Abstract
The ischemic penumbra defined four decades ago has been the main battleground of ischemic stroke. The evolving ischemic penumbra concept has been providing insight for the development of vascular and cellular approaches as well as diagnostic tools for the treatment of ischemic stroke. rtPA thrombolytic therapy to prevent the transition of ischemic penumbra to core has been approved for acute ischemic stroke within 3 hours and was later recommended to extend to 4.5 hours after symptom onset. Mechanical thrombectomy was introduced for the treatment of acute ischemic stroke with a therapeutic window of up to 24 hours after stroke onset. Multiple modalities brain imaging techniques have been developed that provide guidance to define ischemic penumbra for reperfusion therapy in clinical practice. Cellular and molecular dissection of ischemic penumbra has been providing targets for the development of neuroprotective therapy for ischemic stroke. However, the dynamic nature of ischemic penumbra implicates that infarct core eventually expands into penumbra over time without reperfusion, dictating relative short therapeutic windows and limiting the impact of current reperfusion intervention. Entering the 5th decade since the introduction, ischemic penumbra remains the main focus of ischemic stroke research and clinical practice. In this review, we summarized the evolving ischemic penumbra concept and its implication in the development of vascular and cellular interventions as well as diagnostic tools for acute ischemic stroke. In addition, we discussed future perspectives on expansion the campaign beyond ischemic penumbra to develop treatment for ischemic stroke.
Keywords: ischemic stroke, penumbra, reperfusion therapy, neuroprotection, neurovascular unit, oligemia
Introduction
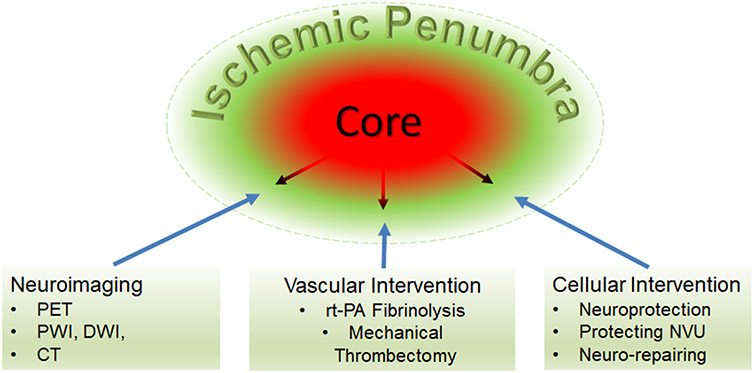
The ischemic penumbra, the curious zone surrounding infarct, was conceptualized 4 decades ago as hypoperfused brain tissue at the level within the thresholds of functional impairment and structural integrity, which has the capacity to recover if blood flow is improved [1-3]. By paired measurements of regional cerebral blood flow (CBF), evoked potential, pH, and extracellular potassium in a baboon model of middle cerebral artery occlusion, Astrup et al identified two thresholds: one of electrical silence when CBF was reduced about 70% and the other of massive release of intracellular K+ when CBF was further decreased to around 10% of the baseline. The ischemic penumbra was defined as “the ischemic brain with the CBF between the upper threshold of electrical failure and the lower threshold of energy and ion pump failure” [2]. Soon after its introduction, ischemic penumbra has become the diagnostic and therapeutic target for ischemic stroke [4]. Looking back since the introducing, the evolving ischemic penumbra concept has been providing the foundation for the development of vascular and cellular interventions for acute ischemic stroke as well as for the development of diagnostic tools to guide reperfusion therapy (Figure 1).
Figure 1.
The evolving ischemic penumbra as the target for the development of vascular and cellular treatments and multiple modalities neuroimaging technologies to define the ischemic penumbra and provide potential guidance for clinical interventions.
Vascular approach targeting ischemic penumbra: from rt-PA fibrinolytic therapy to mechanical thrombectomy
The ischemic penumbra concept predicts that part of the brain region after ischemic stroke is potentially salvageable and that the transition of reversible ischemia to irreversible infarct is a dynamic process as the tolerance of brain tissue to ischemia depends on both the residual blood flow and duration of blood flow disturbance [5]. Ischemic penumbra exists for a short period even in the center of ischemic region, where irreversible necrosis propagates to neighboring tissue over time, indicating that timely reperfusion therapy is the most effective treatment for ischemic stroke.
Recombinant tissue plasminogen activator (rt-PA) is currently the only FDA approved drug for treatment of acute ischemic stroke. The discovery of rt-PA thrombolytic therapy for acute ischemic stroke could be traced back to 1947 when an agent in animal tissues, originally called fibrinokinase, was found to be able to activate plasminogen [6]. However, it has not been extensively studied until the identification and purification of tPA from Bowes melanoma cells in 1977 [7-9]. tPA is the most physiologically active plasminogen activator that predominately produced and secreted from endothelial cell. In the presence of fibrin, both tPA and plasminogen can bind, tPA cleave plasminogen into active plasmin that further induce fibrinolysis and restoration of blood flow. tPA represents one of the greatest success of translation research in the modern medicine. It took less than 10 years from the identification of tPA in the bench to a FDA approved life-saving drug for the treatment of heart attack in 1987 [10-12, 9]. Administration of rt-PA fibrinolytic reperfusion therapy in a timely manner was a major advance in the treatment of acute myocardial infarction although it was lately dominated by percutaneous coronary intervention which has demonstrated superiority over fibrinolytic reperfusion therapy [13].
Reperfusion therapy for ischemic stroke is distinctive and complex given the uniqueness of brain in high-energy expenditure, limited energy storage, complex cellular components, and sophisticated brain structure and functions. tPA thrombolytic therapy was quickly introduced into ischemic stroke with the first clinical trial in 1991 [14, 15]. The subsequent NINDS and ECASS trials demonstrated that intravenous administration of rt-PA treatment within 3 hours after onset has significantly improved morbidity and mortality of ischemic stroke patients [16, 17]. Encouragingly, the results of all neurological rating scales showed approximately a 50% improvement for rt-PA-treated patients as compared to controls [17, 16]. rt-PA thrombolytic intervention has been the most effective therapy for acute ischemic stroke [18, 19]. Nonetheless, the 3-hour therapeutic window dramatically limits the clinical application of rt-PA in ischemic stroke. Great effort has been invested to extend the therapeutic window of rt-PA in ischemic stroke [19, 20]. More than 10 years after the FDA approval of rt-PA for the treatment of acute ischemic stroke, AHA/ASA acute stroke guidelines recommended 3 to 4.5-hour window of intravenous rt-PA treatment in selected ischemic stroke patients based on the results from ECASSIII and SITS-ISTR analysis [21-23].
Even with extended time window, as few as 10% of ischemic stroke patients can be eligible to intravenous rt-PA treatment [24]. Recent studies have indicated that penumbral imaging-based thrombolysis with rt-PA might be feasible up to 24 hours after symptom onset [25, 26]. The narrow therapeutic window, potential hemorrhagic transformation, and low recanalization rate, especially in large arteries, have driven scientists and clinicians to explore alternatives for the treatment of acute ischemic stroke, including intra-arterial reperfusion therapies and mechanical thrombectomy [27, 28]. Similar as percutaneous coronary intervention for acute myocardial infarct, after initial failure, mechanical thrombectomy has been found to be effective with a longer therapeutic window for acute ischemic stroke [29-31]. With the most recent results of DAWN and DEFUSE 3 trials, FDA approved the use of Trevo clot retrieval device in selected ischemic patients within 24 hours after onset in 2018 [32-35]. As compared with rt-PA fibrinolytic therapy, mechanical thrombectomy has a longer therapeutic window with higher reperfusion rate and better functional outcome for acute ischemic stroke. It is still not clear whether it will replace intravenous rt-PA fibrinolytic therapy as the gold standard treatment for acute ischemic stroke in the similar way of percutaneous coronary intervention for acute myocardial infarct. Nonetheless, the slowly expanding therapeutic window for acute ischemic stroke treatment upon the development of novel vascular interventions has been continually improving our knowledge of ischemic penumbra. “Time is brain”, a term coined even before rt-PA trials, emphasizes that the brain tissue is rapidly and irreversibly lost as ischemic stroke progress and that therapeutic interventions should be emergently pursued [36]. However, it is getting clear that the time is not an exclusive factor that dictates the fate of ischemic penumbra. The exact time of stroke onset can be difficult to ascertain in many patients [37]. Accurate identification of patients with salvageable ischemic penumbra is critical for the selection of reperfusion therapy.
Cellular approach targeting ischemic penumbra: from neurocentric focus to integrated view of neurovascular unit and multifunctional actions
In ischemic stroke, occlusion of cerebral arteries dramatically compromises the blood supply of oxygen, glucose, and other nutrients to the brain tissue that leads to subsequent crisis of cellular energy metabolism and loss of neuronal function. The brain is far more vulnerable to ischemia than other organs. Even in the brain, heterogeneous threshold to ischemia has been identified in different species and brain regions [38, 39]. It is plausible that cellular mechanisms underlying heightened vulnerability to ischemia could be identified and blocked [40]. Hibernating mammals have unique capacities to tolerate extreme metabolic challenge, a similar reduction of blood flow caused by ischemic stroke, providing further evidence that support cellular intervention for the treatment of acute ischemic stroke [41]. With the limited impact of reperfusion therapy for acute ischemic stroke, cellular approach has been extensively explored in the last 3 decades.
Ischemic penumbra was first observed based on the relation of evoked potential and CBF. It is not a surprise that the cellular approach was originally focused on neuron exclusively as a neurocentric approach of neuroprotection. It was anticipated that neuroprotection could delay or reverse irreversible neuronal damage in ischemic penumbra hence to provide a cure for acute ischemic stroke or extend the short therapeutic window of reperfusion therapy. Neuroprotection, including pharmacological and non-pharmacological interventions, to attenuate or block pathological cascades and salvage neuronal damage has been investigated in the setting of acute ischemic stroke. Elucidation of ischemic penumbra at molecular and cellular level has been providing insight for the development of cellular intervention for acute ischemic stroke. Glutamate excitotoxicity was among the first identified and most intensively studied ischemic neuronal death mechanisms [41]. Targeting excitotoxicity has been the first explored neuroprotective therapy for ischemic stroke [42]. The subsequent dissection of intricate molecular signaling involving oxidative stress and programmed cell death in ischemic penumbra have yielded additional targets for neuroprotection against ischemic stroke. Nonetheless, the dynamics and potential biphasic roles of these molecular signals have not been fully determined [43]. Most of neuroprotection approaches so far with a single molecular target in a single cell type have been inevitably failed to provide any cure for ischemic stroke in clinical setting [44]. At the cellular level, dissecting the pathophysiology of ischemic penumbra have led to a shift in perspective from a focus on neurons alone to a focus on the complex of neurons, microvessels that supply them, and supportive cells of astrocyte, microglia, and pericyte, defined as the neurovascular unit [45, 46]. Furthermore, mounting evidence in the last decade has demonstrated that peripheral innate and adaptive immune cells such as neutrophils and T cells might play important roles in the pathophysiology of ischemic stroke [47-50]. The original neuroprotective strategy has evolved from targeting a signal pathway in neurons to a holistic multifunctional approach of protecting neurovascular unit and improving cell-cell and cell-extracellular matrix interaction that might ultimately rescue ischemic penumbra and benefits the brain recovery after ischemic stroke [51-53]. Unfortunately, 4 decades after conceptualization, cellular approaches targeting ischemic penumbra has not translated into any success in clinical practice for the treatment of acute ischemic stroke.
The cellular approaches targeting ischemic penumbra have to overcome the same challenge of short time window for reperfusion therapy. In addition, the compromised blood flow in ischemic territory impedes efficient drug delivery for cellular interventions without reperfusion. Nevertheless, the therapeutic agents have potential to reach ischemic penumbra given the relative higher residual CBF in the penumbra than in the core. In addition, the metabolic shift in ischemic penumbra might provide unique environment for penumbral drug delivery for the treatment of acute ischemic stroke [54, 55]. Furthermore, cellular interventions might be combined with vascular interventions to attenuate reperfusion injury and hemorrhagic transformation.
Ischemic penumbra was initially described as tissue of evolving injury surrounding a homogenous central core destined for infarction [3]. Considerable studies in animal models and stroke patients have indicated that infarct core and ischemic penumbra can be of heterogeneity with multiple ‘mini-penumbras’ scatted among and within the evolving infarct core and normal tissue in the complex territory-at-risk, rather than with ischemic penumbra being usually peripheral in location [56]. There is increasing evidence indicating a more complex of ischemic penumbra [43]. Ischemic penumbra is not only passively dying but also actively recovering over time. Activation of NMDA signaling plays a key role in excitotoxic neuronal death after ischemic stroke as well promotes endogenous neurogenesis [57]. Cells that express markers associated with newborn neurons have been found in ischemic penumbra in patients of ischemic stroke [58]. In addition, ischemic stroke can induce long-lasing cellular and hemodynamic changes beyond ischemic penumbra [59]. It is known that focal cerebral ischemia could induce stem cell proliferation in subventricular zone (SVZ) and dentate gyrus, areas beyond ischemic territory [60]. Future dissection of ischemic penumbra and beyond may lead to discovery further treatment to improve function recovery after ischemic stroke.
Multiple modalities neuroimaging to map ischemic penumbra
The goal of stroke treatment is to prevent the transition of ischemic penumbra to infarct, hence, reduce infarct size and improved function outcome of ischemic stroke patients. It is critical to identify ischemic penumbra and map its evolution. Neuroimaging are breakthrough technologies for diagnosis of CNS disorders. There have been remarkable developments in imaging of ischemic penumbra since its original description, using computed tomography (CT), positron-emission tomography (PET), and magnetic resonance imaging (MRI) [61]. The application of these neuroimaging modalities detecting ischemic penumbra has been shedding light on acute management of ischemic stroke patients.
Ischemic penumbra was originally defined by invasive assessment of neuronal function (evoked potential), hemodynamics (CBF), and bioenergetics (pH and potassium level) of the ischemic brain. The development of neuroimaging technologies enables us to evaluate the similar parameters in a noninvasive way. The hemodynamic imaging provided by PET, perfusion CT, and perfusion-weighted MRI (PWI) predicts the brain tissue at risk and possible future of lesion evolution. While the bioenergetic insight provided by PET and diffusion-weighted MRI (DWI) reflects cellular consequence of hemodynamic abnormalities and state of ischemic brain tissue with respect to reversible or irreversibly damage [62, 63]. The mismatch between hemodynamic and bioenergetic imaging has been extensively explored for defining ischemic penumbra in the setting of ischemic stroke.
PET was the first neuroimaging used to study ischemic penumbra. Ischemic penumbra is defined as the brain tissue with reduced CBF but preserved oxygen consumption (CMRO2) and raised oxygen extraction fraction (OEF). PET procedures is still considered the best for recognition of ischemic penumbra [62, 61]. Nonetheless, PET is limited by high operating cost, radioactive tracers, and complex logistics involving a multidisciplinary team that prevent it from clinical practice [63, 61].
Potential clinical application of water diffusion MRI was suggested in 1986 [64]. In 1990, DWI was introduced into ischemic stroke in a cat middle cerebral occlusion model [65, 66]. The reduction of apparent diffusion coefficient (ADC) of water shortly after cerebral ischemia is thought to reflect cytotoxicity, intracellular water accumulation, due to high-energy metabolism failure and loss of ion homeostasis. The mismatch of abnormalities on DWI and PWI has been the most practical in emergency setting for ischemic stroke patients [67]. However, DWI and PWI mismatch only approximates the distinction between infarct core and ischemic penumbra. DWI abnormalities may not necessary represent infarct core as modest ADC declines can be reversed with timely reperfusion therapy [68]. More recently, the intensity of fluid-attenuated inversion recovery (FLAIR) lesion in region of diffusion restriction has been indicated to be associated with time from symptom onset in patients with acute ischemic stroke [69, 70]. DWI-FLAIR mismatch has been suggested for selection of reperfusion therapy in acute ischemic stroke patients [71].
Perfusion CT, a serial CT imaging with an administration of iodinated contrast media, has been increasingly performed for evaluation of the brain parenchyma in the setting of acute ischemic stroke [72]. The cerebral blood volume (CBV), CBF, mean transit time (MTT), and time-to-maximum (Tmax) images derived from perfusion CT has been shown to accurately measure ischemic penumbra and infarct core in patients with acute ischemic stroke [73]. However, there is a lack of consensus and standardization of perfusion CT techniques. Mismatch perfusion CT imaging may not predict ischemic penumbra, thus, have limited impact on reperfusion therapy selection and predicting the outcome in acute ischemic stroke [67].
Clinical-imaging mismatches, such as clinical-diffusion and clinical-CT mismatch, have been developed to predict ischemic stroke outcome based on structure-function correlation between concurrent neuroimaging and clinical findings [74, 75]. However, there is still a lack of a well-validated threshold to define PWI, DWI, and perfusion CT abnormalities. There might be no single perfusion threshold that could accurately distinguish salvageable ischemic penumbra from the brain tissue destined to infarct [76-78]. Nonetheless, neuroimaging techniques have contributed enormously and will continually contribute to improve our understanding of ischemic penumbra.
Targeting territories beyond ischemic penumbra for the treatment of ischemic stroke
When ischemic penumbra was first introduced 4 decades ago, the concept of oligemia was proposed as tissue with low blood flow but unaltered function [1]. Oligemic stage with reduced CBF, elevated oxygen extraction fraction (OEF) and normal CMRO2 has been demonstrated after acute arterial occlusion decades ago [79]. A benign oligemia zone within or surrounding ischemic penumbra has been indicated by neuroimaging that either recovers spontaneously or is under perfused but functioning normally and would survive irrespective of blood flow restoration [80, 81]. The existence of benign oligemia area has also been suggested by sequential neuroimaging study in ischemic stroke patients in which no new ischemic lesions evolved even in the presence of persistent arterial occlusion despite a DWI/PWI indicated acute cortical infarct [82]. The absence of a pH-weighted MRI deficit in benign oligemia regions within PWI hypoperfusion area indicates that energy requirement for normal aerobic metabolism was maintained in the pH-WI-PWI mismatch area [83-85]. It was assumed that the brain tissue in benign oligemia zoon would maintain function for a protracted time and would unlikely convert to ischemic core. Thus, benign oligemia is excluded from reperfusion intervention. Nonetheless, there has been indication that not all oligemia is benign. Viable but metabolically lethargic and non-functional idling (dormant) neurons have been observed in peri-infarct zone at more than 10 years after stroke [86, 87]. Indeed, some of the oligemia tissue, particularly the one due to large artery occlusion/stenosis, may remain dysfunction for a prolonged period without evolving to infarct core [88].
While much attention has been drawn to ischemic penumbra, our understanding of infarct core has been slowly improved over the last several decades. Traditionally, cell death induced by ischemic stroke has been considered exclusively necrotic in nature. Apoptosis and hybrid cell death have also been detected in infarct core [89, 90]. In addition, the core is not just passively dying over time but actively surviving and recovering. In a mouse model of permanent middle cerebral artery occlusion, long term survival of neuronal and vascular cells, regenerative activities and newly generated neuronal cells have been observed in the core region [91]. Consistently, evidence of angiogenesis has been identified in the core [92, 93]. It is speculated that angiogenesis would be essential for brain recovery and repair process including neurogenesis and axonal out-growth [93].
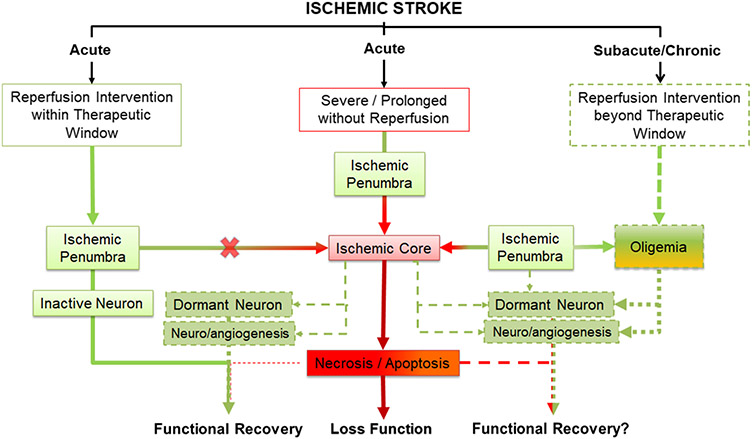
The ever yet slow extending therapeutic window of reperfusion therapy for ischemic stroke and the increasing case reports of beneficial outcome of delayed recanalization beyond 24 hours after stroke symptom onset suggest that delayed recanalization at the chronic stage of ischemic stroke might be effective for some patients (see review [94, 95]). The existence of oligemia zone and the dynamic feature of ischemic penumbra and core after ischemic stroke provide insight for potential mechanisms underlying the delayed reperfusion intervention for ischemic stroke patients. The oligemia tissue with neurological dysfunction may recover if blood flow was restored by reperfusion intervention eventually. Reperfusion intervention even delayed may re-establish function of the surviving neuronal and vascular cells in ischemic core. It is also plausible that delayed reperfusion may improve the neurovascular niche for functional recovery enabled by neurogenesis and angiogenesis (Figure 2). Further investigations are warrant to determine the roles of infarct core, oligemia, and ischemic penumbra dynamics in the outcome of delayed reperfusion intervention for ischemic stroke.
Figure 2.
The schematic depicts three possible outcomes after ischemic stroke. In the center, severe and prolonged ischemic stroke without timely blood flow restoration leads to transition of the ischemic penumbra into infarct core, progressive deterioration of cells in the ischemic penumbra to irreversible cell death through necrosis and apoptosis, and ultimately the loss of function. In the left, reperfusion within the therapeutic window prevents the conversion of ischemic penumbra into infarct core, restores function of inactive neurons in the penumbra, re-establishes function of dormant neuron and neurovascular niche for the neurogenesis and angiogenesis in the core, and thus ultimately leads to full or partial functional recovery. In the right, delayed reperfusion intervention at subacute/chronic phase beyond therapeutic window might restore neuronal function of oligemia brain tissue, re-establishes function of dormant neuron and neurovascular niche for the neurogenesis and angiogenesis in the core, and potentially leads to full or partial functional recovery.
Summary and future perspective
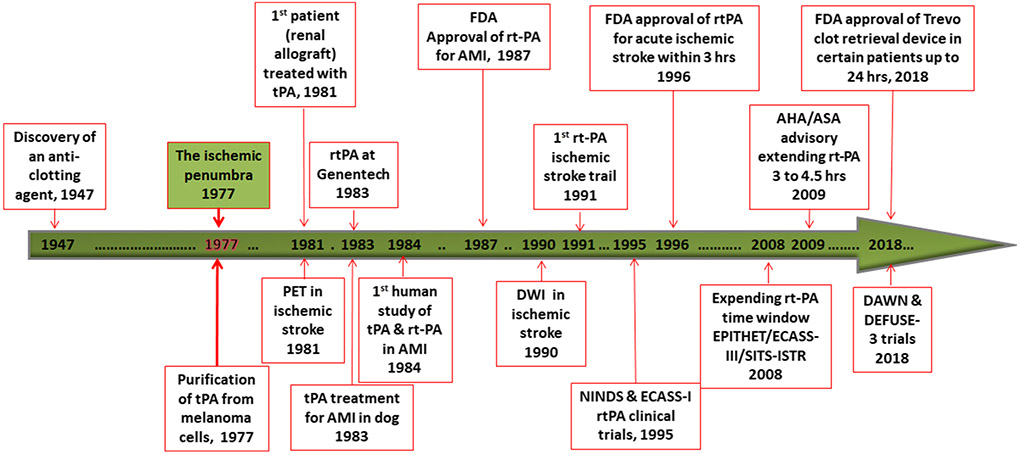
In the last 4 decades, ischemic penumbra has been the main battleground of ischemic stroke that has brought great success for ischemic stroke management (Figure 3). rt-PA thrombolytic therapy has been introduced for acute treatment of ischemic stroke with a therapeutic window of 4.5 hours after symptom onset. Mechanical thrombectomy has been established for acute ischemic stroke treatment with a therapeutic window up to 24 hours after stroke onset. Multiple modalities brain imaging technique has been developed to provide guidance for reperfusion therapy in clinical practice. Cellular and molecular dissection of ischemic penumbra has been providing insight for developing of cellular intervention for ischemic stroke. Without a doubt, ischemic penumbra will still be the main target of ischemic stroke in the near future for research and clinical practice. High-resolution metabolic neuroimaging techniques need to be developed to precisely define ischemic penumbra; rapid and highly effective reperfusion intervention is needed to restore blood flow for the treatment of ischemic stroke with minimal side effect; further understanding of metabolic and cellular/molecular dynamics of ischemic penumbra and cell death pathways is warranted for the development of effective cellular intervention to preserve and reverse ischemic penumbra [96, 97]; combined intervention of cerebrovascular and cellular approach is up to be explored for the treatment of ischemic stroke patients with longer therapeutic window.
Figure 3.
Timeline of major developments of ischemic stroke interventions in related to the introduction of ischemic penumbra concept. AMI, acute myocardial infarction.
Entering the 5th decade since ischemic penumbra was defined, there is increasing evidence to explore therapeutic targets beyond ischemic penumbra. Increased proliferation of neuronal progenitor cells has been observed in areas beyond ischemic territory after stroke in experimental focal cerebral ischemia models and ischemic stroke patients [98, 99]. Dysfunctional oligemia tissue may remain for a long period without evolving to infarct core and restore function if sufficient blood flow was re-established by reperfusion intervention eventually [88]. Long-term survival neuronal and vascular cells as well as neurogenesis and angiogenesis have been observed in infarct core [91, 93]. The mounting evidence supports to expand the campaign beyond ischemic penumbra, which might ultimately break the curse of ischemic penumbra for ischemic stroke patients.
Funding:
This work was partly funded by National Institutes of Health grants R01NS088596 (SY) and R01NS109583 (SY).
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Ethics Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Reference
- 1.Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8(1):51–7. [DOI] [PubMed] [Google Scholar]
- 2.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–5. [DOI] [PubMed] [Google Scholar]
- 3.Symon L The relationship between CBF, evoked potentials and the clinical features in cerebral ischaemia. Acta Neurol Scand Suppl. 1980;78:175–90. [PubMed] [Google Scholar]
- 4.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42(1 Suppl):S7–11. doi: 10.1161/STROKEAHA.110.596684. [DOI] [PubMed] [Google Scholar]
- 5.Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol. 1983;14(3):294–301. doi: 10.1002/ana.410140307. [DOI] [PubMed] [Google Scholar]
- 6.Astrup T, Permin PM. Fibrinolysis in the animal organism. Nature. 1947;159(4046):681. [DOI] [PubMed] [Google Scholar]
- 7.Collen D, Billiau A, Edy J, De Somer P. Identification of the human plasma protein which inhibits fibrinolysis associated with malignant cells. Biochim Biophys Acta. 1977;499(2):194–201. [DOI] [PubMed] [Google Scholar]
- 8.Collen D, Rijken DC, Van Damme J, Billiau A. Purification of human tissue-type plasminogen activator in centigram quantities from human melanoma cell culture fluid and its conditioning for use in vivo. Thromb Haemost. 1982;48(3):294–6. [PubMed] [Google Scholar]
- 9.Collen D, Lijnen HR. The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol. 2009;29(8):1151–5. doi: 10.1161/ATVBAHA.108.179655. [DOI] [PubMed] [Google Scholar]
- 10.Bergmann SR, Fox KA, Ter-Pogossian MM, Sobel BE, Collen D. Clot-selective coronary thrombolysis with tissue-type plasminogen activator. Science. 1983;220(4602):1181–3. [DOI] [PubMed] [Google Scholar]
- 11.Pennica D, Holmes WE, Kohr WJ, Harkins RN, Vehar GA, Ward CA et al. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983;301(5897):214–21. [DOI] [PubMed] [Google Scholar]
- 12.Van de Werf F, Ludbrook PA, Bergmann SR, Tiefenbrunn AJ, Fox KA, de Geest H et al. Coronary thrombolysis with tissue-type plasminogen activator in patients with evolving myocardial infarction. N Engl J Med. 1984;310(10):609–13. doi: 10.1056/NEJM198403083101001. [DOI] [PubMed] [Google Scholar]
- 13.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev Esp Cardiol (Engl Ed). 2017;70(12):1082. doi: 10.1016/j.rec.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Terashi A, Kobayashi Y, Katayama Y, Inamura K, Kazama M, Abe T. Clinical effects and basic studies of thrombolytic therapy on cerebral thrombosis. Semin Thromb Hemost. 1990;16(3):236–41. doi: 10.1055/s-2007-1002675. [DOI] [PubMed] [Google Scholar]
- 15.Levine SR, Brott TG. Thrombolytic therapy in cerebrovascular disorders. Prog Cardiovasc Dis. 1992;34(4):235–62. [DOI] [PubMed] [Google Scholar]
- 16.National Institute of Neurological D, Stroke rt PASSG. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274(13):1017–25. [PubMed] [Google Scholar]
- 18.Molina CA, Saver JL. Extending reperfusion therapy for acute ischemic stroke: emerging pharmacological, mechanical, and imaging strategies. Stroke. 2005;36(10):2311–20. doi: 10.1161/01.STR.0000182100.65262.46. [DOI] [PubMed] [Google Scholar]
- 19.Cheng NT, Kim AS. Intravenous Thrombolysis for Acute Ischemic Stroke Within 3 Hours Versus Between 3 and 4.5 Hours of Symptom Onset. Neurohospitalist. 2015;5(3):101–9. doi: 10.1177/1941874415583116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell BC, Meretoja A, Donnan GA, Davis SM. Twenty-Year History of the Evolution of Stroke Thrombolysis With Intravenous Alteplase to Reduce Long-Term Disability. Stroke. 2015;46(8):2341–6. doi: 10.1161/STROKEAHA.114.007564. [DOI] [PubMed] [Google Scholar]
- 21.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 22.Wahlgren N, Ahmed N, Davalos A, Hacke W, Millan M, Muir K et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372(9646):1303–9. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 23.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP Jr., American Heart Association Stroke C. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Los Rios la Rosa F, Khoury J, Kissela BM, Flaherty ML, Alwell K, Moomaw CJ et al. Eligibility for Intravenous Recombinant Tissue-Type Plasminogen Activator Within a Population: The Effect of the European Cooperative Acute Stroke Study (ECASS) III Trial. Stroke. 2012;43(6):1591–5. doi: 10.1161/STROKEAHA.111.645986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kate M, Wannamaker R, Kamble H, Riaz P, Gioia LC, Buck B et al. Penumbral Imaging-Based Thrombolysis with Tenecteplase Is Feasible up to 24 Hours after Symptom Onset. J Stroke. 2018;20(1):122–30. doi: 10.5853/jos.2017.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shang X, Lin M, Zhang S, Li S, Guo Y, Wang W et al. Clinical Outcomes of Endovascular Treatment within 24 Hours in Patients with Mild Ischemic Stroke and Perfusion Imaging Selection. AJNR Am J Neuroradiol. 2018;39(6):1083–7. doi: 10.3174/ajnr.A5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribo M, Alvarez-Sabin J, Montaner J, Romero F, Delgado P, Rubiera M et al. Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke. 2006;37(4):1000–4. doi: 10.1161/01.STR.0000206443.96112.d9. [DOI] [PubMed] [Google Scholar]
- 28.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke. 2007;38(3):948–54. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 29.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 30.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 31.Hameed A, Zafar H, Mylotte D, Sharif F. Recent Trends in Clot Retrieval Devices: A Review. Cardiol Ther. 2017;6(2):193–202. doi: 10.1007/s40119-017-0098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks MP, Heit JJ, Lansberg MG, Kemp S, Christensen S, Derdeyn CP et al. Endovascular Treatment in the DEFUSE 3 Study. Stroke. 2018;49(8):2000–3. doi: 10.1161/STROKEAHA.118.022147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desai SM, Haussen DC, Aghaebrahim A, Al-Bayati AR, Santos R, Nogueira RG et al. Thrombectomy 24 hours after stroke: beyond DAWN. J Neurointerv Surg. 2018;10(11):1039–42. doi: 10.1136/neurintsurg-2018-013923. [DOI] [PubMed] [Google Scholar]
- 34.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 35.Rao VL, Mlynash M, Christensen S, Yennu A, Kemp S, Zaharchuk G et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab. 2020;40(10):1966–74. doi: 10.1177/0271678X20918816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SH, Lou M, Luo B, Jiang WJ, Liu R. Precision Medicine for Ischemic Stroke, Let Us Move Beyond Time Is Brain. Transl Stroke Res. 2018;9(2):93–5. doi: 10.1007/s12975-017-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wouters A, Lemmens R, Dupont P, Thijs V. Wake-up stroke and stroke of unknown onset: a critical review. Front Neurol. 2014;5:153. doi: 10.3389/fneur.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcoux FW, Morawetz RB, Crowell RM, DeGirolami U, Halsey JH Jr. Differential regional vulnerability in transient focal cerebral ischemia. Stroke. 1982;13(3):339–46. [DOI] [PubMed] [Google Scholar]
- 39.Baltan S, Besancon EF, Mbow B, Ye Z, Hamner MA, Ransom BR. White matter vulnerability to ischemic injury increases with age because of enhanced excitotoxicity. J Neurosci. 2008;28(6):1479–89. doi: 10.1523/JNEUROSCI.5137-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 Suppl):A7–14. [DOI] [PubMed] [Google Scholar]
- 41.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to "cerebral ischemia". J Cereb Blood Flow Metab. 1994;14(2):193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- 42.Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4(2):131–6. [DOI] [PubMed] [Google Scholar]
- 43.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 44.Grupke S, Hall J, Dobbs M, Bix GJ, Fraser JF. Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clin Neurol Neurosurg. 2015;129:1–9. doi: 10.1016/j.clineuro.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35(2):354–6. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 46.del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354(6):553–5. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- 47.Strecker JK, Schmidt A, Schabitz WR, Minnerup J. Neutrophil granulocytes in cerebral ischemia - Evolution from killers to key players. Neurochem Int. 2017;107:117–26. doi: 10.1016/j.neuint.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Selvaraj UM, Stowe AM. Long-term T cell responses in the brain after an ischemic stroke. Discov Med. 2017;24(134):323–33. [PMC free article] [PubMed] [Google Scholar]
- 49.Schabitz WR, Minnerup J. Neutrophils in Acute Stroke Pathophysiology. Stroke. 2019;50(3):e44–e5. doi: 10.1161/STROKEAHA.118.024300. [DOI] [PubMed] [Google Scholar]
- 50.Xie L, Li W, Hersh J, Liu R, Yang SH. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab. 2019;39(11):2268–76. doi: 10.1177/0271678X18792372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Del Zoppo GJ. Toward the neurovascular unit. A journey in clinical translation: 2012 Thomas Willis Lecture. Stroke. 2013;44(1):263–9. doi: 10.1161/STROKEAHA.112.653618. [DOI] [PubMed] [Google Scholar]
- 52.Minnerup J, Schabitz WR. Multifunctional actions of approved and candidate stroke drugs. Neurotherapeutics. 2009;6(1):43–52. doi: 10.1016/j.nurt.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steliga A, Kowianski P, Czuba E, Waskow M, Morys J, Lietzau G. Neurovascular Unit as a Source of Ischemic Stroke Biomarkers-Limitations of Experimental Studies and Perspectives for Clinical Application. Transl Stroke Res. 2020;11(4):553–79. doi: 10.1007/s12975-019-00744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu S, Levine SR, Winn HR. Targeting ischemic penumbra: part I - from pathophysiology to therapeutic strategy. J Exp Stroke Transl Med. 2010;3(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Levine SR, Winn HR. Targeting ischemic penumbra Part II: selective drug delivery using liposome technologies. J Exp Stroke Transl Med. 2011;4(1):16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31(9):1836–51. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14(1):10–8. [DOI] [PubMed] [Google Scholar]
- 58.Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y et al. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103(35):13198–202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu R, Yuan H, Yuan F, Yang SH. Neuroprotection targeting ischemic penumbra and beyond for the treatment of ischemic stroke. Neurol Res. 2012;34(4):331–7. doi: 10.1179/1743132812Y.0000000020. [DOI] [PubMed] [Google Scholar]
- 60.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V et al. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24(3):739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 61.Davis S, Donnan GA. Time is Penumbra: imaging, selection and outcome. The Johann jacob wepfer award 2014. Cerebrovasc Dis. 2014;38(1):59–72. doi: 10.1159/000365503. [DOI] [PubMed] [Google Scholar]
- 62.Heiss WD. Best measure of ischemic penumbra: positron emission tomography. Stroke. 2003;34(10):2534–5. doi: 10.1161/01.STR.0000092396.70827.28. [DOI] [PubMed] [Google Scholar]
- 63.Warach S Measurement of the ischemic penumbra with MRI: it's about time. Stroke. 2003;34(10):2533–4. doi: 10.1161/01.STR.0000092395.19554.9A. [DOI] [PubMed] [Google Scholar]
- 64.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161(2):401–7. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 65.Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J et al. Early detection of regional cerebral ischemia in cats: comparison of diffusion- and T2-weighted MRI and spectroscopy. Magn Reson Med. 1990;14(2):330–46. [DOI] [PubMed] [Google Scholar]
- 66.Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF et al. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176(2):439–45. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- 67.Yuh WT, Alexander MD, Ueda T, Maeda M, Taoka T, Yamada K et al. Revisiting Current Golden Rules in Managing Acute Ischemic Stroke: Evaluation of New Strategies to Further Improve Treatment Selection and Outcome. AJR Am J Roentgenol. 2017;208(1):32–41. doi: 10.2214/AJR.16.16557. [DOI] [PubMed] [Google Scholar]
- 68.Minematsu K, Li L, Sotak CH, Davis MA, Fisher M. Reversible focal ischemic injury demonstrated by diffusion-weighted magnetic resonance imaging in rats. Stroke. 1992;23(9):1304–10; discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 69.Wouters A, Dupont P, Norrving B, Laage R, Thomalla G, Albers GW et al. Prediction of Stroke Onset Is Improved by Relative Fluid-Attenuated Inversion Recovery and Perfusion Imaging Compared to the Visual Diffusion-Weighted Imaging/Fluid-Attenuated Inversion Recovery Mismatch. Stroke. 2016;47(10):2559–64. doi: 10.1161/STROKEAHA.116.013903. [DOI] [PubMed] [Google Scholar]
- 70.Wouters A, Dupont P, Christensen S, Norrving B, Laage R, Thomalla G et al. Association Between Time From Stroke Onset and Fluid-Attenuated Inversion Recovery Lesion Intensity Is Modified by Status of Collateral Circulation. Stroke. 2016;47(4):1018–22. doi: 10.1161/STROKEAHA.115.012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10(11):978–86. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 72.Klug J, Dirren E, Preti MG, Machi P, Kleinschmidt A, Vargas MI et al. Integrating regional perfusion CT information to improve prediction of infarction after stroke. J Cereb Blood Flow Metab. 2021;41(3):502–10. doi: 10.1177/0271678X20924549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heit JJ, Wintermark M. Perfusion Computed Tomography for the Evaluation of Acute Ischemic Stroke: Strengths and Pitfalls. Stroke. 2016;47(4):1153–8. doi: 10.1161/STROKEAHA.116.011873. [DOI] [PubMed] [Google Scholar]
- 74.Kent DM, Hill MD, Ruthazer R, Coutts SB, Demchuk AM, Dzialowski I et al. "Clinical-CT mismatch" and the response to systemic thrombolytic therapy in acute ischemic stroke. Stroke. 2005;36(8):1695–9. doi: 10.1161/01.STR.0000173397.31469.4b. [DOI] [PubMed] [Google Scholar]
- 75.Prosser J, Butcher K, Allport L, Parsons M, MacGregor L, Desmond P et al. Clinical-diffusion mismatch predicts the putative penumbra with high specificity. Stroke. 2005;36(8):1700–4. doi: 10.1161/01.STR.0000173407.40773.17. [DOI] [PubMed] [Google Scholar]
- 76.Nagakane Y, Christensen S, Ogata T, Churilov L, Ma H, Parsons MW et al. Moving beyond a single perfusion threshold to define penumbra: a novel probabilistic mismatch definition. Stroke. 2012;43(6):1548–55. doi: 10.1161/STROKEAHA.111.643932. [DOI] [PubMed] [Google Scholar]
- 77.Sobesky J Refining the mismatch concept in acute stroke: lessons learned from PET and MRI. J Cereb Blood Flow Metab. 2012;32(7):1416–25. doi: 10.1038/jcbfm.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kamalian S, Kamalian S, Konstas AA, Maas MB, Payabvash S, Pomerantz SR et al. CT perfusion mean transit time maps optimally distinguish benign oligemia from true "at-risk" ischemic penumbra, but thresholds vary by postprocessing technique. AJNR Am J Neuroradiol. 2012;33(3):545–9. doi: 10.3174/ajnr.A2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baron JC. Pathophysiology of Acute Cerebral Ischemia: PET Studies in Humans. Cerebrovasc Dis. 1991;1991(1 (Suppl 1)):22–31. doi: 10.1159/000108877. [DOI] [Google Scholar]
- 80.Bandera E, Botteri M, Minelli C, Sutton A, Abrams KR, Latronico N. Cerebral blood flow threshold of ischemic penumbra and infarct core in acute ischemic stroke: a systematic review. Stroke. 2006;37(5):1334–9. doi: 10.1161/01.STR.0000217418.29609.22. [DOI] [PubMed] [Google Scholar]
- 81.Goyal M, Menon BK, Derdeyn CP. Perfusion imaging in acute ischemic stroke: let us improve the science before changing clinical practice. Radiology. 2013;266(1):16–21. doi: 10.1148/radiol.12112134. [DOI] [PubMed] [Google Scholar]
- 82.Bang OY, Lee KH, Kim SJ, Liebeskind DS. Benign oligemia despite a malignant MRI profile in acute ischemic stroke. J Clin Neurol. 2010;6(1):41–5. doi: 10.3988/jcn.2010.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun PZ, Zhou J, Sun W, Huang J, van Zijl PC. Detection of the ischemic penumbra using pH-weighted MRI. J Cereb Blood Flow Metab. 2007;27(6):1129–36. doi: 10.1038/sj.jcbfm.9600424. [DOI] [PubMed] [Google Scholar]
- 84.Guo Y, Zhou IY, Chan ST, Wang Y, Mandeville ET, Igarashi T et al. pH-sensitive MRI demarcates graded tissue acidification during acute stroke - pH specificity enhancement with magnetization transfer and relaxation-normalized amide proton transfer (APT) MRI. Neuroimage. 2016;141:242–9. doi: 10.1016/j.neuroimage.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheung J, Doerr M, Hu R, Sun PZ. Refined Ischemic Penumbra Imaging with Tissue pH and Diffusion Kurtosis Magnetic Resonance Imaging. Transl Stroke Res. 2020. doi: 10.1007/s12975-020-00868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neubauer RA. Idling neurons. Lancet. 1990;335(8699):1217. doi: 10.1016/0140-6736(90)92736-2. [DOI] [PubMed] [Google Scholar]
- 87.Macleod MA, Francis TJ, Smith DJ. Enhancing "idling" neurons. Lancet. 1990;335(8693):860–1. doi: 10.1016/0140-6736(90)90979-f. [DOI] [PubMed] [Google Scholar]
- 88.Motta M, Ramadan A, Hillis AE, Gottesman RF, Leigh R. Diffusion-perfusion mismatch: an opportunity for improvement in cortical function. Front Neurol. 2014;5:280. doi: 10.3389/fneur.2014.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res. 2004;1022(1-2):54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 90.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–9. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 91.Jiang MQ, Zhao YY, Cao W, Wei ZZ, Gu X, Wei L et al. Long-term survival and regeneration of neuronal and vasculature cells inside the core region after ischemic stroke in adult mice. Brain Pathol. 2017;27(4):480–98. doi: 10.1111/bpa.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Abumiya T, Lucero J, Heo JH, Tagaya M, Koziol JA, Copeland BR et al. Activated microvessels express vascular endothelial growth factor and integrin alpha(v)beta3 during focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19(9):1038–50. doi: 10.1097/00004647-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 93.Kanazawa M, Takahashi T, Ishikawa M, Onodera O, Shimohata T, Del Zoppo GJ. Angiogenesis in the ischemic core: A potential treatment target? J Cereb Blood Flow Metab. 2019;39(5):753–69. doi: 10.1177/0271678X19834158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kang R, Gamdzyk M, Tang H, Luo Y, Lenahan C, Zhang JH. Delayed Recanalization-How Late Is Not Too Late? Transl Stroke Res. 2020. doi: 10.1007/s12975-020-00877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Camara R, Matei N, Zhang JH. Evolution of the stroke paradigm: A review of delayed recanalization. J Cereb Blood Flow Metab. 2021;41(5):945–57. doi: 10.1177/0271678X20978861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Datta A, Sarmah D, Mounica L, Kaur H, Kesharwani R, Verma G et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl Stroke Res. 2020;11(6):1185–202. doi: 10.1007/s12975-020-00806-z. [DOI] [PubMed] [Google Scholar]
- 97.Ronaldson PT, Davis TP. Regulation of blood-brain barrier integrity by microglia in health and disease: A therapeutic opportunity. J Cereb Blood Flow Metab. 2020;40(1_suppl):S6–S24. doi: 10.1177/0271678X20951995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K et al. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22(3):299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Macas J, Nern C, Plate KH, Momma S. Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. J Neurosci. 2006;26(50):13114–9. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]