Abstract
Objectives
Recently studies demonstrated that adipose tissue can produce and release complement C3 and serum complement C3 levels were associated with diabetes mellitus, metabolic syndrome and non-alcoholic fatty liver disease (NAFLD). Thus, we plan to investigate the association of complement C3 levels and the presence of metabolic-associated fatty liver disease (MAFLD).
Design
Observational study with a cross-sectional sample.
Setting
This study surveyed 4729 participants in Zhejiang province, China.
Participants
55 participants were excluded for acute infection and 1001 participants were excluded for lack of ultrasonography diagnoses and complete or partial absence of laboratory tests. The final sample size was 3673 participants.
Outcome measures
Spearman correlation analysis was used to examine the correlations between complement C3 levels and variables. Binary logistic regression was carried out to evaluate the association between complement C3 levels and the presence of MAFLD after adjustment for demographic and biochemical variables. Mediation effects were used to explore whether insulin resistance (IR), hyperlipidaemia and obesity mediated the association between complement C3 and MAFLD.
Results
Participants with MAFLD had higher complement C3 levels and complement C3 levels were closely associated with body mass index, waist circumference, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase and homoeostasis model assessment (HOMA)-IR. The presence of MAFLD increased with the increase of complement C3 levels and the presence of MAFLD were highest in the HOMA-IR ≥2.5 participants. We found the OR and Cl of standardised C3 for MAFLD was 1.333 (1.185–1.500), each 1 SD increase in C3 would increase the presence of MAFLD by 33.3%, and obesity partly mediated the effect of complement C3 on the presence of MAFLD.
Conclusions
The present results suggest that complement C3 can be used as a risk factor for the presence of MAFLD after adjustment for confounding variables and obesity may partly mediate the effect of complement C3 on the presence of MAFLD.
Keywords: epidemiology, hepatobiliary disease, diabetes & endocrinology
Strengths and limitations of this study.
This study evaluated whether complement C3 can predict the presence of metabolic-associated fatty liver disease (MAFLD) in Chinese adults.
Regression analyses were applied to analyse the association between complement C3 and MAFLD.
This study based on a large population.
The diagnosis of hepatic steatosis was based on an ultrasonography examination.
This study was a retrospective cross-sectional study and cannot explain causal effects between complement C3 and MAFLD.
Introduction
The diagnosis of non-alcoholic fatty liver disease (NAFLD) excluded alcohol intake, viral hepatitis and autoimmune hepatitis, but ‘excess alcohol’ intake was difficult to determine, and some studies have shown that alcohol increased the risk of liver disease, furthermore some gut microbes produce alcohol, which can also cause liver damage.1 2 Sarin et al3 indicated alcohol-related liver disease and hepatitis B/C-related liver disease developed more rapidly in the context of metabolic dysfunction and obesity. Eslam et al4 pointed out the heterogeneity of major drivers and coexisting disease in NAFLD population is an important barrier to effective drug therapy, and response rates for the investigational drugs range from 20% to 40%, the difference compared with placebos was 10%–20%. To solve these issues, the panel of international experts recently proposed a change in nomenclature to a definition of fatty liver associated with metabolic dysfunction, and proposed new diagnostic criteria which did not exclude patients with alcohol intake or other chronic liver diseases in 2020.4 5 The criteria were based on evidence of hepatic steatosis in the presence of one or more of overweight/obesity, type 2 diabetes mellitus or evidence of metabolic dysregulation.1 6 Thus, the metabolic-associated FLD (MAFLD) links FLD more closely with obesity and metabolic abnormality, and considered other etiologies of FLD.
The complement system was a complex immune network, the progress in recent years transformed the perception of complement an antimicrobial system to a regulator of immunity and tissue homoeostasis.7 8 The complexity of complement system was readily reflected by the multifaceted involvement of complement-driven networks in inflammatory, obesity, insulin resistance (IR), diabetes, neurodegenerative disorders and cancers.8 9 Complement C3 is produced mainly by the liver and consisted of soluble and membrane-bound proteins, can modulate the immune system and promote biological activities, and produce C3a with local and systemic immunologic properties by the complement cascade.10 11 Recent studies demonstrated that complement C3 and several other components of the complement pathway were mainly produced and released from adipose tissue, and then C3a was metabolised to acylation stimulating protein (ASP) which was a potent lipogenic hormone.10 12 The close correlations between complement C3 levels and obesity/IR have been reported in some researches.10 12 13 IR is the first step in a series of events that lead to obesity from simple to complex.10 13 The large cohort studies found that complement C3 could independently predict the development of diabetes mellitus, pre-diabetes and metabolic syndrome.10 14 The cross-sectional study of Xu et al15 found NAFLD participants had higher serum complement C3 levels and complement C3 levels were positively associated with the presence and severity of NAFLD.
Lin et al16 compared the characteristics of MAFLD and NAFLD, found MAFLD patients had higher body mass index (BMI) level, homoeostasis model assessment (HOMA)-IR, lipid and liver enzymes, and indicated MAFLD definition was more practical for identifying patients with high risk of FLD progression than NAFLD definition. Thus, we performed a cross-sectional study based on a large Chinese population to investigate the association of complement C3 levels and the presence of MAFLD.
Methods
Study population
This study was performed among adults who underwent their annual health examinations at the Health Care Centre in the First Affiliated Hospital of Medical College of Zhejiang University between July 2014 and November 2017. A total of 4729 participants were enrolled in this study. Fifty-five participants were excluded for self-reported acute infection within 2 weeks and 1001 participants were excluded for lack of ultrasonography diagnoses and complete or partial absence of laboratory tests. The final sample size was 3673 participants.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Diagnostic criteria
MAFLD is diagnosed based on an ultrasonography diagnosed hepatic steatosis and in the presence of one or more of overweight/obesity (BMI ≥23 in Asians), type 2 diabetes mellitus or evidence of metabolic dysregulation.5 The hepatic steatosis was detected by ultrasonography techniques using a Toshiba Nemio 20 sonography machine with a 3.5 MHz probe (Toshiba, Tokyo, Japan). Hepatic steatosis was diagnosed according to characteristic echo patterns, such as diffuse hyperechogenicity compared with the kidneys, the gradual attenuation of far field ultrasound echo, vascular blurring and poor visualisation of intrahepatic structures.17 The metabolic dysregulation was defined as the presence of at least two of the following at-risk criteria: (A) waist circumference (WC) ≥90/80 cm in men/women; (B) Blood pressure ≥130/85 mm Hg or specific drug treatment; (C) triglycerides (TG) ≥1.70 mmol/L or specific drug treatment; (D) high-density lipoprotein-cholesterol (HDL-C) <1.0 mmol/L for men and <1.3 mmol/L for women or specific drug treatment; (E) Pre-diabetes (ie, fasting glucose (Glu) levels 5.6–6.9 mmol/L, or 2 hour post-load Glu levels 7.8 to 11.0 mmol/L or glycated haemoglobin 5.7% to 6.4%); (F) HOMA-IR score ≥2.5 and (G) high-sensitivity C reactive protein (hsCRP) level >2 mg/L5.
Assessment of demographic and biochemical variables
All participants underwent a physical examination that included demographic data, medical history, blood pressure measurement and health habit inventory. Systolic and diastolic blood pressures (SBP and DBP) were measured by an automated sphygmomanometer with the participants in the sitting position. BMI was calculated as measured weight (kg) divided by height squared (m2).
All venous blood samples were obtained in the morning after a 12-hour fast. The following biochemical parameters included alanine aminotransferase (ALT), aspartate aminotransferase (AST), TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), total bilirubin (TB), γ-glutamyl transpeptidase (GGT), Glu, creatinine (Cr), uric acid (UA) and hsCRP were measured by a Hitachi Two D modules and one P module (DDP) autoanalyzer (Hitachi, Ibaragi, Japan) using assay-specific Roche reagents (Roche Diagnostics, Indianapolis, Indiana, USA), except for Shanghai Shensuoyoufu reagent (Shanghai, Shensuoyoufu, China) for hsCRP. Serum complement C3 levels were assessed using immune-turbidimetric assay by a Hitachi 008 autoanalyzer (Hitachi, Ibaragi, Japan) using Shanghai Zhicheng reagent (Zhicheng, Shanghai, china). Glycated haemoglobin (HbA1c) was determined by Tosoh HLC 723 G8 (Tosoh Bioscience, Tokyo, Japan) using Tosoh original reagent. Insulin (INS) were determined by electrochemiluminescence immunoassay using an ARCHITECT i4000 (Abbott Diagnostics, Abbott Park, Illinois, USA). IR was assessed by the HOMA-IR. HOMA-IR was calculated according to the following equation: HOMA-IR (mIU·mmol/L)=fasting INS (mIU/L)×fasting Glu (mmol/L)/22.5.
Statistical analysis
Continuous normally distributed variables were presented as mean±SD and non-normally distributed variables were presented as median (25th–75th percentile). Categorical variables were expressed as percentages. Differences between/among groups were analysed by Student’s t-test/one-way analysis of variance or the Mann-Whitney U test/Kruskal-Wallis H test for continuous variables, and the χ2 test for categorical variables. Spearman correlation analysis was used to examine the correlations between C3 levels and demographic and laboratory variables. Binary logistic regression was carried out to evaluate the association between C3 levels and the presence of MAFLD after adjustment for demographic and biochemical variables. The mediated effect was calculated by using three regression equations. In the first step, the regression equation between complement C3 and MAFLD was calculated and the coefficient was set as c; in the second step, the regression equation between complement C3 and other variables were calculated and the coefficient was set as a; in the third step, after controlling the influence of C3, the regression equation between other variables and MAFLD were calculated and the coefficient of the variable was set as b. The ratio of mediating effect to total effect is calculated by M= (a×b/c)×100%. Mediation effects were used to explore whether IR, hyperlipidaemia and obesity mediated the association between complement C3 and MAFLD. P<0.05 (two tailed) were considered as statistically significant. All analyses were conducted by SPSS V.22 software (SPSS).
Results
Baseline characteristics of the participants
A total of 3673 participants were divided into two groups: MAFLD group (n=1262) and non-MAFLD group (n=2411). The baseline characteristics of the 3673 participants are shown in table 1. Participants with MAFLD were older and had higher BMI, WC, SBP, DBP, complement C3 levels, hepatic enzymatic variables (ALT, AST and GGT), lipid variables (TG, TC and LDL-C), kidney variables (Cr and UA), Glu, HbA1c, HOMA-IR, hsCRP, diabetes rates, smoke rates and drinking rates, along with lower HDL-C, female and physical exercise rates. The prevalence of diabetes and obesity (BMI >23 kg/m2) were 16.2% and 93.3% in MAFLD participants, compared with only 5.3% and 53.4% in non-MAFLD participants.
Table 1.
Baseline characteristics of the study population
| Characteristics | Non-MAFLD (n=2411) | MAFLD (n=1262) | P value |
| Age (year) | 48.7±9.9 | 50.5±8.9 | <0.001* |
| WC (cm) | 83.2±8.2 | 92.7±7.6 | <0.001* |
| BMI (kg/m2) | 23.6±2.7 | 26.6±2.7 | <0.001* |
| SBP (mm Hg) | 125±18 | 135±17 | <0.001* |
| DBP (mm Hg) | 76±11 | 83±10 | <0.001* |
| ALT (U/L) | 16 (12–23) | 26 (19–38) | <0.001# |
| AST (U/L) | 19 (17–23) | 22 (18–27) | <0.001# |
| GGT (U/L) | 20 (14–34) | 37 (24–58) | <0.001# |
| TB (μmol/L) | 11 (8–14) | 12 (9–15) | <0.001# |
| TG (mmol/L) | 1.17 (0.87–1.68) | 1.96 (1.43–2.87) | <0.001# |
| TC (mmol/L) | 4.67 (4.13–5.28) | 4.94 (4.38–5.54) | <0.001# |
| HDL-C (mmol/L) | 1.28 (1.07–1.53) | 1.05 (0.90–1.23) | <0.001# |
| LDL-C (mmol/L) | 2.66 (2.23–3.16) | 2.84 (2.37–3.35) | <0.001# |
| Cr (μmol/L) | 71 (59–81) | 75 (63–83) | <0.001# |
| UA (μmol/L) | 311 (257–372) | 379 (321–433) | <0.001# |
| Glu (mmol/L) | 4.89±0.90 | 5.50±1.55 | <0.001* |
| HbA1c (%) | 5.5 (5.3–5.7) | 5.7 (5.4–6.1) | <0.001# |
| hsCRP (mg/L) | 1.0 (0.47–1.97) | 1.5 (0.8–2.77) | <0.001# |
| INS (μU/mL) | 6.5 (4.7–9.1) | 10.8 (7.8–14.4) | <0.001# |
| HOMA-IR | 1.39 (0.98–1.99) | 2.52 (1.78–3.57) | <0.001# |
| C3 (mg/dL) | 103 (92–115) | 118 (107–129) | <0.001# |
| Smoke, n (%) | 552 (22.9) | 349 (27.7) | 0.001$ |
| Drinking, n (%) | 427 (17.7) | 308 (24.4) | <0.001$ |
| Female, n (%) | 1087 (45.1) | 331 (26.2) | <0.001$ |
| Physical exercise, n (%) | 832 (34.5) | 346 (27.4) | <0.001$ |
| BMI >23, n (%) | 1287 (53.4) | 1178 (93.3) | <0.001$ |
| Diabetes, n (%) | 128 (5.3) | 205 (16.2) | <0.001$ |
Continuous variables were presented as mean±SD or median (25th–75th percentile). The statistical significance of differences between the non-MAFLD group and MAFLD group were analysed by Student’s t-test (*), the Mann-Whitney U test (#) or the Χ2 test ($).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; C3, complement C3; Cr, creatinine; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; Glu, glucose; HbA1c, glycosylated haemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homoeostasis model assessment of insulin resistance; hsCRP, High sensitivity C reactive protein; INS, insulin; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic associated fatty liver disease; SBP, systolic blood pressure; TB, total bilirubin; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Correlation analyses between complement C3 and other variables
The correlation analyses revealed that complement C3 levels in MAFLD participants were correlated with age (r=−0.084, p=0.003), WC (r=0.159, p<0.001), BMI (r=0.196, p<0.001), SBP (r=0.168, p=0.001), DBP (r=0.133, p<0.001), ALT (r=0.201, p<0.001), AST (r=0.165, p<0.001), GGT (r=0.130, p<0.001), TB (r=−0.058, p=0.040), TG (r=0.140, p<0.001), TC (r=0.151, p<0.001), Cr (r=−0.114, p<0.001), LDL-C (r=0.165, p<0.001), UA (r=0.086, p=0.002), HbA1c (r=0.123, p<0.001), hsCRP (r=0.276, p<0.001), Glu (r=0.105, p<0.001), HOMA-IR (r=0.082, p=0.003). These results suggest that the above-mentioned variables may act as cofactors for the link between complement C3 and MAFLD.
Subgroup analysis of C3 levels and MAFLD
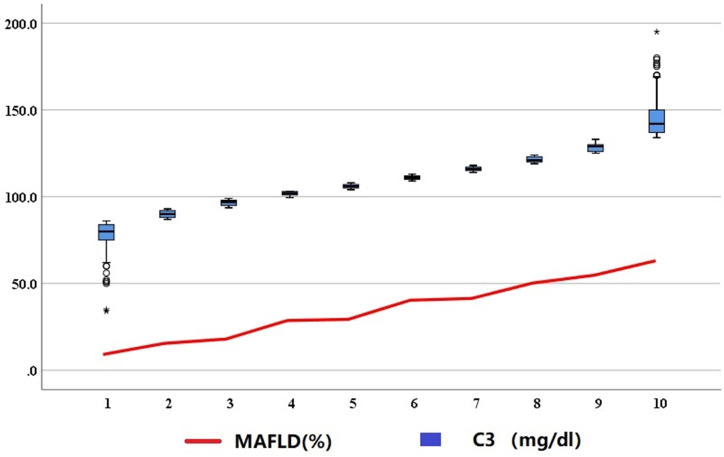
We divided 3673 participants into 10 groups based on the deciles of C3, and found that the presence of MAFLD increased with the increase of complement C3 level (figure 1). This showed complement C3 was closely related to MAFLD. Further subgroup analysis was performed to identify possible cofactors. Serum complement C3 levels and the presence of MAFLD were significantly higher in obesity participants (BMI >23 kg/m2), metabolic abnormalities participants (WC ≥90/80 cm, high blood pressure, high TG, high HOMA-IR, high hsCRP and low HDL-C), diabetes mellitus and pre-diabetes than in lean participants (BMI ≤23 kg/m2), metabolic normal participants and normal Glu metabolism participants. The complement C3 levels and the presence of MAFLD were highest (median 120 mg/dL and 65.7%) in the HOMA-IR ≥2.5 participants among all subgroups. We found that these three states (obesity, metabolic abnormalities and diabetes mellitus) were related to complement C3 and the presence of MAFLD.
Figure 1.
The presence of MAFLD in the decile subgroups of complement C3 levels. Numbers from 1 to 10 represent the decile grouping of complement C3 (from 1st to 10th). MAFLD, metabolic-associated fatty liver disease. * at 1st decile represent the lowest C3 level and * at 10th decile represent the highest C3 level.
The association between complement C3 and the presence of MAFLD
Univariate regression analyses were performed to analyse the associations between complement C3/other variables and the presence of MAFLD in the 3673 participants (table 2). Data in table 2 show that complement C3, age, gender, WC, BMI, SBP, DBP, ALT, AST, GGT, TB, TG, TC, HDL-C, LDL-C, Cr, UA, HbA1c, Glu, INS, HOMA-IR, hsCRP, drinking, physical exercise and smoking (all p<0.05) could impact the presence of MAFLD. Then these variables were included in the binary logistic regression analysis for MAFLD. Since HOMA-IR was calculated by Glu and INS, Glu and INS were not included in binary logistic regression analysis so as not to increase the weight of these two variables. The results of the adjusted binary logistic regression analysis models are shown in table 3. In the adjusted models, the OR and 95% CI for MAFLD was 1.015 (1.009 to 1.021) (table 3, the OR of the covariates was in online supplemental table 1). Thus, each 1 unit increase in complement C3 level would increase the risk of the presence of MAFLD by 1.5% in all participants. To increase the ability of the models to identify risks for the presence of MAFLD, we standardised the complement C3 data, and found the OR and Cl of the standardised complement C3 for MAFLD was 1.333 (1.185–1.500), each 1 SD increase in component level would increase the risk of the presence of MAFLD by 33.3% in all participants. This showed that complement C3 could be used as a risk factor for the presence of MAFLD.
Table 2.
Associations of C3 with the presence of MAFLD by univariate regression analyses
| Characteristics | OR (95% CI) | P value |
| Age | 1.019 (1.012 to 1.026) | <0.001 |
| WC | 1.167 (1.154 to 1.181) | <0.001 |
| BMI | 1.570 (1.518 to 1.624) | <0.001 |
| SBP | 1.033 (1.028 to 1.037) | <0.001 |
| DBP | 1.054 (1.047 to 1.061) | <0.001 |
| ALT | 1.048 (1.042 to 1.054) | <0.001 |
| AST | 1.046 (1.037 to 1.056) | <0.001 |
| GGT | 1.012 (1.010 to 1.014) | <0.001 |
| TB | 1.024 (1.012 to 1.037) | <0.001 |
| TG | 1.971 (1.827 to 2.127) | <0.001 |
| TC | 1.352 (1.253 to 1.459) | <0.001 |
| HDL-C | 0.074 (0.057 to 0.097) | <0.001 |
| LDL-C | 1.310 (1.196 to 1.434) | <0.001 |
| Cr | 1.015 (1.011 to 1.020) | <0.001 |
| UA | 1.008 (1.008 to 1.009) | <0.001 |
| Glu | 1.678 (1.552 to 1.814) | <0.001 |
| HbA1c | 1.977 (1.772 to 2.206) | <0.001 |
| hsCRP | 1.015 (1.002 to 1.027) | 0.019 |
| INS | 1.223 (1.201 to 1.246) | <0.001 |
| HOMA-IR | 2.267 (2.107 to 2.440) | <0.001 |
| C3 | 1.045 (1.040 to 1.049) | <0.001 |
| Smoke | 1.287 (1.102 to 1.504) | 0.001 |
| Drinking | 1.499 (1.270 to 1.769) | <0.001 |
| Female | 0.433 (0.373 to 0.503) | <0.001 |
| Physical exercise | 0.717 (0.617 to 0.832) | <0.001 |
| BMI >23 | 12.248 (9.679 to 15.498) | <0.001 |
| Diabetes | 3.459 (2.742 to 4.365) | <0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; C3, complement C3; Cr, creatinine; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; Glu, glucose; HbA1c, glycosylated haemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homoeostasis model assessment of insulin resistance; hsCRP, High sensitivity C reactive protein; INS, insulin; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic-associated fatty liver disease; SBP, systolic blood pressure; TB, total bilirubin; TC, total cholesterol; TG, triglyceride; UA, uric acid; WC, waist circumference.
Table 3.
Associations of C3 with the presence of MAFLD by multivariate regression analyses
| Characteristics | OR (95% CI) | P value |
| Model 1 | 1.027 (1.022 to 1.032) | <0.001 |
| Model 2 | 1.023 (1.018 to 1.028) | <0.001 |
| Model 3 | 1.021 (1.016 to 1.026) | <0.001 |
| Model 4 | 1.017 (1.011 to 1.022) | <0.001 |
| Model 5 | 1.019 (1.013 to 1.025) | <0.001 |
| Model 6 | 1.015 (1.009 to 1.021) | <0.001 |
Adjusted model 1: Age, WC, BMI, SBP, DBP, sex, drinking, physical exercise and smoke (baseline indexes); model 2: model 1+AST, ALT, GGT and TB liver markers); model 3: model 2+Cr and UA (kidney markers); model 4: model 3+TC, TG, HDL-C, and LDL-C (lipid markers); model 5: model 4+hsCRP; model 6: model 5+HOMA IR and HbA1c.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; Cr, creatinine; DBP, diastolic blood pressure; GGT, γ-glutamyl transpeptidase; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein-cholesterol; HOMA-IR, homoeostasis model assessment-insulin resistance; hsCRP, high-sensitivity C reactive protein; LDL-C, low-density lipoprotein cholesterol; MAFLD, metabolic-associated fatty liver disease; SBP, systolic blood pressure; TB, total bilirubin; TC, total cholesterol; TG, triglycerides; UA, uric acid; WC, waist circumference.
bmjopen-2021-051218supp001.pdf (71.3KB, pdf)
Mediated effects of HOMA-IR/TG/BMI on the association between C3 and NAFLD
IR, hyperlipidaemia and obesity are variables of metabolic abnormalities in MAFLD. IR(HOMA-IR), hyperlipidaemia (TG) and obesity (BMI) and complement C3 were positively associated with the presence of MAFLD (table 2), while complement C3 was positively correlated with HOMA-IR, TG, and BMI, suggesting a mechanistic link between complement C3 and MAFLD, possibly explained by HOMA-IR, TG, or BMI. To explore the internal relationships among HOMA-IR/TG/BMI, C3 and MAFLD, we conducted the mediated effects analysis to explore whether HOMA-IR/TG/BMI mediated the association between complement C3 and the presence of MAFLD. The contribution rate of mediated effects of BMI, TG, and HOMA-IR to the total effect (complement C3 on the presence of MAFLD) were 55.7%, 14.0% and 26.3% (all p<0.001), respectively, this showed obesity significantly partly mediated the effect of complement C3 on the presence of MAFLD compared with TG and HOMA-IR.
Discussion
Participants with MAFLD had higher complement C3 levels, BMI, WC, SBP, DBP, ALT, AST, GGT, TB, TG, TC, LDL-C, Cr, UA, Glu, INS, HbA1c, HOMA-IR, hsCRP, diabetes rates, smoke rates and drinking rates, but lower HDL-C and physical exercise rates, compared with that of participants without MAFLD. Serum complement C3 levels were closely associated with abdominal obesity (BMI and WC), liver enzymology markers (ALT, AST and GGT), and HOMA-IR by spearman correlation analyses. The presence of MAFLD increased with the increase of complement C3 level, and the presence of MAFLD were highest (median 120 mg/dL and 65.7%) in the HOMA-IR ≥2.5 participants. We found the OR and Cl of standardised complement C3 for MAFLD was 1.333 (1.185–1.500), each 1 SD increase in complement C3 levels would increase the risk of the presence of MAFLD by 33.3% by the adjusted binary regression models. Complement C3 can not only directly affect the presence of MAFLD, but also indirectly affect the presence of MAFLD through the mediated effects of obesity (accounted for 55.7%).
The complement system is an innate immune system, playing a key role in host homoeostasis, the regulation of inflammation, and in the defence against pathogens.14 The excessive activation of complement is associated with many autoimmune diseases.18 Complement C3 is mainly produced by the liver and adipocytes/macrophages in adipose tissue and is a key element in three complement activation pathways (classical, alternative and lectin pathways), inflammation and metabolic effects.10 19 20 Complement C3 is involved in metabolic disorders mainly by C3a and ASP.20 C3a has INS-like effects and facilitates TG metabolism, and can trigger a cytokine/chemokine response and mediate IR.10 12 ASP can stimulate synthesis and release of TG, this leads to excessive accumulation of fat in liver cells and disorder of lipid metabolism.21 Adipocytes in addition to complement C3 can also produce complement C3 receptors, mild inflammation of adipose tissue leads to complement C3 activation, which may lead to further deterioration of metabolic complications in obese patients.19 22 Some reports have shown that there is a strong relationship between C3/C3a/ASP and adipose tissue, cardiovascular disease, metabolic syndrome, diabetes, NAFLD and AFLD, implying that complement C3 may play a central role and maybe a powerful predictor or therapeutic target for FLD.10 15 19 23
Our study showed that the serum complement C3 levels in MAFLD patients were significantly higher than that in non-MAFLD patients, and closely associated with obesity, higher TG and HOMA-IR, indicating that the synthesis and release of complement C3 in MAFLD patients were increased under the interaction of various factors. There are some relevant studies on NAFLD and AFLD.3 15 The study of Rensen et al24 indicated 74% of the NAFLD patients showed activated complement C3 and C4d were deposited predominantly around hepatocytes with macrovesicular steatosis, and were accompanied by hepatic neutrophil infiltration, increased interleukin (IL)-8/IL-6 expression, and numbers of apoptotic cells. Patients with Nonalcoholic steatohepatitis (NASH) had a higher presence of activated complement C3 deposition.7 24 Zhong et al23 found complement C3 promoted the accumulation of TG and contributed to liver injury and steatosis by regulating the production of tRNA-derived fragments glycine tRNA-derived fragments (Gly-tRF) in AFLD live. Wlazlo et al7 found C3a were associated with liver fat content and hepatocellular injury in heavy alcohol consumers daily. Complement C3 deficiency has been shown to impart protective effects against ethanol-induced hepatic steatosis and inflammation.23 Animal experiments also found important cross-talk between complement C3 and lipid regulators in alcohol-induced steatosis, and in C3-/- mice liver steatosis was strongly reduced after alcohol exposure.25 26 This suggests that the complement C3 contributes to the development of alcohol-induced fatty liver. The inhibitors of C3 activation or Gly-tRF may provide a precise therapeutic approach for AFLD.
Our study also found that complement C3 levels were positively correlated with common indicators ALT and AST of liver injury, the presence of MAFLD increased with the increase of complement C3 level, and complement C3 was a risk factor for the presence of MAFLD. There are some studies that are close to our results. The study of Jia et al14 first demonstrate that serum complement C3 levels were independently associated with a higher presence of NAFLD and AFLD in adult male population, the presence of NAFLD and AFLD in the highest quartile of complement C3 compared with the lowest quartile was 4.13 and 2.09 times, respectively. The Chinese cross-sectional study of Xu et al15 found that serum complement C3 was independently associated with risk for NAFLD and complement C3 level is positively associated with prevalence and severity of NAFLD. Pan et al27 revealed that complement C3 can be used as a surrogate biomarker of NAFLD in patients with chronic kidney disease with sensitivity 63.9% and specificity 70.1%. Ursini et al18 revealed complement C3 can be used as a surrogate biomarker of NAFLD in rheumatoid arthritis patients with sensitivity 76% and specificity 64%. Therefore, the activation of the component in NAFLD patients was associated with disease occurrence and disease severity, and C3 can be a risk factor for the prevalence of MAFLD.
In addition, we found the presence of MAFLD was highest in the IR participants (HOMA-IR ≥2.5), and obesity, HOMA-IR and TG may partly mediate the effect of complement C3 on the presence of MAFLD by mediate effect. Then, we suggested a mechanistic link between complement C3 and MAFLD which might be explained by metabolic disorder. There are some similarities between these following studies and our study. Castellano-Castillo D et al12 found complement C3 mRNA levels were associated with Glu metabolism and IR, while complement C3 methylation levels were associated with adiposity variables. Al-Domi et al9 found C3 and IR were significantly higher in the obese group than that in the normal body weight, suggested complement C3 may be an independent marker of the chronic inflammatory process and may have a role in the progression of IR during obesity. Himoto et al28 found complement C3 levels were significantly correlated with BMI, HOMA-IR and TG, and increased with the aggravation of liver steatosis and liver fibrosis in chronic hepatitis C patients, they suggested complement C3 levels may reflect obesity, IR and hepatic steatosis. Therefore, we speculated that C3 might be of great significance for the incidence and progression of MAFLD, and the regulation of complement C3, C3a and ASP levels might provide therapeutic targets for the control of hepatic steatosis in MAFLD patients.
Our study has several potential limitations. First, the diagnosis of hepatic steatosis was based on an ultrasonography examination, which may miss mild steatosis. Second, this study was a retrospective cross-sectional study not a prospective study. Third, our study was limited to Chinese Han adults, and our study may not be applicable to other ethnic groups and children. Therefore, prospective, multicentre and multiracial clinical research need to be further carried out.
In conclusion, our results first demonstrated a significant correlation between complement C3 levels and MAFLD. Thus, complement C3 was a risk factor of the presence of MAFLD after adjustment for confounding variables and obesity may partly mediate the effect of complement C3 on the presence of MAFLD.
Supplementary Material
Footnotes
LF and YZ contributed equally.
Contributors: LF and W-LW conceived and designed the research, LF and YZ collected the data. LF summarised all data and did statistical analysis. LF and YZ drafted the manuscript. LF responsibled for the overall content as the guarantor. All authors contributed to the article and approved the submitted version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All free text entered below will be published.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This work was approved by the Ethics Committee of the First Affiliated Hospital of Medical College at Zhejiang University (Ethics Approval Ref: 2019-1486) and informed consent was obtained from participants.
References
- 1.Zheng KI, Fan J-G, Shi J-P, et al. From NAFLD to MAFLD: a "redefining" moment for fatty liver disease. Chin Med J 2020;133:2271–3. 10.1097/CM9.0000000000000981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fouad Y, Waked I, Bollipo S, et al. What’s in a name? Renaming 'NAFLD' to 'MAFLD'. Liver Int 2020;40:1254–61. 10.1111/liv.14478 [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Eslam M, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2020;5:167–228. 10.1016/S2468-1253(19)30342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslam M, Sarin SK, Wong VW-S, et al. The Asian Pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 2020;14:889–919. 10.1007/s12072-020-10094-2 [DOI] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 2020;73:202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 6.Eslam M, Sanyal AJ, George J, et al. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020;158:1999–2014. 10.1053/j.gastro.2019.11.312 [DOI] [PubMed] [Google Scholar]
- 7.Wlazlo N, van Greevenbroek MMJ, Ferreira I, et al. Activated complement factor 3 is associated with liver fat and liver enzymes: the CODAM study. Eur J Clin Invest 2013;43:679–88. 10.1111/eci.12093 [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis G, Reis ES, Mastellos DC, et al. Novel mechanisms and functions of complement. Nat Immunol 2017;18:1288–98. 10.1038/ni.3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Domi HA, Al Haj Ahmad RM. Association between complement component C3 and body composition: a possible obesity inflammatory biomarker for insulin resistance. Asia Pac J Clin Nutr 2017;26:1082–7. 10.6133/apjcn.012017.02 [DOI] [PubMed] [Google Scholar]
- 10.Ursini F, Abenavoli L. The emerging role of complement C3 as a biomarker of insulin resistance and cardiometabolic diseases: preclinical and clinical evidence. Rev Recent Clin Trials 2018;13:61–8. 10.2174/1574887112666171128134552 [DOI] [PubMed] [Google Scholar]
- 11.Strey CW, Markiewski M, Mastellos D, et al. The proinflammatory mediators C3a and C5a are essential for liver regeneration. J Exp Med 2003;198:913–23. 10.1084/jem.20030374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellano-Castillo D, Moreno-Indias I, Fernandez-Garcia JC, et al. Complement factor C3 methylation and mRNA expression is associated to BMI and insulin resistance in obesity. Genes 2018;9 10.3390/genes9080410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ursini F, Grembiale A, Naty S, et al. Serum complement C3 correlates with insulin resistance in never treated psoriatic arthritis patients. Clin Rheumatol 2014;33:1759–64. 10.1007/s10067-013-2366-4 [DOI] [PubMed] [Google Scholar]
- 14.Jia Q, Li C, Xia Y, et al. Association between complement C3 and prevalence of fatty liver disease in an adult population: a cross-sectional study from the Tianjin chronic low-grade systemic inflammation and health (TCLSIHealth) cohort study. PLoS One 2015;10:e0122026. 10.1371/journal.pone.0122026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu C, Chen Y, Xu L, et al. Serum complement C3 levels are associated with nonalcoholic fatty liver disease independently of metabolic features in Chinese population. Sci Rep 2016;6:23279. 10.1038/srep23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Huang J, Wang M, et al. Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver Int 2020;40:2082–9. 10.1111/liv.14548 [DOI] [PubMed] [Google Scholar]
- 17.Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of hepatology 2010; 18:163-166). J Dig Dis 2011;12:38–44. 10.1111/j.1751-2980.2010.00476.x [DOI] [PubMed] [Google Scholar]
- 18.Ursini F, Russo E, Mauro D, et al. Complement C3 and fatty liver disease in rheumatoid arthritis patients: a cross-sectional study. Eur J Clin Invest 2017;47:728–35. 10.1111/eci.12798 [DOI] [PubMed] [Google Scholar]
- 19.Copenhaver M, CY Y, Hoffman RP. C3 and C4, and the metabolic syndrome. Curr Diabetes Rev 2019;15:44–8. [DOI] [PubMed] [Google Scholar]
- 20.Paglialunga S, Fisette A, Yan Y, et al. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab 2008;294:E521–9. 10.1152/ajpendo.00590.2007 [DOI] [PubMed] [Google Scholar]
- 21.Barbu A, Hamad OA, Lind L, et al. The role of complement factor C3 in lipid metabolism. Mol Immunol 2015;67:101–7. 10.1016/j.molimm.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 22.Ricklin D, Reis ES, Mastellos DC, et al. Complement component C3 - The "Swiss Army Knife" of innate immunity and host defense. Immunol Rev 2016;274:33–58. 10.1111/imr.12500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong F, Hu Z, Jiang K, et al. Complement C3 activation regulates the production of tRNA-derived fragments Gly-tRFs and promotes alcohol-induced liver injury and steatosis. Cell Res 2019;29:548–61. 10.1038/s41422-019-0175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rensen SS, Slaats Y, Driessen A, et al. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology 2009;50:1809–17. 10.1002/hep.23228 [DOI] [PubMed] [Google Scholar]
- 25.Bykov I, Junnikkala S, Pekna M, et al. Complement C3 contributes to ethanol-induced liver steatosis in mice. Ann Med 2006;38:280–6. 10.1080/07853890600664608 [DOI] [PubMed] [Google Scholar]
- 26.Pritchard MT, McMullen MR, Stavitsky AB, et al. Differential contributions of C3, C5, and decay-accelerating factor to ethanol-induced fatty liver in mice. Gastroenterology 2007;132:1117–26. 10.1053/j.gastro.2007.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan B, Wan X, Ma M, et al. Complement C3 and nonalcoholic fatty liver disease in chronic kidney disease patients: a pilot study. Kidney Blood Press Res 2020;45:61–9. 10.1159/000504172 [DOI] [PubMed] [Google Scholar]
- 28.Himoto T, Hirakawa E, Fujita K, et al. Complement component 3 as a surrogate hallmark for metabolic abnormalities in patients with chronic hepatitis C. Ann Clin Lab Sci 2019;49:79–88. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-051218supp001.pdf (71.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. All free text entered below will be published.