Abstract
Background
Substantial variations in the prevalence of mild cognitive impairment (MCI) and its subtypes have been reported, although mostly in geographically defined developed countries and regions. Less is known about MCI and its subtypes in rural areas of less developed central China.
Aims
The study aimed to compare the prevalence of MCI and its subtypes in residents aged 65 years or older in urban and rural areas of Hubei Province, China.
Methods
Participants aged 65 years or older were recruited between 2018 and 2019. Inperson structured interviews and clinical and neuropsychological assessments were performed at city health community centres and township hospitals.
Results
Among 2644 participants without dementia, 735 had MCI, resulting in a prevalence of 27.8% for total MCI, 20.9% for amnestic MCI (aMCI) and 6.9% for non-amnestic MCI (naMCI). The prevalence of MCI in urban and rural areas was 20.2% and 44.1%, respectively. After adjusting for demographic factors, the prevalence of total MCI, aMCI and naMCI differed significantly between rural and urban areas (adjusted odds ratio (OR) 2.10, 1.44 and 3.76, respectively). Subgroup analysis revealed an association between rural socioeconomic and lifestyle disadvantage and MCI and its subtypes.
Conclusions
Our findings suggest that the prevalence of MCI among urban residents in central China is consistent with that in other metropolis areas, such as Shanghai, but the prevalence in rural areas is twice that in urban areas. Prospective studies and dementia prevention in China should focus on rural areas.
Keywords: brief psychiatric rating scale, risk factors, neurocognitive disorders, cohort studies
Introduction
Mild cognitive impairment (MCI) is considered an intermediate condition between healthy ageing and early dementia.1 2 Its prevalence differs widely across countries and regions.3 4 It ranges from 4% to 19% among people aged ≥65 years, depending on the definition used.2 5 Given that more people with MCI progress to dementia than cognitively normal (CN) people do,6 prevention appears to be crucial, and MCI may be the best period for early intervention.1 7 Risk factors, especially the modifiable ones, would be the right targets for effective prevention intervention, but they may differ among countries and regions, depending on genetics, demographics, lifestyle and culture.8 Furthermore, modified interventions may need to accommodate different cultures and environments.9 Thus, a complete understanding of MCI prevalence, risk factors and complicated progression in subnations will guide precise prevention interventions.2 9
Moreover, it is increasingly recognised that MCI is heterogeneous but can be classified into two main clinical functional subtypes, amnestic MCI (aMCI) and non-amnestic MCI (naMCI), depending on memory impairment.1 Previous studies have demonstrated that different MCI subtypes are attributed to different dementia types.10 Individuals with aMCI are at higher risk of Alzheimer’s disease (AD) dementia,2 with 10% to 20% of them developing AD annually,11 while naMCI subtypes tend to progress to non-AD dementia, such as vascular dementia.10 Therefore, recognising the heterogeneity and directing specific interventions to homogeneous subgroups are important, as these could both improve diagnostic specificity and avoid indiscriminate intervention.
China has the largest old population worldwide.12 Although a few population-based studies exist on MCI, aMCI and naMCI, these were mostly performed in metropolises. When studies were performed in both rural and urban areas, MCI prevalence was higher in rural than in urban areas.13 However, there are no reports on MCI functional subtypes in older individuals from rural areas in central China. Given the marked differences in demographic and socioeconomic characteristics, lifestyle and medical resources across regions in China, it may be valuable to explore the differences in MCI and its functional subtypes and reveal the related factors in less developed central China.
This study aimed to estimate the prevalence of MCI, including its functional subtypes (aMCI and naMCI), among community-dwelling individuals aged ≥65 years in central China with rural and urban samples and to analyse the associated factors. We hypothesised that there would be (1) urban–rural disparities in MCI and its functional subtypes among community-dwelling older adults and (2) differences between individuals with and without MCI/MCI subtypes in terms of social relationship and lifestyle behaviours.
Methods
Study design and participants
A cross-sectional survey was conducted in Hubei Province between 2018 and 2020, a less developed region in central China based on annual per capita income in 2017. Sampling followed a tiered process. First, two urban districts in Wuhan, a metropolis, and two rural townships in the Dabie Mountains area were selected at random. Second, 6 neighbourhoods within 2 urban districts and 14 villages within 2 rural townships were sampled at random. Third, all residents aged ≥65 years within the selected neighbourhoods and villages were identified according to the health records archived at the health centres and were invited to participate in the study. China has been providing free annual health screening for residents aged ≥65 years since 2015. Potential participants were excluded if they (1) were not traceable; (2) showed mental retardation or severe schizophrenia on their medical record; (3) had life-threatening illness; or (4) had severe problems of hearing, vision or communication and were not able to participate actively in the neuropsychological assessments. Written informed consent was obtained from each participant.
Sample size
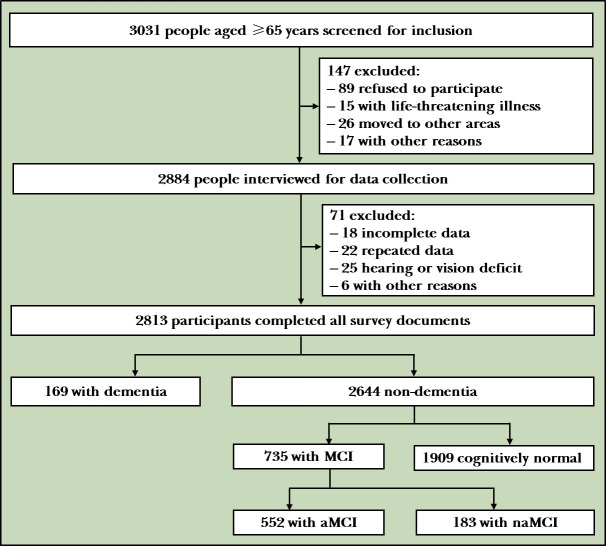
We calculated the sample size according to the following formula: , where n is the sample size; p is the expected prevalence rate; q=1−p; d is the allowable error and a fraction of p, which is generally taken as 0.1p, 0.15p or 0.2p; and Z1−α/2 is the statistics of significance test, when α=0.05, Z1−α/2=1.96 and when α=0.01, Z1−α/2=2.58. Based on published MCI prevalence rates ranging from 15% to 30% and the prevalence rate of MCI among old adults in the Wuhan community, we determined p=27.91%. If d=0.1p and the test level α=0.05, n is about 1034 people. Owing to the large sampling error of cluster sampling, it was increased by 50% of the original basis; therefore, n amounts to around 1551 people. Figure 1 shows the flowchart of subject recruitment. Among 3031 old residents, 147 were excluded owing to severe life-threatening illness or other reasons, 71 were unable to complete the survey owing to severe hearing, vision deficit or other reasons, and 2813 eligible participants completed the full-length survey.
Figure 1.
Flowchart of the study. aMCI, amnestic mild cognitive impairment; MCI, mild cognitive impairment; naMCI, non-amnestic mild cognitive impairment.
Inperson interviews
To collect comprehensive health information, participants were interviewed face to face by trained research assistants at city community health centres and township hospitals. Demographics (age, sex, educational level and residence area), social relationships (marital, living, sibling, friend and neighbour status), lifestyle behaviours (smoking, drinking, eating a healthy diet, physical and cognitive activities), health conditions (insomnia and constipation) and medical history (clinically diagnosed hypertension and diabetes) were collected using a dedicated form. Physical measures (height, weight and blood pressure) were performed by nurses.
Definition of risk factors
Current smoking referred to smoking ≥1 cigarette/day within the last 6 months, consistent with the definition of the 2010 National Smoking Survey of Chinese adults.14 Drinking was defined as consuming >14 drinks/week for men (7 drinks/week for women) for ≥1 year and one drink was defined as 14 g of pure alcohol, which equates to a 150 mL glass of wine, a 350 mL can of beer or two shots of spirits.15 Having a healthy diet was defined by eating fresh fish once a week.16 Physical activities were estimated based on the recall of the frequency and time spent on nine activities, such as walking, dancing, jogging or playing sports in a typical week, according to the guidelines for Chinese adults.17 Cognitive activities were defined as activities like reading, writing, playing chess/poker/mahjong or using an app for playing games ≥3 times/week for ≥30 min/activity.15 18 For self-reported insomnia, participants were asked about ‘trouble falling asleep’, ‘dreaming’, ‘sleep time <6 h/day’, ‘early morning awakening’, ‘daytime sleepiness’ and ‘dissatisfaction with sleep’. For each positive response, if the characteristic has been present for ≥4 weeks, it was defined as self-reported insomnia.19 Body mass index (BMI) was calculated as weight divided by the square of height (kg/m2) and BMI ≥24.0 kg/m2 was classified as overweight.20 Hypertension was self-reported known diagnosis and/or use of antihypertensive medication within 2 weeks prior to the study.21 Diabetes was self-reported diagnosis previously determined by a healthcare professional or current use of antidiabetic medications.22
Neuropsychological tests
Neuropsychological assessments were performed by junior neuropsychologists and trained medical graduate students. A standardised neuropsychological battery containing five domains was used: memory—Auditory Verbal Learning Test23; language—Boston Naming Test24 and Category Fluency (Animals) Test25; attention—Trail Making Test A26 and Digit Span Forward27; executive function—Trail Making Test B26 and Digit Span Backwards27; and visuospatial skills—Clock-Drawing Test.28 Global cognition was evaluated using the Mini-Mental State Examination18 and Montreal Cognitive Assessment-Basic Scale.29 Daily activities were assessed using the Lawton and Brody Activities of Daily Living (ADL) Questionnaire.30 The Clinical Dementia Rating (CDR) Scale31 was used to assess cognitive level. Mood was assessed with the Chinese Geriatric Depression Scale.32
Diagnoses of MCI and MCI subtypes
The expert panel consisting of a board-certified neurologist (RW) and a neuropsychologist (YJG) from two Third-class hospitals and two experts with expertise in dementia (DL and GC) from the Brain Science and Advanced Technology Institute conducted the consensus diagnosis regarding the presence or absence of dementia after reviewing the medical, neurological and neuropsychological assessment data, as well as other pertinent data, using the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.33 Only those who were not diagnosed with dementia were considered for a diagnosis of MCI. MCI was diagnosed based on the Petersen’s criteria33: (1) concern of a cognitive change by the participant, informant or clinician based on the information obtained during the clinical interview, with CDR=0.5; (2) objective impairment for any neuropsychological test within a cognitive domain (ie, performance falling greater than 1.5 standard deviation (SD) outside the age-adjusted normative mean; and (3) essentially normal functional activities (determined from ADL evaluation). Participants with MCI were then divided into two subtypes (aMCI or naMCI) according to the cognitive impairment profile. Participants with MCI who performed poorly in the memory domain were considered as having aMCI, whereas those who performed poorly in other cognitive domains other than memory were considered as having naMCI.34
Statistical methods
All data were double-checked by two researchers and screened for anomalies (eg, outliers, missing data and violations of statistical assumption) using descriptive and exploratory data analysis methods and then analysed using IBM SPSS Statistics for Windows V.26. Continuous variables were denoted as mean (SD) or median (interquartile range, IQR), while categorical variables were expressed as absolute (n) and relative frequency (%). Rural/urban subgroups were compared in terms of basic demographic and other characteristics using independent sample t test, Mann-Whitney U test and χ2 test, as appropriate. The prevalences of MCI and MCI subtypes were calculated and described with 95% CI and analysed using χ2 test for total, rural and urban participants. Group comparisons of the prevalences of MCI and MCI subtypes were analysed using multiple logistic regression analysis. Demographic variables (eg, sex, educational level and age) were also examined for possible associations with cognitive outcome variables and, when significant, were adjusted for in the multiple logistic regression model. Finally, using demographic variables, lifestyle behaviours, social relationships and health status as independent variables, and cognitive outcome as the dependent variable, multivariate logistic regression analysis with the forward unconditional selection method was performed to examine the potential risk factors for each subtype. The CN population was used as the reference group for all regression models. Odds ratios (ORs) were calculated to identify significant independent variables associated with the dependent variable (eg, MCI, aMCI and naMCI). All p values were two-tailed, and the statistical significance level was set at 0.05.
Results
Characteristics of study participants
Table 1 details the selected characteristics of the study participants. About half of the participants (50.1%) were women, 839 (31.8%) were from remote rural areas, and the participants had an average of 9 (5–12) years of education. The average age was 70 (67–75) years, and 1230 (46.5%) were aged 60–69 years and 1128 (42.7%) were aged 70–79 years. Rural and urban participants differed in most surveyed categories, including sex, age, educational level, diet pattern, fish consumption, cognitive activity, physical activity, social network, marital status, smoking, drinking, living arrangements, obesity, diabetes and insomnia. However, there was no significant difference in the prevalence of hypertension between urban and rural samples.
Table 1.
Participant characteristics by residential areas
| Characteristics | Total, n (%) | Urban, n (%) | Rural, n (%) | χ2/Z | P value |
| Number of participants | 2644 | 1805 (68.2) | 839 (31.8) | ||
| Demographics | |||||
| Female | 1325 (50.1) | 965 (53.5) | 360 (42.9) | 25.52 | <0.001 |
| Age (years)*, median (IQR) | 70 (67–75) | 70 (67–75) | 70 (67–74) | 717 546.53 | 0.030 |
| Age groups | |||||
| 60–69 | 1230 (46.5) | 837 (46.4) | 393 (46.8) | 16.15 | <0.001 |
| 70–79 | 1128 (42.7) | 744 (41.2) | 384 (45.8) | ||
| ≥80 | 286 (10.8) | 224 (12.4) | 62 (7.4) | ||
| Education (years)*, median (IQR) | 9 (5–12) | 11 (9–15) | 2 (0–6) | 143 464.51 | <0.001 |
| Education (years) groups | |||||
| <6 | 940 (35.6) | 243 (13.5) | 697 (83.1) | 1229.32 | <0.001 |
| 6–12 | 1166 (44.1) | 1031 (57.1) | 135 (16.1) | ||
| >12 | 538 (20.3) | 531 (29.4) | 7 (0.8) | ||
| BMI level (n=2458) | |||||
| Underweight (<18.5) | 136 (5.5) | 71 (4.1) | 65 (9.2) | 32.14 | <0.001 |
| Normal (18.5–23.9) | 1234 (50.2) | 868 (49.6) | 366 (51.7) | ||
| Overweight (24.0–27.9) | 855 (34.8) | 629 (35.9) | 226 (31.9) | ||
| Obesity (≥28.0) | 233 (9.5) | 182 (10.4) | 51 (7.2) | ||
| Marital status | |||||
| Unmarried | 581 (22.0) | 276 (15.3) | 305 (36.4) | 148.19 | <0.001 |
| Social connections | |||||
| Living alone | 383 (14.5) | 147 (8.1) | 236 (28.1) | 184.67 | <0.001 |
| Having at least one sibling alive | 1818 (68.8) | 1371 (76.0) | 447 (53.3) | 137.13 | <0.001 |
| Having close friends | 1703 (64.4) | 1370 (75.9) | 333 (39.7) | 327.61 | <0.001 |
| Lifestyle behaviours | |||||
| Smoking (yes) | 793 (30.0) | 467 (25.9) | 326 (38.9) | 45.98 | <0.001 |
| Drinking (yes) | 655 (24.8) | 362 (20.1) | 293 (34.9) | 67.93 | <0.001 |
| Fresh fish consumption (yes) | 2190 (82.8) | 1644 (91.1) | 546 (65.1) | 272.30 | <0.001 |
| Physical activities (yes) | 2129 (80.5) | 1587 (87.9) | 542 (64.6) | 198.63 | <0.001 |
| Cognitive activities (yes) | 1777 (67.2) | 1471 (81.5) | 306 (36.5) | 526.84 | <0.001 |
| Comorbidities | |||||
| Insomnia (yes) | 1372 (51.9) | 868 (48.1) | 504 (60.1) | 32.94 | <0.001 |
| Overweight and obesity (yes)† | 1088 (44.3) | 811 (46.3) | 277 (39.1) | 10.65 | 0.001 |
| Hypertension (yes) | 1618 (61.2) | 1096 (60.7) | 522 (62.2) | 0.54 | 0.462 |
| Diabetes (yes) | 449 (17.0) | 354 (19.6) | 95 (11.3) | 27.92 | <0.001 |
*Continuous variables (eg, age and education).
†Percentage of overweight and obesity (yes) on the total of BMI level (n=2458).
Prevalence of MCI and MCI subtypes by residential areas
Table 2 shows that the prevalences of MCI, aMCI and naMCI were 27.8%, 20.9% and 6.9%, respectively. The prevalences of MCI (44.1% vs 20.2%, χ2=162.73, p<0.001), aMCI (32.9% vs 15.3%, χ2=107.47, p<0.001) and naMCI (11.2% vs 4.9%, χ2=34.99, p<0.001) in rural areas were more than twice that in urban areas. After controlling for demographic confounders, the difference in the prevalence of MCI between rural and urban areas remained, with an adjusted OR of 2.10 (95% CI: 1.62 to 2.72, χ2=31.76, p<0.001). Similarly, differences in the prevalence of aMCI (OR=1.44, 95% CI: 1.09 to 1.91, χ2=6.60, p=0.01) and naMCI (OR=3.76, 95% CI: 2.33 to 5.79, χ2=31.30, p<0.001) remained significant.
Table 2.
Prevalences (95% CI) of MCI and MCI subtypes in total, urban and rural participants
| MCI | aMCI | naMCI | ||||
| n | Prevalence (95% CI)% | n | Prevalence (95% CI)% | n | Prevalence (95% CI)% | |
| Total | 735 | 27.8 (26.1 to 29.5) | 552 | 20.9 (19.3 to 22.4) | 183 | 6.9 (6.0 to 7.9) |
| Urban | 365 | 20.2 (18.4 to 22.1) | 276 | 15.3 (13.6 to 17.0) | 89 | 4.9 (3.9 to 5.9) |
| Rural | 370 | 44.1 (40.7 to 47.5) | 276 | 32.9 (29.7 to 36.1) | 94 | 11.2 (9.1 to 13.3) |
| χ2 | 162.73 | 107.47 | 34.99 | |||
| P value | <0.001 | <0.001 | <0.001 | |||
aMCI, amnestic mild cognitive impairment; MCI, mild cognitive impairment; naMCI, non-amnestic mild cognitive impairment.
Factors associated with MCI, aMCI and naMCI by residential area
Multivariable logistic regression analysis revealed different factors independently associated with MCI or MCI subtypes (table 3). For all participants, older age was significantly associated with greater odds of MCI and its functional subtypes, whereas high educational level was only associated with developing aMCI. Having close friends and performing cognitive activities were protective factors against all MCIs. Insomnia and no fresh fish consumption were risk factors for both MCI and aMCI. Being female was a risk factor, but overweight was a protective factor for naMCI.
Table 3.
Logistic regression models for MCI, aMCI and naMCI by residential areas
| Total OR (95% CI) | Urban OR (95% CI) | Rural OR (95% CI) | |||||||
| MCI | aMCI | naMCI | MCI | aMCI | naMCI | MCI | aMCI | naMCI | |
| Sex (female) | 1.29* (1.05 to 1.60) |
1.77** (1.40 to 2.26) |
1.72** (1.21 to 2.45) |
0.61** (0.47 to 0.79) |
0.39** (0.28 to 0.54) |
0.65 (0.41 to 1.03) |
1.64* (1.15 to 2.35) |
1.24 (0.82 to 1.87) |
2.60** (1.51 to 4.48) |
| Age, years | 1.08** (1.06 to 1.10) |
1.09** (1.07 to 1.11) |
1.07** (1.04 to 1.10) |
1.08** (1.05 to 1.10) |
1.09** (1.07 to 1.12) |
1.05 (0.91 to 1.09) |
1.07** (1.04 to 1.11) |
1.07* (1.03 to 1.12) |
1.08* (1.03 to 1.14) |
| Education, years | 0.96 (0.94 to 1.18) |
0.96** (0.93 to 0.98) |
0.99 (0.95 to 1.02) |
0.98 (0.95 to 1.02) |
0.94* (0.90 to 0.98) |
1.06 (0.99 to 1.12) |
0.97 (0.92 to 1.02) |
0.93* (0.88 to 0.99) |
1.07 (0.99 to 1.15) |
| Having close friends | 0.67** (0.54 to 0.84) |
0.70** (0.55 to 0.91) |
0.68* (0.48 to 0.98) |
0.98 (0.74 to 1.31) |
1.04 (0.74 to 1.46) |
0.60* (0.38 to 0.94) |
0.60* (0.42 to 0.87) |
0.60* (0.40 to 0.91) |
0.96 (0.58 to 1.60) |
| Fish consumption | 0.24** (0.18 to 0.30) |
0.17** (0.13 to 0.22) |
0.71 (0.44 to 1.13) |
0.34** (0.23 to 0.49) |
0.27** (0.18 to 0.41) |
0.61 (0.24 to 1.57) |
0.17** (0.12 to 0.25) |
0.12** (0.08 to 0.17) |
0.55* (0.31 to 0.97) |
| Cognitive activities | 0.47** (0.38 to 0.59) |
0.51** (0.39 to 0.68) |
0.52** (0.35 to 0.77) |
0.47** (0.35 to 0.63) |
0.46* (0.32 to 0.66) |
0.66 (0.38 to 1.15) |
0.49** (0.33 to 0.71) |
0.59* (0.38 to 0.91) |
0.38* (0.20 to 0.71) |
| Insomnia | 3.05** (2.46 to 3.78) |
5.04** (3.85 to 6.59) |
1.37 (0.98 to 1.91) |
5.70** (4.27 to 7.61) |
20.39** (12.62 to 32.93) |
1.51 (0.96 to 2.37) |
0.75 (0.52 to 1.08) |
1.59** (1.06 to 2.39) |
1.23 (0.73 to 2.06) |
| Overweight† | 1.03 (0.84 to 1.27) |
0.90 (0.71 to 1.14) |
0.60** (0.40 to 0.90) |
0.74 (0.57 to 1.07) |
0.69 (0.50 to 1.14) |
0.97 (0.63 to 1.51) |
0.51** (0.34 to 0.76) |
0.61** (0.39 to 0.96) |
0.34** (0.17 to 0.66) |
*p<0.05, **p<0.001.
†The body mass index for overweight is greater than or equal to 24.0; it includes both overweight and obesity.
aMCI, amnestic mild cognitive impairment; MCI, mild cognitive impairment; naMCI, non-amnestic mild cognitive impairment.
For urban and rural areas, the risk factors were inconsistently associated with MCI, aMCI and naMCI. For example, in urban areas, being male, older age, no fresh fish consumption, cognitive inactivity and insomnia were identified as risk factors for MCI and aMCI. In rural areas, both cognitive activities and overweight were protective factors against MCI and MCI subtypes. Having close friends was a protective factor for MCI (OR=0.60, 95% CI: 0.42 to 0.87, p<0.05) and aMCI (OR=0.60, 95% CI: 0.40 to 0.91, p<0.05), as well as having fresh fish consumption (MCI: OR=0.17, 95% CI: 0.12 to 0.25, p<0.001; aMCI: OR=0.12, 95% CI: 0.08 to 0.17, p<0.001), whereas insomnia (OR=1.59, 95% CI: 1.06 to 2.39, p<0.001) only increased the risk of aMCI in rural areas. Women had a higher risk of progressing to MCI (OR=1.64, 95% CI: 1.15 to 2.35, p<0.05) and naMCI (OR=2.60, 95% CI: 1.51 to 4.48, p<0.001), but sex (OR=1.24, 95% CI: 0.82 to 1.87, p>0.05) was not associated with aMCI in rural areas. Hypertension, diabetes, drinking and smoking habits and physical activities were neither associated with MCI nor any of its subtypes. These results adhered to the comparison between urban and rural areas.
Discussion
Main findings
The current study reports the prevalences of MCI and its functional subtypes in multi-regional rural and urban communities of individuals aged ≥65 years in Hubei Province in central China; this is the first such report in the English literature to date. Our findings suggest that the prevalence of MCI among urban residents in central China was consistent with that in other metropolises in China, such as Shanghai, but the prevalence in rural areas was twice that in urban areas in our study, which resulted in an overall higher MCI prevalence (27.8%) than the Shanghai Aging Study (SAS) (20.1%)35 and other Chinese findings in 2018 (14.71%).13 Interestingly, the prevalence of aMCI in urban areas was higher than that in SAS but that of naMCI was lower. However, the prevalences of aMCI and naMCI in rural areas were much higher than that in urban areas, with OR=1.44 and OR=3.76, respectively. In our study, aMCI was the most predominant subtype of MCI in total, urban and rural populations, congruent with other studies.4 36 Considering the correlation between MCI subtypes and the progression of dementia types,2 10 11 as well as the high vulnerability of rural older adults with MCI to developing various dementia types, future dementia prevention strategies should identify naMCI and non-AD dementia in rural areas and pay attention to early-stage memory changes in older individuals.
This study found a marked difference in several factors associated with MCI and its subtypes between rural and urban areas. Being female was a risk factor for naMCI and MCI in rural areas and a protective factor against aMCI and MCI in urban areas. To explain this discrepancy, we further performed the analysis of characteristic variables stratified by sex and area (online supplemental table S1). The samples were divided into four groups: (1) urban–male, (2) urban–female, (3) rural–male and (4) rural–female. In rural areas, older women had the lowest educational level, higher prevalence rate of hypertension and obesity and lower proportion of fish consumption. On the other hand, compared with men, older urban women had a relatively high level of education (median: 9 years, IQR 9–12), healthy lifestyles (less smoking and drinking and more physical activities) and better health conditions (lower ratio of hypertension, diabetes and obesity) (online supplemental table S1). As women have a longer life expectancy than men,37–39 rural-dwelling older women should be considered a priority for the prevention of dementia and MCI.
gpsych-2021-100564supp001.pdf (73.3KB, pdf)
Having close friends mainly protected against aMCI and affected MCI in rural areas, whereas its effect was only evident against naMCI in urban areas (table 3). In China, especially in rural areas, older people are seldom re-employed after retirement but participate in community groups; however, they face obstacles in obtaining ideal care from their families, since the children increasingly move to cities for better education and work opportunities.38 39 In this study, the number of older individuals without a spouse was higher in rural than in urban areas (36.4% vs 15.3%); having contact with friends is a critical aspect of social relationships, which is accepted as a protective factor for cognition.9 Further studies should clarify the content of social relationships in this respect.
Being overweight was a protective factor against MCI and MCI subtypes in rural areas, but not in urban areas, in this study. Controversies on the associations between BMI and the risk of cognitive impairment still persist.40 Some studies found that high BMI in late life protects against dementia,41 but increases the risk of aMCI and naMCI in older adults.42 Therefore, watching nutrition and managing weight in older individuals in rural areas are necessary; however, the further studies should confirm whether appropriate overweight can help reduce the risk of progression of MCI to dementia.
Limitations
This study had several limitations. First, this was a cross-sectional rather than a longitudinal study, and some findings should be interpreted with caution. Some factors may be associated with survival rather than the development of the disease. Second, the factors of lifestyle behaviours and social relationships were self-reported, which may be affected by recall bias and deviate from the actual situation. Third, the study explored the relationship between the associated factors and the prevalence of MCI (including aMCI and naMCI) using singular focus, without considering simultaneously the combined influences of several factors that tend to co-occur (eg, increased physical activity but poor diet or the combination of physical exercise with cognitive stimulation may occur). Nonetheless, the findings of our study provide a firm basis for future studies. More longitudinal, population-based cohort studies and randomised clinical trials on the effectiveness of specific interventions addressing modifiable risk factors are needed in the future.
Implications
We found that the prevalence of MCI in rural areas is twice that in urban areas, although in urban areas the prevalence was close to those reported in a previous epidemiology study in more developed cities, such as Shanghai.35 As basic medical services are relatively less established and disease diagnosis is delayed in rural areas, our study suggests that the focus of appropriate programmes on dementia prevention and treatment should be shifted to rural populations. In addition, Hubei is an economically underdeveloped area in central China, and our samples had relatively lower educational levels. Almost a third of recruited participants in this study had no access to secondary education. Therefore, special attention should be paid to memory changes among low educational level population.
Biography
Dr Dan Liu obtained a bachelor's degree in Clinical Medicine from Tongji Medical University, China, in 1999, a master's degree in Clinical Medicine from Huazhong University of Science and Technology in 2004, and a PhD degree in Clinical Medicine from Huazhong University of Science and Technology, China, in 2012. She has been working in Wuhan University of Science and Technology (WUST) since 1999 and is working as a resident doctor with expertise in neurologic disorders and cognitive and emotional health at the Brain Science and Advanced Technology Institute in WUST. Her main research interest includes longitudinal study on cognitively impaired/demented old adults.

Footnotes
Contributors: DL and LL contributed to the conception of the study. QW, RW, LL, LA, CC, MZ, BZ and XG contributed to data collection and collation. GC and YO helped to explain the principles of informed consent. LX and DL contributed to the management and development of the project. LL performed the data analyses and wrote the manuscript. YZ helped perform the analysis with constructive discussions and modified the manuscript.
Funding: The study was funded by the National Natural Science Foundation of China (71774127, 81771488, 81870901).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the ethics committee of our university (No. 201845).
References
- 1.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 2005;62:1160–3. discussion 1167. 10.1001/archneur.62.7.1160 [DOI] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 3.Alex W, Shannon, L. M, et al. Defining mild cognitive impairment: disparity of incidence and prevalence estimates with variable operationalized definitions. Alzheimers Dement 2009. 10.1016/j.jalz.2009.04.392 [DOI] [Google Scholar]
- 4.Li X, Ma C, Zhang J, et al. Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc 2013;61:2111-2119. 10.1111/jgs.12552 [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Lu FC, Department of Disease Control Ministry of Health, PR China . The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci 2004;17 Suppl:1–36. [PubMed] [Google Scholar]
- 6.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet 2006;367:1262–70. 10.1016/S0140-6736(06)68542-5 [DOI] [PubMed] [Google Scholar]
- 7.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 2002;288:2271–81. 10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters R, Booth A, Rockwood K, et al. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open 2019;9:e022846. 10.1136/bmjopen-2018-022846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–46. 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol 2009;66:1447–55. 10.1001/archneurol.2009.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehrner J, Gufler R, Guttmann G, et al. Annual conversion to Alzheimer disease among patients with memory complaints attending an outpatient memory clinic: the influence of amnestic mild cognitive impairment and the predictive value of neuropsychological testing. Wien Klin Wochenschr 2005;117:629–35. 10.1007/s00508-005-0428-6 [DOI] [PubMed] [Google Scholar]
- 12.China Health Statistics Yearbook Committee . 2019 statistical bulletin on national economic and social development, 2019.
- 13.Xue J, Li J, Liang J, et al. The prevalence of mild cognitive impairment in China: a systematic review. Aging Dis 2018;9:706–15. 10.14336/AD.2017.0928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Zhang M, Yang L, et al. Prevalence and patterns of tobacco smoking among Chinese adult men and women: findings of the 2010 national smoking survey. J Epidemiol Community Health 2017;71:154–61. 10.1136/jech-2016-207805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G, Luo C, Cui Y, et al. Clustering of multiple health risk behaviors and its association with diabetes in a Southern Chinese adult population: a cross-sectional study. PeerJ 2020;8:e9025. 10.7717/peerj.9025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eating fish cuts risk of dementia. BMJ 2002;325. [Google Scholar]
- 17.Bureau for Disease Control and Prevention MoH, People’s Republic of China . Physical activity guideline for Chinese adults. Beijing: People’s Medical Publishing House, 2011. [Google Scholar]
- 18.Li X, Ma C, Zhang J, et al. Prevalence of and potential risk factors for mild cognitive impairment in community-dwelling residents of Beijing. J Am Geriatr Soc 2013;61:2111–9. 10.1111/jgs.12552 [DOI] [PubMed] [Google Scholar]
- 19.Gureje O, Oladeji BD, Abiona T, et al. The natural history of insomnia in the Ibadan study of ageing. Sleep 2011;34:965–73. 10.5665/SLEEP.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji CY, Chen TJ, Working Group on Obesity in China (WGOC) . Empirical changes in the prevalence of overweight and obesity among Chinese students from 1985 to 2010 and corresponding preventive strategies. Biomed Environ Sci 2013;26:1–12. 10.3967/0895-3988.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 21.Liu LS. Chinese guidelines for the management of hypertension. Chinese Journal of Hypertension 2010;2011:701–8. in Chinese. [PubMed] [Google Scholar]
- 22.American Diabetes Association . Standards of medical care in diabetes--2014. Diabetes Care 2014;37 Suppl 1:S14–80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 23.Guo QH, Sun YM, PM Y, et al. Norm of auditory verbal learning test in the normal aged in China community. Chinese Journal of Clinical Psychology 2007:132–4. in Chinese. [Google Scholar]
- 24.Wechsler D. Wechsler memory Scale—Revised 1987.
- 25.Zhao Q, Guo Q, Hong Z. Clustering and switching during a semantic verbal fluency test contribute to differential diagnosis of cognitive impairment. Neurosci Bull 2013;29:75–82. 10.1007/s12264-013-1301-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reitan RM. Validity of the TRAIL making test as an indicator of organic brain damage. Percept Mot Skills 1958;8:271–6. 10.2466/pms.1958.8.3.271 [DOI] [Google Scholar]
- 27.Wechsler DA. WAIS-R manual—Wechsler adult intelligence Scale-revised 1981.
- 28.Wolf-Klein GP, Silverstone FA, Levy AP, et al. Screening for Alzheimer's disease by clock drawing. J Am Geriatr Soc 1989;37:730–4. 10.1111/j.1532-5415.1989.tb02234.x [DOI] [PubMed] [Google Scholar]
- 29.Chen K-L, Xu Y, Chu A-Q, et al. Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J Am Geriatr Soc 2016;64:e285–90. 10.1111/jgs.14530 [DOI] [PubMed] [Google Scholar]
- 30.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. 10.1093/geront/9.3_Part_1.179 [DOI] [PubMed] [Google Scholar]
- 31.Chang Y-L, Bondi MW, McEvoy LK, et al. Global clinical dementia rating of 0.5 in MCI masks variability related to level of function. Neurology 2011;76:652–9. 10.1212/WNL.0b013e31820ce6a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu HF, Lee HC, Wing YK, et al. Reliability, validity and structure of the Chinese geriatric depression scale in a Hong Kong context: a preliminary report. Singapore Med J 1994;35:477. [PubMed] [Google Scholar]
- 33.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–8. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- 34.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–94. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 35.Xie LQ, Yang BX, Liao YH. Sleep disturbance in older adults with or without mild cognitive impairment and its associated factors residing in rural area, China. J Geriatr Psychiatry Neurol 2020;24. 10.1177/0891988720964249 [DOI] [PubMed] [Google Scholar]
- 36.Ding D, Zhao Q, Guo Q, et al. Prevalence of mild cognitive impairment in an urban community in China: a cross-sectional analysis of the Shanghai aging study. Alzheimers Dement 2015;11:300–9. 10.1016/j.jalz.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Xiao LD, Wang K, et al. Cognitive impairment and associated factors in rural elderly in North China. J Alzheimers Dis 2020;77:1241–53. 10.3233/JAD-200404 [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Zeng Y, Wang L, et al. Urban–Rural differences in long-term care service status and needs among home-based elderly people in China. Int J Environ Res Public Health 2020;17:1701. 10.3390/ijerph17051701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Guan Y, Shi Z, et al. Sex differences in the prevalence of and risk factors for cognitive impairment no dementia among the elderly in a rural area of northern China: a population-based cross-sectional study. Neuroepidemiology 2019;52:25–31. 10.1159/000493141 [DOI] [PubMed] [Google Scholar]
- 40.Qu Y, Hu H-Y, Ou Y-N, et al. Association of body mass index with risk of cognitive impairment and dementia: a systematic review and meta-analysis of prospective studies. Neurosci Biobehav Rev 2020;115:189–98. 10.1016/j.neubiorev.2020.05.012 [DOI] [PubMed] [Google Scholar]
- 41.Li J-Q, Tan L, Wang H-F, et al. Risk factors for predicting progression from mild cognitive impairment to Alzheimer's disease: a systematic review and meta-analysis of cohort studies. J Neurol Neurosurg Psychiatry 2016;87:476–84. 10.1136/jnnp-2014-310095 [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Zhao M, Han Z, et al. Association of body mass index with amnestic and non-amnestic mild cognitive impairment risk in elderly. BMC Psychiatry 2017;17:334. 10.1186/s12888-017-1493-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gpsych-2021-100564supp001.pdf (73.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.