Abstract
Background
Prognostic models for malignant pleural mesothelioma (MPM) have been limited to demographics, symptoms, and laboratory values. We hypothesize higher accuracy using both tumor and patient characteristics. The Mesothelioma Prognostic Test (MPT) and molecular subtype based on Claudin-15 to Vimentin (C/V) expression ratio are molecular signatures associated with survival. Tumor volume (TV) has improved performance compared to clinical staging, while neutrophil-to-lymphocyte ratio (NLR) is prognostic for MPM.
Methods
Tumor specimens and clinical data were collected prospectively from patients who underwent extrapleural pneumonectomy (EPP) or pleurectomy and decortication (PD) during 2007–2014. MPT and C/V ratio were determined by RT-qPCR, while TV was assessed from pre-operative scans. Risk groups were derived from combinations of adverse factors based on the Cox model. Predictive accuracy was assessed using Harrell’s c-index.
Results
MPT, molecular subtype, TV, and NLR were independently prognostic in EPP patients (N=191), suggesting equal weighting in a final three-group model (c=0.644). In the PD cohort (N=193), MPT poor risk combined with TV>200 cc was associated with triple the risk compared to other subgroups (hazard ratio=2.94, 95% CI: 1.70–5.09, p<0.001) persisting when adjusted for molecular subtype, NLR, performance status and serum albumin to yield a final three-group model (c=0.641). The EPP and PD models achieved higher accuracy than published models (c≤0.584, c≤0.575) and pathological staging (c=0.554, c=0.571).
Conclusion(s)
The novel models utilize pre-treatment parameters obtained from minimally invasive biopsy, imaging and blood tests to evaluate the expected outcome of each type of surgery in newly diagnosed patients and improve stratification on clinical trials
Keywords: Mesothelioma, RT-qPCR tests, Prediction, Surgery, Survival
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive malignancy usually resulting from prior asbestos exposure, affecting roughly 2,000 people annually in the US.1, 2 Extirpative surgery such as extra-pleural pneumonectomy (EPP) or pleurectomy and decortication (PD), followed or preceded by chemotherapy, with or without radiation therapy, is associated with 15–20% five-year survival in selected patients.3,4 First-line chemotherapy was limited to cisplatin and pemetrexed5 for many years, as phase II and III trials evaluating various chemotherapeutic, biologic, and/or immunologic therapies were generally unsuccessful, except the addition of bevacizumab.6, 7 The first-line standard of care changed only recently to nivolumab plus ipilimumab based on significant and clinically meaningful improvements in overall survival for unresectable MPM.8
Treatment decisions for MPM are generally based on physician assessment and clinical staging to determine patient status, disease extent and surgical resectability.9 Clinical staging relies on imaging studies (tumor volume and disease extent by chest CT, metastatic lesions by PET) and is usually supplemented by diagnostic biopsy for histological subtype (epithelioid, biphasic or sarcomatoid) and surgical staging of lymph nodes (mediastinoscopy or EBUS).10 Patient symptoms, performance status, and laboratory tests are also considered in clinical staging as chest pain, cachexia and chest deformity are often associated with advanced disease.10 Aside from clinical findings of advanced cancer, clinical staging is limited due to the absence of an accepted prognostic algorithm and insufficient accuracy in predicting pathological stage or patient outcome.11 Moreover, inclusion criteria for most clinical trials are limited to ambulatory status and histological diagnosis, regardless of stage or other factors, potentially explaining the lack of success and making it very difficult to compare cohorts within or across trials.12 Similarly, selection criteria for surgery as well as surgical approaches are arbitrary and vary greatly among centers.13
We previously proposed a prognostic algorithm for post-operative patients by combining a molecular expression signature or Mesothelioma Prognostic Test (MPT), histological subtype and lymph node status as independent parameters.14, 15 Subsequently, we showed tumor volume (TV) assessed from pre-operative CT could be used to improve prognostic performance compared to clinical staging.16 Others have proposed serum albumin, neutrophil-to-lymphocyte ratio (NLR), White Blood Count (WBC), hemoglobin and other blood parameters as prognostic factors for MPM.17–24
In differential expression analyses of 211 transcriptomes from primary tumors,25 we identified four molecular clusters related to the histological spectrum from epithelioid to sarcomatoid subtypes, closely reflecting tumor heterogeneity and epithelial to mesenchymal transition in MPM. Notably, cluster E was associated with significantly longer survival compared to clusters B-E, B-S and S, and the expression ratio of Claudin-15 (CLDN15) and Vimentin (VIM) (C/V ratio) could be used to discriminate among the four clusters.
Our previous model required extirpative surgery to establish definitively histological subtype and lymph node status.14 However, molecular tests may be performed using small tumor biopsies obtained surgically or under image guidance.26 With the ultimate goal of establishing reliable clinic-pathological stratification prior to surgery or definitive treatment, we have combined existing and novel prognostic markers to validate and extend the post-operative algorithm as well as develop a pre-operative model using a combination of readily obtainable parameters. We compared model performance to the major algorithms published for MPM prognosis.18–20
Materials and Methods
Patients and specimens
Between 2007 and 2014, 686 MPM patients underwent primary resections at a single academic medical center, including 293 EPPs and 298 PDs. All patients had mediastinal staging pre-operatively and were excluded from surgery if positive nodes were found. Among patients who consented to an IRB-approved protocol (Dana-Farber Cancer Institute Protocol 98–063) for prospective collection, tumor specimens were collected at surgery as discarded tissue and fresh frozen, stored and annotated by the institutional tumor bank. Frozen banked specimens were available for 253 EPP and 258 PD patients. We limited the present analysis to 384 patients who underwent resection without neoadjuvant chemotherapy. Clinical and pathologic characteristics (demographics, performance status, symptoms, histology, stage, survival) with pre-operative laboratory values (complete blood count, metabolic panel) were collected prospectively in a separate database independently from surgical specimens and imaging findings.
Tumor volume
Radiological assessment was performed by an experienced thoracic radiologist (RRG) with expertise in imaging and qualitative analyses of MPM who was masked to all case annotation. Image analysis included assessment of tumor distribution, invasion into adjacent structures, clinical staging by the current system27 and measurement of interlobular fissural thickening at the maximum thickness. Quantitative and qualitative radiological assessments were previously described.16 All but 10 patients had pre-operative imaging available for radiological assessment. Majority of imaging data for volumetric assessment was calculated from segmentation of CT scans (n=183, 49%) with 5mm (148) and 3mm (35) slices or diagnostic quality CT scans acquired from PET-CT for attenuation correction with 3.75mm thickness (n=160, 43%). All PET-CT scans were acquired without contrast, whereas a minority of CT scans (n=79, 21%) was acquired after contrast enhancement. Thirty-one (8%) MRI scans were contrast enhanced with clear delineation of tumor allowing for segmentation.
RNA extraction and Real-Time quantitative PCR
Molecular analysis was performed following the end of specimen collection without access to imaging, clinical, pathologic and outcome data. RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) and RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions and quantified using an ND-1000 spectrophotometer ( Thermo Fisher Scientific, Wilmington, DE). RNA integrity number ≥7 was considered indicative of RNA integrity using the Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). Two hundred nanograms of total RNA were reverse-transcribed using Superscript VILO Master Mix (Thermo Fisher Scientific, Waltham, MA). Real-time quantitative PCR (RT-qPCR) was conducted using the PowerUp SYBR Green fluorometry-based detection system (Thermo Fisher Scientific, Waltham, MA) to establish the relative expression levels of six genes using primers (forward and reverse) for TM4SF1, PKM2, ARHGDIA, COBLL1 as published,14 CLDN15 (5’-ACTCCCTGGGCGTCTACAAC-3’ and 5’-ATGGCGGTGATCATGAGTG-3’), and VIM (5’-GACAACCTGGCCGAGGAC-3’ and 5’-AAGATTGCAGGGTGTTTTCG-3’). Controls containing water instead of template were run in multiple wells on each reaction plate.
Molecular tests
The expression level for each gene was obtained from the CT (threshold cycle) value at which the quantity of the amplified gene reaches the exponential phase. The Mesothelioma Prognostic Test (MPT) was calculated as a geometric mean of three gene-pair ratios (TM4SF1/PKM2, TM4SF1/ARHGDIA, COBLL1/ARHGDIA) to assign each tumor specimen as good (geometric mean >1) or poor (geometric mean <1) risk as previously described.14, 15 The C/V ratio was formed by the relative expression levels of CLDN15 to VIM in the comparative CT equation given by 2^[CT(CLDN15) - CT(VIM)], analogous to the gene expression ratios for MPT.14
To translate C/V ratio from RT-qPCR into molecular subtype, a subset of 59 EPP patients with sequenced transcriptomes25 was analyzed using RNAseq as the gold standard to differentiate between clusters E and non-E (B-E, B-S, S) (Supplementary Figure 1A). ROC analysis28 (Supplementary Figure 1B) showed a threshold of 0.3 obtained 80% sensitivity and 88% specificity for assigning each tumor specimen to cluster E (C/V ratio >0.3) or cluster non-E (C/V ratio <0.3).
Statistical analysis
Clinical, pathologic and outcome data were linked to imaging and molecular results after all data collection was completed. Distribution of patient and tumor characteristics was compared between surgery and molecular groups using Fisher’s exact test and Wilcoxon rank sum test. Overall survival was measured from the resection date and estimated using the Kaplan-Meier method. Proportional hazards regression was used in univariable and multivariable models to assess survival difference between patient groups by the score test and to estimate the hazard ratio (HR) associated with each adverse factor. All p-values were based on a two-sided hypothesis. Published cutoffs and adjacent ranges of TV16 and NLR17, 21–24 were evaluated to identify clinically practical thresholds using HR as the effect size. Forward selection based on residual chi-square of the score test was applied at a 0.10 level to determine the additional contribution of prognostic factors in the EORTC19, CALGB20 and Australian18 models using their published thresholds for age and laboratory values (WBC, hemoglobin, serum albumin). Any characteristic with fewer than 10 deaths in a reference or comparison group were not analyzed. The relative magnitude of HRs in a multivariable model was used to weight the prognostic contribution of each clinical or molecular characteristic for translating every combination of adverse factors into a total score. Predictive accuracy of risk models was measured by the agreement between observed and predicted outcomes using the c-index.29 Data analysis was performed using SAS 9.4 (SAS Institute, Cary, NC) and R 4.0.3 (R Foundation, Vienna, Austria).
Results
Table 1 shows clinical, pathologic and molecular characteristics for 191 and 193 patients in the EPP and PD cohorts, respectively. EPP patients were younger than those who underwent PD (median 64 vs. 70 years, p < 0.001) with proportionally fewer females (12% vs. 26%, p < 0.001). Their tumors were more extensive with larger volume (median 311 vs. 141 cc, p < 0.001) and greater pathological involvement of lymph nodes (61% vs. 42%, p < 0.001). Although the histological distribution was very similar in both cohorts (57% vs. 56% epithelioid, p = 0.918), the favorable molecular subtype was less frequent among EPP than PD patients (17% vs. 25% cluster E, p = 0.059). Molecular cluster E was associated with younger age, female gender, epithelioid histology, lower NLR, and higher hemoglobin (Supplementary Table 1). The molecular prognosis based on MPT was generally good in both cohorts, with a higher proportion of good risk among EPP than PD patients (93% vs. 80%, p < 0.001). MPT good risk was associated with less chest pain, epithelioid histology, negative nodes, and higher hemoglobin (Supplementary Table 2). Unsurprisingly, the favorable molecular subtype and MPT good risk were strongly correlated (23% (good risk) vs. 6% (poor risk) cluster E, p = 0.003). Median follow-up was 24.7 and 51.8 months among 17 (9%) and 28 (15%) surviving patients in the EPP and PD cohorts, respectively.
Table 1.
Patient and tumor characteristics based on final specimens
| EPP(N=191) | PD (N=193) | |
|---|---|---|
| No. (%) | No. (%) | |
|
Age at surgery, years Median (range) ≥49 years |
64 (18–81) 173 (91%) |
70 (30–86) 185 (96%) |
|
Gender Male Female |
169 (88%) 22 (12%) |
143 (74%) 50 (26%) |
|
ECOG performance status 0 1 2 |
42 (22%) 142 (74%) 7 (4%) |
53 (27%) 136 (70%) 4 (2%) |
|
Symptoms at presentation Weight loss >5% Chest pain |
78 (41%) 65 (34%) |
74 (38%) 76 (39%) |
|
Pathological histological subtype Epithelioid Biphasic Sarcomatoid Desmoplastic |
109 (57%) 71 (37%) 10 (5%) 1 (<1%) |
109 (56%) 70 (36%) 13 (7%) 1 (<1%) |
|
Pathological lymph node stage pN0 pN1 pN2 pNx |
74 (39%) 36 (19%) 81 (42%) |
111 (58%) 8 (4%) 54 (28%) 20 (10%) |
|
Tumor volume (TV)a, cc Median (range) >200 cc |
311 (<1–4285) 118 (65%) |
141 (<1–4437) 75 (39%) |
|
Mesothelioma prognosis test (MPT) Good risk Poor risk |
177 (93%) 14 (7%) |
155 (80%) 38 (20%) |
|
Molecular subtype test Cluster E Cluster non-E (BE/BS/S) |
32 (17%) 159 (83%) |
48 (25%) 145 (75%) |
|
Neutrophil:Lymphoctye ratio (NLR) Median (range) NLR >3 |
3.9 (0.3–29.0) 136 (71%) |
3.2 (0.9–20.5) 107 (55%) |
|
White blood count, x109/L Median (range) |
8.0 (3.9–17.1) | 7.2 (3.0–25.1) |
|
Hemoglobin, g/L Median (range) |
127 (67–164) | 126 (69–173) |
|
Serum albumin, g/L Median (range) |
39 (21–49) | 39 (10–48) |
Tumor volume missing for 10 patients (EPP 9, PDC 1).
Post-operative models:
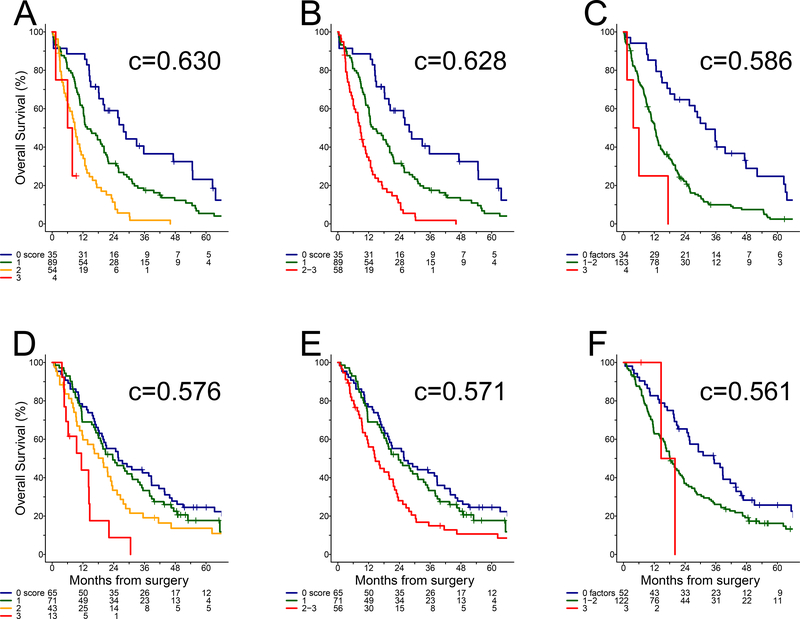
The prognostic value of TV was assessed initially in the EPP cohort when added to the original three-parameter model14 combining MPT, histological subtype and lymph node status (Table 2). While larger TV was generally associated with worse outcome, a cutoff of 200 cc was identified based on effect size. Adding TV to the multivariable model with the original parameters (Table 2), the prognostic contribution of lymph node status was modest (HR = 1.32) whereas MPT, histological subtype, and TV were associated with survival differences (HRs ranging from 1.78 to 2.15) corresponding to approximately doubling of risk. Therefore, we proposed a revised three-parameter model by summing the number of adverse features among MPT poor risk, non-epithelioid histology and TV >200 cc to derive the Mesothelioma Prognosis Score (MPS) with 0–3 range. As only four (2%) EPP patients had a non-epithelioid tumor larger than 200 cc and classified as poor risk by MPT, the worst risk groups with MPS 2–3 were combined to obtain a three-group post-operative model with virtually no loss of predictive accuracy (c = 0.628, 95% CI: 0.586–0.671) compared to the four-group model (c = 0.630, 95% CI: 0.587–0.672). Both forms of the MPS (Figure 1A–B) were more accurate for predicting outcome of EPP patients than the original three-parameter model (c = 0.586, 95% CI: 0.551–0.621) (Figure 1C) and pathological stage using the current27 (c = 0.554, 95% CI: 0.505–0.603) or previous30 (c = 0.527, 95% CI: 0.483–0.570) AJCC criteria (Supplementary Figure 2A–B).
Table 2.
Survival analysis of EPP patients
| Adverse factor | HR | 95% CI | p-value |
|---|---|---|---|
| Multivariable analysis of post-operative characteristics (N=191, 174 deaths) | |||
| MPT poor risk | 1.78 | 1.00–3.17 | 0.049 |
| Non-epithelioid histology | 2.15 | 1.57–2.95 | <0.001 |
| pN+ stage | 1.32 | 0.96–1.82 | 0.091 |
| TV >200 cc | 1.93 | 1.37–2.71 | <0.001 |
| Univariable analysis of pre-operative characteristics (N=191, 174 deaths) | |||
| MPT poor risk | 1.75 | 0.99–3.10 | 0.053 |
| Molecular cluster non-E | 2.33 | 1.47–3.68 | <0.001 |
| NLR >3 | 1.83 | 1.30–2.58 | <0.001 |
| TV >200 cc* | 1.75 | 1.26–2.44 | <0.001 |
| Multivariable analysis of pre-operative characteristics (N=182, 166 deaths) | |||
| MPT poor risk | 2.08 | 1.16–3.71 | 0.012 |
| Molecular cluster non-E | 2.35 | 1.47–3.77 | <0.001 |
| NLR >3 | 1.92 | 1.35–2.72 | <0.001 |
| TV >200 cc* | 1.81 | 1.29–2.53 | <0.001 |
TV missing for 9 patients
Figure 1. Overall survival.
(A) EPP cohort: MPS four-group model, (B) EPP cohort: MPS three-group model, (C) EPP cohort: original three-parameter model (D) PD cohort: MPS four-group model, (E) PD cohort: MPS three- group model, (F) PD cohort: original three-parameter model.
Although the MPS segregated PD patients by survival, the four-group (c = 0.576, 95% CI: 0.530–0.622) or three-group models (c = 0.571, 95% CI: 0.525–0.617) did not perform as well as in the EPP cohort. The most favorable groups with 0 or 1 adverse features had almost overlapping survival curves, while patients with MPS 2–3 corresponding to intermediate and worst risks showed clearer separation (Figure 1D–E). The original three-parameter model performed poorly (c = 0.561, 95% CI: 0.521–0.600) in the PD cohort (Figure 1F), as only 3 (2%) patients were identified as poor risk. In contrast, the latest (c = 0 571, 95% CI: 0.523–0.618) and previous (c = 0.554, 95% CI: 0.508–0.601) staging systems27, 30 (Supplementary Figure 2C–D) provided virtually no discrimination among all disease stages beyond Stage 1.
Novel models:
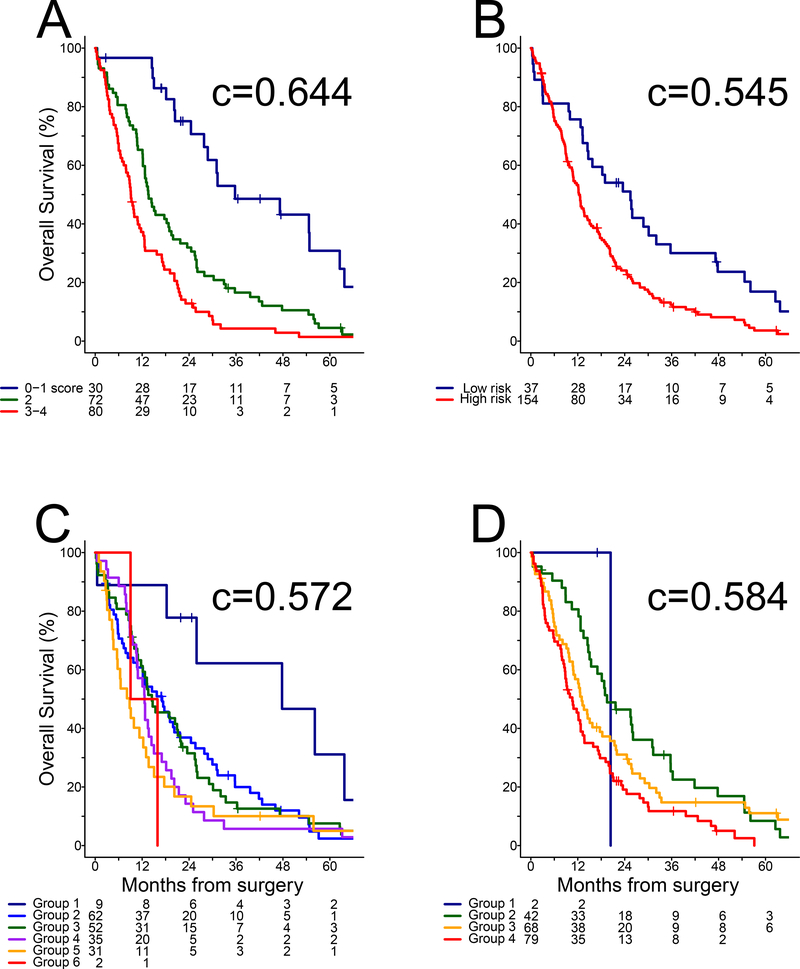
Previously proposed variables were evaluated to develop a prognostic algorithm that does not require surgical pathology for histology or lymph node status. The MPT, molecular subtype test, NLR and TV were associated with outcome of EPP patients in univariable analysis and remained significant in multivariable analysis (Table 3), confirming the choice of candidates for a novel algorithm. Age, gender, ECOG performance status, WBC, hemoglobin and serum albumin were significantly associated with outcome in univariable analysis (Supplementary Table 3), but none were independently prognostic when added to the multivariable model. The significant variables were associated with survival differences of a similar magnitude (HRs ranging from 1.81 to 2.35), suggesting a scoring system with equal weighting of each prognostic factor. Specifically, a value of 1 was assigned to each of MPT poor risk, cluster non-E, NLR >3 and TV >200 cc but a value of 0 otherwise, yielding total scores of 0–4. As only 5 (3%) EPP patients presented pre-operatively with no adverse factor and 3 (2%) had all four, the lowest and highest scores were pooled with adjacent values to obtain a final pre-operative model comprised of three groups with 0–1, 2 and 3–4 adverse factors, respectively (Figure 2A). The novel pre-operative model with three risk groups (c = 0.644, 95% CI: 0.602–0.687) outperformed the EORTC (c = 0.545, 95% CI: 0.508–0.583), CALGB (c = 0.572, 95% CI: 0.521–0.622) and Australian models (c = 0.584, 95% CI: 0.542–0.627) in the EPP cohort (Figure 2B–D).
Table 3.
Survival analysis of PD patients
| Adverse factor | HR | 95% CI | p-value |
|---|---|---|---|
| Univariable analysis of pre-operative characteristics (N=193, 165 deaths) | |||
| MPT poor risk | 1.28 | 0.87–1.88 | 0.212 |
| Molecular cluster non-E | 1.66 | 1.15–2.41 | 0.007 |
| NLR >3 | 1.54 | 1.13–2.11 | 0.007 |
| TV >200 cc* | 1.59 | 1.16–2.19 | 0.004 |
| Multivariable analysis of pre-operative characteristics (N=193, 165 deaths) | |||
| MPT poor risk & TV >200 cc | 2.51 | 1.44–4.38 | <0.001 |
| Molecular cluster non-E | 1.58 | 1.08–2.31 | 0.017 |
| NLR >3 | 1.50 | 1.09–2.06 | 0.013 |
| ECOG performance status 1–2 | 1.53 | 1.07–2.71 | 0.017 |
| Serum albumin ≥43 g/L | 1.68 | 1.70–2.64 | 0.023 |
TV missing for 1 patient
Figure 2. Overall survival in EPP cohort:
(A) novel pre-operative model derived in Table 2, (B) EORTC model, (C) CALGB model, and (D) Australian model.
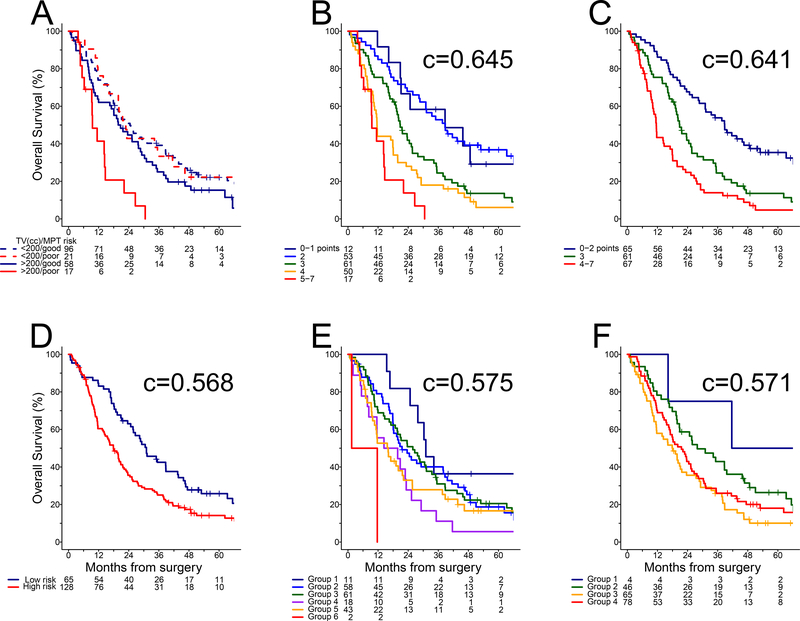
The MPT was not associated with outcome of PD patients in univariable analysis, while the molecular subtype test, NLR and TV were significant (Table 3). Subgroup analysis showed the MPT effect was limited to larger tumors (Figure 3A), namely MPT poor risk and TV >200 cc was associated with three times higher risk of death compared to MPT good risk and/or TV ≤200 cc (HR = 2.94, 95% CI: 1.70–5.09). Fitting an interaction term in a multivariable model (Table 3), HR estimates suggested weighting MPT poor risk in the presence of TV >200 cc three times as much as each of the other adverse factors. Among clinical and laboratory characteristics associated with outcome in univariable analysis (Supplementary Table 3), ECOG performance status and serum albumin were independently prognostic in the multivariable model (Table 3). Therefore, a value of 3 was assigned to MPT poor risk and TV >200 cc, while a value of 1 was assigned to each of cluster non-E, NLR >3, ECOG performance status 1–2, and serum albumin ≤43 g/L, but a value of 0 otherwise. Interestingly, total score of 0 was not observed, indicating all PD patients presented pre-operatively with at least one adverse factor. Stratifying by total score into five groups (Figure 3B), the survival curves virtually overlapped between scores of 1 and 2, while separation was modest between scores of 4 and 5–7. Therefore, we proposed pooling the lowest and highest risks with adjacent values to obtain a final pre-operative model with total scores of 0–2, 3 and 4–7, respectively (Figure 3C). The parsimonious model with three risk groups showed minimal loss of predictive accuracy (c = 0.641, 95% CI: 0.599–0.683) compared to the initial pre-operative model with five groups (c = 0.645, 95% CI: 0.602–0.687). If ECOG performance status and serum albumin were excluded, the reduced model had lower accuracy based on four groups (c = 0.610, 95% CI: 0.568–0.652) with total score of 3–5 as worst risk (data not shown). The EORTC (c = 0.568, 95% CI: 0.528–0.607), CALGB (c = 0.575, 95% CI: 0.530–0.620) and Australian models (c = 0.571, 95% CI: 0.524–0.617) did not perform well in the PD cohort (Figure 3D–F).
Figure 3. Overall survival in PD cohort:
(A) MPT risk groups by TV, (B) novel pre-operative model derived in Table 3, (C) novel pre-operative model with three groups, (D) EORTC model, (E) CALGB model, and (F) Australian model.
Discussion
The MPT was developed originally as a molecular signature to differentiate between good and poor prognosis in MPM patients who undergo EPP. The signature was validated in independent cohorts31 and combined with pathologic parameters to develop a post-operative algorithm for improved stratification based on survival after surgery.14 We present herein a prospective study to examine the original algorithm that incorporates the MPT, histological subtype, and lymph node status together with TV as prognostic markers in surgical patients. In addition to TV, the MPT and histological subtype remained predictive in multivariable analysis of the EPP cohort with similar HRs validating the published results.14 Notably, the MPS was more accurate than the current staging edition27 for stratifying EPP patients into survival groups. Although individual parameters of the MPS were not all validated in the PD cohort, the predictive accuracy was nevertheless as high as the current staging scheme27 but the AJCC system did not provide any discrimination among more advanced disease beyond Stage 1 (Supplementary Figure 2C).
The eight-year duration of patient enrollment was a transitional period for surgical approaches to MPM. During the 1990s, the most common operation was EPP, while PD was reserved for palliation in unresectable patients or for those considered medically unfit or who had refused EPP. Since the 2000s, PD was offered to some patients as the surgery of choice. PD is a more variable procedure than EPP and potentially not as complete in removing every possible surface of the pleura. All patients undergoing extirpative surgery in the current era had negative mediastinal nodes as part of pre-operative work-up. Also, the extent of lymph nodes collected during PD may be smaller compared to EPP, culminating in a combination of factors potentially limiting the utility as a prognostic factor in contemporary patients. In our study population, the clinical distribution between EPP and PD patients was mostly balanced, but demographic and tumor characteristics were different, with older ages, more women, lower tumor burden and earlier stages in the PD cohort. The simplest explanation for inability to validate the MPT in the PD subgroup with TV ≤200 cc is that the molecular signature was developed originally for EPP during an era when larger tumors were resected. Nevertheless, the MPS still stratified EPP and PD patients at least as well as existing staging systems27,30 if not better, thus validating the algorithm for pathological staging and survival prediction of post-operative patients.
Other prognostic tests and biomarkers have been proposed for MPM, including NLR, WBC, platelet count, hemoglobin and serum albumin. For example, the Western Australian group developed a clinical prediction model based on ECOG performance status, weight loss status, hemoglobin and serum albumin, but their study was limited by inclusion of mostly non-surgical patients without reference to pathological staging or defined histological diagnosis.18 In our study, only ECOG performance status and serum albumin were sufficiently robust to add prognostic value to the pre-operative variables identified for a novel model, at least in the PD cohort. Interestingly, their decision tree approach identified a threshold of serum albumin at 43 g/L that showed higher predictive accuracy in our final model compared to commonly used cutoffs of 30 or 35 g/L. We suspect that majority of laboratory tests were particularly abnormal in older patients with more advanced cancer in the Australian cohort and thus less meaningful in our surgical population.
This study has several limitations. The imaging data used to assess TV showed heterogeneity stemming from variability in slice thickness and radiology modality as well as availability of contrast-enhanced images. As the degree to which imaging parameters affected volumetric assessment was not assessed, future work is needed to explore how much the comparative differences influence prognosis. Moreover, volumetric assessment may not be feasible outside academic centers due to lack of specialized radiology expertise. Molecular testing was performed using frozen specimens that may not be readily available in community settings. Although additional studies are needed to validate the C/V ratio in formalin-fixed paraffin-embedded tissues, the MPT was successfully validated in a multi-center collection of MPM tumors,32 indicating the gene expression ratio test performed well in formalin-fixed paraffin-embedded specimens. In addition, statistical modeling is limited by the observed distribution of study cohorts, as very few patients were identified with extreme risk scores, for example, no PD patient presented pre-operatively with only non-adverse factors. Although the lowest and highest risk groups may be defined by artifacts of pooling small patient numbers, the ordering of prognosis is nevertheless valid even if less than optimal at extremes of the survival distribution in the whole population.
While a reliable pathological staging system is useful for informing patients about future prognosis and treatment options after surgery, it does not inform clinicians or patients whether surgery is indicated in the first place. Due to variability of response to neoadjuvant chemotherapy, the study cohorts were limited to treatment-naive patients to define a representative population of newly diagnosed cases who are candidates for extirpative surgery. As minimally invasive tests would better serve patients pre-treatment, we have replaced histological subtype and lymph node status determined at surgery by pre-operative novel tests that are prognostic singly and in combination. Histological subtype was substituted by an RNA signature to distinguish cluster E from non-E tumors,25 while lymph node status was replaced with NLR based on standard blood counts. Specifically, we confirmed the prognostic value of NLR using a threshold of 317, 21, 23, 24 in univariable and multivariable analyses, whereas smaller effects were associated with a cutoff of 522. We thus propose stratifying patients into three risk groups for surgery outcome based on TV from chest CT or MRI using radiomics tools that integrate with routine radiology workflow, NLR from a differential blood count, ECOG performance status, and serum albumin from a basic metabolic panel as well as two simple gene expression signatures respectively utilizing 4 and 2 genes from a pleural biopsy of tumor. The novel algorithm, MPM Risk Score (MRiS), may be used for stratifying patients pre-treatment to allow a more rational interpretation of trial data and to inform surgical prognosis depending on the procedure. Median survival estimates in order of increasing risk were 36, 14 and 9 months for EPP patients (Figure 2A), and 39, 21 and 12 months for those who underwent PD (Figure 3C). MRiS demonstrates that while prognostic factors for MPM patients undergoing surgery are the same, their effects have different magnitude and are affected by additional markers of clinical status depending on the procedure.
Although the eventual outcome of individual patients may be influenced by post-operative treatment, the prognostic utility of pathological and clinical staging systems such as MRiS is the upfront stratification of risk groups based on pre-treatment variables. While the proposed clinical staging tool needs to be validated in a multi-center setting, the components of MRiS are readily available on most patients so it can be put into clinical practice to serve as a reference of expected survival for a newly diagnosed patient considering extirpative surgery. We have created an online tool which can be used to input the pre-operative variables for calculating MRiS scores and expected median survival associated with each type of surgery (https://mris.brighamandwomens.org/). Although MRiS did not perform statistically better than the CALGB and Australian models solely based on 95% CIs of the c-index, the study was not designed with high power for model comparison. Remarkedly, only the lowest risk is identified clearly by Group 1 of the CALGB (Figures 2C & 3E) and Australian models (Figures 2D & 3F), whereas intermediate-risk and high-risk groups have overlapping and/or intersecting survival curves despite representing 94% or more of each cohort. Lack of survival discrimination beyond the lowest risk is also a feature of pathological staging systems (Supplementary Figure 2A–D), reminding us that a single statistical summary such as the c-index may not necessarily convey a comprehensive evaluation of prognostic algorithms. In contrast, a more balanced distribution of risk groups is obtained by MRiS (Figures 2A & 3C), while maintaining clear survival separation across all timepoints. Nevertheless, the c-indices associated with MRiS suggest the need for additional markers to achieve c = 0.7 generally accepted for clinical utility. Notably, PD-L1 expression has been shown to be a negative prognostic marker33–36 that is increasingly relevant due to the advent of immunotherapy for MPM8. Expanding the panel of molecular markers will be critical in future directions for assessing the utility of MRiS in neoadjuvant and unresectable settings.
Supplementary Material
Acknowledgments
Funding This work was supported by grants to RB from the National Cancer Institute (R01 CA120528), and the International Mesothelioma Program at Brigham and Women’s Hospital. The study sponsors played no role in the study design, collection, analysis, interpretation of data, writing of the report, or decision to submit the paper for publication.
Disclosure: Dr. Bueno reports research grants and clinical trials support from MedGenome, Roche, Verastem, Genentech, Merck, Gritstone, Epizyme, Siemens, National Institutes of Health, and Department of Defense. In addition, Dr. Bueno holds 4 patents through Brigham and Women’s Hospital and equity in Navigation Sciences. The other authors disclose no potential conflicts of interest.
Abbreviations
- MPM
malignant pleural mesothelioma
- EPP
extra-pleural pneumonectomy
- PD
pleurectomy and decortication
- TV
tumor volume
- NLR
neutrophil-to-lymphocyte ratio
- WBC
white blood count
- C/V ratio
Claudin-15 (CLDN15) and Vimentin (VIM) expression ratio
- RT-qPCR
real-time quantitative PCR
- MPT
Mesothelioma Prognosis Test
- HR
hazard ratio
- PS
Mesothelioma Prognosis Score
- MRiS
MPM Risk Score
- AJCC
American Joint Committee on Cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keshava HB, Tang A, Siddiqui HU, et al. Largely Unchanged Annual Incidence and Overall Survival of Pleural Mesothelioma in the USA. World J Surg 2019;43:3239–3247. [DOI] [PubMed] [Google Scholar]
- 2.Raja S, Murthy SC, Mason DP. Malignant pleural mesothelioma. Curr Oncol Rep 2011;13:259–264. [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54–63; discussion 63–55. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg 2014;260:577–580; discussion 580–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636–2644. [DOI] [PubMed] [Google Scholar]
- 6.Eberst G, Anota A, Scherpereel A, et al. Health-Related Quality of Life Impact from Adding Bevacizumab to Cisplatin-Pemetrexed in Malignant Pleural Mesothelioma in the MAPS IFCT-GFPC-0701 Phase III Trial. Clin Cancer Res 2019;25:5759–5765. [DOI] [PubMed] [Google Scholar]
- 7.Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405–1414. [DOI] [PubMed] [Google Scholar]
- 8.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375–386. [DOI] [PubMed] [Google Scholar]
- 9.Bueno R, Opitz I, Taskforce IM. Surgery in Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1638–1654. [DOI] [PubMed] [Google Scholar]
- 10.Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol 2012;7:1631–1639. [DOI] [PubMed] [Google Scholar]
- 12.Tsao AS, Lindwasser OW, Adjei AA, et al. Current and Future Management of Malignant Mesothelioma: A Consensus Report from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol 2018;13:1655–1667. [DOI] [PubMed] [Google Scholar]
- 13.Rice D Standardizing surgical treatment in malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon GJ, Dong L, Yeap BY, et al. Four-gene expression ratio test for survival in patients undergoing surgery for mesothelioma. J Natl Cancer Inst 2009;101:678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon GJ, Jensen RV, Hsiao LL, et al. Using gene expression ratios to predict outcome among patients with mesothelioma. J Natl Cancer Inst 2003;95:598–605. [DOI] [PubMed] [Google Scholar]
- 16.Gill RR, Yeap BY, Bueno R, et al. Quantitative Clinical Staging for Patients With Malignant Pleural Mesothelioma. J Natl Cancer Inst 2018;110:258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abakay O, Tanrikulu AC, Palanci Y, et al. The value of inflammatory parameters in the prognosis of malignant mesothelioma. J Int Med Res 2014;42:554–565. [DOI] [PubMed] [Google Scholar]
- 18.Brims FJ, Meniawy TM, Duffus I, et al. A Novel Clinical Prediction Model for Prognosis in Malignant Pleural Mesothelioma Using Decision Tree Analysis. J Thorac Oncol 2016;11:573–582. [DOI] [PubMed] [Google Scholar]
- 19.Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145–152. [DOI] [PubMed] [Google Scholar]
- 20.Herndon JE, Green MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723–731. [DOI] [PubMed] [Google Scholar]
- 21.Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923–1929. [DOI] [PubMed] [Google Scholar]
- 22.Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res 2010;16:5805–5813. [DOI] [PubMed] [Google Scholar]
- 23.Kao SC, Vardy J, Chatfield M, et al. Validation of prognostic factors in malignant pleural mesothelioma: a retrospective analysis of data from patients seeking compensation from the New South Wales Dust Diseases Board. Clin Lung Cancer 2013;14:70–77. [DOI] [PubMed] [Google Scholar]
- 24.Ozyurek BA, Ozmen O, Ozdemirel TS, et al. Relation between neutrophil/lymphocyte ratio and primary tumor metabolic activity in patients with malign pleural mesothelioma. Clin Respir J 2018;12:646–651. [DOI] [PubMed] [Google Scholar]
- 25.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet 2016;48:407–416. [DOI] [PubMed] [Google Scholar]
- 26.De Rienzo A, Dong L, Yeap BY, et al. Fine-needle aspiration biopsies for gene expression ratio-based diagnostic and prognostic tests in malignant pleural mesothelioma. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17:310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. Eighth ed. . New York: Springer International Publishing; 2017. [Google Scholar]
- 28.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology 2003;229:3–8. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE Jr., Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med 1984;3:143–152. [DOI] [PubMed] [Google Scholar]
- 30.Edge SB, Byrd DR, Compton CC, et al. Cancer Staging Manual 7th. New York: Springer; 2010. [Google Scholar]
- 31.Gordon GJ, Rockwell GN, Godfrey PA, et al. Validation of genomics-based prognostic tests in malignant pleural mesothelioma. Clin Cancer Res 2005;11:4406–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Rienzo A, Cook RW, Wilkinson J, et al. Validation of a Gene Expression Test for Mesothelioma Prognosis in Formalin-Fixed Paraffin-Embedded Tissues. J Mol Diagn 2017;19:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brosseau S, Danel C, Scherpereel A, et al. Shorter Survival in Malignant Pleural Mesothelioma Patients With High PD-L1 Expression Associated With Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin Lung Cancer 2019;20:e564–e575. [DOI] [PubMed] [Google Scholar]
- 34.Cedres S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Combaz-Lair C, Galateau-Salle F, McLeer-Florin A, et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol 2016;52:9–18. [DOI] [PubMed] [Google Scholar]
- 36.Thapa B, Salcedo A, Lin X, et al. The Immune Microenvironment, Genome-wide Copy Number Aberrations, and Survival in Mesothelioma. J Thorac Oncol 2017;12:850–859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.