Abstract
Worldwide, thousands of cases of multisystem inflammatory syndrome in children (MIS-C) have already been reported in children. Evidence regarding neonatal MIS-C is limited. We present the first case report of a neonate presenting within 48 hours of life with predominant abdominal signs mimicking surgical abdomen. Clinical picture comprised fever, multiorgan dysfunction (gastrointestinal, cardiorespiratory, hepatic and dermatological), positive inflammatory markers, high ferritin and high D-dimer levels. Cardiac enzyme N-terminal-pro-B-type natriuretic peptide as well as D-dimer levels were elevated. Blood, urine, stool and cerebrospinal fluid cultures were sterile. Positive anti-SARS-CoV-2 IgG in both the mother and the infant, along with an epidemiological evidence of maternal contact with COVID-19, clinched the diagnosis of MIS-C. Immunomodulatory drugs (intravenous immunoglobulin and systemic steroids) were administered and showed good clinical response. A high index of suspicion of MIS-C in critically ill neonates can improve outcomes.
Keywords: COVID-19, dermatology, infectious diseases, neonatal and paediatric intensive care
Background
Since December 2019, the spread of SARS-CoV-2 has triggered a major health crisis worldwide. Evidence suggests that children generally have mild symptoms.1 Few months after the onset of this pandemic, several publications described a hyperinflammatory process in children associated with COVID-19, with features similar to Kawasaki’s disease. Children presented with fever, multiorgan dysfunction and positive inflammatory markers, and the process was labelled as multisystem inflammatory syndrome in children (MIS-C).2–6 As per definition proposed by the WHO, the diagnosis of MIS-C should be considered among children aged from 0 year to 19 years, with characteristics of typical or atypical Kawasaki disease or shock.7
Worldwide, thousands of cases of MIS-C have already been reported in children. The exact mechanism is not known but is assumed to be a postinfectious immune dysregulation seen 4–6 weeks after exposure to COVID-19. Similar manifestations have also been described in neonates. Unlike older children, the mechanism is unique in neonates as COVID-19 infection and the subsequent inflammatory reaction leading to MIS-C occur in two different individuals. Maternal infection may trigger a hyperinflammatory syndrome in neonates secondary to transplacental transfer of antibodies. To date, only four case reports involving five neonates have been described with presentations of MIS-C in the neonatal period.8–11 Recently, Pawar et al described a case series of 20 neonates with similar features.12
The majority of the neonates described earlier have presented with predominantly myocardial or respiratory involvement. We present the first case report of a neonate presenting within 48 hours of life with predominant abdominal signs mimicking surgical abdomen and having multisystem involvement. Informed consent was obtained from the parents.
Case presentation
A singleton male infant 39 weeks of gestational age with a birth weight of 3300 g was delivered by elective caesarean section to a 34-year-old female primigravida. The mother did not have any comorbidity, had good antenatal care, negative TORCH (Toxoplasmosis, Other agents, Rubella, Cytomegalovirus, Herpes) serologies, normal antenatal ultrasonography and no risk factor for sepsis. The infant did not require resuscitation, and Apgar scores were 8 and 10 at 1 and 5 min, respectively. Delayed cord clamping was carried out, along with immediate skin-to-skin care, in the delivery room. Breast feeding was initiated and he was roomed in with the mother. At 44 hours of life, he had one episode of fever of 38.3°C (101°F), along with one episode of non-bilious vomiting and abdominal distension, necessitating shifting the infant to nursery for observation. Suspecting early-onset sepsis, blood culture was drawn and intravenous antibiotics (ampicillin and gentamicin) were initiated. In view of persistent fever, vomiting and progressive abdominal distension, he was referred to our neonatal intensive care unit.
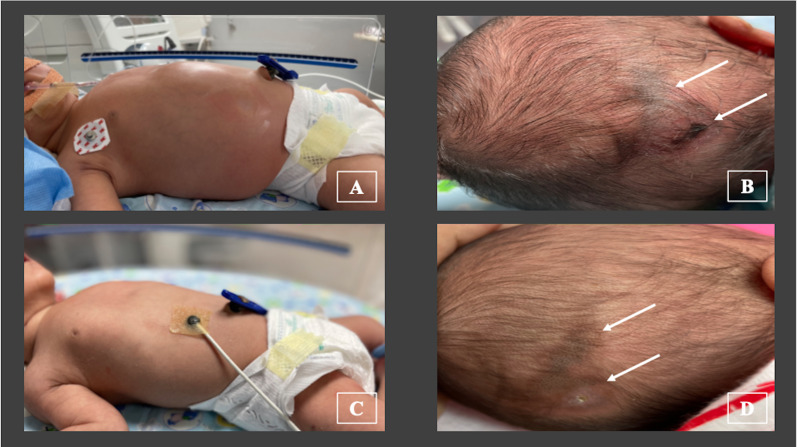
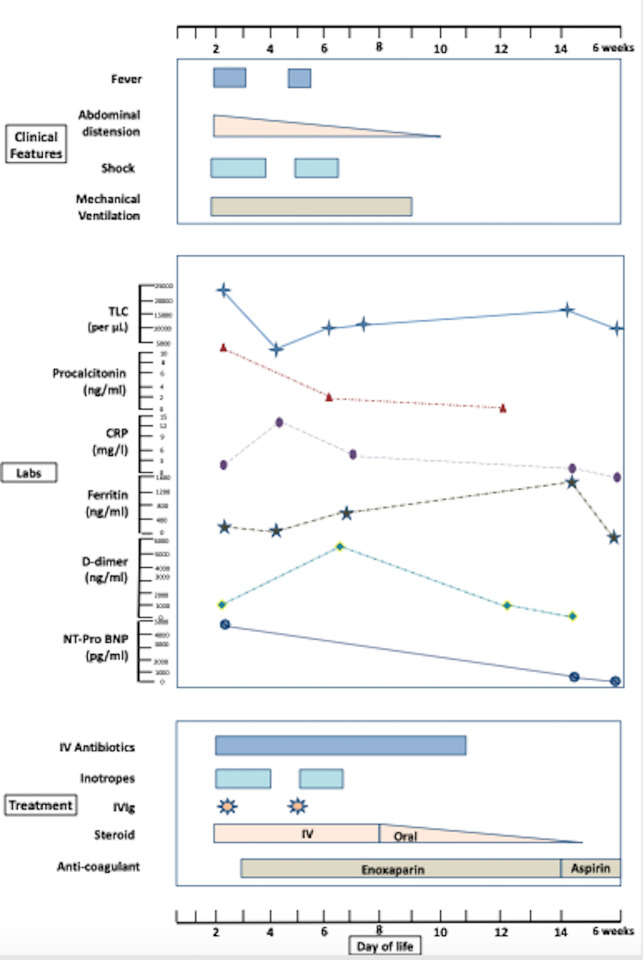
At admission, the infant was lethargic and had fever of 38.0°C (100.4°F), heart rate of 176 beats/min, respiratory rate of 68 breaths/min, grunting, cold peripheries, prolonged capillary refill time of >3 s, mean blood pressure of 36 mm Hg and preductal oxygen saturation of 97%. The abdomen was markedly distended and shiny (figure 1A), along with visible bowel loops and absent bowel sounds. The abdomen was tender, but there was no abdominal discolouration. He had passed stool once within 24 hours. There was no skin rash, conjunctival redness or other mucosal involvement. There was no oedema, lymphadenopathy or dysmorphism. There was no history of loose motions or seizures. The clinical course, along with appropriate investigations and management, is depicted in figure 2. The infant was electively intubated in view of respiratory distress and abdominal distension and was continued on mechanical ventilation (patient-triggered ventilation mode, volume guarantee of 4 mL/kg). Intravenous paracetamol (15 mg/kg) was administered, following which fever subsided. Normal saline bolus (10 mL/kg over 30 min) was administered and then inotropic support (injection dopamine at 10 mcg/kg/min) was initiated, following which blood pressures were within the expected range. In view of multiorgan involvement and shock, antibiotics were upgraded to piperacillin–tazobactum and netilmicin. Causes considered were early-onset sepsis, intestinal atresia, necrotising enterocolitis (NEC) and Hirschsprung disease (HD). In view of maternal contact with COVID-19 4 weeks before delivery, MIS-C was also suspected.
Figure 1.
(A) Marked abdominal distension (on day 2 of life), with visible bowel loops and shiny abdominal wall. (B) Firm, indurated ulcer with erythema noted on the occiput (on day 5 of life) (marked by bold arrows). (C) Abdomen on day 13 of life: no distension, no visible bowel loops, normal coloured wall. (D) Healed ulcer on the occiput at 6 weeks of life: soft, no induration, no erythema (marked by bold arrows).
Figure 2.
Clinical course of the neonate (as per day of life). Upper box depicts clinical features; middle box depicts laboratory investigations; lower box depicts treatment provided. CRP, C reactive protein; IVIg, intravenous immunoglobulin; Labs, laboratory investigations; NT-Pro BNP, N-terminal-pro-B-type natriuretic peptide; TLC, total leucocyte count.
Investigations
Laboratory investigations (online supplemental table 1) showed white blood cell count was 23 940 cells/µL with neutrophil:lymphocyte count ratio being 3.2, with normal platelet count (339 000/µL). Arterial blood gas showed pH 7.18, partial pressure of carbon dioxide of 47 mm Hg, partial pressure of oxygen of 42 mm Hg, bicarbonate 15.5 mmol/L, base excess of −10.9 and lactate 3.6 mmol/L. Inflammatory markers were raised (procalcitonin 10.76 ng/mL (normal range: <0.5 ng/mL) and ferritin 156.4 ng/mL (normal range: 10–200 ng/mL)). C reactive protein was within normal limits (2.1 mg/L) initially, which rose to 13.7 mg/L within 48 hours. Liver enzymes were mildly deranged (alanine aminotransferase 42 units/L (normal range: 0–40 units/L), aspartate aminotransferase 78 units/L (normal range: 0–40 units/L)); coagulation parameters were abnormal (prothrombin time 17 s, activated partial thromboplastin time (aPTT) 80.8 s); D-dimer was high (1331 ng/mL, normal range: 0–500 ng/mL). Renal function, electrolytes and thyroid function tests were within normal limits. Ultrasonography of the abdomen showed mild ascites. Cranium ultrasonography was not suggestive of intracranial bleed or parenchymal lesion. Echocardiography showed normal cardiac functions and normal coronary arteries without any evidence of structural malformations. Cardiac enzyme N-terminal-pro-B-type natriuretic peptide was elevated (4297 pg/mL, normal range: <125 pg/mL), so was lactate dehydrogenase (LDH) (764 units/L, normal range: 10–25 units/L). Electrocardiography showed normal sinus rhythm. Cerebrospinal fluid (CSF) analysis was not suggestive of meningitis. Blood, urine, stool and CSF cultures were sterile. Chest X-ray showed normal inflation with minimal pulmonary infiltrates bilaterally. Abdominal X-ray showed dilated small and large intestines with absence of rectal gas with no radiological evidence of intestinal atresia or NEC.
bcr-2021-246579supp001.pdf (42.6KB, pdf)
Suspecting maternal antibody induced MIS-C, work-up was carried out. Both mother and infant’s nasopharyngeal reverse transcriptase PCR (RT-PCR) tests for COVID-19 were negative. Infant’s rectal swab for PCR (RT-PCR) test for COVID-19 was also negative. A rapid serological test (Abbot Architect, positive >1.4 S/C) of peripheral blood of the mother as well as the infant was performed, and results were non-reactive for anti SARS-CoV-2 IgM and reactive for IgG. The mother’s antibody titres were 35.7 units, whereas antibody titres in the infant were 30.3 units. The mother was not vaccinated against COVID-19. There was a history of contact of mother with COVID-19 4 weeks before delivery, but she was asymptomatic. In view of outborn delivery and no risk factor for COVID-19 during delivery, placental examination could not be carried out.
Further treatment, outcome and follow-up
Clinical picture comprising fever, multiorgan dysfunction, positive inflammatory markers, high ferritin and D-dimer levels, along with epidemiological evidence of maternal contact with COVID-19 and positive serologies in both mother and infant fitted into a hyperinflammatory process probably MIS-C as per Centers for Disease Control and Prevention (CDC) and WHO criteria.2 7
Intravenous immunoglobulin (IVIg) was administered (2 g/kg over 12 hours) on day 2 (figure 2). Along with IVIg, intravenous methylprednisolone (1 mg/kg/dose 12 per hour) was initiated. Fever subsided within 24 hours and inotropes were tapered and stopped over the next 48 hours. He was kept nil per os. TPN (Total parenteral nutrition) was initiated. Considering surgical causes, a paediatric surgeon’s opinion was sought. In view of hypotension and abnormal coagulation, contrast study was withheld and conservative management was continued. He had a recurrence of fever and shock on day 5. New-onset skin lesions were noticed on the occiput (erythematous ulcer with induration, figure 1B). There were no other skin lesions or other evidence of bed sores. Blood gas showed metabolic acidosis. Following the aforementioned clinical worsening, a repeat dose of IVIg (2 g/kg) was administered. There was good clinical as well as biochemical response. There was no recurrence of fever or shock during the hospital stay. He was extubated successfully to room air on day 9 of life. Intravenous steroids were continued for 7 days and then changed to oral form (prednisolone). Steroids were administered for a total duration of 3 weeks (1 week intravenous+2 weeks oral). In view of high D-dimer levels with prolonged aPTT, injectable enoxaparin was started (1 mg/kg two times per day, subcutaneously) and continued for 2 weeks. Aspirin (3 mg/kg once a day, orally) was started after enoxaparin and continued for 4 weeks. There was no clinical bleeding during the course of the aforementioned medications. Gradually, abdominal distension subsided (figure 1C). Minimal feeds were started on day 10, which he tolerated well. Enteral feeds were progressively increased and he was on full enteral feeds on day 14 of life. There was no recurrence of feed intolerance. Skin lesions were managed conservatively with topical antibiotic (mupirocin), along with dry care and posture changes. Intravenous antibiotics were administered for 10 days.
He was discharged on day 16 of life on oral steroids (tapering course), injectable enoxaparin, oral ranitidine, oral vitamin D and on exclusive breast feeding. Repeat laboratory parameters showed an improving trend with declining procalcitonin, ferritin, LDH and D-dimer levels. Repeat echocardiography after 2 and 4 weeks were normal without any dilatation of the coronaries. There was no abdominal distension or feed intolerance; hence, further work-up for HD was withheld. On follow-up at 45 days, the infant was on exclusive breast feeds; weight was 4.4 kg; and steroid as well as aspirin were stopped. D-dimer, ferritin and coagulation parameters were almost normalised. The skin lesion on the occiput had healed (figure 1D).
Discussion
MIS-C is an evolving entity with varied presentations in the paediatric age group.3–6 Literature data regarding neonatal MIS-C is quite limited.8–12 This entity is unique in neonates. Because infection and subsequent hyperinflammatory process could have occurred in two different individuals (infection in mother and MIS-C in neonate) or else transplacental inflammation that could have triggered this hyperinflammatory process.8 13–16 The exact etiopathogenesis is still debatable. Unlike previous case reports, we report a case of MIS-C in a neonate with predominant abdominal signs mimicking surgical abdomen. Although the mother was asymptomatic and RT-PCR for SARS-CoV-2 was negative, epidemiological contact with a patient with COVID-19 and positive antibodies (anti SARS-CoV-2 IgG) in the mother and the baby clinched the diagnosis. As per the current WHO and CDC criteria, our index case met all the essential criteria to be labelled as MIS-C.2 7
Orlanski-Meyer et al have described an infant with predominant gastrointestinal (GI) signs which presented at 8 weeks of life.17 Unlike our case, this infant presented beyond the neonatal period and had different presenting features such as diarrhoea, vomiting and bloody stools. GI symptoms are commonly seen in older infants and children with MIS-C.3 None of the neonatal case reports published had predominant GI manifestations. The case series described by Pawar et al had six neonates (30%) with GI features.12
Typical skin manifestation in the form of an indurated ulcer seen in our case has been described in literature. This could be due to coagulation dysfunction and might have occurred due to ischaemic changes. Similar skin lesions have been described by Kappanayil et al.10 The case series by Pawar et al also reported one neonate with peeling of skin on palms and lips.12 Similar to previous reports, skin lesion in our index case healed with conservative measures. Presence of such skin lesions in a symptomatic neonate can point towards MIS-C.
In view of MIS-C and multiorgan dysfunction (GI, cardiac, respiratory, haematological, hepatic and dermatological), we administered IVIg two times, along with systemic steroids. There is a lack of substantial evidence regarding use of these medications in this age group. Early outcomes of large observational studies regarding the efficacy of these drugs in children have conflicting results.18 19 Most of the case reports and case series describing MIS-C in neonates have used the aforementioned drugs and have shown good clinical response. However, further studies are needed in this age group to prove their efficacy.
So, this case report should increase awareness among paediatricians, neonatologists as well as obstetricians regarding such atypical presentations and suspect MIS-C in critically ill neonates with maternal history of COVID-19 infection or epidemiological contact. A high index of suspicion can lead to early diagnosis and good outcomes.
Learning points.
Multisystem inflammatory syndrome should be considered as a differential diagnosis in all critically ill neonates, particularly with maternal history of COVID-19 infection or epidemiological contact.
Typical skin lesions can help in diagnosing multisystem inflammatory syndrome.
Immunomodulatory drugs (intravenous immunoglobulin and systemic steroids) show good clinical response in such cases.
Footnotes
Contributors: GA and SW: involved in clinical care of the patient, supervised data collection, conceived and wrote the initial draft, and reviewed and finalised the final manuscript. AA and SKS: involved in clinical care of the patient, co-ordinated the data collection and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from parent(s)/guardian(s).
References
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088–95. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C). Available: https://www.cdc.gov/mis-c/hcp/ [Accessed 25 Jun 2021].
- 3.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334–46. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med 2020;383:347–58. 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771–8. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074–87. 10.1001/jama.2021.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. Geneva: WHO, 2021. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [Google Scholar]
- 8.Khaund Borkotoky R, Banerjee Barua P, Paul SP, et al. COVID-19-Related potential multisystem inflammatory syndrome in childhood in a neonate presenting as persistent pulmonary hypertension of the newborn. Pediatr Infect Dis J 2021;40:e162–4. 10.1097/INF.0000000000003054 [DOI] [PubMed] [Google Scholar]
- 9.Divekar AA, Patamasucon P, Benjamin JS. Presumptive neonatal multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Am J Perinatol 2021;38:632–6. 10.1055/s-0041-1726318 [DOI] [PubMed] [Google Scholar]
- 10.Kappanayil M, Balan S, Alawani S, et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: a case report. Lancet Child Adolesc Health 2021;5:304–8. 10.1016/S2352-4642(21)00055-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaiba LA, Hadid A, Altirkawi KA, et al. Case report: neonatal multi-system inflammatory syndrome associated with SARS-CoV-2 exposure in two cases from Saudi Arabia. Front Pediatr 2021;9:652857. 10.3389/fped.2021.652857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawar R, Gavade V, Patil N, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children 2021;8:572. 10.3390/children8070572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diriba K, Awulachew E, Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res 2020;25:39. 10.1186/s40001-020-00439-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blumberg DA, Underwood MA, Hedriana HL, et al. Vertical transmission of SARS-CoV-2: what is the optimal definition? Am J Perinatol 2020;37:769–72. 10.1055/s-0040-1712457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020;11:3572. 10.1038/s41467-020-17436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarty KL, Tucker M, Lee G, et al. Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection. Pediatrics 2021;147:e2020010132. 10.1542/peds.2020-010132 [DOI] [PubMed] [Google Scholar]
- 17.Orlanski-Meyer E, Yogev D, Auerbach A, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome Coronavirus-2 in an 8-week-old infant. J Pediatric Infect Dis Soc 2020;9:781–4. 10.1093/jpids/piaa137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son MBF, Murray N, Friedman K, et al. Multisystem Inflammatory Syndrome in Children - Initial Therapy and Outcomes. N Engl J Med 2021;385:23–34. 10.1056/NEJMoa2102605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021;385:11–22. 10.1056/NEJMoa2102968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bcr-2021-246579supp001.pdf (42.6KB, pdf)