress respiratory syndrome (ARDS) could develop chronic pain and it would be possible that patients with a severe type of COVID-19 disease could develop similar complications [16]. Moreover, the psychological burden of ICU admission and stay of a patient can affect also his/her relatives who can develop symptoms of PTSD as well [17].
Here, we reviewed the clinical studies on neurocognitive disorders in Post-COVID patients. Studies cited in this narrative review were discovered through PubMed searches. PubMed was searched for clinical articles published in the last two years related to neurocognitive disorders, covid-19 survivors, physical cognitive, and mental health disorders. Based on these data, we proposed an overview of the COVID-19 health consequences by focusing on three important aspects: neurological disorders, physical health, and nutritional status.
The post-COVID syndrome
The Post-COVID Syndrome includes persistent symptoms related to residual inflammation, organ damage, non-specific effects from the hospitalization post-intensive care syndrome, social isolation, or impact on pre-existing health conditions [18–20].
Post-COVID syndrome could be due to various mechanisms such as post-ICU syndrome, post-viral fatigue syndrome, permanent organ damage, or others [21]. Even if Long COVID was initially thought to be limited to survivors of hospital care and to those admitted to the ICU, it is now evident that most cases are described even in those who were not hospitalized or who did not immediately seek medical care [22–24].
The most frequent alterations include headache, dizziness, balance and coordination disorders, difficulty in attention, concentration, and memory, as well as chronic fatigue, insomnia, changes in taste and smell, depression, and anxiety. These physical, psychological, and neurocognitive symptoms are close to those present in post-traumatic stress disorder (PTSD) [25, 26].
Several studies observed persistent symptoms and unexpected substantial organ dysfunction after SARS-CoV-2 infection in an increasing number of patients after recovering from their initial illness [27–29].
Data from a prospective cohort study on 270 COVID-19 survivors confirmed a post-COVID-19 syndrome in half of the patients experiencing symptoms such as fatigue and respiratory (dyspnea) or neurological complaints, 10–14 weeks after disease onset [30, 31] showed that 76% of hospitalized COVID-19 survivors reported at least one symptom that persisted, with fatigue or muscle weakness being the most frequently reported symptom, 6 months after illness onset.
Among ICU COVID-19 survivors several patients face impairments regarding their cognitive and mental health or physical function far beyond their hospital discharge [32].
Data from a UK study on the post-discharge impact of COVID‐19 infection on the health status of 100 survivors (32 ICU) revealed that: fatigue was the most common reported symptom in both ICU COVID-19 survivors (72%) and COVID-19 survivors (60%), followed by breathlessness (66% and 43%, respectively) and psychological distress (47% and 24%, respectively). Moreover, data showed a clinically significant HR-QoL in both ICU COVID-19 survivors (69%) and COVID-19 survivors (46%). Sixty percent of the ICU COVID-19 survivors and 15% of the COVID-19 survivors remained off‐sick from work after 4 weeks or more since discharge [29].
Data collected among symptomatic adults tested in outpatient settings (patients with mild COVID-19 without hospitalization) reported that 94% experienced one or more symptoms (cough 43%, fatigue 35%, or shortness of breath 29%) after infection onset, resulting in prolonged illness [28]. Persistent symptoms (such as anosmia/ageusia, dyspnea, or asthenia) have been reported in two-thirds of patients with non-critical COVID-19 [30]. The ongoing COVID-19 pandemic and the occurrence of Post-COVID syndrome has highlighted the PICS issue and the complex rehabilitation needs for people with severe illness and long ICU stays as well as for COVID-19 survivors that have not been hospitalized. The persistence of various symptoms in Long-COVID patients is a major health issue worldwide. Monitoring and treatment of patients with post-COVID syndrome are necessary to ensure rehabilitation and recovery of general functions. These findings support the need for a multidisciplinary approach to the care of this vulnerable population and to conduct research studies during 1–2 years of follow-up, as is currently happening in the UK and USA [31, 33, 34] (see Table 1).
Table 1.
Studies of Physical health, Neurological disorders, and Nutritional status during the COVID-19 pandemic
| References | Design | Country | Sample size (N) | Male (%) | Mean age (years) | Categories (health care workers, patient, hospitalized/ICU, general public) | Physical health | Neurological disorders | Nutritional status | COVID-19 status | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||||||
| [90] | Cross-sectional study | UK | 431.051 | 55.8 | 57.2 | Hospitalized | n.a | psychological distress, neuroticism | n.a | 908 hospitalized for COVID-19 | Association of lower cognitive function based on a test of verbal and numerical reasoning with a higher risk of COVID-19 |
| [91] | Observational study | Italy | 50 | n.a | Hospitalized | DEPENDENCE for motor or respiratory functions | n.a | (90%) dysphagia and need of a modified diet’s consistency or nasogastric feeding (45%) high risk and (26%) moderate risk of malnutrition BMI improvement 14 (43.7%) | 50 (100%) positive to COVID-19 | three-step nutritional protocol to ensure optimal nutritional status and improve clinical outcomes in COVID19 patients in a rehabilitation unit | |
| [92] | Web-based cross-sectional survey | Italy | 2291 | 25.3 | 30.0 | General public | n.a | poor sleep quality (57.1%), high anxiety (32.1%), high distress (41.8%), PTSD symptomatology (7.6%) | n.a | Positive to COVID-19 9 (0.4%) | Correlation between epidemic with anxiety, sleep disorders and PTSD |
| [93] | Multicenter study | India | 906 | 35.7 | 29 | Healthcare workers | Headache 289 (31.9%), Throat pain 304 (33.6%), no symptoms 302 (33.3%) | moderate to very-severe depression 48 (5.3%), moderate to extremely severe anxiety 79 (8.7%), moderate to extremely severe stress 20 (2.2%), and moderate to severe levels of psychological distress 34 (3.8%), Lethargy 241(26.6%), Insomnia 190(21.0%) | n.a | n.a | significant association between the prevalence of physical symptoms and psychological outcomes among healthcare workers during the COVID-19 outbreak |
| [94] | Web-based cross-sectional survey | Italy | 2291 | 25.3 | 30.0 | General public | n.a |
Higher risk of psychopathological symptoms in females (OR 2,32) younger than 50 years (OR > 1,68). Higher risk of developing anxiety was higher in females (OR = 3,10 younger than 50 (OR > 1,47) and undergraduates (OR = 1,68) Higher risk of PTSD symptomatology associated with female (OR 2,39) |
n.a | 9 (0.4%) positive to COVID-19 | COVID-19 pandemic can be related to anxiety, changes in mood, high psychopathological symptomatology, and could be associated with the development of PTSD |
| [95] | Web-based cross-sectional survey | China | 7236 | 45.4 | 35.3 | Healthcare workers, General public | n.a |
Generalized anxiety disorder, depressive symptoms and was significantly higher in general public, Healthcare workers reported highest rate of poor sleep quality. Generalized anxiety disorder 2540 (35.1%), depressive symptoms 1454 (20.1%), poor sleep quality 1317 (18.2%) |
n.a | n.a | Major mental health burden during COVID outbreak |
| [96] | Online survey | Canada | 1908 | 19.6 | 42 | n.a |
40.5% of inactive and 22,4% of active individual became less active 33% of inactive and 40.3% of active became more active 28.3% of inactive altered their type of activity, 39.6% of active maintained their type |
Inactive scored significantly lower on the mental health assessment than the active participants, however there is no significant difference in generalized anxiety.; inactive participants that were more active indicated higher levels of social, emotional and psychological health, and lower levels of anxiety; inactive with mild anxiety were more physically active than participants with moderate anxiety; inactive participants with severe anxiety spent fewer minutes in outdoor physical activity than individuals with low or mild anxiety | n.a | n.a | Strong association between physical activity and well-being; |
| [3] | Observational study | Italy | 143 | 62.9 | 56.5 | Hospitalized | Post covid-19: 53.1% of individuals showed fatigue, 43.4% dyspnoea, 27.3% joint pain, 21.7% chest pain, Fatigue 53% | n.a | n.a | 143 (100%) patient’s post-recovery from COV19 | This study found that in patients who had recovered from COVID-19, 87.4% reported persistence of at least 1 symptom, particularly fatigue and dyspnea |
| [97] | Web-based cross-sectional survey | China | 994 | 14.5 | Aged 25– 40 years (63.4%) | Healthcare workers | n.a | (36.9%) subthreshold mental health disturbances, (34.4%) mild disturbances, (22.4%) moderate disturbances, and (6.2%) had severe disturbance | n.a | None | These findings emphasize the importance of being prepared to support frontline workers through mental health interventions at times of widespread crisis |
| [98] | Web-based cross-sectional survey | China | 9684 | 23.3 | 813 (64.7%) were aged 26–40 y | Healthcare workers | 67 (60.9%) had fever, 66 (60.0%) myalgia or fatigue, 62 (56.4%) cough, 55 (50%) sore throat, 50 (45.5%) muscle ache | 634 (50.4%) depression,560 (44.6%) anxiety, 427(34%) insomnia, 899 (71.5%) distress | n.a | 110 (1%) positive to COVID-19 | These findings suggest that front-line healthcare workers exposed to COVID-19 have a high risk of developing unfavorable mental health outcomes and may need psychological support |
| [99] | Observational study | India | 470 | 31.7 | 31 | Healthcare workers, General public | n.a |
60 (14.5%) participants showed anxiety, 42 (8.9%) depression, 31 (6.6%) stress, 36 (7.7%) post-traumatic stress disorder Anxiety was higher among nonmedical health care workers than medical personnel (20.7% versus 10.8%; adjusted prevalence ratio, 1.85 [95% CI, 1.15–2.99]; P = 0.011) Higher mean DASS-21 anxiety and stress subscale scores and higher IES-R total and subscale scores were observed in nonmedical health care workers |
n.a | None | Nonmedical health care personnel are at highest risk for psychological distress during the COVID-19 outbreak. Early psychological interventions targeting this vulnerable group may be beneficial |
| [100] | Online survey | UK | 153 | 48 | 71 | General public | n.a |
39 (31%) presented altered mental status, comprising 9 (23%) patients with unspecified encephalopathy and 7 (18%) encephalitis. 23 (59%) with altered mental status fulfilled the clinical case definitions for psychiatric diagnoses as classified by the notifying psychiatrist or neuropsychiatrist, and 21 (92%) of these were new diagnoses 6 (26%) had a neurocognitive (dementia-like) syndrome, and four (17%) had an affective disorder |
n.a |
114 (92%) confirmed 5 (4%) probable 5 (4%) possible |
This study identified acute presentations of new-onset complications of COVID-19. Ischemic stroke was common in our cohort |
| [101] | Observational study | China | 1738 | Survey 1: 32.7; Survey 2: 25 | Survey 1: 21,4–30,8y (53,1%); Survey 2: 21,4–30,8y (46,5%) | General public | n.a, |
Measured by DASS-21 Mean score (SD), survey 1: 7,76 (7,74) stress, 6,16 (6,57) anxiety, 6,25 (7,16) depression; survey 2: 7,86 (7,93) stress, 6,15 (6,94) anxiety, 6,38 (7,39) depression. NS comparison between surveys |
n.a | n.a | Protective factors included high level of confidence in doctors, perceived survival likelihood and low risk of contracting COVID-19, satisfaction with health information, personal precautionary measures. Governments should focus on effective methods of disseminating unbiased COVID-19 knowledge, correct containment methods, availability of essential services/commodities, and sufficient financial support |
| [102] | Web-based cross-sectional survey | China | 2182 | 35.8 | 18-60y, n = 2,101 (96,3%) | Healthcare workers, General public | n.a | Medical health workers showed higher prevalence rates of insomnia (38.4 vs. 30.5%, p < 0.01), anxiety (13.0 vs. 8.5%, p < 0.01), depression (12.2 vs. 9.5%; p = 0.04), somatization (1.6 vs. 0.4%; p < 0.01), and obsessive–compulsive symptoms (5.3 vs. 2.2%; p < 0.01) than nonmedical health workers | n.a | n.a | During the COVID-19 outbreak, medical health workers had psychosocial problems and risk factors for developing them. They needed attention and recovery programs |
| [46] | Observation study | China | 214 | 40.7 | 52.7 | Hospitalized | Compared with patients with non-severe infection, patients with more severe infection had skeletal muscle injury 17 (19.3%) vs 6 (4.8%) |
78 patients (36.4%) had neurologic manifestations: Compared to patients with non-severe infection, patients with more severe infection had neurologic manifestations, such as acute cerebrovascular diseases 5 (5.7%) vs 1 (0.8%), impaired consciousness 13 (14.8%) vs 3 (2.4%) |
n.a | 214 (100%) positive to COVID-19 | During the pandemic, patients with neurologic manifestations should have SARS-CoV2 infection suspected, to avoid delayed diagnosis or misdiagnosis and to lose the ability to treat and prevent transmission |
| [56] | Observational study | China | 201 | 63.7 | 51 | Hospitalized |
188 (93.5%) had Fever, 163 (81.1%) cough, 80 (39.8%) dyspnea, and 65 (32.3%) had fatigue or myalgia 66 (32.8%) had fever with fatigue, myalgia, or headache; |
7 (3.5%) had nervous system disease | n.a | 201 (100%) positive to COVID-19 | Older age was associated with a high risk of developing ARDS and death: this may have been due to a weaker immune response from this category |
| [57] | Editorial | Europe | n.a | n.a | n.a | n.a | n.a | n.a | COVID-19 patient may have altered nutritional status characterized by malnutrition and body weight loss | n.a | The nutritional approach in SARS-CoV-2 patients in the ICU, internal medicine ward and general health care should not be underestimated, and dietary intervention should be an important part of the care provided to these patients.. |
| [54] | Observational study | Denmark | 498,151 | 32,775 (6.5%) | 43 | Hospitalized and non- hospitalized | Increased risk of receiving hospital diagnoses of dyspnoea and venous thromboembolism for SARS-CoV-2-positive individuals compared with negative individuals | No increased risk of serious complications of SARS-CoV-2 infection, ischaemic stroke, encephalitis, psychoses | n.a | 1310 positive to COVID-19 | For those with SARS-CoV-2 who do not require hospitalization, the risk of having serious complications such as venous thromboembolism, ischaemic stroke, and psychoses is low |
Potential tools to evaluate outcomes in long-COVID and post-COVID patients: our proposal
Cognitive, physical, and psychological dysfunction reported by COVID19 patients can have profound effects on the HR-QoL [32].
We proposed a multimodal process as well as the sequence of several aspects of the health-related quality of life (HRQoL) contributing to the impact of the disease on cognitive, physical, and nutritional outcomes by considering the set of the following tools:
The Short Form Health Survey 36 (SF-36), which is a short questionnaire (36 items) that evaluates eight dimensions: physical functioning (10 items), social functioning (2 items), limitations due to physical problems (4 items), limitations due to emotional problems (3 items), mental health (5 items), energy/vitality (4 items), pain (2 items) and perception of general health (6 items) [35]. The SF-36 investigates the health changes of an individual compared to the previous year. As the tool evaluates the state of health in general, it is suitable for studies in the general population and transversal or longitudinal investigations on specific diseases, and treatments. Due to its characteristic of a general questionnaire, it needs to be accompanied by specific questionnaires when studying patient populations.
The Barthel Index, developed to measure improvements in individuals with a chronic disability who underwent rehabilitation programs, is commonly used in post-ARDS patients [36].
The Psychological General Well-Being Index (PGWBI) is designed for providing an index for measuring subjective well-being or suffering. It is composed of 22 items to assess anxiety, depression, positive well-being, self-control, general health, and vitality[37].
The EuroQoL [38]. It represents the attempt to develop a standardized, general tool for describing and evaluating HRQoL regardless of the specific disease. It is a questionnaire consisting of five dimensions and an analog self-assessment scale.
The Pittsburgh Sleep Quality Index (PSQI), for assessing sleep quality[39].
The Mini-Mental Test investigates the neurocognitive and functional state through simple targeted questions as well as small graphical tasks. It explores different domains of brain function, such as orientation, memory, attention and calculation, the ability to recall certain acquisitions, language, etc. [40].
The Brief Pain Inventory (BPI) rapidly assesses the severity of pain and its impact on functioning [41].
PTSS-14 (Post Traumatic Stress Syndrome 14 items) is a screening instrument to identify the patients who developed the Syndrome [42].
HADS (Hospital Anxiety and Depression Scale) to evaluate the level of depression and anxiety of the patients discharged at home [43].
MNA (Mini Nutritional Assessment) a nutritional educational program to assess nutritional status in patients in healthcare settings, appears ideal for patients with COVID -19, alongside a clinical and Para clinical evaluation [44, 45].
Focus on three aspects to manage COVID-19 survivors
We underline three important aspects to manage COVID-19 survivors: (1) neurological disorders, (2) physical health, (3) nutritional status.
(1) Neurological disorders
Accumulated pieces of evidence highlighted that SARS-CoV-2 affected the nervous system [46–48]. In patients with a severe form of the disease, neurological manifestations were more evident. As reported by Mao et al. [46], neurologic manifestations affected or the central nervous system (CNS) (dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, and seizure), or the peripheral nervous system (PNS) (nerve pain and impairment of vision, test, and smell) or the skeletal muscular apparatus (injury). Among CNS alterations, the acute cerebrovascular disease was more evident in older patients and with severe infection and included cerebral hemorrhage and ischemic stroke diagnosed by clinical symptoms and head CT. Carfi et al. [3] demonstrated that worsened quality of life was observed in 44.1% of patients, and 87.4% reported persistence of at least 1 symptom, particularly fatigue and dyspnoea. The pathologic mechanism underlying the CNS invasion of SARS-CoV-2 is presumably like that of other respiratory viruses. Specifically, SARS-COV-2 can invade the CNS through the hematogenous or retrograde neuronal route. Since SARS-CoV-2 infects a large part of the world's population, understanding the potential neurologic implications of COVID-19 will help clinicians to identify and intervene in neurologic morbidity during and after the pandemic.
(2) Physical health
Patients who have undergone intensive care after discharge may experience a post-intensive care syndrome (PICS) characterized by physical, mental, cognitive [1], and nutritional problems [49]. The impact of ICU on physical function can impair daily activities, involving the neuromuscular, cardio-respiratory, and skeletal systems: these individuals very often report inability to return to work, musculoskeletal weakness and difficulty walking, impaired lung and respiratory function [50–53].
Most of the complications due to COVID-19 described were related to hospitalized patients and therefore not associated with patients who received home care. Few studies have evaluated the presence of complications in patients positive for SARS-Cov-2 who did not require hospital care but were still positive for SARS-Cov-2. In a population-based cohort study in Denmark, it was found that the risk of severe complications after COVID-19 in non-hospitalized patients about 6 months after the infection is very low, but these still have a higher risk for venous thrombotic events than people without disease and negative for SARS-Cov-2 [54]. There are currently no other studies evaluating the long-term effects of the virus in non-hospitalized patients beyond six months of infection.
(3) Nutritional status
Previous studies have highlighted the poor nutritional status of patients upon admission and during their stay in intensive care. The greater propensity for malnutrition and wasting is more visible in these critically ill patients, due to their developed metabolic disorders [49]. Nutritional status has long been considered an important factor that can influence the outcome of various infectious diseases, including viral pneumonia caused by SARS-CoV2 (COVID-19) [55, 56]. In COVID-19 patients, an altered nutritional status characterized by malnutrition and loss of body weight can be found and due to various causes [57, 58], including dyspnoea, anorexia, dysphagia, nausea, vomiting, diarrhea, increased energy requirements [59] advanced age, frailty, comorbidity [57] and prolonged hospital stay in ICU [58]. Currently, there are no specific dietary guidelines for post-COVID-19 patients with PICS disorders. However, several aspects could be considered to improve the impairments of cognitive functions. Eating habits can affect cognitive abilities [60]: unbalanced diets can have an overall negative impact on cognitive and mental health [61–63], negatively affecting the ability to reason, attention, and memory [61, 62, 64] and promote dementia and depression [65–68]. Greater adherence to a diet that includes healthy foods such as vegetables, fruits, seafood, lean meats, and whole grains, reduces the likelihood of suffering from depression or anxiety [69–71]. Nutrients such as vitamins (B1, B6, B12, B9, C, E, D), polyphenols, ω-3 fatty acids, minerals (iron, zinc, selenium), and foods with a low glycemic index have inhibitory action against oxidative stress and neuroinflammation [72, 73], and positively influence cognitive function [68, 74–84]. In this regard, several studies have reported that greater adherence to the Mediterranean diet was associated with an improvement in cognitive function and a reduced risk of cognitive impairment [83, 85–89]. It would be useful to monitor body composition, using methods such as bio impedance-analysis (BIA) or plicometry, and the nutritional status using the MNA [44, 45], to offer the most adequate nutritional support that contributes to reduce physical and cognitive complications both in post-hospitalization and in the long term.
Conclusions
The Sars-Cov-2 is an invisible enemy that makes us feel constantly under threat, it can infect people at any time, and this can generate different responses in subjects: anxiety, depression, panic, sleep, concentration disorders, and fatigue. All normal and legitimate reactions, however, must be contained to try to limit the effects, allowing us to better face the emergency we are experiencing. COVID-19 survivors, after clinical recovery, may have neurocognitive damage that should not be underestimated, and the extent and duration of which is not yet known. As we reported, also the COVID-19 survivors (without hospitalization), reported the post- COVID-19 syndrome. The most frequent alterations found are headache, balance and coordination disorders, difficulty in attention, insomnia, changes in taste and smell, depression, anxiety, physical and nutritional dysfunctions. The isolation, the hospitalization, the drama of the health emergency could have been decisive in the onset of some of these symptoms. Overall, the impact of post- COVID-19 syndrome should be considered as the potential cause of a delayed pandemic that may have a major public health impact in the medium to long term. Thus, preventive interventional approaches mitigating social impact should be considered as an integral part of the response to the crisis during pandemics. Moreover, the involvement of specific health professional figures is needed even after the pandemic, to manage and care for an increased number of patients (Fig. 1).
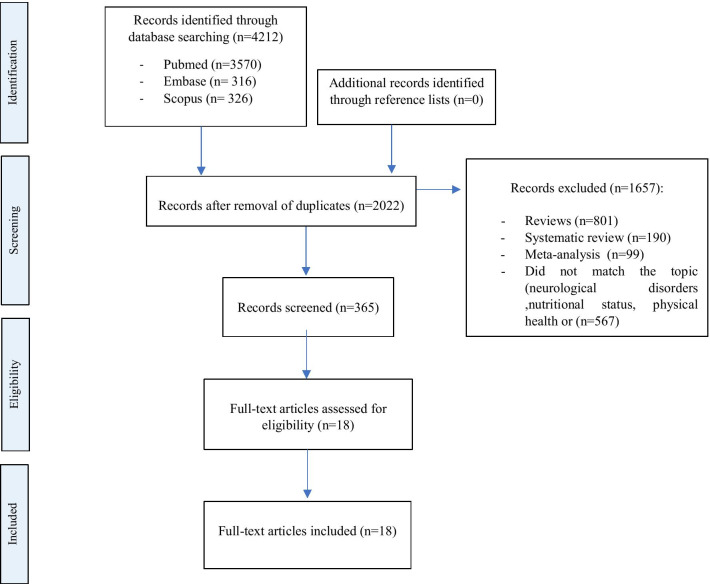
Fig. 1.
PRISMA flow diagram
Acknowledgements
The authors are grateful to Alessandra Trocino and Mrs. Maria Cristina Romano from the National Cancer Institute of Naples for providing excellent bibliographic service and assistance.
Abbreviations
- PICS
Post-intensive care syndrome
- ICUs
Intensive care units
- PTSD
Post-traumatic distress disorder
- ARDS
Acute respiratory distress syndrome
- HRQoL
Health-related quality of life
- SF-36
Short Form Health Survey 36
- PGWBI
The Psychological General Well-Being Index
- PSQI
The Pittsburgh Sleep Quality Index
- BPI
Brief Pain Inventory
- PTSS-14
Post Traumatic Stress Syndrome 14 items
- HADS
Hospital Anxiety and Depression Scale
- MNA
Mini Nutritional Assessment
- BIA
Bio impedance-analysis
Authors' contributions
The present review was mainly written by AC and SB. All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azoulay E, Vincent JL, Angus DC, Arabi YM, Brochard L, Brett SJ, Citerio G, Cook DJ, Curtis JR, Dos Santos CC, et al. Recovery after critical illness: putting the puzzle together-a consensus of 29. Crit Care. 2017;21(1):296. doi: 10.1186/s13054-017-1887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Intern Med. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carfi A, Bernabei R, Landi F. Gemelli against C-P-ACSG: persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanno LK, Casale T, Demoly P. Coronavirus disease (COVID)-19: world health organization definitions and coding to support the allergy community and health professionals. J Allergy Clin Immunol Pract. 2020;8(7):2144–2148. doi: 10.1016/j.jaip.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neufeld KJ, Leoutsakos JS, Yan H, Lin S, Zabinski JS, Dinglas VD, Hosey MM, Parker AM, Hopkins RO, Needham DM. Fatigue symptoms during the first year following ARDS. Chest. 2020;158(3):999–1007. doi: 10.1016/j.chest.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MNM, Sarker MS. A review of coronavirus 2019 (Covid-19), a life threating disease all over the world. World Cancer Res J. 2020;7:e1586. [Google Scholar]
- 7.Sidiras G, Patsaki I, Karatzanos E, Dakoutrou M, Kouvarakos A, Mitsiou G, Routsi C, Stranjalis G, Nanas S, Gerovasili V. Long term follow-up of quality of life and functional ability in patients with ICU acquired weakness: a post hoc analysis. J Crit Care. 2019;53:223–230. doi: 10.1016/j.jcrc.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Kamdar BB, Suri R, Suchyta MR, Digrande KF, Sherwood KD, Colantuoni E, Dinglas VD, Needham DM, Hopkins RO. Return to work after critical illness: a systematic review and meta-analysis. Thorax. 2020;75(1):17–27. doi: 10.1136/thoraxjnl-2019-213803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffiths J, Hatch RA, Bishop J, Morgan K, Jenkinson C, Cuthbertson BH, Brett SJ. An exploration of social and economic outcome and associated health-related quality of life after critical illness in general intensive care unit survivors: a 12-month follow-up study. Crit Care. 2013;17(3):R100. doi: 10.1186/cc12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davidson JE, Jones C, Bienvenu OJ. Family response to critical illness: postintensive care syndrome-family. Crit Care Med. 2012;40(2):618–624. doi: 10.1097/CCM.0b013e318236ebf9. [DOI] [PubMed] [Google Scholar]
- 11.Ely EW. The ABCDEF bundle: science and philosophy of how ICU liberation serves patients and families. Crit Care Med. 2017;45(2):321–330. doi: 10.1097/CCM.0000000000002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haines KJ, Sevin CM, Hibbert E, Boehm LM, Aparanji K, Bakhru RN, Bastin AJ, Beesley SJ, Butcher BW, Drumright K, et al. Key mechanisms by which post-ICU activities can improve in-ICU care: results of the international THRIVE collaboratives. Intensive Care Med. 2019;45(7):939–947. doi: 10.1007/s00134-019-05647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chippa V, Aleem A, Anjum F. Post Acute Coronavirus (COVID-19) Syndrome. [Updated 2021 Oct 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK570608/.
- 14.Al-Jahdhami I, Al-Naamani K, Al-Mawali A. The post-acute COVID-19 syndrome (long COVID) Oman Med J. 2021;36(1):e220. doi: 10.5001/omj.2021.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, Cook JR, Nordvig AS, Shalev D, Sehrawat TS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vittori A, Lerman J, Cascella M, Gomez-Morad AD, Marchetti G, Marinangeli F, Picardo SG. COVID-19 pandemic acute respiratory distress syndrome survivors: pain after the storm? Anesth Analg. 2020;131(1):117–119. doi: 10.1213/ANE.0000000000004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meagher T. Long COVID: an early perspective. J Insur Med. 2021;49(1):19–23. doi: 10.17849/insm-49-1-1-5.1. [DOI] [PubMed] [Google Scholar]
- 18.Carta MG, Orru G, Scano A, Coghe F, Nunnari G, Facchini G, Numis FG, Berretta M. In the face of the SARS-CoV-2 outbreak, do people suffering from oncological disease need specific attention? Eur Rev Med Pharmacol Sci. 2020;24(7):3434–3436. doi: 10.26355/eurrev_202004_20794. [DOI] [PubMed] [Google Scholar]
- 19.Hayes JP, Vanelzakker MB, Shin LM. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front Integr Neurosci. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC, Pujol JC, Klaser K, Antonelli M, Canas LS, et al. Attributes and predictors of long COVID. Nat Med. 2021;27(4):626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortinovis M, Perico N, Remuzzi G. Long-term follow-up of recovered patients with COVID-19. Lancet. 2021;397(10270):173–175. doi: 10.1016/S0140-6736(21)00039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moreno-Perez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jimenez J, Asensio S, Sanchez R, Ruiz-Torregrosa P, Galan I, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378–383. doi: 10.1016/j.jinf.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, Banerjee A. Post-COVID syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwu CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38:65. doi: 10.11604/pamj.2021.38.65.27366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dönmez E, Temiz G, Dulger Z, Bayram Z, Doger BNB, Acar O, Demirci NS. The effects of Covid-19 phobia on quality of life: a cross-sectional study of cancer patients. World Cancer Res J. 2021;8:e1965. doi: 10.32113/wcrj_20215_1965. [DOI] [Google Scholar]
- 27.Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, Thompson JL, Chandrasekhar R, Ely EW, Brummel NE. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018;46(9):1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenforde MW, Kim SS, Lindsell CJ, Billig Rose E, Shapiro NI, Files DC, Gibbs KW, Erickson HL, Steingrub JS, Smithline HA, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network: United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, Walshaw C, Kemp S, Corrado J, Singh R, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, Flament T, Ferreira-Maldent N, Bruyere F, Stefic K, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall M. The lasting misery of coronavirus long-haulers. Nature. 2020;585(7825):339–341. doi: 10.1038/d41586-020-02598-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Cheng J, Zou J, Duan L, Campbell JE. Health-related quality of life of hospitalized COVID-19 survivors: an initial exploration in Nanning City, China. Soc Sci Med. 2021;274:113748. doi: 10.1016/j.socscimed.2021.113748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirelli U, Taibi R, Chirumbolo S. Post COVID syndrome: a new challenge for medicine. Eur Rev Med Pharmacol Sci. 2021;25(12):4422–4425. doi: 10.26355/eurrev_202106_26154. [DOI] [PubMed] [Google Scholar]
- 34.Perrella A, Carannante N, Berretta M, Rinaldi M, Maturo N, Rinaldi L. Novel coronavirus 2019 (Sars-CoV2): a global emergency that needs new approaches? Eur Rev Med Pharmacol Sci. 2020;24(4):2162–2164. doi: 10.26355/eurrev_202002_20396. [DOI] [PubMed] [Google Scholar]
- 35.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 37.Grossi E, Groth N, Mosconi P, Cerutti R, Pace F, Compare A, Apolone G. Development and validation of the short version of the Psychological General Well-Being Index (PGWB-S) Health Qual Life Outcomes. 2006;4:88. doi: 10.1186/1477-7525-4-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.EuroQol G. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 41.Caraceni A, Mendoza TR, Mencaglia E, Baratella C, Edwards K, Forjaz MJ, Martini C, Serlin RC, de Conno F, Cleeland CS. A validation study of an Italian version of the Brief Pain Inventory (Breve Questionario per la Valutazione del Dolore) Pain. 1996;65(1):87–92. doi: 10.1016/0304-3959(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 42.Parker AM, Nikayin S, Bienvenu OJ, Needham DM. Validity of the posttraumatic stress symptoms-14 instrument in acute respiratory failure survivors. Ann Am Thorac Soc. 2017;14(6):1047–1048. doi: 10.1513/AnnalsATS.201702-112LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 44.Guigoz Y. The Mini Nutritional Assessment (MNA) review of the literature: what does it tell us? J Nutr Health Aging. 2006;10(6):466–485. [PubMed] [Google Scholar]
- 45.Haraj NE, El Aziz S, Chadli A, Dafir A, Mjabber A, Aissaoui O, Barrou L, El Kettani EHC, Nsiri A, Al Harrar R, et al. Nutritional status assessment in patients with Covid-19 after discharge from the intensive care unit. Clin Nutr ESPEN. 2021;41:423–428. doi: 10.1016/j.clnesp.2020.09.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nordvig AS, Fong KT, Willey JZ, Thakur KT, Boehme AK, Vargas WS, Smith CJ, Elkind MSV. Potential neurologic manifestations of COVID-19. Neurol Clin Pract. 2021;11(2):e135–e146. doi: 10.1212/CPJ.0000000000000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021;748:135694. doi: 10.1016/j.neulet.2021.135694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javid Mishamandani Z, Norouzy A, Hashemian SM, Khoundabi B, Rezaeisadrabadi M, Safarian M, Nematy M, Pournik O, Jamialahmadi T, Shadnoush M, et al. Nutritional status of patients hospitalized in the intensive care unit: a comprehensive report from Iranian hospitals, 2018. J Crit Care. 2019;54:151–158. doi: 10.1016/j.jcrc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Fan E, Dowdy DW, Colantuoni E, Mendez-Tellez PA, Sevransky JE, Shanholtz C, Himmelfarb CR, Desai SV, Ciesla N, Herridge MS, et al. Physical complications in acute lung injury survivors: a two-year longitudinal prospective study. Crit Care Med. 2014;42(4):849–859. doi: 10.1097/CCM.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denehy L, Berney S, Whitburn L, Edbrooke L. Quantifying physical activity levels of survivors of intensive care: a prospective observational study. Phys Ther. 2012;92(12):1507–1517. doi: 10.2522/ptj.20110411. [DOI] [PubMed] [Google Scholar]
- 52.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 53.van der Schaaf M, Beelen A, Dongelmans DA, Vroom MB, Nollet F. Poor functional recovery after a critical illness: a longitudinal study. J Rehabil Med. 2009;41(13):1041–1048. doi: 10.2340/16501977-0443. [DOI] [PubMed] [Google Scholar]
- 54.Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, Christiansen CF, Thomsen RW, Pottegård A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021;21(10):1373–1382. doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen ML. Changing patterns of infectious disease. Nature. 2000;406(6797):762–767. doi: 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- 56.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P. Endorsed by the EC: ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller R, Englund K. Clinical presentation and course of COVID-19. Clevel Clin J Med. 2020;87(7):384–388. doi: 10.3949/ccjm.87a.ccc013. [DOI] [PubMed] [Google Scholar]
- 59.Wischmeyer PE. Nutrition therapy in sepsis. Crit Care Clin. 2018;34(1):107–125. doi: 10.1016/j.ccc.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez Garcia RM, Jimenez Ortega AI, Lopez Sobaler AM, Ortega RM. Nutrition strategies that improve cognitive function. Nutr Hosp. 2018;35(Spec No6):16–19. doi: 10.20960/nh.2281. [DOI] [PubMed] [Google Scholar]
- 61.Beilharz JE, Maniam J, Morris MJ. Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7(8):6719–6738. doi: 10.3390/nu7085307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8):815. doi: 10.3390/nu9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Owen L, Corfe B. The role of diet and nutrition on mental health and wellbeing. Proc Nutr Soc. 2017;76(4):425–426. doi: 10.1017/S0029665117001057. [DOI] [PubMed] [Google Scholar]
- 64.Cook R, O’Dwyer N, Parker H, Donges C, Cheng H, Steinbeck K, Cox E, Franklin J, Garg M, Rooney K, O’Connor H. Iron deficiency anemia, not iron deficiency, is associated with reduced attention in healthy young women. Nutrients. 2017;9(11):1216. doi: 10.3390/nu9111216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71(1 Suppl):171S–175S. doi: 10.1093/ajcn/71.1.171S. [DOI] [PubMed] [Google Scholar]
- 66.Derbyshire E. Do omega-3/6 fatty acids have a therapeutic role in children and young people with ADHD? J Lipids. 2017;2017:6285218. doi: 10.1155/2017/6285218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller JW, Harvey DJ, Beckett LA, Green R, Farias ST, Reed BR, Olichney JM, Mungas DM, DeCarli C. Vitamin D status and rates of cognitive decline in a multiethnic cohort of older adults. JAMA Neurol. 2015;72(11):1295–1303. doi: 10.1001/jamaneurol.2015.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Warthon-Medina M, Moran VH, Stammers AL, Dillon S, Qualter P, Nissensohn M, Serra-Majem L, Lowe NM. Zinc intake, status and indices of cognitive function in adults and children: a systematic review and meta-analysis. Eur J Clin Nutr. 2015;69(6):649–661. doi: 10.1038/ejcn.2015.60. [DOI] [PubMed] [Google Scholar]
- 69.Jacka FN, Mykletun A, Berk M, Bjelland I, Tell GS. The association between habitual diet quality and the common mental disorders in community-dwelling adults: the Hordaland Health study. Psychosom Med. 2011;73(6):483–490. doi: 10.1097/PSY.0b013e318222831a. [DOI] [PubMed] [Google Scholar]
- 70.Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O'Reilly SL, Nicholson GC, Kotowicz MA, Berk M. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167(3):305–311. doi: 10.1176/appi.ajp.2009.09060881. [DOI] [PubMed] [Google Scholar]
- 71.Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A, Schlatter J, Lahortiga F, Serra Majem L, Martinez-Gonzalez MA. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- 72.Devassy JG, Leng S, Gabbs M, Monirujjaman M, Aukema HM. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv Nutr. 2016;7(5):905–916. doi: 10.3945/an.116.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miquel S, Champ C, Day J, Aarts E, Bahr BA, Bakker M, Banati D, Calabrese V, Cederholm T, Cryan J, et al. Poor cognitive ageing: vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res Rev. 2018;42:40–55. doi: 10.1016/j.arr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 74.Schlogl M, Holick MF. Vitamin D and neurocognitive function. Clin Interv Aging. 2014;9:559–568. doi: 10.2147/CIA.S51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369(9557):208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 76.Aparicio Vizuete A, Robles F, Rodriguez-Rodriguez E, Lopez-Sobaler AM, Ortega RM. Association between food and nutrient intakes and cognitive capacity in a group of institutionalized elderly people. Eur J Nutr. 2010;49(5):293–300. doi: 10.1007/s00394-009-0086-y. [DOI] [PubMed] [Google Scholar]
- 77.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 2: macronutrients. J Nutr Health Aging. 2006;10(5):386–399. [PubMed] [Google Scholar]
- 78.Kim SH, Park YM, Choi BY, Kim MK, Roh S, Kim K, Yang YJ. Associations of serum levels of vitamins A, C, and E with the risk of cognitive impairment among elderly Koreans. Nurs Res Pract. 2018;12(2):160–165. doi: 10.4162/nrp.2018.12.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Markiewicz-Zukowska R, Gutowska A, Borawska MH. Serum zinc concentrations correlate with mental and physical status of nursing home residents. PLoS ONE. 2015;10(1):e0117257. doi: 10.1371/journal.pone.0117257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andruchow ND, Konishi K, Shatenstein B, Bohbot VD. A lower ratio of omega-6 to omega-3 fatty acids predicts better hippocampus-dependent spatial memory and cognitive status in older adults. Neuropsychology. 2017;31(7):724–734. doi: 10.1037/neu0000373. [DOI] [PubMed] [Google Scholar]
- 81.Guo XM, Liu H, Qian J. Daily iron supplementation on cognitive performance in primary-school-aged children with and without anemia: a meta-analysis. Int J Clin Exp Med. 2015;8(9):16107–16111. [PMC free article] [PubMed] [Google Scholar]
- 82.Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1: micronutrients. J Nutr Health Aging. 2006;10(5):377–385. [PubMed] [Google Scholar]
- 83.Scarmeas N, Anastasiou CA, Yannakoulia M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018;17(11):1006–1015. doi: 10.1016/S1474-4422(18)30338-7. [DOI] [PubMed] [Google Scholar]
- 84.Seetharaman S, Andel R, McEvoy C, Dahl Aslan AK, Finkel D, Pedersen NL. Blood glucose, diet-based glycemic load and cognitive aging among dementia-free older adults. J Gerontol A Biol Sci Med Sci. 2015;70(4):471–479. doi: 10.1093/gerona/glu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katsiardanis K, Diamantaras AA, Dessypris N, Michelakos T, Anastasiou A, Katsiardani KP, Kanavidis P, Papadopoulos FC, Stefanadis C, Panagiotakos DB, et al. Cognitive impairment and dietary habits among elders: the Velestino Study. J Med Food. 2013;16(4):343–350. doi: 10.1089/jmf.2012.0225. [DOI] [PubMed] [Google Scholar]
- 86.Kesse-Guyot E, Andreeva VA, Lassale C, Ferry M, Jeandel C, Hercberg S, Galan P, Group SVMR Mediterranean diet and cognitive function: a French study. Am J Clin Nutr. 2013;97(2):369–376. doi: 10.3945/ajcn.112.047993. [DOI] [PubMed] [Google Scholar]
- 87.Gardener S, Gu Y, Rainey-Smith SR, Keogh JB, Clifton PM, Mathieson SL, Taddei K, Mondal A, Ward VK, Scarmeas N, et al. Adherence to a Mediterranean diet and Alzheimer's disease risk in an Australian population. Transl Psychiatry. 2012;2:e164. doi: 10.1038/tp.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80(18):1684–1692. doi: 10.1212/WNL.0b013e3182904f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McEvoy CT, Guyer H, Langa KM, Yaffe K. Neuroprotective diets are associated with better cognitive function: the health and retirement study. J Am Geriatr Soc. 2017;65(8):1857–1862. doi: 10.1111/jgs.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Batty GD, Deary IJ, Luciano M, Altschul DM, Kivimaki M, Gale CR. Psychosocial factors and hospitalisations for COVID-19: prospective cohort study based on a community sample. Brain Behav Immun. 2020;89:569–578. doi: 10.1016/j.bbi.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brugliera L, Spina A, Castellazzi P, Cimino P, Arcuri P, Negro A, Houdayer E, Alemanno F, Giordani A, Mortini P, et al. Nutritional management of COVID-19 patients in a rehabilitation unit. Eur J Clin Nutr. 2020;74(6):860–863. doi: 10.1038/s41430-020-0664-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. doi: 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chew NWS, Lee GKH, Tan BYQ, Jing M, Goh Y, Ngiam NJH, Yeo LLL, Ahmad A, Ahmed Khan F, Napolean Shanmugam G, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun. 2020;88:559–565. doi: 10.1016/j.bbi.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forte G, Favieri F, Tambelli R, Casagrande M. The enemy which sealed the world: effects of COVID-19 diffusion on the psychological state of the Italian population. J Clin Med. 2020;9(6):1802. doi: 10.3390/jcm9061802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lesser IA, Nienhuis CP. The impact of COVID-19 on physical activity behavior and well-being of Canadians. Int J Environ Res Public Health. 2020;17(11):3899. doi: 10.3390/ijerph17113899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kang L, Ma S, Chen M, Yang J, Wang Y, Li R, Yao L, Bai H, Cai Z, Xiang Yang B, et al. Impact on mental health and perceptions of psychological care among medical and nursing staff in Wuhan during the 2019 novel coronavirus disease outbreak: a cross-sectional study. Brain Behav Immun. 2020;87:11–17. doi: 10.1016/j.bbi.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lai X, Wang M, Qin C, Tan L, Ran L, Chen D, Zhang H, Shang K, Xia C, Wang S, et al. Coronavirus disease 2019 (COVID-2019) infection among health care workers and implications for prevention measures in a Tertiary Hospital in Wuhan, China. JAMA Netw Open. 2020;3(5):E209666. doi: 10.1001/jamanetworkopen.2020.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan BYQ, Chew NWS, Lee GKH, Jing M, Goh Y, Yeo LLL, Zhang K, Chin HK, Ahmad A, Khan FA, et al. Psns for prevention measures in a Tertiary Hospital in Wuhan, psychological impact of the COVID-19 pandemic on health care workers in Singapore. Ann Intern Med. 2020;173(4):317–320. doi: 10.7326/M20-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, Sultan M, Easton A, Breen G, Zandi M, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang CY, Pan RY, Wan XY, Tan YL, Xu LK, McIntyre RS, Choo FN, Tran B, Ho R, Sharma VK, et al. A longitudinal study on the mental health of general population during the COVID-19 epidemic in China. Brain Behav Immun. 2020;87:40–48. doi: 10.1016/j.bbi.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang WR, Wang K, Yin L, Zhao WF, Xue Q, Peng M, Min BQ, Tian Q, Leng HX, Du JL, et al. Mental health and psychosocial problems of medical health workers during the COVID-19 epidemic in China. Psychother Psychosom. 2020;89(4):242–250. doi: 10.1159/000507639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.