Abstract
The demand for novel and renewable sources of energy has increased as a result of rapid population growth, limited sources of bioenergy, and environmental pollution, caused by excessive use of fossil fuels. The need to meet future energy demands have motivated researchers to search for alternative and sustainable sources of energy. The bioconversion of lignocellulosic waste (agricultural and food waste) into biofuels shows competitive promises. Lignocellulosic waste is easily accessible and has a large enzyme system that can be immobilised onto nano-matrices. Consequently, resulting in higher biofuel production and process efficiency. However, the excessive production cost of the current procedures, which involve physical, chemical, and enzymatic reactions, is limited. The use of nanomaterials has recently been shown to concentrate lignocellulosic waste, therefore, reviewing the quest for efficient production of sustainable and cost-effective development of bioenergy from lignocellulosic wastes. This review paper explores the advanced strategies of using nanobiotechnology to combine enzyme-conjugated nanosystems for the cost-effective production of sustainable bioenergy solutions. This research will help to develop an inexpensive, eco-friendly technology for biofuels production and also help overcome the environmental burden of lignocellulosic waste worldwide.
Keywords: Biofuel, Sustainable energy, Nanobiotechnology, Enzymes, Clean energy technology
Introduction
Fossil fuels are nonrenewable energy sources that pollute the environment significantly. The increase in population growth and industrialization has increased the energy consumption unprecedentedly (Bhatia et al. 2021a). The search for renewable and efficient sources of bioenergy has been of interest to reduce pollution and also ensure the wellbeing and survival of future generations. The sustainable development of future energy sources is also one of the major priorities listed in the Sustainable Development Goals 2030 Agenda (https://sdgs.un.org/2030agenda2021). A viable alternative to fossil fuels is the use of biofuels as the latter does not cause as much pollution and can be produced from materials that are going to wastelands. Therefore, it results in the reduction of waste production while generating useful and clean energy. Biofuels can be categorized into first, second, and third generations depending upon their source/origin, starch, cellulose/agro-industrial waste, and algae, respectively (Gong et al. 2021). The first generation of biofuels includes the production of biofuels using food-based materials. In contrast, second-generation biofuels and third-generation biofuels are derived from lignocellulosic wastes and algal cells, respectively. Biofuels are mainly composed of alkanes, alcohol, gases, and fuels from a biological origin. Most of these components are also present in current fossil fuels used as an energy source, which emphasizes the advantage of using biofuels. Therefore, the conversion of biowaste to bioenergy using technology is one of the approaches that can be used to fulfill the energy demands of society (Bhatia et al. 2018). Currently, the global biofuel market size for biofuels has been evaluated to be at 135.7 billion U.S. dollars in 2019, with a compound annual growth rate of 6.9%, estimated to increase to 8.5% by 2027 (http://www.grandviewresearchanalysis/biofuelmarket2021).
More than 60% of plant biomass on earth is lignocellulose, making it an abundant resource for future bioenergy. Lignocellulosic agro-industrial wastes can be transformed into biofuels through the use of enzymes such as β-glucosidase, xylanase, laccase, lipase, cellobiohydrolases, and cellulases (Fang et al. 2012). Currently, the bioconversion of lignocellulosic waste to usable cellulase involves tedious, inefficient, and expensive traditional processes. Many factors, such as lignin content, the crystallinity of cellulose, and particle size, limit the digestibility of the hemicellulose and cellulose present in the lignocellulosic biomass. Therefore, novel, modest, and effective approaches to access lignocellulosic biomass are required (Verma et al. 2011). The use of enzymes plays an essential role in the production of biofuels. However, the application of enzymes in biofuel production has some significant limitations such as reusability and increased cost. The two main advantages of immobilization are that it increases the surface area, reusability and the stability which reduces the overall cost significantly. The cellulase and lipases are used to convert lignocelluloses into easily digestible forms to produce biofuels (Ghadi et al. 2015). The conversion of lignocellulosic biomass into biofuel for energy includes various steps from pre-treatment, saccharification, fermentation. Finally, distillation methods may vary from conventional approaches such as physical, chemical methods to novel methods of using enzymes to break down the complex carbohydrates into simpler ones. Most commonly, lignin is entirely removed from the pre-treatment mixture. One of the significant limitations in the commercial production of biofuels is the high operational costs, optimum conditions required for pre-treatment. The cost-effective production of biofuels can be achieved using modified/saccharified substrate obtained using enzymatic preparations conjugated with nanomaterials as a future strategy (Xu et al. 2020).
Moreover, conjugations with modified matrices have increased porosity and better surface area with high stability and reusability (Ganesan et al. 2021).The covalent immobilization provides a stable solution for sustainable yields of biofuel. Biocatalysts have a limitation of leaching in case of adsorption or in the presence of detergents that can be improved by using a hetero functional support matrix. One advanced approach is to use nanomaterials in combination with lignocellulosic waste that increases the surface area and thus leads to more enzyme loading capacity and better conversion to biofuel. In the present review, we present a detailed analysis to study categories of lignocellulosic wastes, fabrication of nanomaterial-based lignocellulosic waste, and summarize recent applications involving immobilization of enzymes on various nanocomposites for the production of biofuels. Hence this review provides insights into sustainable and renewable biofuel production. It will help in translating the technology from laboratory to commercial scale.
Source of lignocellulosic waste
The two main sources of lignocellulosic waste are of agricultural origin or domestic origin. In countries like India, China, the USA, and Australia, agriculture is practiced on a large scale, and approximately one-third of the food produced is wasted as agricultural waste. Some examples include fruits, vegetables, roots, and tubers. Waste from agriculture is usually used as fertilizer, thrown away in the rubbish, or burnt away. Several studies have converted lignocellulosic waste into a functional product by treatment and conversion (Ravindran et al. 2016). The pre-treatment techniques involving lignocellulosic-based raw material involve the complex disrupting structure by mechanical, physical, chemical, and combination methods. Therefore, agricultural waste is a good source of lignocellulosic waste. Moreover, for the production of biofuel, municipal and industrial solid wastes also play a significant role. A recent study reported that integrated bioconversion of cellulose-enriched municipal solid waste could be used as an alternative. It can also be utilized in association with garbage disposal, organic waste, and household by-products which make it environmentally friendly. Still, the cost of processing is high (Achinas and Euverink 2016).
Lignocellulosic waste is composed of three major polymers (cellulose, hemicellulose, lignin), proteins, lipid components (waxes, chlorophyll), non-structural sugars, and small amounts of pectin. The composition of lignocellulosic waste varies with plant species, age, stage of growth, and season (Bhatia et al. 2020). The most abundant lignocellulosic biomass component is cellulose. Cellulose represents 40–60% in weight and consists of β-D-glucopyranose units linked via β-(1,4) glycosidic bonds cellobiose as the fundamental repeating unit. The cellulose chains of 500–1400 D-glucose units are arranged together to form microfibrils which are packed together to form cellulose fibrils. 20–30% of the biomass consists of hemicellulose which is amorphous with little physical strength and hydrolyzed by diluted acids. The second most abundant polymer present is lignin, which provides hydrophobicity and structural rigidity and acts as a barrier between enzyme and cellulose.
Lignocellulose consists of the compact crystalline complex made up of microfibers of polysaccharides which consists of layers of lignin that protect the polysaccharides against the attack of hydrolytic enzymes, external factors and also provides stability to the complex structure. Cellulose, hemicellulose filaments, and pectin most commonly form the micro-polysaccharide fibers. The lignocellulosic residues based on the agro-industrial activity are classified into two main categories: those in which the primary carbon source is lignocellulose and the other is in which simple carbohydrates such as mono and disaccharides are also present in addition to lignocellulose (Villas-Boas et al. 2002). The presence of cellulose, hemicelluloses, and lignin functional groups make lignocellulosic fibers very effective bio-sorbents due to the ability of carboxylic (primarily present in hemicelluloses, lignin, and pectin), phenolic (lignin and extractives), and to a certain extent hydroxylic (cellulose, hemicelluloses, lignin, extractives, and pectin) and carbonyl group (lignin) to bind heavy metal ions from polluted water (Rangabhashiyam and Balasubramanian 2019).
Use of nanotechnology in conjugating lignocellulosic mass and enzymes
The use of enzymes for the conversion of lignocellulosic mass into biofuels is a novel approach to produce sustainable energy. When these enzymes are immobilized on nanomaterials, the resulting biocatalysts show improved overall efficiency due to their reusability, enzyme loading support, and overall low cost (Verma et al. 2013, 2020). Some of the nanomaterials tested include simple carbon nanotubes, nanofibers, nanocomposites to chitosan, and protein or carbohydrate supports (Verma et al. 2013, 2020). The types of nanomaterials prepared by using the lignocellulose as biomass is described in Table 1. A plethora of enzymes are used to convert cellulose mass into biofuels and these include lipase, xylanase, pectinase, cellulase, and lignin peroxidases for breaking down cellulose, hemicelluloses, and lignin (Binod et al. 2019). Chitosan is a starch polymer by conjugation of β-1–4 connected D-glucosamine. Biobased polymers such as protein and carbohydrates have recently been encouraged due to their easy biodegradability, compatibility, immunogenicity, and antibacterial properties. Furthermore, other polymers such as alginate, chitosan, albumin, keratin, sericin, fibroin, gelatin, and collagen have also been used to immobilize enzymes to convert cellulosic mass into biofuels (Verma et al. 2020). Another primary strategy is the green approach to use phytogenic nanoparticles as it has the advantage of producing no or significantly less pollution (Selim et al. 2020). Out of all strategies used for enzyme immobilization, compared to entrapment or covalent binding, adsorption is the most efficient approach (Verma et al. 2020).
Table 1.
Recent studies on using lignocellulosic biomass on conjugation with nano-materials
| Sr. No. | Type of biomass | Nanomaterial | Strategy for fabrication | References |
|---|---|---|---|---|
| 1 | Lignin | Carbon quantum dots (CQDs) | Thermal-based method | Figueiredo et al. (2018) |
| 2 | Lignin | Carbon quantum dots (CQDs) | Microwave one pot strategy for fabrication | Si et al. (2018) |
| 3 | Lignocellulosic | Lignin nanoparticles (LNPs) | Enzymatic pretreatment | Tian et al. (2017) |
| 4 | Cellulose | Carbon nanofibres | Thermal treatment | Zhou et al. (2020) |
| 5 | Cellulose | Porous carbon nanofibres with ZnO and NiO | Biological energy device | Liu et al. (2015) |
| 6 | Cellulose | Potassium citrate | Jiang et al. (2016) | |
| 7 | Lignin–Cellulose | Carbon nanofibres | Novel biocapacitor | Cao et al. (2019) |
| 8 | Lignin | Smart CLEAs | Nanofibres and Nanocrystalline | Rai et al. (2018); Sheldon (2018) |
| 9 | Lignin | Nanofibrils | Deep entectic solvent at 160°–180° | Shen et al. (2020) |
| 10 | Cellulose from sunflower stalk/banana peel/wheat straw | Lignin containing cellulose nanofibers | Solala et al. (2019) |
Fabrication of nanoconjugated lignocellulosic mass
Lignin and cellulose are the primary component of wood and is abundant on Earth. They have been considered as a clean and green alternative for renewable energy production. Nano-biomaterials combined with lignocellulosic mass are an economical alternative for renewable energy sources without adverse environmental effects (Zhu et al. 2016). Moreover, due to high biocompatibility, the specialized lignocellulosic nano-biomaterial provides potential applications in the biomedical, electronics, and energy sectors (Richter et al. 2015). An advantage of lignocellulosic nano-materials is that they are non-toxic nanomaterials compared to conventional nanomaterials. Lignin nanoparticles with advanced and tunable properties using carbon quantum dots (CQDs) possess good fluorescent properties that can be used for energy sources (Figueiredo et al. 2018). (Si et al. 2018) (Fig. 1) developed a one-pot strategy for developing the lignin nanoparticles. One of the significant challenges in fabricating lignocellulosic nanoparticles (LNPs) is to change the recalcitrant structure of lignocelluloses to develop sustainable solutions for biorefineries. The use of cellulolytic enzyme lignin obtained after enzymatic pre-treatment has been explored for lignocellulosic biorefineries by Tian et al. (2017). Three types of pre-treated cellulolytic enzyme lignin (CEL) from agricultural corn waste, hardwood poplar, and softwood pine have been evaluated by dialysis to obtain 90.9, 81.8, and 41.0% of yields, respectively (Tian et al. 2017). A similar approach has been used for obtaining silk, chitosan, and starch nanoparticles. This process is one of the best alternatives for the fabrication of nanocomposites. Conventional biorefinery setups use steam as pre-treatment with the significant lignin valorization problem. However, this enzyme-based method can provide controlled lignin nanoparticles with improved hydrophobic modifications (Kumar et al. 2012; Qian et al. 2014). Structural properties have been evaluated using a transmission electron microscope and scanning electron microscope. These particles have been found with a wide range of pH, one of the essential properties for imparting stability and light scattering (Richter et al. 2015). To keep the lignin structure intact, steam pre-treatment has been followed by extracting lignin residues using dimethylsulfoxide, and subsequent dialysis has been performed (Tian et al. 2017) without much change in hydrophobic properties high enzyme loading capacity.
Fig. 1.
One-pot strategy for fabrication of lignin nanoparticles from rice straw as lignocellulosic biomass
In Fig. 1, the procedure for fabricating lignin nanoparticles using rice straw as a substrate is studied (Si et al. 2018). Carbon-based helical carbon nanomaterials to porous and stacked conformations can be used depending upon the requirement nanomaterials can be classified based on dimensions ranging from zero sizes in case of carbon-based quantum dots (Li et al. 2015; Zhao et al. 2015), one dimensional in case of carbon nanotubes, two-dimensional graphene (Allen et al. 2017) . Carbon nanofibers have been considered essential for fabricating one-dimensional nanoparticles (Zheng et al. 2014; Wang et al. 2018; Zhou et al. 2020). Biological energy storage devices using natural materials for reducing non-renewable resources have been proposed in recent studies. In a recent study, it was proposed the use of the fabricated carbon nanofibers bridged to porous carbon nanosheets with bacterial cellulose and potassium citrate (Jiang et al. 2016). This has been evaluated as ultra-high energy density with a high cycling life. In a similar study, novel supercapacitor has been fabricated using lignin-cellulose-based carbon nanofibers (Cao et al. 2019). Biomass has been used to produce biofuels due to a diverse range of polysaccharides like cellulose, inulin, chitosan, lignin, and aquatic carbohydrates from microalgae that can act as feedstock for biorefineries (Sheldon 2018). Smart ferromagnetic cross-linked enzyme aggregates (CLEAs) can be another type of nanoparticle for the application of biofuels (Sheldon 2018). Lignin has been used for conversion into commodity chemicals (Sheldon 2018). The transformation of lignocellulosic biomass into nanocellulose has been widespread worldwide and categorized as nanofibers and nanocrystals (Rai et al. 2018). Another similar study on the fabrication of lignin nanoparticles followed after deep eutectic solvent pre-treatment includes 160–140°C for 6 h (Shen et al. 2020). Structural motifs of lignin have been evaluated using C13 NMR spectra, and after these treatments, the degradation and recondensation, and cleavage of carbon–oxygen and carbon–carbon bonds occur (Shen et al. 2020).
Lignin-containing cellulose nanofibrils (LCNFs) are more advanced materials with 1% of lignin in cellulose. These LCNFs have been tried with wheat straw, banana pulp, and even bark (Solala et al. 2019). The nano-biocatalyst mixture comprises a fabrication method employed on lignocellulosic mass for better digestibility (Rai et al. 2018). Conventional techniques include various ways such as acid catalysts and liquid acid catalysis to use dilute acid 1–10% at high temperature (Shahbaz and Zhang 2010) associated with corrosion issues solid acid catalysts to use solids that can exchange ions (Sritrakul et al. 2018). However, the enzymatic catalysis method is the most novel method with endoglucanase, exoglucanases, cellobiohydrolases, acetylesterase, xylanase, galactomannans, and hemicelluloses (Rozenfelde et al. 2017). Thus lignocellulosic/waste biomass due to abundance can be efficiently converted into nanoconjugates for easy conversion to biofuels. This provides an excellent method to strengthen the biorefinery concept.
Production of biofuels using immobilized enzymes conjugated with nanomaterials
The use of biocatalysts to speed up the reactions such as pyrolysis, catalytic cracking fermentation, and trans-esterification involved in producing biofuels/biodiesel from biomass has been recently reviewed (Ganesan et al. 2021). Enzymes such as esterase’s and lipases can be used for transesterification and lipolysis reactions. Immobilizing these enzymes on different matrices such as alumina, silica gel, ceramics, and polymers enhances catalytic activity, selectivity, and comfortable recovery (Hama et al. 2018). In addition, the increased surface area of nanomaterials results in more active sites and easy biofuel recovery with less energy loss (Ganesan et al. 2021).
Appropriate use of recyclable carriers and matrices for the immobilization of enzymes is another potential area of research in biofuel production (Table 2). Tosylate cloisite 30B has been suggested as a novel support for removing lipase from Candida rugosa to produce biodiesel from waste frying oil (Nezhad and Aghaei 2021). Covalent immobilization of the lipase was performed with a yield of 93.6%. All the physicochemical parameters were optimized for carrying out the transesterification reactions. Stability and reusability studies have shown the immobilized enzyme to be stable for 10 cycles up to 30 days of storage with 70.6% residual activity (Nezhad and Aghaei 2021). However, one of the significant limitations in biotechnological reactions involving enzymes is the high cost involved due to less reusability and stability (Bezerra et al. 2020).
Table 2.
List of enzymes and immobilization matrix conjugated with nanomaterials for biofuels production
| Sr. No. | Name of enzyme | Name of microorganism | Immobilization matrix | Application | References |
|---|---|---|---|---|---|
| 1 | Lipase | Candida rugosa | Tosylated cloisite | Biodiesel from waste cooking oil | Nezhad and Aghaei (2021) |
| 2 | Cellic Ctec2 Novozymes with xylanase and cellulase | Commercial preparation | Magnetic graphene oxide | Lignocellulosic mass hydrolysis for second generation ethanol | Paz-cederno et al. (2021) |
| 3 | Lipase AH-F2 | Aspergillus terreus | Iron oxide and polydopamine nanoparticles | High temperature and pH range adaptability for conversion of biodiesel from waste cooking oil | Touqeer et al. (2020) |
| 4 | Lipase | Bacillus subtilis | Iron oxide | Biofuel production | Lyu et al. (2019) |
| 5 | Lignin | Rhodococcus sp. YHY01 | Biodiesel from palm oil fatty acid methyl esters | Bhatia et al. (2019a, b, 2021a, b ) | |
| 6 | β-Mannanase | Streptomyces Alg S25 | Chitosan nanoparticles | Cost effective production processing of lignocellulosic waste | Mohapatra (2021) |
| 7 | Lipase | Rhizopus oryzae | Magnetic nanoparticles with mesoporous silica containing aldehyde and amine group | Biodiesel production from olive oil | Esmi et al. (2021) |
| 8 | β-Glucosidase | Aspergillus versicolor | Manganese oxide nanoparticles | Hydrolysis of cellobiose to give cellulosic ethanol | Huang et al. (2021) |
| 9 | Cellulases | Aspergillus fumigatus | Ferric oxide magnetic nanoparticles | Saccharification of rice straw | Kaur et al. (2021) |
| 10 | Lipase |
Saitozyma podzolica zwy 2–3 Burkholderia pyrrolica wz 103 |
Magnetic ferric oxide nanoparticles with glutaraldehyde | One step transesterification for biodiesel production | Cao et al. (2021) |
Xylanases, cellulases are the enzymes to process lignocellulosic biomass into second-generation ethanol with less stability. One of the most effective immobilization matrixes for these enzymes is graphene oxide that involves immobilization using carbodiimide and N-hydroxysuccinimide. Residual activities of endoglucanase, xylanase, β-glucosidase, and β-xylosidase activities of 70, 66, 88, and 70%, respectively, after ten cycles of hydrolysis (Paz-cederno et al. 2021). Moreover, magnetic nanoparticles provide easier separation and downstream processing. Various advancements in transesterification reactions involving enzyme-mediated have been discussed and reviewed (Bhatia et al. 2021a). Iron oxide nanoparticles have been used in combination with polydopamine to immobilize lipase from Aspergillus terreus AH-F2 lipase (Touqeer et al. 2020). Immobilized lipase exhibited better pH adaptability with a 92% yield for biodiesel production from waste cooking oil (Touqeer et al. 2020). In a similar study by Mijone et al. 2020a; Mijone et al. 2020b iron oxide was utilized to immobilize lipase extracted from Burkholderia cepacia using polysiloxane polyvinyl alcohol matrix and the optimum conversion yield (96.5%) has also been observed (Mijone et al. 2020a,2020b). Lipase obtained from Bacillus subtilis has been immobilized with ferric oxide nano dispersed magnetite microspheres (Lyu et al. 2019).
β-Mannanase is another powerful enzyme to break down complex mannan as a significant component in the cell wall of Chlorella and several seaweed species. β-Mannanase extracted from novel Streptomyces species AlgS25 has been immobilized using chitosan nanoparticles (Mohapatra 2021). This enzyme has displayed maximum activity at a wide range of pH and temperature with low Km (4.94 mg/mL) and high Vmax (93.5 U/mg protein) toward galactomannan with 81.2% residual activity after 10 cycles (Mohapatra 2021). Synthesis of fatty acid methyl esters from olive oil by transesterification reaction has been carried out employing magnetic nanoparticles (Esmi et al. 2021). In this study, lipase extracted from Rhizopus oryzae was immobilized using magnetic nanoparticles containing active amine and aldehyde groups by adsorption and covalent linkage. The highest yield of biodiesel (84.6%) has been observed using the aldehydic carrier compared to others (Esmi et al. 2021). β-glucosidase is another essential enzyme catalyzing the hydrolysis of cellobiose and thus crucial in ethanol production using cellulose. A novel β-glucosidase has been purified from Aspergillus Versicolor, followed by immobilization using manganese oxide with the highest enzyme activity (813 U/mL) to hydrolyze cellobiose (Huang et al. 2021). Lignin constitutes a significant part of the lignocellulosic mass in plants, along with xylose and glucose-Rhodococcus sp. YHY01 has been used to utilize lignin and subsequent conversion into biodiesel from barley straw (Bhatia et al. 2019a, 2021b, 2021c; Bhatia et al. 2019a). This study has provided insights into the utilization of lignin that is the second most abundant lignocellulosic waste. Bhatia et al. (2021b) have studied the use of Rhodococcus sp. YHY01 as a microbial cell factory for biodiesel production from waste cooking oil. Response surface methodology has been employed to find the best interactive effect of variables such as ammonium chloride, sodium chloride, and polyethylene glycol. Most of the studies for the production of biofuels involve lab-based setup, and very few have been translated at the industrial level.
Strategies for pre-treatments of lignocellulosic waste using enzymes
The pre-treatment of lignocellulosic waste is one of the most challenging steps in the manufacturing of usable lignocellulosic substrates for biofuels. Various techniques have been developed and tested for the pre-treatment of the lignocellulosic substrate. Among those, biological pre-treatment involving the use of microbes and enzymes for the degradation at the first stage of hydrolysis is the most common. This is usually combined with other different pre-treatment methods (Tanjore et al. 2015). In the second stage (also known as enzymatic saccharification), cellulase enzymes are then added to convert cellulose into oligomers and sugar monomers. The various chemical mediators and enzymes are needed to remove the biological and physiological barriers and effective biological pre-treatment. In addition, to increase access by opening the cell wall matrix and expanding tiny pores, various enzymes can be used (Amin et al. 2017).
Each of the common pre-treatment approaches falls under the four categories of physical, chemical, physicochemical, and biological methods. As a result, different products and yields can be obtained from each pre-treatment approach, and each method has its advantages and disadvantages. The various techniques, such as physical pre-treatment techniques, including mechanical tasks of different kinds of light and ultrasonic pre-treatment, are used to improve the availability of hydrolyzable polymers inside lignocellulosic material. Compound pre-treatment strategies are utilized to increase the biodegradation of the complex material more frequently than organic or physical pre-treatment techniques. It incorporates alkaline and acid pre-treatment (Jönsson and Martín 2016). Biological pre-treatment is completed through the use of microorganisms or catalysts. Microbiological treatment is done by utilizing microscopic organisms such as bacteria (Actinomycetes) or fungi (white-rot fungi). It is a cost-effective strategy. However, its rate is delayed for mechanical purposes. Another drawback is that the microorganisms themselves use some part of the sugar as an initial step. Biological pre-treatment can be utilized or combined with another pre-treatment technique when the biomass has low lignin content (Amin et al. 2017). In several pre-treatment methods, many lignocelluloses derived inhibitors such as furfural are produced, damaging cell membranes, DNA and inhibiting glycolysis and TCA cycle in the cell (Seo et al. 2016). Genetic engineering method is employed or used to reduce furfural toxicity. In this method, the salvage pathway genes pncB and nadE were overexpressed in isobutanol producing strain which increases the consumption of glucose and conversion of furfural to furfuryl alcohol and leads to the formation of NAD and NADH, essential for furfural tolerance and the overexpression of NAD salvage pathway enzymes increases the patience for the inhibitor (Song et al. 2017).
Enzymes used for pre-treatment
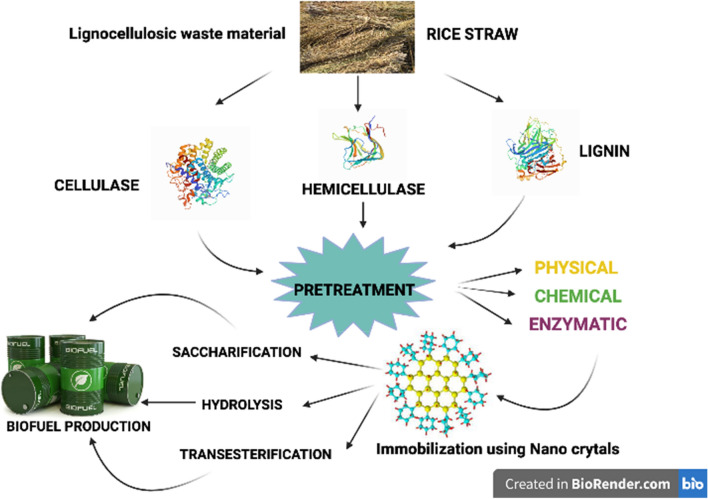
Lignocellulosic biomass constitutes plants (which are mainly unutilized) during enzymatic treatment. It includes residue from various sectors like agriculture, wood, municipal waste, forest, and paper waste. It is primarily composed of cellulose, hemicellulose, and lignin. Lignocellulosic waste can be used for the synthesis of biofuels and many bio-based materials. But for that process, lignocellulosic waste must be pre-treated to make it lignin-free. For pre-treatment, various physical, chemical, biological methods have been applied recently, but they are costly, and they also tend to produce toxins. These problems have been overcome by the use of the new cost-effective and eco-friendly method. This approach is extensively used in various sectors such as biofuel production. This method involves the usage of nanomaterials due to the small size of nanomaterials. It allows easy penetration into the cell wall of lignocellulosic biomass and interacts with cellulose and hemicellulose to release its monomeric and oligomeric sugars. Nanomaterial used for the pre-treatment has been known to improve the chemistry of biomass at the molecular level. For enzymatic hydrolysis, nanomaterial has been used to immobilize enzymes like cellulases, hemicellulose, and ligninolytic enzymes (Arora et al. 2020). Enzymes are the biocatalyst that is primarily used in the food sector to maintain a safe environment as a substitute to the earlier food processing procedures. The enzymes have applications in all the fields and industries such as pharmaceutical, cosmetic industry, and even some enzyme produces various compounds such as tannins which are used in the treatment of tannery effluents and pre-treatment of animal feed containing tannin (Kumar et al. 2017). Enzymes have been extracted from edible plants, animals, microorganisms.
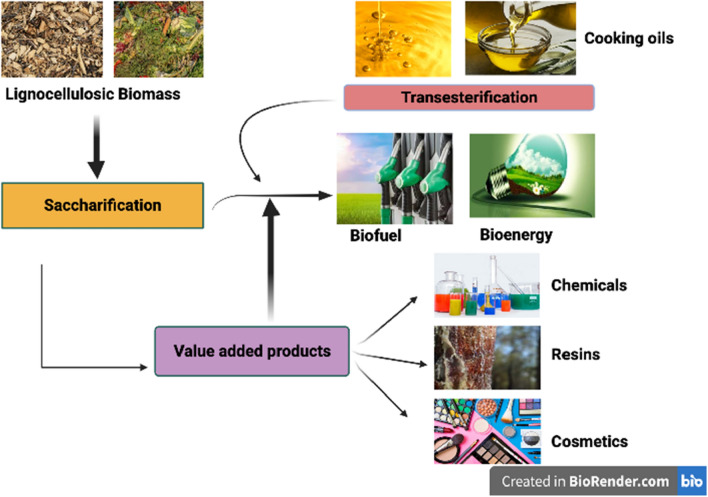
For lignocellulose degradation, the most common enzymes are laccase (EC 1.10.3.2), lignin peroxidase (EC 1.11.1.13), and cellulase. In a recent study, it was found that the enzymes such as feruloyl esterase (EC 3.1.1.73), aryl-alcohol oxidase (EC 1.1.3.7), lipases (EC 3.1.1.3), xylanase (EC 3.2.1.8), and catechol 2,3-dioxygenase (EC 1.13.11.2) have an indirect role in the ligninolytic enzyme process for degradation of environmental pollutants (Ozer et al. 2020). In Fig. 2, the steps for the production of value-added products by using enzymes and lignocellulose as a substrate. The degradation of cellulose and hemicellulose involves various enzymatic pathways, making it a complex process (Aarti et al. 2015). The various aerobic and anaerobic microorganisms are used for the degradation of cellulose and hemicellulose. Still, a different set of enzymes such as acetyl xylan and esterase are utilized to degrade hemicelluloses (Vazquez et al. 2007).
Fig. 2.
Applications of lignocellulosic biomass
Lignin enzymes
The lignin-degrading enzymes are classified into two major groups; lignin-degrading auxiliary enzymes and lignin modifying enzymes. Lignin degrading enzymes cannot completely degrade the lignin and requires an additional enzyme for complete degradation. Various microorganisms produce lignin modifying enzymes known as ligninolytic enzymes. These can be categorized as laccase and heme-containing peroxidase, including lignin, manganese, and versatile peroxidase (Ozer et al., 2019). The primary function of these ligninolytic enzymes is to degrade the organic pollutants and compounds containing lignocellulosic waste and also treats the industrial waste and other xenobiotic compounds via the biodegradation process (Shi et al. 2013). In the Table 2, the feedstock used to produce various enzymes such as laccase, cellulase, lipase, protease, and pectinase using different microbial strains is shown.
Laccase
Laccase (EC 1.10.3.2) is present in microorganisms such as bacteria, fungi, and actinomycetes, and its structure consists of copper-containing extracellular enzymes made up of monomeric, dimeric, and tetrameric glycoproteins. The enzyme yields water as the by-product, and molecular oxygen as co-substrate that is required for the catalysis. The primary source of this enzyme is fungi but other sources also include bacteria, insects, and plants. Fungal laccases are mainly extracellular enzymes extracted from the residual compost of industrial production of edible mushrooms such as Agaricus bisporus, Trametes versicolor, and Pleurotus ostreatus (Pandohee et al. 2014). This enzyme is primarily utilized in food, pharmaceutical, and environmental industries and the bleaching of paper pulp. Laccase also can act on lignocellulosic substances, which contain phenolic, non-phenolic, and significantly residual compounds for its detoxification and degradation (Kumar et al. 2019).
Xylanases
One of the most abundant renewable biomasses presents on Earth is lignocellulose, and its main constituents are cellulose, hemicelluloses, and lignin, along with smaller amounts of pectin, protein, etc. Xylanase represents enzymes responsible for the complete hydrolysis of the linear polysaccharide β-1,4-xylan into simpler compounds. The source of xylanase includes fungi, bacteria, yeast and snails. A recent study reported that xylanases produced by bacteria and actinomycetes (Bacillus sp., Pseudomonas sp, Streptomyces sp.) are effective in a broader pH range between 5 and 9 and the optimum temperature for xylanase activity between 35 °C and 60 °C (Motta et al. 2013). Xylanases are produced either by solid-state fermentation or submerged fermentation. The use of solid-state fermentation as a method for the production of xylanase has an advantage over submerged fermentation (Almanaa et al. 2020). It is more economically feasible and requires simple fermentation equipment with low requirements of aeration and agitation for enzyme production. The use of metagenomics and service of advanced tools of microbiology such as next-generation sequencing, site-directed mutagenesis, fusion protein, enzyme engineering for increasing the activity and production increases the efficacy of the enzymes (Bhatia et al. 2020). The primary application of xylanase is in the food and feed industry, where it is used for the pre-treatment to improve the digestibility of ruminant feeds and facilitates composting (Ran et al. 2019).
Methods for pre-treatment
The pre-treatment methods are categorized into physical, chemical, and biological techniques. The harsh chemical substances and high conventional heating strategies utilized for biomass pre-treatment require broad energy measures and are not eco-friendly. Besides, these pre-treatment strategies lead to the development of various unfortunate mixes, for example, aliphatic acid, vanillic acid, uronic acid, 4-hydroxybenzoic acid, phenol, furaldehyde, cinnamaldehyde, and formaldehyde, which may all interfere with the development of the fermentative microorganisms during fermentation (Ravindran and Jaiswal 2016). Rising advancements for pre-treatment of lignocellulosic biomass, include non- ionizing radiation (microwaves), ionizing radiation (gamma beam, electron beam, pulsed homogenization), and ultrasound. For the most part, every essential pre-treatment is categorized as physical, synthetic, and biological.
Biological pre-treatment
The main aim of the biological method is to deconstruct lignin structures in the cell wall using microbes or using enzymes isolated from these microbes, which can be used as a catalyst. Bacteria such as Actinomycetes and fungi such as white-rot fungi (Trametes orientalis) can generate various enzymes that are helpful in the degradation of lignocellulosic waste (Amin et al. 2017). In this method, lignin is degraded into monomeric units. It also decomposes the prominent linkages present between hemicellulose or lignin and cellulose, decreasing the activation energy and increasing the reaction rate at lower temperatures (Vasco-Correa et al. 2016). White rot fungi are considered the most potent microorganism for this treatment. It degrades lignin and can produce enzymes laccase and peroxidase that helps in lignin oxidation (Vasco-Correa et al. 2016; Yang et al. 2007). A recent study found that the consortium of white-rot fungus (Trametes Orientalis) and brown rot fungus (Fomitopsis pinicola), when used for corncob lignin treatment, showed that white-rot fungus efficiently degraded lignin into its constituents. In contrast, brown rot fungus promoted the breakdown of guaiacyl units and indicated the synergetic effect of fungi in lignin degradation (You et al. 2019). The significant limitations of this method are the new and limited use of microorganisms, and this process is very time-consuming. It requires a large amount of space for this treatment.
Production of biofuel and bioenergy using enzymes
Biofuels is a promising alternative to the commercial petroleum diesel that helps in the conservation of fossil fuels. Biodiesel production also contributes to waste management by utilization, including kitchen waste, sewage for potential methanogens, plant and animal wastes. Nowadays, lignocellulosic plant biomass is utilized as an alternative for biofuels as it contains a large amount of carbon and decreases our need to consume fossil fuels. It comprises almost half of the entire photosynthetic plant material that is used for biofuels/bioenergy production. These are also the most significant waste piles, including dried plant leaves, wood shaves, logs, newspapers, kitchen oils, wheat straw, corn cobs, bran, bagasse amongst others. (Sanderson 2011). For biofuel production, agro-industrial wastes generated from crop cultivation and food processing are also a potential raw material and thus dramatically promote the sustainable development of the economy. The structure of lignocellulose contains lignin which has the property of noncompliance; therefore, it is a slow biodegradable compound. Thus delignification, followed by saccharification of lignocellulosic waste to sugars is essential for further fermentation to biofuel. These processes essentially take place in the presence of microbial enzymes. Pre-treatment of the waste followed by microbial catalyst conversion, enzymatic hydrolysis is the process that releases monomeric sugars from the structural carbohydrates, cellulose, and hemicellulose in lignocellulosic biomass. Due to the recalcitrant property of lignocellulosic biomass, pre-treatment is an essential step to break down the complex lignin-cellulose structures. However, a setback to this step is that pre-treated lignocellulose hikes up the cost (Verma et al. 2016). Lignocellulosic material is the most abundant renewable source and is a suitable raw material for various human sustainability applications, including agriculture and forestry waste materials. This makes it a second-generation biofuel as there is no competition for land and water or even food materials for biofuel production. Waste raw materials are processed for successful development for regular diesel alternatives, thus partaking in Environment safety and Global Health.
Several enzymes and microbial strains are used in degradation of lignocellulose waste. The steps include delignification, saccharification, and fermentation to produce environmentally friendly biofuels and other essential commodities. Cellulase secreted from the fungi Trichoderma reesei has shown high efficiency in the hydrolysis of cellulosic substrates like wheat straw. Still, without pre-treatment, the process is not feasible, cost becomes insufficiently high. Later fermentation is carried out using T. guizhouense FTG2/TUCIM 4455 (Grujic et al. 2019). Basidiomycete fungi produces hydrolytic enzymes like cellulase and xylanase using two fungi strains, Bjerkandera adusta and Pycnoporus sanguineus, using cedarwood, oak sawdust, and wheat straw as substrate. The white-rot fungal group of basidiomycetes disintegrates all wood components by secreting xylanase and cellulases and shows optimum growth on the lignocellulose substrate (Quiroz-Castañeda et al. 2011).
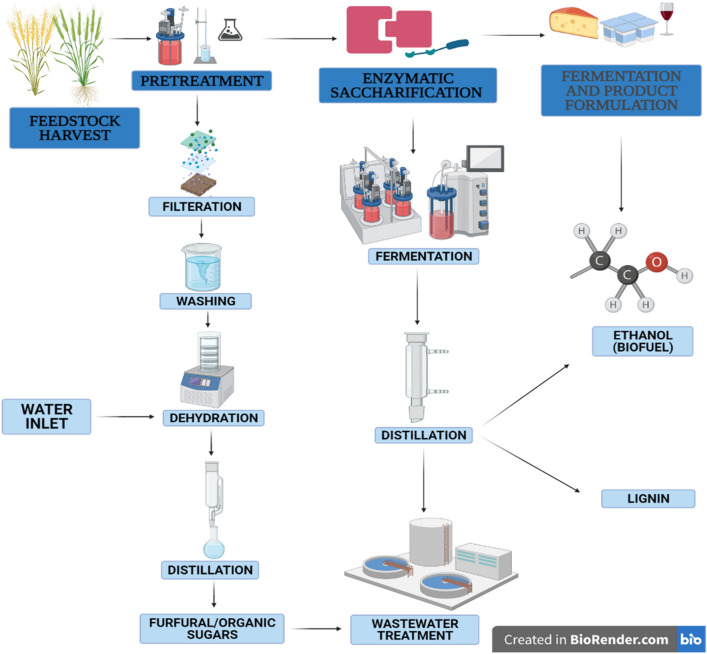
Usage of waste fiber sludge from pulp mills and lignocellulosic biorefineries has been used for the combined production of biofuels and enzymes. This decreases the biocatalyst cost and is a feasible method since fiber sludge has carbohydrates in high concentration compared to lignin. This method is economical and environmentally friendly for producing biofuels and other organic, inorganic chemicals; however, it does suffer from low energy efficiency due to the high amount of moisture in the waste fiber sludge. In the Fig. 3, the steps for the production of biofuel are shown. Hydrogen is also a potential bioenergy source, highly efficient, non-toxic, and quickly produced from the feedstock. It is a sustainable source of energy and can be utilized as an alternative to electricity. Feedstock from the agro-industrial origin is acted upon by enzymes (cellulases and xylanase) produced by Pseudomonas sp. and other fungal and bacterial species including Trichoderma sp., Aspergillus sp., and Clostridium thermocellum (Cheng and Chang 2011). Cellulase is an essential biocatalyst comprised of endo-β-1,4-D-glucanase, exo-β-1,4-D-glucanase, and β-1,4-D-glucosidase, converted cellulose fibers soluble sugar for biofuels production. The endo-β-1,4-D-glucanase binds to the amorphous part of cellulose, causing fragmentation of cellulosic materials; exo-β-1,4-D-glucanase hydrolyzes crystalline cellulose into simple sugars such as cellobiose and cello dextrin. Finally, b-glucosidase degrades cello oligosaccharides into glucose. (Li et al. 2011; Soni et al. 2010).
Fig. 3.
Steps for the production of biofuel
Lipase-based enzymes play a vital role in converting waste from the kitchen, such as cooking oils, to produce biofuels and free fatty acids (FFAs) by transesterification-esterification of lipids (Lam et al. 2010). The enzymatic route has an advantage over commercial chemical synthesis as it is economically and environmentally sustainable. Lipase as the catalyst is used either as whole-cell catalysts or immobilized lipases. The common organisms for lipase production include Wild-type filamentous fungus Rhizopus oryzae containing homogenous cell-bound lipases (Balasubramaniam et al. 2012) and recombinant Aspergillus oryzae, and Escherichia coli produce intracellular lipases (Takaya et al. 2011; Yan et al. 2012). Saccharomyces cerevisiae and Pichia pastoris yeast cells were used as recombinant yeast whole-cell catalysts in the form of intracellular enzymes. In Fig. 4, the steps for the production of ethanol by enzymatic saccharification and pre-treatment of the substrate are studied.
Fig. 4.
Steps for the production of ethanol by enzymatic saccharification and pre-treatment of the feedstock
The synthesis of biodiesel recombinant P. pastoris yeast whole-cell catalysts was constructed with an intracellular expression of Thermomyces lanuginosus lipase (TLL) (Yan et al. 2014). A study reported that P. pastoris has a high cellular density and greater expression level of the extracellular enzyme due to less hyper-glycosylation. In this study, lipase and biodiesel are produced in the same pot to decrease production and enzyme extraction costs. Thermomyces lanuginosus lipase is selected as it is widely used for biodiesel production. For the development of biodiesel, the two processes used are concurrent transesterification–esterification and stepwise hydrolysis-esterification. Hydrolysis-esterification is more economical and gives 87% recovery of biodiesel. Liquid enzymes produce a 47% biodiesel yield over the whole-cell catalyst with just a 7% yield. But in the stepwise hydrolysis-esterification process, there is a collective 56% yield of biodiesel using both liquid and whole-cell enzymes (Faruque et al. 2020). While most of the enzyme produced by P. pastoris expressing TLL) is extracellular enzymes, some intracellular enzymes are also processed, secreted post hydrolysis of acylglycerides, thus not neglected despite its lower activity (Yan et al. 2014). Intracellular enzymes are essential as these FFAs are small molecules and tend to penetrate inside the cell. With intracellular enzymes, there is a rise in biodiesel yield.
Furthermore, household waste such as pasta, rice, flour, cereal, meat, fish, bones, and garden waste can be converted into biofuel using simultaneous saccharification and fermentation method and cellulose activity was checked (Sotiropoulos et al. 2019). In recent findings, it was found that there are synergistic proteins that are produced by aerobic microorganisms that have been shown to have more excellent hydrolysis activity and boosting effect on cellulase activity, and the lytic polysaccharide monooxygenases (LPMOs) is one such protein (Zhao et al. 2016). LPMOs showcase synergistic effects with cellulases. This results in lesser enzyme load during bioconversion of cellulosic materials. The synergistic effects can also be increased using different carbohydrate-binding molecules, plant/bacterial expansions, or expansions-like proteins. Genes encoding synergistic effect can create a recombinant strain of microorganism, a potential research area to develop more efficient microbial strains for biodiesel production. In a recent study, it was found that the integration of LPMOs genes from Themobifida fusca into designer cellulosomes resulted in a 2.6-fold increase in the release of soluble sugars in comparison to free cellulases (Zhao et al. 2016).
Xylans, a trendy set of enzymes, also known as hemicellulases, and the enzymes used for the hydrolysis of xylan include endoxylanases and β-xylosidases (collectively called xylanases). The role of endoxylanases is to act on the homopolymeric backbone of 1,4-linked β-d-xylopyranose and produce xylooligomers, while β-xylosidases act on these xylooligomers releasing xylose for the production of xylanase bacteria, fungi, marine algae, yeast, protozoa, crustaceans, snails, protozoa, and insects. Various Bacillus sp. have shown a high amount of xylanase production. These are used to hydrolyze and delignify lignocellulosic material to produce environmentally friendly transport fuels. For the generation of biofuels such as xylitol and ethanol, the synergism of xylanase with ligninase, glucanase, xylosidase, glucosidase, mannanase, etc. is used by utilizing lignocellulose biomass as a substrate (Burlalcu et al. 2016).
Laccase plays a significant role in the delignification of pulp and forms reactive radicals with lignin or activating lignocellulosic fibers. The best way to produce biofuels is using an enzyme cocktail (xylanase, cellulose, and laccase) for enzymatic hydrolysis and degradation (Pennacchio et al. 2018).
For biofuel production, old landfills with softwood and newspapers are prime lignocellulosic-derived waste sites and use fungal enzymes like fungal peroxidases (lignin peroxidase) for hydrolysis and saccharification and lignin-degrading bacterial strain Agrobacterium species. The methanogens from sewage sludge are also utilized to produce biogas in stirred bioreactors, while fungal peroxidase is degrading newspapers and increases methane yield by 20% in newspaper reactor. At the same time, bacterial strain acts on both the wood lignin and newspaper. There are high rates of biogas production from landfill waste in the initial stage. The slow production of gas is insufficient for energy generation after the quickly depleted matter is exhausted. The two major contributors of the climate change are carbon dioxide and methane as a potential greenhouse gas, which are the components of the biogas. A recent experimental analysis found that a 92% increase in bioreactor production was found, which consists of Agrobacterium species.
Cellulases and hemicellulases in biofuel production
Saccharification is the critical process in the conversion of lignocellulosic mass into fermentable sugars such as glucose. Cellulases and hemicellulases are biocatalysts used to hydrolysis glycosidic bonds in lignocellulosic waste (Verma et al. 2020). The high cost involved with enzymes necessitated immobilization to retrieve and reuse the enzymes to increase the process’s economic feasibility. A cellulase recycling and another strategy for the financial management of the operation have been reviewed (Gomes et al. 2021). It has been discussed that recycling cellulases during the process can reduce the consumption of cellulases. Thus, reduction in the overall cost of the process (Oliveira et al. 2018). Solid-phase interaction has been evaluated for checking affinity by cellulose-binding domains in the case of free enzymes. However, in immobilization, various matrices such as chitosan and magnetic nanoparticles have been discussed. Some recent modification strategies involving 3D printing have been employed for desired configuration and reactor processing (Rodríguez-Restrepo and Orrego 2020; Singh et al. 2020; He et al. 2021). Consolidated bioprocessing for combining cellulose production, harvesting, hydrolysis, and fermentation in a single step is another strategy for better ethanol yield (Gomes et al. 2021).
Biodiesel production using catalysts or transesterification reactions from used oils has been discussed (Esmaeilli et al. 2021). Cellulases comprised of endocellulases, exocellulases and β-glucosidases produced by various bacterial and fungal sources viz. Bacillus, Cellulomonas, Thermomonospora, Caldicellulosiruptor, Erwinia, Clostridium, Trichoderma, Penicillium, and Aspergillus (Singhvi and Kim 2020). Metagenomic analysis and similar approaches have resulted in the characterization of novel cellulases and hemicellulase genes (Gavande et al. 2021). In this study, microbial diversity of thermotolerant microbial consortium has been carried out for lignocellulolytic enzymes (Gavande et al. 2021). This can make the cost-effective production of biofuel a reality.
Global markets
Lignocellulose is the most abundant raw material for bioenergy production as fossil fuels are depleting at an alarming rate. Due to the harms of greenhouse gases, there has been a rise in interest in bioenergy fuels from sources such as sugars, starches, and lignocellulosic materials. This has led to the production of bioethanol in the USA and worldwide. Bioethanol is primarily produced from corn starch feedstocks, while in Brazil, biofuel is mainly made from sugarcane juice and molasses. Together, these countries account for 89% of the current global bioethanol production (Limayem and Ricke 2012). Several countries have initiated new alternatives for gasoline from renewable feedstocks. In the North American hemisphere, bioethanol has been extracted from starch sources such as corn.
In contrast, biofuel has been primarily provided from sugars, including sugarcane and sugar beets in the South American hemisphere. While European countries are deploying extensive efforts to increase their 5% worldwide bioethanol production, biodiesel produced in Europe, primarily in France and Germany, remains by far more substantial and accounts for approximately 56% of the global output, mainly because of the rising importance of diesel engines and feedstock opportunity costs. Although most of the remaining countries in the world collectively account for only 5% of the global bioethanol production, China, Thailand, and India are continuing to invest substantially in agricultural biotechnology and emerge as potential biofuel producers (Limayem and Ricke 2012). The agro-industrial wastes containing abundant cellulosic fibers and carbohydrates such as grape pomace, sugar beet pomace, barley, and rice straw, corncobs, sunflower stalks, and heads, cotton waste, brewer's spent grain, forest residues, etc. act as a alternative substrate, for the production of bioethanol (Petrova and Ivanova 2010). Combining lignocellulose with nanotechnological advancements would be a beneficial step towards sustainable energy, both cost-effective and eco-friendly (Rai et al. 2019).
The use of nanotechnology is recommended but the cost of enzymes is high. Enzyme immobilized functionalized magnetic nanomaterials are used as reuse of nanobiocatalysts in reactions would be cost-effective, and it also does not require pre-treatment methods which reduces its cost and also increases the yield of fermentable sugars as the surface area is increased due to the small size of nanomaterials (Arora et al. 2020). A new process known as Lignin-first has emerged in biorefining research in which a reductive catalyst for the hydrolysis of lignin polymers is used. It results in a low molecular weight of lignin with high cellulose concentration pulp (Rai et al. 2016). Despite their advantages, there are concerns about excessive and uncontrolled use of such nanomaterials, which would lead to adverse effects on human health and the environment and thus limit nanoparticles’ exposure. The significant limitations of using nanomaterials include maintaining the structural stability of nanobiocatalysts during the biochemical reactions. Hence, immobilized enzymes with functional efficiency and enhanced reproducibility are used as alternatives despite their expensiveness (Rai et al. 2016).
The current production of lignocellulosic wastes is from municipal wastes, agricultural wastes and forest wastes (Tu and Hallett 2019). Giant reed (Arundo donax) is a perennial lignocellulosic grass, currently being regarded as an emerging bioenergy crop, with high biomass yield and low input requirement and the studies are ongoing on the suitability of giant reed biomass as a substrate for second-generation biofuels, as well as on the prototyping of the production of high-quality inoculum for the anaerobic digestion (Popp et al. 2016).
Problems encountered in nanomaterials
Nanotechnology has added the advantage of a larger surface area and more enzyme loading capacity. But some disadvantages have limited their use for large-scale production of biofuels. Nanomaterial conjugated enzymes refer to immobilization of nanocomposites by covalent bonds, Vander Waals forces as ionic bonds or entrapment (Misson et al., 2015). The small size of most nanomaterials may interfere with downstream processing and may add up the product’s final cost. Moreover, the small size will cause problems uploading the reactor. However, magnetic nanoparticles are easier to separate, and biofuels can be quickly recovered. Another point of concern is the complicated preparation of magnetic nanoparticles which adds up the cost. High cost is one of the major problems encountered in the case of employing nanomaterials in biofuel production. An increase in viscosity affecting the rheology of the medium for presentation using bioreactor adversely impacts production (Zhong et al. 2020). Toxicity remains another point that has to be taken into account. Any kind of toxicity associated with nanoparticles to microbial cells makes it more difficult to employ at a large scale. (Sekoai et al. 2019). Economic feasibility, technological advancements, and environmental impact will decide the viability of biofuel projects using sustainable and renewable substrates (Moustakas 2019). Still, translation of the process to the commercial level depends on the project's financial viability and biofuels.
Future prospects
Microfluidics is one of the recent approaches involving miniaturization of the whole lab on a small chip to deal with current challenges in biofuel technology (Zhang et al. 2016a,2016b). This field of science involves a multidisciplinary approach using and analyzing fluids at the microscale. The role of microfluidics can help in all upstream processing steps such as screening efficient catalysts, effective nanomaterial for support, medium optimization, and engineering of the conditions for better yields of biofuels using lignocellulosic waste materials. Various strategies of using microfluidics biofuel production have been studied and used in the future (Banerjee et al. 2019). For example, a multiplexed energy-producing model with a high continuous flow with minimum resistance can help establish efficient energy systems for microbial microfluidic cells. More such systems should be encouraged for pilot-scale studies, and results by employing valid subsidies to the users can impede the process of bringing a revolution in the bioenergy sector.
Conclusion
Bioenergy using inexpensive sources is one of the most critical aspects of reducing environmental pollution, which is a significant global concern across the world. This is the most environmentally beneficial method of producing biofuels as a clean and renewable energy source. Nanotechnology for combining enzymes with nanoparticles results in better yield due to high surface area, high reusability of enzymes and easy downstream processing. However, translation of process from lab scale to industrial level needs to overcome some limitations as financial viability and optimal loading of enzymes for production at bioreactor level. This review provides valuable insights about the present status and prospects along with problems encountered in this cost-effective novel technology for the production of clean and green bioenergy.
Acknowledgements
The authors would like to thank the Prof. Prem Kumar Khosla, Chancellor, Shoolini University of Biotechnology and Management Sciences, Solan, Himachal Pradesh, India and the School of Biotechnology here for providing the laboratory and technical facilities in the field of Enzyme Technology. We would also like to acknowledge Dr. Purnima Bali (Assistant Professor, English); Mr. Neeraj Pizar, Assistant Director, Scientific Writing Cell, Shoolini University for their valuable inputs during the preparation of the manuscript.
Author contributions
Conceptualization, PK, MT, PK and SK; formal analysis, SK, PK and JP; data curation; SK, PK and JP; writing-original draft preparation, PK, MT, DT, SM, GKB and PK; writing-review and editing, SK, PK JP, PK and MT All authors have read and agreed to the published version of the manuscript.
Funding
There is no funding source for this study.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not have any studies with human participants or animals performed by any of the authors.
Consent to participate
Not Applicable.
Consent for publication
All the authors mutually agreed that the work should be published in the 3 Biotech.
Footnotes
Parneet Kaur, Meenu Thakur and Divya Tondan have contributed equally.
Contributor Information
Pradeep Kumar, Email: pradeep.kumar@shooliniuniversity.com.
Saurabh Kulshrestha, Email: sourabhkulshreshtha@shooliniuniversity.com.
References
- Aarti C, Arasu MV, Agastian P. Lignin degradation: a microbial approach. Indian J Biol Sci. 2015;1:119–127. doi: 10.22205/sijbs/2015/v1/i3/100405. [DOI] [Google Scholar]
- Achinas S, Euverink GJW. Consolidated briefing of biochemical ethanol production from lignocellulosic biomass. Electron J Biotechnol. 2016;23:44–53. doi: 10.1016/j.ejbt.2016.07.006. [DOI] [Google Scholar]
- Allen DT, Lorenz CD. A novel method for constructing continuous intrinsic surfaces of nanoparticles. J Mol Model. 2017;23:219. doi: 10.1007/s00894-017-3378-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almanaa TN, Vijayaraghavan P, Alharbi NS, Kadaikunnan S, Khaled JM, Alyahya SA. Solid-state fermentation of amylase production from Bacillus subtilis D19 using agro-residues. J King Saud Univ Sci. 2020;32(2):1555–1561. doi: 10.1016/j.jksus.2019.12.011. [DOI] [Google Scholar]
- Amin FR, Khalid H, Zhang H, et al. Pre-treatment methods of lignocellulosic biomass for anaerobic digestion. AMB Expr. 2017 doi: 10.1186/s13568-017-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora A, Nandal P, Singh J, Verma ML. Nanobiotechnological advancements in lignocellulosic biomass pre-treatment. Mater Sci Energ Technol. 2020;3:308–318. doi: 10.1016/j.mset.2019.12.003. [DOI] [Google Scholar]
- Balasubramaniam B, Perumal AS, Jayaraman J, Mani J, Ramanujam P. Comparative analysis for the production of fatty acid alkyl esterase using whole cell biocatalyst and purified enzyme from Rhiz opus oryzae on waste cooking oil (sunflower oil) Waste Manag. 2012;32:1539–1547. doi: 10.1016/j.wasman.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Kumar SPJ, Mehendale N, Sevda S, Garlapati VK. Intervention of microfluidics in biofuel and bioenergy sector: technological considerations and future prospects. Renew and Sustain Energy Rev. 2019;101:548–558. doi: 10.1016/j.rser.2018.11.040. [DOI] [Google Scholar]
- Bezerra RM, Monteiro RR, Neto DM, da Silva FF, de Paula RC, de Lemos TL, Fechine PB, Correa MA, Bohn F, Gonçalves LR, Dos Santos JC. A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone. Application in the immobilization of lipase from Thermomyces lanuginosus. Enzyme Microb Technol. 2020 doi: 10.1016/j.enzmictec.2020.109560. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Joo HS, Yang YH. Biowaste-to-bioenergy using biological methods—a mini-review. Energy Convers Manag. 2018;177:640–660. doi: 10.1016/j.enconman.2018.09.090. [DOI] [Google Scholar]
- Bhatia SK, Gurav R, Choi TR, Han YH, Park YL, Park JY, et al. Bioconversion of barley straw lignin into biodiesel using Rhodococcus sp. YHY01. Bioresour Technol. 2019 doi: 10.1016/j.biortech.2019.121704. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Gurav R, Choi TR, Jung HR, Yang SY, et al. Effect of synthetic and food waste-derived volatile fatty acids on lipid accumulation in Rhodococcus sp. YHY01 and the properties of produced biodiesel. Energy Convers Manag. 2019;192:385–395. doi: 10.1016/j.enconman.2019.03.081. [DOI] [Google Scholar]
- Bhatia SK, Jagtap SS, Bedekar AA, Bhatia RK, Patel AK, Pant D, Banu JR, Rao CV, Kim YG, Yang YH. Recent developments in pre-treatment technologies on lignocellulosic biomass: effect of key parameters, technological improvements, and challenges. Bioresour Technol. 2020;300:122724. doi: 10.1016/j.biortech.2019.122724. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Bhatia RK, Jeon JM, Pugazhendhi A, Awasthi MK, et al. An overview on advancements in biobased transesterification methods for biodiesel production: oil resources, extraction, biocatalysts, and process intensification technologies. Fuel. 2021;285:119117. doi: 10.1016/j.fuel.2020.119117. [DOI] [Google Scholar]
- Bhatia SK, Gurav R, Choi YK, Lee HJ, Kim SH, et al. Rhodococcus sp. YHY01 a microbial cell factory for the valorization of waste cooking oil into lipids a feedstock for biodiesel production. Fuel. 2021 doi: 10.1016/j.fuel.2021.121070. [DOI] [Google Scholar]
- Bhatia SK, Palai AK, Kumar A, Bhatia RK, Patel AK, Thakur VK, Yang YH. Trends in renewable energy production employing biomass-based biochar. Bioresour Technol. 2021;340:125644. doi: 10.1016/j.biortech.2021.125644. [DOI] [PubMed] [Google Scholar]
- Binod P, Gnansounou E, Sindhu R, Pandey A. Enzymes for second generation biofuels: recent developments and future perspectives. Bioresour Technol Rep. 2019;5:317–325. doi: 10.1016/j.biteb.2018.06.005. [DOI] [Google Scholar]
- Burlacu A, Cornea CP, Israel-Roming F. Microbial xylanase: a review. Series F Biotechnol. 2016;20:335–342. [Google Scholar]
- Cao Q, Zhu M, Chen J, Song Y, Li Y, Zhou J. Novel Lignin-cellulose-based carbon nanofibers as high-performance supercapacitors. ACS Appl Mater Interfaces. 2019;12:1210–1221. doi: 10.1021/acsami.9b14727. [DOI] [PubMed] [Google Scholar]
- Cao X, Xu H, Li F, Zou Y, Ran Y, et al. One step direct transesterification of wet yeast for biodiesel production catalyzed by magnetic nanoparticle immobilized lipase. Renew Energy. 2021;171:11–21. doi: 10.1016/j.renene.2021.02.065. [DOI] [Google Scholar]
- Cheng CL, Chang JS. Hydrolysis of lignocellulosic feedstock by novel cellulases originating from Pseudomonas sp. CL3 for fermentative hydrogen production. Biores Technol. 2011;102(18):8628–8634. doi: 10.1016/j.biortech.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Esmaeilli H, Nourafkan E, Nakisa M, Ahmed W. Emerging nanotechnologies for renewable energy. Amsterdam: Elsevier; 2021. Application of nanotechnology for biofuel production; pp. 1–24. [Google Scholar]
- Esmi F, Nematian T, Salehi Z, Khodadadi AA, Dalai AK. Amine and aldehyde functionalized mesoporous silica on magnetic nanoparticles for enhanced lipase immobilization, biodiesel production, and facile separation. Fuel. 2021;291:120126. doi: 10.1016/j.fuel.2021.120126. [DOI] [Google Scholar]
- Fang J, Shijirbaatar A, Lin DH, Wang DJ, Shen B, Sun PD, Zhou ZQ. Stability of co-existing ZnO and TiO2 nanomaterials in natural water: aggregation and sedimentation mechanisms. Chemosphere. 2012;184:1125–1133. doi: 10.1016/j.chemosphere.2017.06.097. [DOI] [PubMed] [Google Scholar]
- Faruque MO, Razzak SA, Hossain MM. Application of heterogeneous catalysts for biodiesel production from microalgal oil-a review. Catalysts. 2020;10(9):1025. doi: 10.3390/catal10091025. [DOI] [Google Scholar]
- Figueiredo P, Lintinen K, Hirvonen JT, Kostiainen MA, Santos HA. Properties and chemical modifications of lignin: towards lignin-based nanomaterials for biomedical applications. Prog Mater Sci. 2018;93:233–269. doi: 10.1016/j.pmatsci.2017.12.001. [DOI] [Google Scholar]
- Ganesan R, Maniganandan S, Shanmugam S, Chandramohan VP, Sindhu R, Kim H, Brindhadevi K, Pugzhendhi A. A detailed scrutinize on panorama of catalysts in biodiesel synthesis. Sci Total Env. 2021 doi: 10.1016/j.scitotenv.2021.145683. [DOI] [Google Scholar]
- Gavande PV, Basak A, Sen S, Lepcha K, Murmu N, et al. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci Rep. 2021;11:3032. doi: 10.1038/s41598-021-82163-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadi A, Tabandeh F, Mahjoub S, Mohsenifar A, Roshan FT, Alavije R. Fabrication and characterization of core-shell magnetic chitosan nanoparticles as a novel carrier for immobilization of Burkholderia cepacia lipase. J Oleo Sci. 2015;64(4):423–430. doi: 10.5650/jos.ess14236.PMid:25833452. [DOI] [PubMed] [Google Scholar]
- Gomes D, Cunha J, Zanuso E, Teixeira J, Domingues L. Strategies towards reduction of cellulases consumption: debottlenecking the economics of lignocellulosics valorization processes. Polysaccharides. 2021;2:287–310. doi: 10.3390/polysaccharides2020020. [DOI] [Google Scholar]
- Gong C, Singh A, Singh P, Singh A. Anaerobic digestion of agri-food wastes for generating biofuels. Indian J Microbiol. 2021 doi: 10.1007/s12088-021-00977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grujić M, Dojnov B, Potočnik I, Atanasova L, Duduk B, Srebotnik E, Druzhinina IS, Kubicek CP, Vujčić Z. Superior cellulolytic activity of Trichoderma guizhouense on raw wheat straw. World J Microbiol Biotechnol. 2019;35(12):1. doi: 10.1007/s11274-019-2774-y. [DOI] [PubMed] [Google Scholar]
- Hama S, Noda H, Kondo A. How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr Opin Biotechnol. 2018;50:57–64. doi: 10.1016/j.copbio.2017.11.001. [DOI] [PubMed] [Google Scholar]
- He B, Chang P, Zhu X, Zhang S. Anemone-inspired enzymatic film for cellulose heterogeneous catalysis. Carbohydr Polym. 2021;260:117795. doi: 10.1016/j.carbpol.2021.117795. [DOI] [PubMed] [Google Scholar]
- http://www.grandviewresearchanalysis/biofuelmarket. Accessed 4 June 2021
- https://sdgs.un.org/2030agenda. Accessed 20 June 2021
- Huang C, Feng Y, Patel G, Xu XQ, Qian J, Liu Q, Kai GY. Production, immobilization and characterization of beta-glucosidase for application in cellulose degradation from a novel Aspergillus versicolor. Int J Biol Macromol. 2021;177:437–446. doi: 10.1016/j.ijbiomac.2021.02.154. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Yan J, Wu X, Shan D, Zho Q, Jiang L, Yang D, Fan Z. Facile synthesis of carbon nanofibers-bridged porous carbon nanosheets for high-performance supercapacitors. J Power Sour. 2016;307:190–198. doi: 10.1016/j.jpowsour.2015.12.081. [DOI] [Google Scholar]
- Jönsson LJ, Martín C. Pre-treatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol. 2016;199:103–112. doi: 10.1016/j.biortech.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Kaur P, Taggar MS, Kalia A. Characterization of magnetic nano particle-immobilized cellulases for enzymatic saccharification of rice straw. Biomass Conver Bioref. 2021;11(3):955–969. doi: 10.1007/s13399-020-00628-x. [DOI] [Google Scholar]
- Kumar L, Chandra R, Chung PA, Saddler J. Can the same steam pre-treatment conditions be used for most softwoods to achieve good, enzymatic hydrolysis and sugar yields? Bioresour Technol. 2012;101:7827–7833. doi: 10.1016/j.biortech.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Kumar P, Pandey D, Thakur V, Thakur A, Chand D. Hyperproduction of tannin acylhydrolase in submerged fermentation from Aspergillus fumigatus. JAM. 2017;3(2):60–77. [Google Scholar]
- Kumar V, Chandra R, Yadav S. Extremophilic enzymatic processing of lignocellulosic feedstocks to bioenergy. Cham: Springer; 2019. Extremophilic ligninolytic enzymes; pp. 115–154. [Google Scholar]
- Lam MK, Lee KT, Mohamed AR. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: a review. Biotechnol Adv. 2010;28:500–518. doi: 10.1016/j.biotechadv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Li Z, Li X, Wang Y, Wang YD, Wang F, Jiang JC. Expression and characterization of recombinant Rhizopus oryzae lipase for enzymatic biodiesel production. Bioresour Technol. 2011;102:9810–9813. doi: 10.1016/j.biortech.2011.07.070. [DOI] [PubMed] [Google Scholar]
- Li PJ, Xia JL, Shan Y, Nie ZY, Su DL, Gao QR, Zhang C, Ma YL. Optimizing production of pectinase from orange peel by Penicillium oxalicum PJ02 using response surface methodology. Waste Biomass Valor. 2015;6:13–22. doi: 10.1007/s12649-014-9317-4. [DOI] [Google Scholar]
- Limayem A, Ricke SC. Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog Energy Combust Sci. 2012;38(4):449–456. doi: 10.1016/j.pecs.2012.03.002. [DOI] [Google Scholar]
- Liu Y, Zhou J, Chen L, Zhang P, Fu W, Zhao H, Ma Y, Pan X, Zhang Z, Han W, Xie E. Highly flexible freestanding porous carbon nanofibres for electrodes material of high performance for carbon supercapacitors. CS Appl Mater Interfaces. 2015;42:23515–23520. doi: 10.1021/acsami.5b06107. [DOI] [PubMed] [Google Scholar]
- Lyu J, Li Z, Men J, Jiang R, Tang G, Zhou Y, et al. Covalent immobilization of Bacillus subtilis lipase A on Fe3O4 nanoparticles by aldehyde tag: an ideal immobilization with minimal chemical modification. Process Biochem. 2019 doi: 10.1016/j.procbio.2019.03.017. [DOI] [Google Scholar]
- Mijone PD, Bôas RN, Bento HB, Reis CE, de Castro HF. Coating and incorporation of iron oxides into a magnetic-polymer composite to be used as lipase support for ester syntheses. Renew Energy. 2020;149:1167–1173. doi: 10.1016/j.renene.2019.10.100. [DOI] [Google Scholar]
- Mijone PD, Boas RNV, Bento HBS, Reis CER, de Castro HF (2020) Coating and incorporation
- Misson M, Zhang H, Jin B. Nanobiocatalyst advancements and bioprocessing applications. J R Soc Interface. 2015;12:20140891. doi: 10.1098/rsif.2014.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra BR. Characterization of β-mannanase extracted from a novel Streptomyces species Alg-S25 immobilized on chitosan nanoparticles. Biotechnol Biotechnol Equip. 2021;35(1):150–161. doi: 10.1080/13102818.2020.1858158. [DOI] [Google Scholar]
- Motta FL, Andrade CC, Santana MH. A review of xylanase production by the fermentation of xylan: classification, characterization and applications. Sustainable degradation of lignocellulosic biomass-techniques, applications and commercialization. Intech. 2013 doi: 10.5772/53544. [DOI] [Google Scholar]
- Moustakas K. A review of recent developments in renewable and sustainable energy systems: key challenges and future perspectives. Renew Sustain Energy Rev. 2019;119:109418. doi: 10.1016/j.rser.2019.109418. [DOI] [Google Scholar]
- Nezhad MK, Aghaei H. Tosylated cloisite as a new heterofunctional carriers for covalent immobilization of lipase and its utilization for production of biodiesel from waste frying oil. Renew Energy. 2021;164:876–888. doi: 10.1016/j.renene.2020.09.117. [DOI] [Google Scholar]
- Oliveira C, Romani A, Gomes D, Cunha JT, Gama FM, et al. Recombinant family 3 carbohydrate binding module as a new additive for enhanced enzymatic saccharification of whole slurry from autohydrolyzed Eucalyptus globules wood. Cellulose. 2018;25:2502–2514. doi: 10.1007/s10570-018-1722-6. [DOI] [Google Scholar]
- Ozer A, Sal FA, Belduz AO, Kirci H, Canakci S. Use of feruloyl esterase as laccase-mediator system in paper bleaching. Appl Biochem Biotech. 2020;190(2):721–731. doi: 10.1007/s12010-019-03122-x. [DOI] [PubMed] [Google Scholar]
- Pandohee J, Stevenson PG, Conlan XA, Zhou XR, Jones OA. Off-line two-dimensional liquid chromatography for metabolomics: an example using Agaricus bisporus mushrooms exposed to UV irradiation. Metabolomics. 2014;11(4):939–951. doi: 10.1007/s11306-014-0749-4. [DOI] [Google Scholar]
- Paz-Cedeno FR, Carceller JM, Iborra S, Donato RK, Godoy AP, et al. Magnetic graphene oxide as a platform for the immobilization of cellulases and xylanases: ultrastructural characterization and assessment of lignocellulosic biomass hydrolysis. Renew Energy. 2021;164:491–501. doi: 10.1016/j.renene.2020.09.059. [DOI] [Google Scholar]
- Pennacchio A, Ventorino V, Cimini D, Pepe O, Schiraldi C, Inverso M, Faraco V. Isolation of new cellulase and xylanase producing strains and application to lignocellulosic biomasses hydrolysis and succinic acid production. Bioresour Technol. 2018;259:325–333. doi: 10.1016/j.biortech.2018.03.027. [DOI] [PubMed] [Google Scholar]
- Petrova P, Ivanova V. Perspectives for the production of bioethanol from lignocellulosic materials. Biotechnol Biotechnol Equip. 2010;24:529–546. doi: 10.1016/j.enconman.2010.08.013. [DOI] [Google Scholar]
- Popp J, Harangi-Rákos M, Gabnai Z, Balogh P, Antal G, Bai A. Biofuels and their co-products as livestock feed: global economic and environmental implications. Mole. 2016;21(3):285. doi: 10.3390/molecules21030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Deng Y, Qiu X, Li H, Yang D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014;16:2156–2163. doi: 10.1039/C3GC42131G. [DOI] [Google Scholar]
- Quiroz-Castañeda RE, Pérez-Mejía N, Martínez-Anaya C, et al. Evaluation of different lignocellulosic substrates for the production of cellulases and xylanases by the basidiomycete fungi Bjerkandera adusta and Pycnoporus sanguineus. Biodegradation. 2011;22:565–572. doi: 10.1007/s10532-010-9428-y. [DOI] [PubMed] [Google Scholar]
- Rai M, Dos Santos JC, Soler MF, Franco Marcelino PR, Brumano LP, Ingle AP, Gaikwad S, Gade A, da Silva SS. Strategic role of nanotechnology for production of bioethanol and biodiesel. Nanotechnol Rev. 2016;5(2):231–250. doi: 10.1515/ntrev-2015-0069. [DOI] [Google Scholar]
- Rai M, Ingle AP, Pandit R, Paralikar P, Biswas JK, Da Silva SS. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal Rev. 2018;61(1):1–26. doi: 10.1080/01614940.2018.1479503. [DOI] [Google Scholar]
- Rai M, Ingle AP, Pandit R, Paralikar P, Biswas JK, da Silva SS. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal Rev. 2019;61(1):1–26. doi: 10.1080/01614940.2018.1479503. [DOI] [Google Scholar]
- Ran T, Saleem AM, Shen Y, Ribeiro GO, Jr, Beauchemin KA, Tsang A, Yang W, McAllister TA. Effects of a recombinant fibrolytic enzyme on fiber digestion, ruminal fermentation, nitrogen balance, and total tract digestibility of heifers fed a high forage diet. J Anim Sci. 2019;97(8):3578–3587. doi: 10.1093/jas/skz216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangabhashiyam S, Balasubramanian P. The potential of lignocellulosic biomass precursors for biochar production: performance, mechanism and wastewater application-a review. Ind Crops and Prod. 2019;128:405–423. doi: 10.1016/j.indcrop.2018.11.041. [DOI] [Google Scholar]
- Ravindran R, Jaiswal A. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol. 2016;199:92–102. doi: 10.1016/j.biortech.2015.07.106. [DOI] [PubMed] [Google Scholar]
- Richter AP, Brown JS, Bharti B, Wang A, Gangwal S, Houck K, Cohen EA, Hubal VN, Paunov S, Stoyanov D, Velev OD. An environmentally benign antimicrobial nanoparticle based on a silver infused lignin core. Nat Nanotechnol. 2015;10:817–823. doi: 10.1038/nnano.2015.141. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Restrepo YA, Orrego CE. Immobilization of enzymes and cells on lignocellulosic materials. Environ Chem Lett. 2020;18(3):787–806. doi: 10.1007/s10311-020-00988-w. [DOI] [Google Scholar]
- Rozenfelde L, Puíe M, Krûma I, Poppele I, Matjuðkova N, Vederòikovs N, Rapoport A. Enzymatic hydrolysis of lignocellulose for bioethanol production. Proc Latvian Acad Sci. 2017;71:275–279. doi: 10.1515/prolas-2017-0046. [DOI] [Google Scholar]
- Sanderson K. Lignocellulose: a chewy problem. Nature. 2011;474:S12–S14. doi: 10.1038/474S012a. [DOI] [PubMed] [Google Scholar]
- Sekoai PT, Ouma CNM, Preez SPD, Modisha P, Engelbrecht N, Bessasobov DG, Ghimire A. Applications of nanoparticles in biofuels an overview. Fuel. 2019;237:380–397. doi: 10.1016/j.fuel.2018.10.030. [DOI] [Google Scholar]
- Selim KA, Easa SM, El-Diwany A. The Xylose metabolizing yeast Spathaspora passalidarum is a promising genetic treasure for improving bioethanol production. Fermentation. 2020;6(1):33. doi: 10.3390/fermentation6010033. [DOI] [Google Scholar]
- Seo HM, Jeon JM, Lee JH, Song HS, Joo HB, Park SH, Choi KY, Kim YH, Park K, Ahn J, Lee H, Yang YH. Combinatorial application of two aldehyde oxidoreductases on isobutanol production in the presence of furfural. J Ind Microbiol Biotechnol. 2016;43(1):37–44. doi: 10.1007/s10295-015-1718-2. [DOI] [PubMed] [Google Scholar]
- Shahbaz A, Zhang B. Dilute and concentrated acid hydrolysis of lignocellulosic biomass. Bioalcohol Prod. 2010 doi: 10.3390/polym13172886. [DOI] [Google Scholar]
- Sheldon RA. The road to biorenewable carbohydrates to commodity chemicals. ACS Sustain Chem Eng. 2018;6:4464–4480. doi: 10.1021/acssuschemeng.8b00376. [DOI] [Google Scholar]
- Shen XJ, Chen T, Wang H-M, Mei Q, Yue F, Sun S, Wen SL, Yaan TQ, Sun RC. Structural and morphological transformations of lignin macromolecules during bio-based deep entectic solvent (DES) pre-treatment. ACS Sust Chem Eng. 2020;8:2130–2137. doi: 10.1021/acssuschemeng.9b05106?ref=pdf. [DOI] [Google Scholar]
- Shi Y, Chai L, Tang C, Yang Z, Zhang H, Chen R, Chen Y, Zheng Y. Characterization and genomic analysis of kraft lignin biodegradation by the beta-proteobacterium Cupriavidus basilensis B-8. Biotechnol Biofuels. 2013;6(1):1–4. doi: 10.1186/1754-6834-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si M, Zhang J, He Y, Yang Z, Yan X, Liu M, Zhuo S, Wargs MX, Gao C, Chai L, Shi Y. Synchronous and rapid preparation of lignin nanoparticles and carbon quantum dots from natural lignocelluloses. Green Chem RSC. 2018 doi: 10.1039/C8GC00744F. [DOI] [Google Scholar]
- Singh N, Dhanya BS, Verma ML. Nano-immobilized biocatalysts and their potential biotechnological applications in bioenergy production. Mater Sci Energy Technol. 2020;3:808–824. doi: 10.1016/j.mset.2020.09.006. [DOI] [Google Scholar]
- Singhvi M, Kim BS. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechnology approach. Energies. 2020;13(5300):1–20. doi: 10.3390/en13205300. [DOI] [Google Scholar]
- Solala J, Ilesias MC, Yeresin MS. On the potential of lignin containing cellulose nanofibre (LCNPs) a review on properties and applications. Cellulose. 2019;27:1853–1877. doi: 10.1007/s10570-019-02899-8. [DOI] [Google Scholar]
- Song HS, Jeon JM, Kim HJ, Bhatia SK, Sathiyanarayanan G, Kim J, Hong JW, Hong YG, Choi KY, Kim YG, Kim W. Increase in furfural tolerance by combinatorial overexpression of NAD salvage pathway enzymes in engineered isobutanol-producing E. coli. Bioresour Technol. 2017;245:1430–1435. doi: 10.1016/j.biortech.2017.05.197. [DOI] [PubMed] [Google Scholar]
- Soni R, Nazir A, Chadha BS. Optimization of cellulase production by a versatile Aspergillus fumigatus fresenius strain (AMA) capable of efficient deinking and enzymatic hydrolysis of Solka floc and bagasse. Ind Crops Prod. 2010;31:277–283. doi: 10.1016/j.indcrop.2009.11.007. [DOI] [Google Scholar]
- Sotiropoulos A, Vourka I, Erotokritou A, Novakovic J, Panaretou V, Vakalis S, Thanos T, Moustakas K, Malamis D. Combination of decentralized waste drying and SSF techniques for household biowaste minimization and ethanol production. Waste Manag. 2019;52:353–359. doi: 10.1016/j.wasman.2016.03.047. [DOI] [PubMed] [Google Scholar]
- Sritrakul N, Nitisinprasert S, Keawsompong S. Evaluation of dilute acid pre-treatment for bioethanol fermentation from sugarcane bagasse pith. Agri Nat Resour. 2018;51(6):512–519. doi: 10.1016/j.anres.2017.12.006. [DOI] [Google Scholar]
- Takaya T, Koda R, Adachi D, Nakashima K, Wada J, Bogaki T, Ogino C, Kondo A. Highly efficient biodiesel production by a whole-cell biocatalyst employing a system with high lipase expression in Aspergillus oryzae. Appl Microbiol Biotechnol. 2011;90:1171–1177. doi: 10.1007/s00253-011-3186-6. [DOI] [PubMed] [Google Scholar]
- Tanjore D, Shi J, Wyman CE. Dilute acid and hydrothermal pre-treatment of cellulosic biomass. In: Simmons B, editor. Chemical and biochemical catalysis for next generation biofuels. Cambridge: Royal Society of Chemistry; 2015. pp. 64–88. [Google Scholar]
- Tian D, Hu J, Chandra RP, Saddler JN, Lu C. Valorizing recalcitrant cellulolytic enzyme lignin via lignin nanoparticles fabrication in an integrated biorefinery. ACS Sustain Chem Eng. 2017;5:2702–2710. doi: 10.1021/acssuschemeng.6b03043. [DOI] [Google Scholar]
- Touqeer T, Mumtaz MW, Mukhtar H, Irfan A, Akram S, Shabbir A, et al. Fe3O4-PDA-lipase as surface functionalized nano biocatalyst for the production of biodiesel using waste cooking oil as feedstock: characterization and process optimization. Energies. 2020;13(1):177. doi: 10.3390/en13010177. [DOI] [Google Scholar]
- Tu WC, Hallett JP. Recent advances in the pre-treatment of lignocellulosic biomass. Current opinion in green and sustainable chemistry. Elsevier. 2019;20:11–17. doi: 10.1016/j.cogsc.2019.07.004. [DOI] [Google Scholar]
- Vasco-Correa J, Ge X, Li Y. Biological pre-treatment of lignocellulosic biomass. Biomass fractionation technologies for a lignocellulosic feedstock based biorefinery. Elsevier. 2016 doi: 10.1016/B978-0-12. [DOI] [Google Scholar]
- Vazquez M, Oliva M, Tellez-Luis SJ, Ramírez JA. Hydrolysis of sorghum straw using phosphoric acid: evaluation of furfural production. Bioresour Technol. 2007;98(16):3053–3060. doi: 10.1016/j.biortech.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Verma N, Bansal NC, Kumar V. Pea peel waste: a lignocellulosic waste and its utility in cellulose production by Trichoderma reesei under solid state cultivation. BioResources. 2011;6:1505–1519. doi: 10.15376/biores.6.2.1505-1519. [DOI] [Google Scholar]
- Verma ML, Barrow CJ, Puri M. Nanobiotechnology as a novel paradigm for enzyme immobilization and stabilization with potential applications in biodiesel production. Appl Microbiol Biotechnol. 2013;97:23–39. doi: 10.1007/s00253-012-4535-9. [DOI] [PubMed] [Google Scholar]
- Verma N, Kumar V, Bansal MC. Comparative view on microbial consumption of agro-based lignocellulosic waste biomass in sustainable production of cellulases. Biomass Convers Biorefin. 2020 doi: 10.1007/s13399-020-00617-0. [DOI] [Google Scholar]
- Villas-Boas SG, Esposito E, Mitchell DA. Microbial conversion of lignocellulosic residue for production of animal feeds. Anim Feed Sci Tech. 2002;98:1–12. doi: 10.1023/A:1025105506004. [DOI] [Google Scholar]
- Wang H, Yang X, Wu Q, Zhang Q, Chen H, Jing H, Wang T, Mi SB, Rogach AL, Niu C. Encapsulating silica/antimony into porous electrospun carbon nanofibers with robust structure stability for high-efficiency lithium storage. ACS Nano. 2018;12(4):3406–3416. doi: 10.1021/acsnano.7b09092. [DOI] [PubMed] [Google Scholar]
- Xu B, Zhong R, Qiao H. The impact of biofuel consumption on CO2 emissions: a panel data analysis for seven selected G20 countries. Energy Environ. 2020;3:1498–1514. doi: 10.1177/0958305X20915426. [DOI] [Google Scholar]
- Yan JY, Li AT, Xu Y, Ngo Thao PN, Phua SC, Li Z. Efficient production of biodiesel from waste grease: one-pot esterification and transesterification with tandem lipases. Bioresour Technol. 2012;123:332–337. doi: 10.1016/j.biortech.2012.07.103. [DOI] [PubMed] [Google Scholar]
- Yan J, Zheng X, Du L, Li S. Integrated lipase production and in situ biodiesel synthesis in a recombinant Pichia pastoris yeast: an efficient dual biocatalytic system composed of cell free enzymes and whole cell catalysts. Biotechnol Biofuels. 2014;7(1):1–8. doi: 10.1186/1754-6834-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–1788. doi: 10.1016/j.fuel.2006.12.013. [DOI] [Google Scholar]
- You T, Li X, Wang R, Zhang X, Xu F. Effects of synergistic fungal pre-treatment on structure and thermal properties of lignin from corncob. Bioresour Technol. 2019;272:123–129. doi: 10.1016/j.biortech.2018.09.145. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yan S, Yuan D, Alici G, Nguyen NT, Ebrahimi Warkiani M, Li W. Fundamentals and applications of inertial microfluidics: a review. Lab Chip. 2016;16(1):10–34. doi: 10.1039/C5LC01159K. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xie Y, He X, Li X, Hu J, Ruan Z, Zhao S, Peng N, Liang Y. Comparison of high-titer lactic acid fermentation from NaOH- and NH3-H2O2-pre-treated corncob by Bacillus coagulans using simultaneous saccharification and fermentation. Sci Rep. 2016;6:37245. doi: 10.1038/srep37245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang L, Li X, Jia X, Liu L, Zeng J, et al. Functionalized graphene oxide nanoparticles for cancer cell specific delivery of antitumor drug. Bioconjugate Chem. 2015;26:128–136. doi: 10.1021/bc5005137. [DOI] [PubMed] [Google Scholar]
- Zhao X, Xiong L, Zhang M, Bai F. Towards efficient bioethanol production from agricultural and forestry residues: exploration of unique natural microorganisms in combination with advanced strain engineering. Bioresour Technol. 2016;215:84–91. doi: 10.1016/j.biortech.2016.03.158. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhao J, Xu F, Li Y. Pre-treatment of lignocellulosic biomass for enhanced biogas production. Prog Energy Combust Sci. 2014;42:35–53. doi: 10.1016/j.pecs.2014.01.001. [DOI] [Google Scholar]
- Zhong L, Feng Y, Wang G, Wang Z, Bilal M, Lv H, Jia S, Cui J. Production and use of immobilized lipases in/on nanomaterials: a review from waste to biodiesel production. Int J Biol Macromol. 2020;152:207–222. doi: 10.1016/j.ijbiomac.2020.02.258. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang Y, Gong C, Liu B, Wei G. Production structural design, functional control and broad application of carbon nanofiber based nanomaterials, a comprehensive review. Chem Eng J. 2020;402:126–189. doi: 10.1016/j.cej.2020.126189. [DOI] [Google Scholar]
- Zhu P, Weng Z, Li X, Liu X, Wu S, Young KWK, Wang X, Cui Z, Yang X, Chui PK. Biomedical applications of functionalized ZnO nanomaterials: from biosensors to bioimaging. Adv Mater Interface. 2016;3(1):1500494. doi: 10.1002/admi.201500494. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.